Environmental Conditions Affect Striped Red Mullet (Mullus surmuletus) Artisanal Fisheries
Abstract
1. Introduction
2. Material and Methods
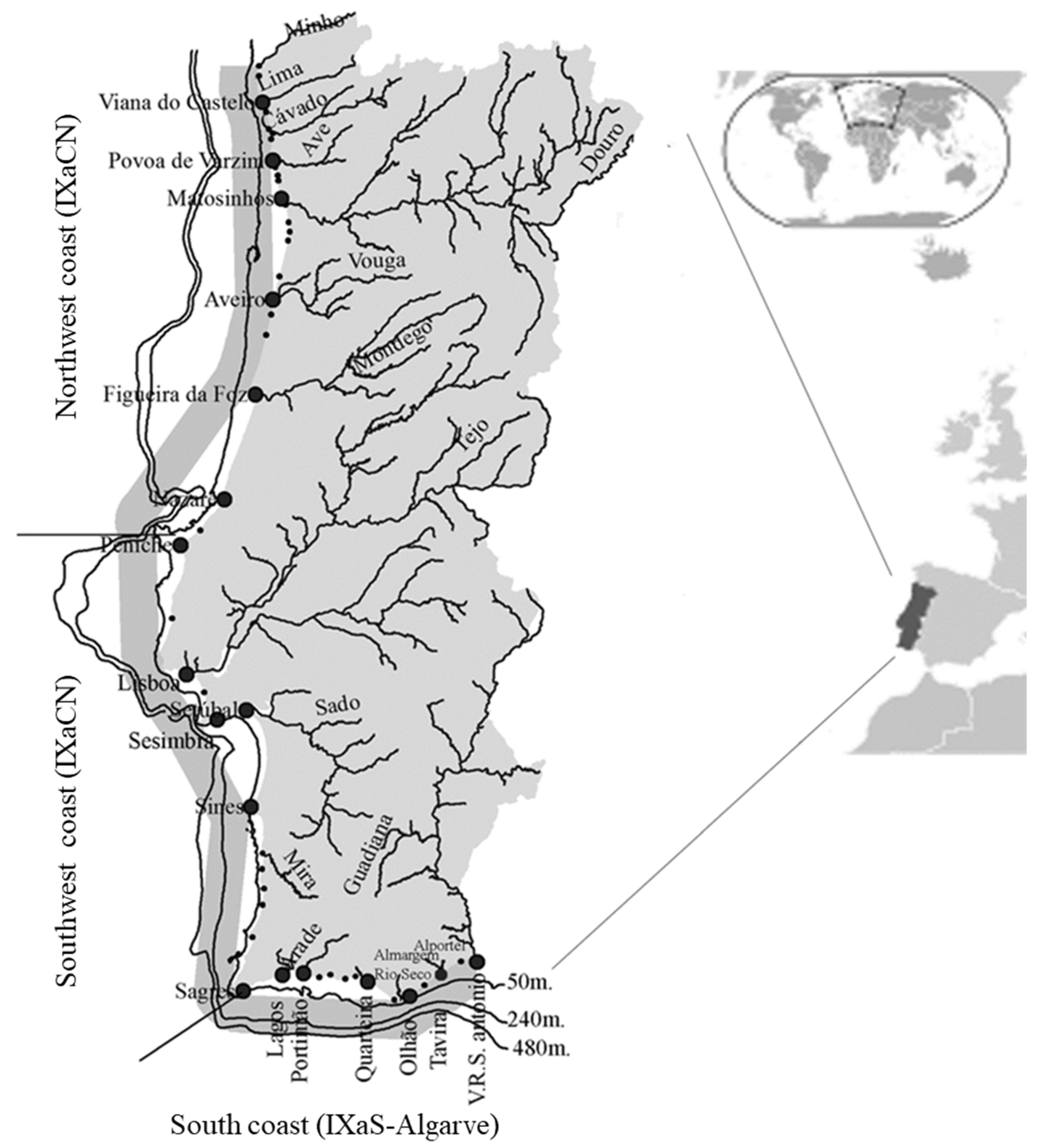
2.1. Study Sites
2.2. Data Acquisition
- (1)
- due to the lack of satellite data near the coast, the ports/harbors coordinates were moved 6 nautical miles into the territorial sea where most mullet artisanal fishery occur (Gaspar and Pereira 2014 [17]);
- (2)
- a buffer with a radius of 36 km was created around each port (polygon shapefile);
- (3)
- the raster values contained within each polygon for a given period of time and port were averaged using the “isectpolyrst” tool of Geospatial Modeling Environment (http://www.spatialecology.com/gme/isectpolyrst.htm (accessed on 10 October 2010));
- (4)
- the data was averaged by fishing area (IXaCN, IXaCS, IXaS-Algarve) using port data (Figure 1).
2.3. Time-Series Analyses (Statistical Models and Assumptions)
3. Results
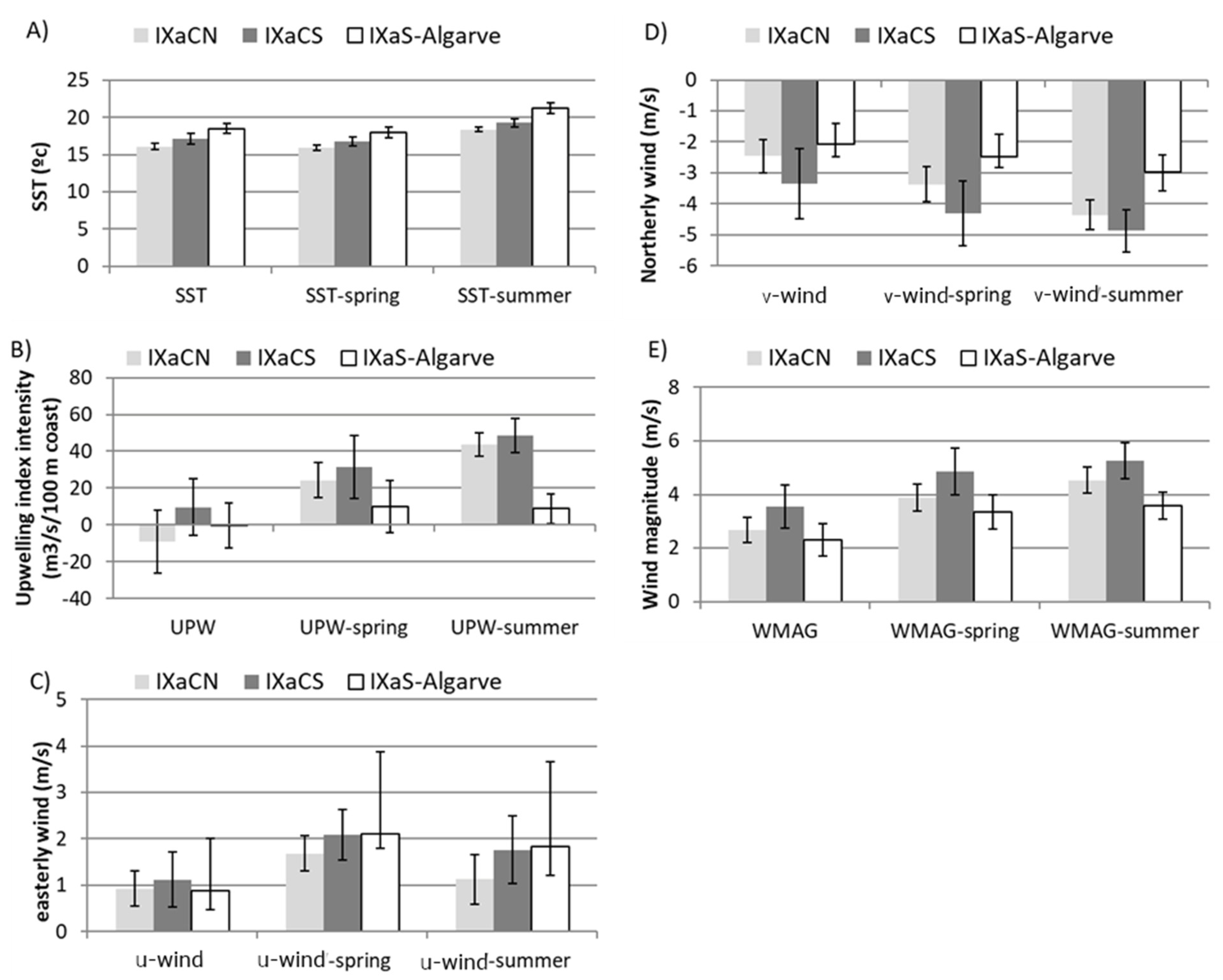
3.1. Ancillary Data
3.2. Brief Description of the Oceanographic Features in the ICES IXa Subdivisions
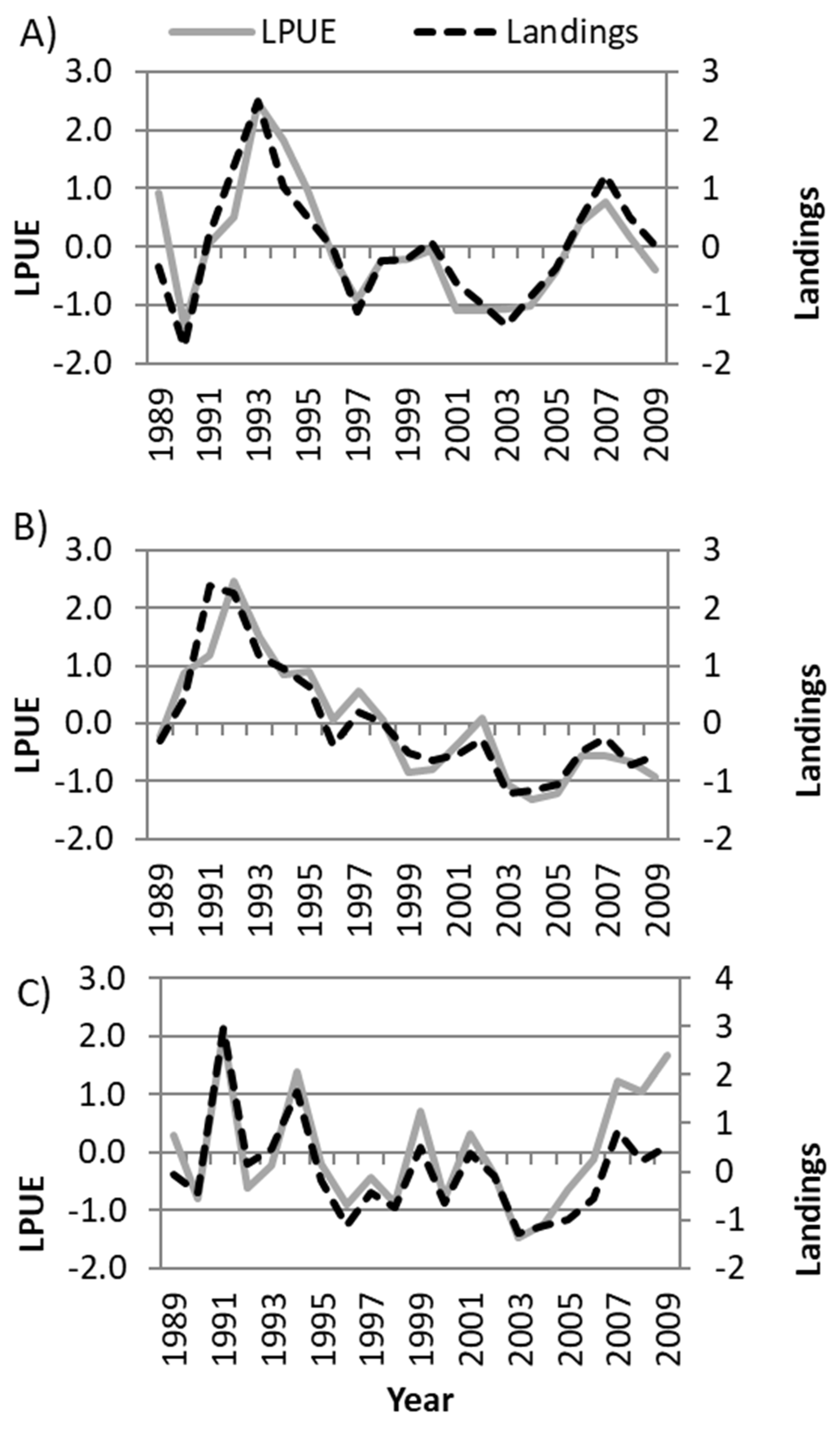
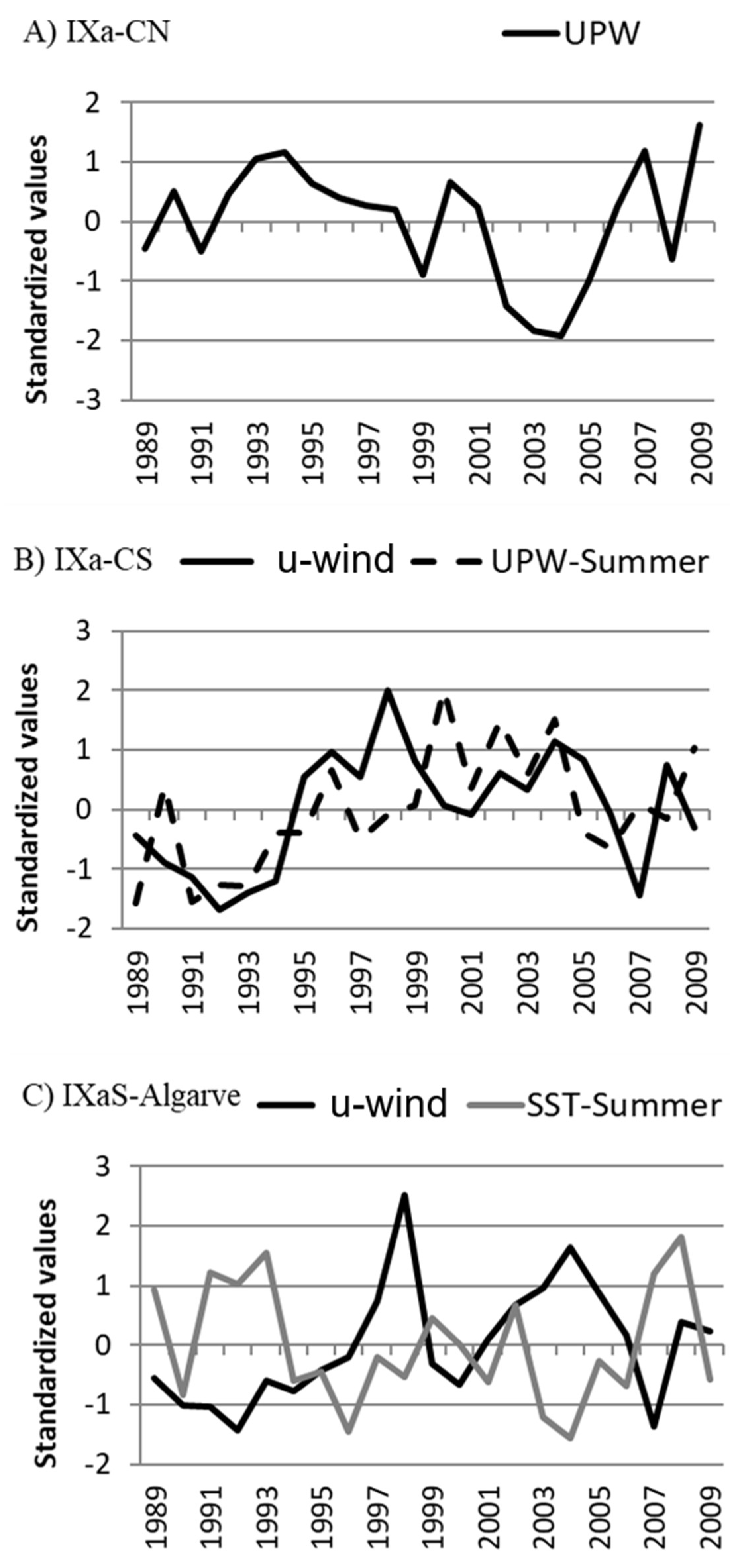
3.3. Environmental–Fisheries Relationship
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durand, M.H.; Mendelssohn, R. How to detect a change both on global and local scale in oceanographic time series. In Local vs. Global Changes in Upwelling Systems; Durand, M.H., Cury, P., Mendelssohn, R., Roy, C., Bakun, A., Pauly, D., Eds.; Office de la Recherche Scientifique et Technique Outre-Mer (ORSTOM): Paris, France, 1998; pp. 45–78. [Google Scholar]
- Santos, A.M.P.; Borges, M.D.F.; Groom, S. Sardine and horse mackerel recruitment and upwelling off Portugal. ICES J. Mar. Sci. 2001, 58, 589–596. [Google Scholar] [CrossRef]
- Lehodey, P.; Alheit, J.; Barange, M.; Baumgartner, T.; Beaugrand, G.; Drinkwater, K.; Frontentin, J.-M.; Hare, S.R.; Ottersen, G.; Roy, R.I.; et al. Climate variability, fish and fisheries. J. Climate. 2006, 19, 5009–5030. [Google Scholar] [CrossRef]
- Levi, D.; Andreoli, M.G.; Bonanno, A.; Fiorentino, F.; Garofalo, G.; Mazzola, S.; Norrito, G.; Patti, B.; Pernice, G.; Ragonese, S.; et al. Embedding sea surface temperature anomalies into the stock recruitment relationship of red mullet (Mullus barbatus L. 1758) in the Strait of Sicily. Sci. Mar. 2003, 67, 259–268. [Google Scholar] [CrossRef]
- Pinnegar, J.K.; Cheung, W.W.L.; Jones, M.; Merino, G.; Turrell, B.; Reid, D. Impacts of climate change on fisheries. MCCIP Sci. Rev. 2013, 302–317. [Google Scholar] [CrossRef]
- Lasker, R. Field criteria for survival of anchovy larvae: The relation between inshore chlorophyll maximum layers and successful first feeding. Fish. Bull. 1975, 73, 453–678. [Google Scholar]
- Cury, P.; Roy, C. Optimal Environmental Window and Pelagic Fish Recruitment Success in Upwelling Areas. Can. J. Fish. Aquat. Sci. 1989, 46, 670–680. [Google Scholar] [CrossRef]
- Cushing, D.H. Towards a Science of Recruitment in Fish Populations; Ecology Institute: Oldendorf/Luhe, Germany, 1996. [Google Scholar]
- Ottersen, G.; Planque, B.; Belgrano, A.; Post, E.; Reid, P.C.; Stenseth, N.C. Ecological effects of the North Atlantic Oscillation. Oecologia 2001, 128, 1–14. [Google Scholar] [CrossRef]
- Myers, R.A. When do environment-recruitment correlations work? Rev. Fish Biol. Fish. 1998, 82, 85–305. [Google Scholar]
- ICES. Report of the Working Group on Assessment of New MoU Species (WGNEW), 11–15 October 2010; ICES CM 2010/ACOM: 21; ICES HQ: Copenhagen, Denmark, 2010; 185p. [Google Scholar]
- Bentes, L.M.C.F. Crescimento, Reprodução e Ecologia Alimentar de Mullus surmuletus L. 1758, Salmonete, na costa Sudoeste de Portugal. Relatório de Estágio do curso de Licenciatura em Biologia Marinha e Pescas; Universidade do Algarve: Faro, Portugal, 1996; 65p. [Google Scholar]
- Arslan, M.; İşmen, A. Age, growth and reproduction of Mullus surmuletus (Linnaeus, 1758) in Saros Bay (Northern Aegean Sea). Medit. Env. 2013, 19, 217–233. [Google Scholar]
- Mahé, K.; Coppin, F.; Vaz, S.; Carpentier, A. Striped red mullet (Mullus surmuletus, Linnaeus, 1758) in the eastern English Channel and southern North Sea: Growth and reproductive biology. J. Appl. Ichthyol. 2013, 29, 1067–1072. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Pinnegar, J.; Merino, G.; Jones, M.C.; Barange, M. Review of climate change impacts on marine fisheries in the UK and Ireland. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 368–388. [Google Scholar] [CrossRef]
- García–Rodríguez, M.; de Oceanografía, M.I.E.; Fernández, A.; Esteban, A. Biomass response to environmental factors in two congeneric species of Mullus, M. barbatus and M. surmuletus, off Catalano–Levantine Mediterranean coast of Spain: A preliminary approach. Anim. Biodivers. Conserv. 2011, 34, 113–122. [Google Scholar] [CrossRef]
- Gaspar, M.B.; Pereira, F. Pequena Pesca na Costa Continental Portuguesa: Caracterização Sócio-Económica, Descrição da Actividade e Identificação de Problemas; Projecto União Europeia, FEDER: Mestreech, The Netherlands, 2014; 265p. [Google Scholar]
- Leitão, F.; Baptista, V.; Erzini, K.; Zeller, D. Reconstructed catches and trends for mainland Portugal fisheries between 1938 and 2009: Implications for sustainability, domestic fish supply and imports. Fish. Res. 2014, 155, 35–50. [Google Scholar] [CrossRef]
- Leitão, F.; Alms, V.; Erzini, K. A multi-model approach to evaluate the role of environmental variability and fishing pressure in sardine fisheries. J. Mar. Syst. 2014, 139, 128–138. [Google Scholar] [CrossRef]
- Leitão, F.; Baptista, V. The discard ban policy, economic trends and opportunities for the Portuguese fisheries sector. Mar. Policy 2016, 75, 75–83. [Google Scholar] [CrossRef]
- Leitão, F.; Roa-Ureta, R.H.; Cánovas, F. Vulnerability of Fisheries to Climate Change. Front. Mar. Sci. 2020, 7, 613793. [Google Scholar] [CrossRef]
- Leitão, F.; Baptista, V.; Erzini, K. Reconstructing discards profiles of unreported catches. Sci. Mar. 2018, 82, 39–49. [Google Scholar] [CrossRef]
- UE. REGULAMENTO (UE) 2022/515 DO CONSELHO de 31 de março de 2022. Jornal Oficial da União Europeia (PT). L, 104/2. 2022. Available online: https://eur-lex.europa.eu/legal-content/PT/TXT/?uri=CELEX:32022R0515 (accessed on 1 June 2023).
- Relvas, P.; Barton, E.; Dubert, J.; Oliveira, P.B.; Peliz, Á.; da Silva, J.; Santos, A.M.P. Physical oceanography of the western Iberia ecosystem: Latest views and challenges. Prog. Oceanogr. 2007, 74, 149–173. [Google Scholar] [CrossRef]
- Bettencourt, A.; Bricker, S.B.; Ferreira, J.G.; Franco, A.; Marques, J.C.; Melo, J.J.; Nobre, A.; Ramos, L.; Reis, C.S.; Salas, F.; et al. Typology and Reference Conditions for Portuguese Transitional and Coastal Waters Development of Guidelines for the Application of the European Union Water Framework Directive; Instituto da Agua (INAG)—Institute of Marine Science (IMAR): Lisbon, Portugal, 2004; 98p, Available online: http://www.ecowin.org/TICOR/ (accessed on 1 June 2023).
- Solow, A.R. Fisheries recruitment and the North Atlantic Oscillation. Fish. Res. 2002, 54, 295–297. [Google Scholar] [CrossRef]
- Ullah, H.; Leitão, F.; Baptista, V.; Chícharo, L. An analysis of the impacts of climatic variability and hydrology on the coastal fisheries, Engraulis encrasicolus and Sepia officinalis, of Portugal. Ecohydrol. Hydrobiol. 2012, 12, 337–352. [Google Scholar] [CrossRef]
- Maynou, F. Impact of NAO on Mediterranean fisheries. In Hydrological, Socioeconomic and Ecological Impacts of the North Atlantic Oscillation in the Mediterranean Region; Advances in Global Change Research; Vicente-Serrano, S., Trigo, R., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 46. [Google Scholar] [CrossRef]
- Baptista, V.; Leitão, F. Commercial catch rates of Spisula solida reflect local environmental conditions on the coast of Portugal. J. Mar. Syst. 2014, 130, 79–89. [Google Scholar] [CrossRef]
- Baptista, V.; Blasco, I.P.; Bueno-Pardo, J.; Teodósio, M.A.; Leitão, F. Environmental variability and fishing effects on artisanal flatfish fisheries along the Portuguese coast. Front. Mar. Sci. 2022, 9, 844158. [Google Scholar] [CrossRef]
- Hurrell, J.W. Decadal Trends in the North Atlantic Oscillation: Regional Temperatures and Precipitation. Science 1995, 269, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.; Hoffman, R.; Ardizzone, J.; Leidner, S.M.; Jusem, J.C.; Smith, D.K.; Gombos, D. A Cross-calibrated, Multiplatform Ocean Surface Wind Velocity Product for Meteorological and Oceanographic Applications. Bull. Am. Meteorol. Soc. 2011, 92, 157–174. [Google Scholar] [CrossRef]
- Roberts, J.J.; Best, B.D.; Dunn, D.C.; Treml, E.A.; Halpin, P.N. Marine Geospatial Ecology Tools: An integrated framework for ecological geoprocessing with ArcGIS, Python, R., MATLAB, and C++. Environ. Model. Softw. 2010, 25, 1197–1207. [Google Scholar] [CrossRef]
- Rueda, F.P.; Álvarez, A.J.L.; Flórez, G.L.; Herrero, J.F.; Benito, H.R.; Menéndez, M.A. Selectividad de la Beta para el Salmonete, Mullus surmuletus, en la Costa Asturiana e Implicaciones para la Gestión Pesquera; CEP Report of Project PRESPO; European Union: Geneva, Switzerland, 2011; p. 13. [Google Scholar]
- Hilborn, R.; Walters, C.J. Quantitative Fisheries Stock Assessment, Choice, Dynamics and Uncertainty; Chapman & Hall: London, UK, 1992. [Google Scholar]
- Santos, M.N.; Erzini, E.; Díasz, A.; Manzano, C. Catálogo de Espécies de Peixes de Interesse Comercial da Costa Sul Atlântica da Península Ibérica; Junta de Andalucía: Andalucia, Spain, 2007; ISBN 84-8474-219-9. [Google Scholar]
- Mendes, B.; Fonseca, P.; Campos, A. Selectividade em Sacos de Redes de Arrasto para Seis Espécies de Peixes, na Costa Sudoeste Portuguesa. Relat. Cient. Téc. IPIMAR, Série digital. Nº 11, 19p. 2004. Available online: https://www.ipma.pt/resources.www/docs/publicacoes.site/docweb/2004/Reln11final.pdf (accessed on 19 June 2023).
- Cherif, M.; Zarrad, R.; Gharbi, H.; Missaoui, H.; Jarboui, O. Some biological parameters of the red mullet, Mullus barbatus L., 1758, from the Gulf of Tunis. Acta Adriat 2007, 48, 131–144. [Google Scholar]
- Yimin, Y.; Al-Husaini, M.; Al-Baz, A. Use of generalized linear models to analyze catch rates having zero values: The Kuwait driftnet fishery. Fish. Res. 2001, 53, 151–168. [Google Scholar] [CrossRef]
- King, M. Fisheries Biology, Assessment and Management; Blackwell Science: Hoboken, NJ, USA, 1995; 341p. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analysing Ecological Data; Springer: New York, NY, USA, 2007; 672p. [Google Scholar]
- Zuur, A.F.; Tuck IDBailey, N. Dynamic factor analysis to estimate common trends in fisheries time series. Can. J. Fish. Aquat. Sci. 2003, 60, 542–552. [Google Scholar] [CrossRef]
- Zuur, A.F.; Fryer, R.J.; Jolliffe, I.T.; Dekker, R.; Beukema, J.J. Estimating common trends in multivariate time series using dynamic factor analysis. Environmetrics 2003, 14, 665–685. [Google Scholar] [CrossRef]
- Fréon, P.; Mullon, C.; Pichon, G. CLIMPROD: Experimental Interactive Software for Choosing and Fitting Surplus Production Models Including Environmental Variables; FAO Computerized Information Series (Fisheries); No. 5; FAO: Rome, Italy, 1993. [Google Scholar]
- Santos, A.M.P.; Borges, M.F.; Crato, N.; Mendes, H.; Mota, B. Sardine regime shifts off Portugal: A time series analysis of catches and wind conditions. Sci. Mar. 2003, 67, 235–244. [Google Scholar] [CrossRef]
- Oliveira, P.B.; Stratoudakis, Y. Satellite-derived conditions and advection patterns off Iberia and NW Africa: Potential implications to sardine recruitment dynamics and population structuring. Remote Sens. Environ. 2008, 112, 3376–3387. [Google Scholar] [CrossRef]
- Oliveira, P.B.; Angelica, M.M.; Fernandes, J.; Castro, J.; Cruz, T. Near shore oceanographic conditions off SW Portugal in summer 2006 and 2007 from satellite and in situ data. In Proceedings of the 2nd MERIS/(A) ATSR User Workshop, Frascati, Italy, 22–26 September 2008; pp. 1–5. [Google Scholar]
- Peterman, R.M.; Bradford, M.J. Wind Speed and Mortality Rate of a Marine Fish, the Northern Anchovy (Engraulis mordax). Science 1987, 235, 354–356. [Google Scholar] [CrossRef]
- Jenkins, G.P. Influence of climate on fishery recruitment of a temperate, seagrass-associated fish, the king George whiting Sillaginoides punctate. Mar. Ecol. Prog. Ser. 2005, 2887, 263–271. [Google Scholar] [CrossRef]
- Kermorvant, C.; Caill-Milly, N.; Sous, D.; Paradinas, I.; Lissardy, M.; Liquet, B. Detecting the effects of inter-annual and seasonal changes in environmental factors on the striped red mullet population in the Bay of Biscay. J. Sea Res. 2021, 169, 102008. [Google Scholar] [CrossRef]
- Planque, B.; Frédou, T. Temperature and the recruitment of Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 1999, 56, 2069–2077. [Google Scholar] [CrossRef]
- Pepin, P. Effect of Temperature and Size on Development, Mortality, and Survival Rates of the Pelagic Early Life History Stages of Marine Fish. Can. J. Fish. Aquat. Sci. 1991, 48, 503–518. [Google Scholar] [CrossRef]
- Teixeira, C.M.; Gamito, R.; Leitão, F.; Cabral, H.N.; Erzini, K.; Costa, M.J. Trends in landings of fish species potentially affected by climate change in Portuguese fisheries. Reg. Environ. Chang. 2014, 14, 657–669. [Google Scholar] [CrossRef]
- Maltby, K.M.; Rutterford, L.A.; Tinker, J.; Genner, M.J.; Simpson, S.D. Projected impacts of warming seas on commercially fished species at a biogeographic boundary of the European continental shelf. J. Appl. Ecol. 2020, 57, 2222–2233. [Google Scholar] [CrossRef]
- Gamito, R.; Teixeira, C.; Costa, M.J.; Cabral, H. Climate-induced changes in fish landings of different fleet components of Portuguese fisheries. Reg. Environ. Chang. 2013, 13, 413–421. [Google Scholar] [CrossRef]
- Gamito, R.; Teixeira, C.M.; Costa, M.J.; Cabral, H.N. Are regional fisheries’ catches changing with climate? Fish. Res. 2015, 161, 207–216. [Google Scholar] [CrossRef]
- Teixeira, C.M.; Gamito, R.; Leitão, F.; Murta, A.G.; Cabral, H.N.; Erzini, K.; Costa, M.J. Environmental influence on commercial fishery landings of small pelagic fish in Portugal. Reg. Environ. Chang. 2016, 16, 709–716. [Google Scholar] [CrossRef]
- Fiorentino, F.; Badalamenti, F.; D’Anna, G.; Garofalo, G.; Gianguzza, P.; Gristina, M.; Pipitone, C.; Rizzo, P.; Fortibuoni, T. Changes in spawning-stock structure and recruitment pattern of red mullet, Mullus barbatus, after a trawl ban in the Gulf of Castellammare (central Mediterranean Sea). ICES J. Mar. Sci. 2008, 65, 1175–1183. [Google Scholar] [CrossRef]
- Agostini, V.N.; Bakun, A. ‘Ocean triads’ in the Mediterranean Sea: Physical mechanisms potentially structuring reproductive habitat suitability (with example application to European anchovy, Engraulis encrasicolus). Fish. Ocean. 2002, 11, 129–142. [Google Scholar] [CrossRef]
- DQEM. Diretiva Quadro Estratégia Marinha, Estratégia Marinha para a Subdivisão do Continente; Ministerio da Agriculture, do Mar, do Ambiente, e do ordenamento do Território: Lisboa, Portugal, 2014; 906p. [Google Scholar]
- Albo-Puigserver, M.; Bueno-Pardo, J.; Pinto, M.; Monteiro, J.N.; Ovelheiro, A.; Teodósio, M.A.; Leitão, F. Ecological sensitivity and vulnerability of fishing fleet landings to climate change across regions. Sci. Rep. 2022, 12, 17360. [Google Scholar] [CrossRef]
- Bueno-Pardo, J.; Nobre, D.; Monteiro, J.N.; Sousa, P.M.; Costa, E.F.S.; Baptista, V.; Ovelheiro, A.; Vieira, V.M.N.C.S.; Chícharo, L.; Gaspar, M.; et al. Climate change vulnerability assessment of the main marine commercial fish and invertebrates of Portugal. Sci. Rep. 2021, 11, 2958. [Google Scholar] [CrossRef]

| Northwestern (IXaCN) | ||||
| DFA | GLS | MAFA | Probability | |
| UPW | sig. (+) | sig. (+) | sig. (+) | high |
| Southwestern (IXaCS) | ||||
| FE | sig. (+) | sig. (+) | sig. (+) | high |
| u-wind | sig. (−) | sig. (−) | sig. (−) | high |
| UPW-summer | n.s. | sig. (−) | sig. (−) | high |
| UW-spring | sig. (−) | n.s. | n.s. | low |
| South Algarve (IXaS-Algarve) | ||||
| SST | sig. (+) | n.s. | n.s. | low |
| SST-spring | n.s. | sig. (+) | n.s. | low |
| SST-summer | sig. (+) | sig. (+) | sig. (+) | high |
| u-wind | sig. (−) | sig. (−) | sig. (−) | high |
| UPW-summer | sig. (−) | n.s. | n.s. | low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitão, F. Environmental Conditions Affect Striped Red Mullet (Mullus surmuletus) Artisanal Fisheries. Oceans 2023, 4, 220-235. https://doi.org/10.3390/oceans4030015
Leitão F. Environmental Conditions Affect Striped Red Mullet (Mullus surmuletus) Artisanal Fisheries. Oceans. 2023; 4(3):220-235. https://doi.org/10.3390/oceans4030015
Chicago/Turabian StyleLeitão, Francisco. 2023. "Environmental Conditions Affect Striped Red Mullet (Mullus surmuletus) Artisanal Fisheries" Oceans 4, no. 3: 220-235. https://doi.org/10.3390/oceans4030015
APA StyleLeitão, F. (2023). Environmental Conditions Affect Striped Red Mullet (Mullus surmuletus) Artisanal Fisheries. Oceans, 4(3), 220-235. https://doi.org/10.3390/oceans4030015







