Seasonal Upwelling Conditions Modulate the Calcification Response of a Tropical Scleractinian Coral
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Environmental Conditions and Seawater Carbonate Chemistry at the Sampling Site
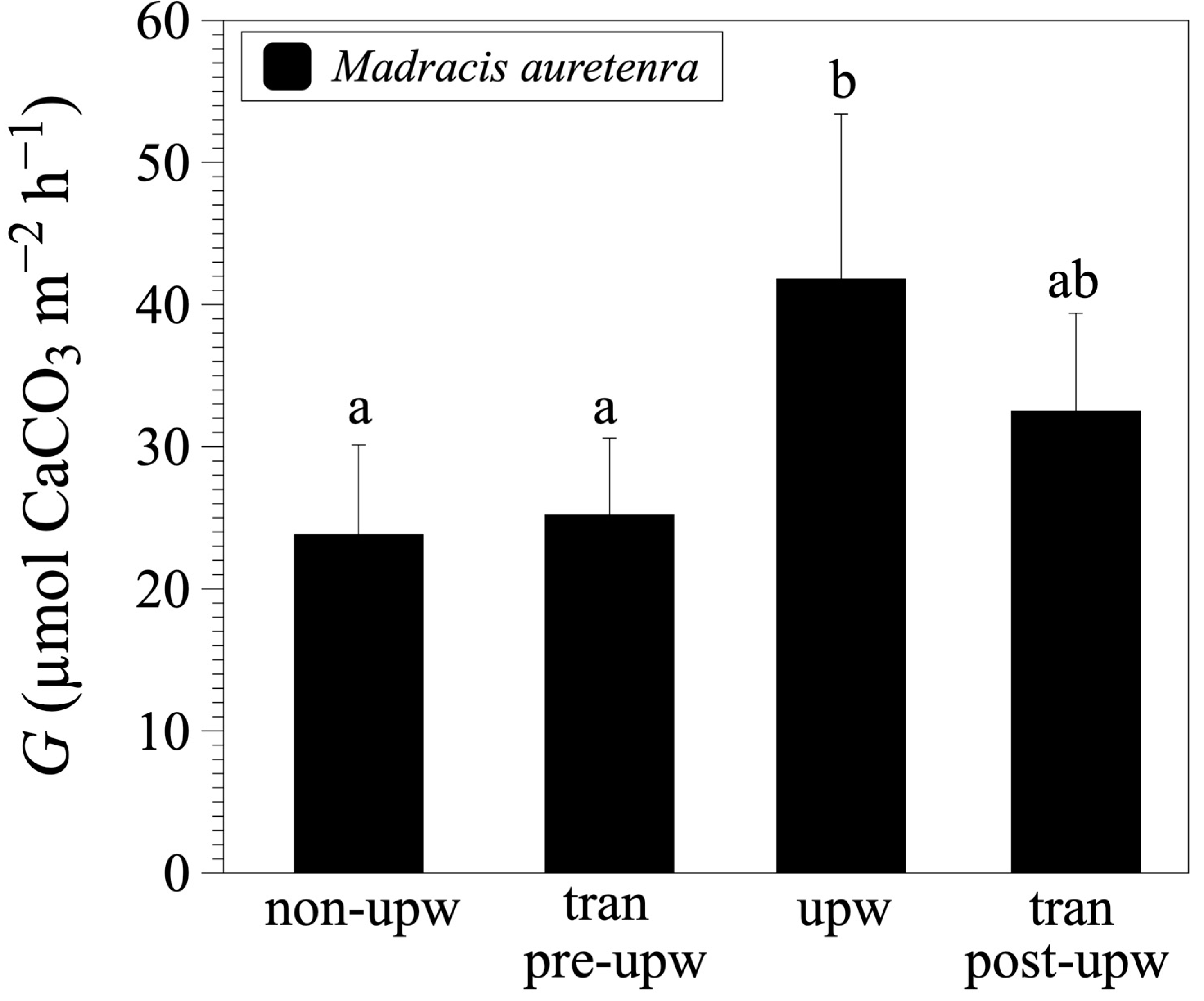
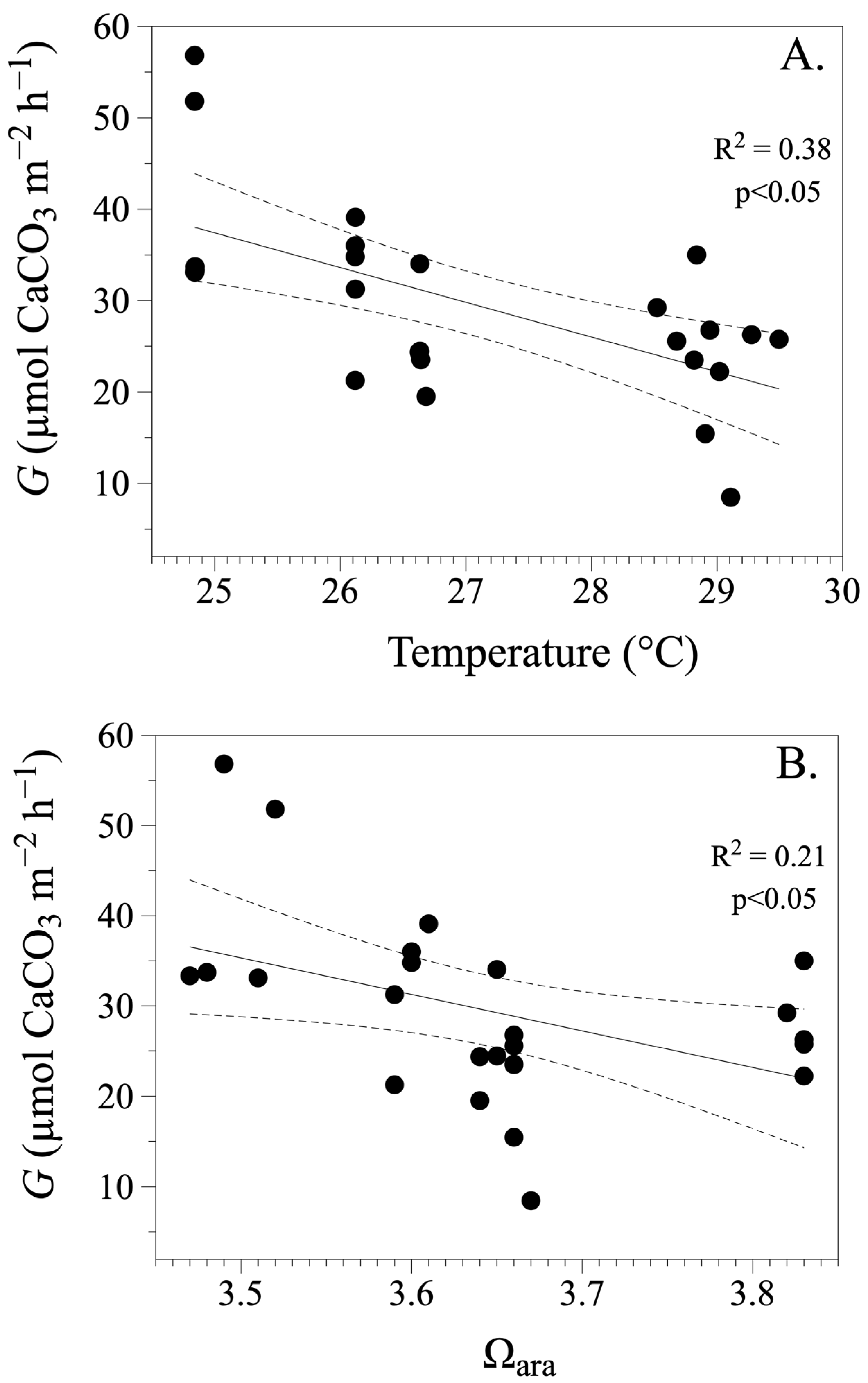
3.2. Calcification and Dissolution Rates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef]
- Cornwall, C.E.; Comeau, S.; Kornder, N.A.; Perry, C.T.; van Hooidonk, R.; DeCarlo, T.; Pratchett, M.S.; Anderson, K.D.; Brown, N.; Carpenter, R.; et al. Global declines in coral reef calcium carbonate production under ocean acidification and warming. Proc. Natl. Acad. Sci. USA 2021, 118, e2015265118. [Google Scholar] [CrossRef]
- Caldeira, K.; Wickett, M.E. Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef]
- Gattuso, J.; Frankignoulle, M.; Bourge, I.; Romaine, S.; Buddemeier, R.W. Effect of calcium carbonate saturation of seawater on coral calcification. Glob. Planet. Change 1998, 18, 37–46. [Google Scholar] [CrossRef]
- Kleypas, J.A.; Buddemeier, R.W.; Archer, D.; Gattuso, J.P.; Langdon, C.; Opdyke, B.N. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 1999, 284, 118–120. [Google Scholar] [CrossRef]
- Langdon, C.; Atkinson, M.J. Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J. Geophys. Res. 2005, 110, C09S07. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Guinotte, J.M.; Orr, J.; Cairns, S.; Freiwald, A.; Morgan, L.; George, R. Will human-induced changes in seawater chemistry alter the distribution of deep-sea scleractinian corals? Front. Ecol. Environ. 2006, 4, 141–146. [Google Scholar] [CrossRef]
- Eyre, B.D.; Cyronak, T.; Drupp, P.; De Carlo, E.H.; Sachs, J.P.; Andersson, A.J. Coral reefs will transition to net dissolving before end of century. Science 2018, 359, 908–911. [Google Scholar] [CrossRef]
- Feely, R.A.; Sabine, C.L.; Lee, K.; Berelson, W.; Kleypas, J.; Fabry, V.J.; Millero, F.J. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 2004, 305, 362–366. [Google Scholar] [CrossRef]
- González-Barrios, F.J.; Álvarez-Filip, L. A framework for measuring coral species-specific contribution to reef functioning in the Caribbean. Ecol. Indic. 2018, 95, 877–886. [Google Scholar] [CrossRef]
- Allemand, D.; Ferrier-Pagès, C.; Furla, P.; Houlbrèque, F.; Puverel, S.; Tambutté, R.E.; Tambutté, S.; Zoccola, D. Biomineralisation in reef-building corals: From molecular mechanisms to environmental control. Comptes. Rendus. Palevol. 2004, 3, 453–467. [Google Scholar] [CrossRef]
- McCulloch, M.; Falter, J.; Trotter, J.; Montagna, P. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Change 2012, 2, 623–627. [Google Scholar] [CrossRef]
- Holcomb, M.; Venn, A.A.; Tambutté, E.; Tambutté, S.; Allemand, D.; Trotter, J.; Mcculloch, M. Coral calcifying fluid pH dictates response to ocean acidification. Sci. Rep. 2015, 4, 5207. [Google Scholar] [CrossRef] [PubMed]
- Raybaud, V.; Tambutté, S.; Ferrier-Pagès, C.; Reynaud, S.; Venn, A.A.; Tambutté, É.; Nival, P.; Allemand, D. Computing the carbonate chemistry of the coral calcifying medium and its response to ocean acidification. J. Theor. Biol. 2017, 424, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Jury, C.P.; Whitehead, R.F.; Szmant, A.M. Effects of variations in carbonate chemistry on the calcification rates of Madracis auretenra (=Madracis mirabilis sensu Wells, 1973): Bicarbonate concentrations best predict calcification rates. Glob. Change Biol. 2010, 16, 1632–1644. [Google Scholar] [CrossRef]
- Leung, J.Y.S.; Zhang, S.; Connell, S.D. Is Ocean acidification really a threat to marine calcifiers? A systematic review and meta-analysis of 980+ studies spanning two decades. Small 2022, 18, 2107407. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- Albright, R.; Takeshita, Y.; Koweek, D.A.; Ninokawa, A.; Wolfe, K.; Rivlin, T.; Nebuchina, Y.; Young, J.; Caldeira, K. Carbon dioxide addition to coral reef waters suppresses net community calcification. Nature 2018, 555, 516–519. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Duarte, C.M.; Gattuso, J.P. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Change Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.N.; Singh, G.G. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms: Biological responses to ocean acidification. Ecol. Lett. 2010, 13, 1419–1434. [Google Scholar] [CrossRef] [PubMed]
- Hennige, S.J.; Wicks, L.C.; Kamenos, N.A.; Perna, G.; Findlay, H.S.; Roberts, J.M. Hidden impacts of ocean acidification to live and dead coral framework. Proc. R. Soc. B 2015, 282, 20150990. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.E.; Wickes, L.; Deegan, D.; Etnoyer, P.J.; Cordes, E.E. Growth and feeding of deep-sea coral Lophelia pertusa from the California margin under simulated ocean acidification conditions. PeerJ 2018, 6, e5671. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, K.E.; Langdon, C.; Uthicke, S.; Humphrey, C.; Noonan, S.; De’ath, G.; Okazaki, R.; Muehllehner, N.; Glas, M.S.; Lough, J.M. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Change 2011, 1, 165–169. [Google Scholar] [CrossRef]
- Kurihara, H.; Watanabe, A.; Tsugi, A.; Mimura, I.; Hongo, C.; Kawai, T.; Reimer, J.D.; Kimoto, K.; Gouezo, M.; Golbuu, Y. Potential local adaptation of corals at acidified and warmed Nikko Bay, Palau. Sci. Rep. 2021, 11, 11192. [Google Scholar] [CrossRef]
- Glazier, A.; Herrera, S.; Weinnig, A.; Kurman, M.; Gómez, C.E.; Cordes, E. Regulation of ion transport and energy metabolism enables certain coral genotypes to maintain calcification under experimental ocean acidification. Mol. Ecol. 2020, 29, 1657–1673. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M.; Rodolfo-Metalpa, R.; Martin, S.; Ransome, E.; Fine, M.; Turner, S.M.; Rowley, S.J.; Tedesco, D.; Buia, M.C. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 2008, 454, 96–99. [Google Scholar] [CrossRef]
- Enochs, I.C.; Manzello, D.P.; Kolodziej, G.; Noonan, S.H.; Valentino, L.; Fabricius, K.E. Enhanced macroboring and depressed calcification drive net dissolution at high-CO2 coral reefs. Proc. R. Soc. B 2016, 283, 20161742. [Google Scholar] [CrossRef]
- Sánchez-Noguera, C.; Stuhldreier, I.; Cortés, J.; Jiménez, C.; Morales, Á.; Wild, C.; Rixen, T. Natural Ocean acidification at Papagayo upwelling system (north Pacific Costa Rica): Implications for reef development. Biogeosciences 2018, 15, 2349–2360. [Google Scholar] [CrossRef]
- Spreter, P.M.; Reuter, M.; Mertz-Kraus, R.; Taylor, O.; Brachert, T.C. Calcification response of reef corals to seasonal upwelling in the northern Arabian Sea (Masirah Island, Oman). Biogeosciences 2022, 19, 3559–3573. [Google Scholar] [CrossRef]
- Ramajo, L.; Valladares, M.; Astudillo, O.; Fernández, C.; Rodríguez-Navarro, A.B.; Watt-Arévalo, P.; Núñez, M.; Grenier, C.; Román, R.; Aguayo, P.; et al. Upwelling intensity modulates the fitness and physiological performance of coastal species: Implications for the aquaculture of the scallop Argopecten purpuratus in the Humboldt Current System. Sci. Total Environ. 2020, 745, 140949. [Google Scholar] [CrossRef] [PubMed]
- Feely, R.A.; Sabine, C.L.; Hernandez-Ayon, J.M.; Ianson, D.; Hales, B. Evidence for upwelling of corrosive ‘acidified’ water onto the continental shelf. Science 2008, 320, 1490–1492. [Google Scholar] [CrossRef] [PubMed]
- Gruber, N.; Hauri, C.; Lachkar, Z.; Loher, D.; Frölicher, T.L.; Plattner, G.K. Rapid progression of ocean acidification in the California Current System. Science 2012, 337, 220–223. [Google Scholar] [CrossRef]
- Manzello, D.P. Coral growth with thermal stress and ocean acidification: Lessons from the eastern tropical Pacific. Coral Reefs 2010, 29, 749–758. [Google Scholar] [CrossRef]
- Manzello, D.P.; Kleypas, J.A.; Budd, D.A.; Eakin, C.M.; Glynn, P.W.; Langdon, C. Poorly cemented coral reefs of the Eastern Tropical Pacific: Possible insights into reef development in a high-CO2 world. Proc. Natl. Acad. Sci. USA 2008, 105, 10450–10455. [Google Scholar] [CrossRef]
- Glynn, P.W.; Alvarado, J.J.; Banks, S.; Cortés, J.; Feingold, J.S.; Jiménez, C.; Maragos, J.E.; Martínez, P.; Maté, J.L.; Moanga, D.A.; et al. Eastern Pacific coral reef provinces, coral community structure and composition: An overview. In Coral Reefs of the Eastern Tropical Pacific; Glynn, P.W., Manzello, D.P., Enochs, I.C., Eds.; Springer: Amsterdam, The Netherlands, 2017; Volume 8, pp. 107–176. [Google Scholar]
- Rueda-Roa, D.T.; Muller-Karger, F.E. The southern Caribbean upwelling system: Sea surface temperature, wind forcing and chlorophyll concentration patterns. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2013, 78, 102–114. [Google Scholar] [CrossRef]
- Rueda-Roa, D.; Ezer, T.; Muller-Karger, F. Description and mechanisms of the mid-year upwelling in the Southern Caribbean Sea from remote sensing and local data. JMSE 2018, 6, 36. [Google Scholar] [CrossRef]
- Santos, F.; Gómez-Gesteira, M.; Varela, R.; Ruiz-Ochoa, M.; Días, J.M. Influence of upwelling on SST trends in La Guajira system. J. Geophys. Res. Oceans 2016, 121, 2469–2480. [Google Scholar] [CrossRef]
- Andrade, C.A.; Barton, E.D. The Guajira upwelling system. Cont. Shelf Res. 2005, 25, 1003–1022. [Google Scholar] [CrossRef]
- Paramo, J.; Correa, M.; Núñez, S. Evidencias de desacople físico-biológico en el sistema de surgencia en La Guajira, Caribe colombiano. Rev. Biol. Mar. Oceanogr. 2011, 46, 421–430. [Google Scholar] [CrossRef]
- Correa-Ramirez, M.; Rodriguez-Santana, Á.; Ricaurte-Villota, C.; Paramo, J. The Southern Caribbean upwelling system off Colombia: Water masses and mixing processes. Deep Sea Res. Part I Oceanogr. Res. Pap. 2020, 155, 103145. [Google Scholar] [CrossRef]
- Garzón-Ferreira, J. Bahía de Chengue, Parque Nacional Natural Tayrona, Colombia. In Caribbean Coral Reef, Seagrass and Mangrove Sites; Unesco: Paris, France, 1998; pp. 115–125. [Google Scholar]
- Gómez-López, D.I.; Acosta-Chaparro, A.; Gonzalez, J.D.; Sanchez, L.; Navas-Camacho, R.; Alonso, D. Reporte del Estado de Los Arrecifes Coralinos y PASTOS marinos en Colombia (2016–2017); Serie de Publicaciones Generales del Invemar #101; INVEMAR: Santa Marta, Colombia, 2018; 100p. [Google Scholar]
- Garzón-Ferreira, J.; Díaz, J.M. The Caribbean coral reefs of Colombia. In Latin American Coral Reefs; Cortés, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 275–301. [Google Scholar] [CrossRef]
- Díaz, J.M.; Barrios, L.M.; Cendales, M.H.; Garzón-Ferreira, J.; Geister, J.; López-Victoria, M.; Ospina, G.; Parra-Velandia, F.; Pinzón, J.; Vargas-Angel, B.; et al. Áreas Coralinas de Colombia; Serie Publicaciones Especiales No. 5; INVEMAR: Santa Marta, Colombia, 2000; p. 176. [Google Scholar]
- Arévalo-Martínez, D.L.; Franco Herrera, A. Características oceanográficas de la surgencia frente a la ensenada de Gaira, Departamento de Magdalena, época seca menor de 2006. Bol. Inv. Mar. Cost. 2016, 37, 131–162. [Google Scholar] [CrossRef]
- Bayraktarov, E.; Pizarro, V.; Wild, C. Spatial and temporal variability of water quality in the coral reefs of Tayrona National Natural Park, Colombian Caribbean. Environ. Monit. Assess. 2014, 186, 3641–3659. [Google Scholar] [CrossRef]
- Locke, J.M.; Weil, E.; Coates, K.A. A newly documented species of Madracis (Scleractinia: Pocilloporidae) from the Caribbean. Proc. Biol. Soc. Wash. 2007, 120, 214–226. [Google Scholar] [CrossRef]
- Sebens, K.P.; Witting, J.; Helmuth, B. Effects of water flow and branch spacing on particle capture by the reef coral Madracis mirabilis (Duchassaing and Michelotti). J. Exp. Mar. Biol. Ecol. 1997, 211, 1–28. [Google Scholar] [CrossRef]
- Hurd, C.L. Water motion, marine macroalgal physiology, and production. J. Phycol 2000, 36, 453–472. [Google Scholar] [CrossRef]
- Carvalho, V.F.; Silva, J.; Kerr, R.; Anderson, A.B.; Bastos, E.O.; Cabral, D.; Gouvêa, L.P.; Peres, L.; Martins, C.D.L.; Silveira-Andrade, V.M.; et al. When descriptive ecology meets physiology: A study in a South Atlantic rhodolith bed. J. Mar. Biol. Ass. 2020, 100, 347–360. [Google Scholar] [CrossRef]
- Dickson, A.; Sabine, C.L.; Christian, J. Guide to Best Practices for Ocean CO2 Measurement; PICES Special Publication; PICES, 2007. [Google Scholar]
- Bernal, C.; Sánchez-Cabeza, J.A.; Martínez-Galarza, R.A.; Gómez, M.; Norzagaray-López, O. Determinación de Carbono Inorgánico Disuelto en Agua de Mar Utilizando Analizador Automático con Detección Infrarrojo—AIRICA.; REMARCO: Santa Marta, Colombia, 2021; 18p, Available online: https://remarco.org/manual-ao/ (accessed on 15 March 2022).
- Pierrot, D.; Lewis, E.; Wallace, D. MS Excel Program Developed for CO2 System Calculations. ORNL/CDIAC-105a; Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy: Oak Ridge, TN, USA, 2006.
- Mehrbach, C.; Culberson, C.H.; Hawley, J.E.; Pytkowicx, R.M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 1973, 18, 897–907. [Google Scholar] [CrossRef]
- Dickson, A.G.; Millero, F.J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. 1987, 34, 1733–1743. [Google Scholar] [CrossRef]
- Leuker, T.J.; Dickson, A.G.; Keeling, C.D. Ocean pCO2 calculated from dissolved inorganic carbon, alkalinity, and equations for K1 and K2. Validation based on laboratory measurements of CO2 in gas and seawater at equilibrium. Mar. Chem. 2000, 70, 105–119. [Google Scholar] [CrossRef]
- Dickson, A.G. Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K. Deep Sea Res. Part A. Oceanogr. Res. Pap. 1990, 37, 755–766. [Google Scholar] [CrossRef]
- Lee, K.; Millero, F.J.; Byrne, R.H.; Feely, R.A.; Wanninkhof, R. The recommended dissociation constants for carbonic acid in seawater. Geophys. Res. Lett. 2000, 27, 229–232. [Google Scholar] [CrossRef]
- Smith, S.V.; Key, G.S. Carbon dioxide and metabolism in marine environments1. Limnol. Oceanogr. 1975, 20, 493–495. [Google Scholar] [CrossRef]
- Chisholm, J.R.M.; Gattuso, J.-P. Validation of the alkalinity anomaly technique for investigating calcification and photosynthesis in coral reef communities. Limnol. Oceanogr. 1991, 36, 1232–1239. [Google Scholar] [CrossRef]
- Fajardo, G. Surgencia costera en las proximidades de la península colombiana de La Guajira. Bol. Cien. CIOH 1979, 2, 7–10. [Google Scholar] [CrossRef]
- Ávila-Melean, R. Dióxido de carbono total y su relación con el oxígeno disuelto en las aguas del Golfo de Santa Fe, Estado Sucre, Venezuela. Bol. Inst. Oceanogr. Univ. Oriente 1976, 15, 133–139. [Google Scholar]
- Zhang, J.Z.; Millero, F.J. The chemistry of the anoxic waters in the Cariaco Trench. Deep-Sea Res. 1993, 40, 1023–1041. [Google Scholar] [CrossRef]
- Astor, Y.M.; Lorenzoni, L.; Thunell, R.; Varela, R.; Muller-Karger, F.; Troccoli, L.; Taylor, G.T.; Scranton, M.I.; Tappa, E.; Rueda, D. Interannual variability in sea surface temperature and fCO2 changes in the Cariaco Basin. Deep Sea Res. Part II Top. Stud. Oceanogr. 2013, 93, 33–43. [Google Scholar] [CrossRef]
- Hernández-Guerra, A.; Joyce, T.M. Water masses and circulation in the surface layers of the Caribbean at 66°W. Geophys. Res. Lett. 2000, 27, 3497–3500. [Google Scholar] [CrossRef]
- Anthony, K.R.N.; Kleypas, J.; Gattuso, J.-P. Coral reefs modify their seawater carbon chemistry—Implications for impacts of ocean acidification. Glob. Change Biol. 2011, 17, 3655–3666. [Google Scholar] [CrossRef]
- Cyronak, T.; Andersson, A.J.; Langdon, C.; Albright, R.; Bates, N.R.; Caldeira, K.; Carlton, R.; Corredor, J.E.; Dunbar, R.B.; Enochs, I.; et al. Taking the metabolic pulse of the world’s coral reefs. PLoS ONE 2018, 13, e0190872. [Google Scholar] [CrossRef] [PubMed]
- Marubini, F.; Ferrier–Pages, C.; Cuif, J. Suppression of skeletal growth in scleractinian corals by decreasing ambient carbonate-ion concentration: A cross-family comparison. Proc. R. Soc. Lond. B 2003, 270, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Glynn, P.W. Coral growth in upwelling and nonupwelling areas off the Pacific coast of Panamá. J. Mar. Res. 1977, 35, 567–585. [Google Scholar]
- Porter, J.W.; Battey, J.F.; Smith, G.J. Perturbation and change in coral reef communities. Proc. Natl. Acad. Sci. USA 1982, 79, 1678–1681. [Google Scholar] [CrossRef]
- Saxby, T.; Dennison, W.; Hoegh-Guldberg, O. Photosynthetic responses of the coral Montipora digitata to cold temperature stress. Mar. Ecol. Prog. Ser. 2003, 248, 85–97. [Google Scholar] [CrossRef]
- Schneider, K.; Erez, J. The effect of carbonate chemistry on calcification and photosynthesis in the hermatypic coral Acropora eurystoma. Limnol. Oceanogr. 2006, 51, 1284–1293. [Google Scholar] [CrossRef]
- Steller, D.; Hernandez-Ayón, J.; Riosmena-Rodríguez, R.; Cabello-Pasini, A. Effect of temperature on photosynthesis, growth and calcification rates of the free-living coralline alga Lithophyllum margaritae. Cienc. Mar. 2007, 33, 441–456. [Google Scholar] [CrossRef]
- de Putron, S.J.; McCorkle, D.C.; Cohen, A.L.; Dillon, A.B. The impact of seawater saturation state and bicarbonate ion concentration on calcification by new recruits of two Atlantic corals. Coral Reefs 2011, 30, 321–328. [Google Scholar] [CrossRef]
- Marubini, F.; Thake, B. Bicarbonate addition promotes coral growth. Limnol. Oceanogr. 1999, 44, 716–720. [Google Scholar] [CrossRef]
- Herfort, L.; Thake, B.; Taubner, I. Bicarbonate stimulation of calcification and photosynthesis in two hermatypic corals. J. Phycol. 2008, 44, 91–98. [Google Scholar] [CrossRef]
- Okazaki, R.R.; Swart, P.K.; Langdon, C. Stress-tolerant corals of Florida Bay are vulnerable to ocean acidification. Coral Reefs 2013, 32, 671–683. [Google Scholar] [CrossRef]
- Roberty, S.; Béraud, E.; Grover, R.; Ferrier-Pagès, C. Coral productivity is co-limited by bicarbonate and ammonium availability. Microorganisms 2020, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Chaparro, A.; González, J.D.; Navas-Camacho, R.; Sánchez, L. Estado de las Formaciones Coralinas del Parque Nacional Natural Tayrona; Informe Técnico de Monitoreo Ecosistémico ITF#2; Invemar: Santa Marta, Colombia, 2018; 55p. [Google Scholar]
- Chollett, I.; Mumby, P.J.; Cortés, J. Upwelling areas do not guarantee refuge for coral reefs in a warming ocean. Mar. Ecol. Prog. Ser. 2010, 416, 47–56. [Google Scholar] [CrossRef]
- Sawall, Y.; Harris, M.; Lebrato, M.; Wall, M.; Feng, E.Y. Discrete pulses of cooler deep water can decelerate coral bleaching during thermal stress: Implications for artificial upwelling during heat-stress events. Front. Mar. Sci. 2020, 7, 720. [Google Scholar] [CrossRef]
- Arias-González, J.E.; Baums, I.B.; Banaszak, A.T.; Prada, C.; Rossi, S.; Hernández-Delgado, E.A.; Rinkevich, B. Editorial: Coral reef restoration in a changing world: Science-based solutions. Front. Mar. Sci. 2022, 9, 919603. [Google Scholar] [CrossRef]

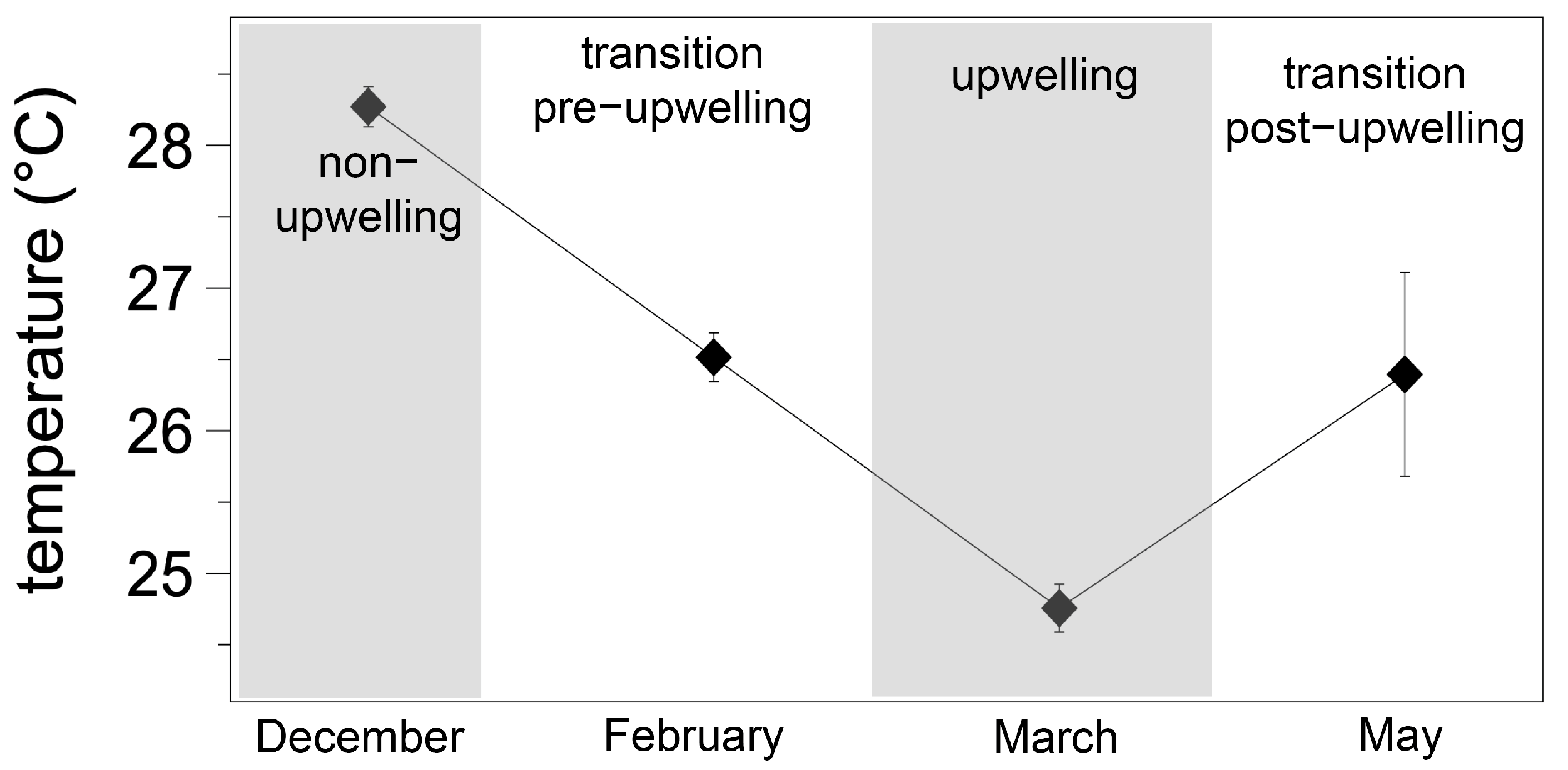

| Date | Temp (°C) | Salinity | AT (µmol kg−1) | DIC (µmol kg−1) | pHT | pCO2 (µatm) | HCO3− | CO32− | Ωara |
|---|---|---|---|---|---|---|---|---|---|
| 12/2/20 | 28.97 | 33.80 | 2369 | 2042 | 8.03 | 427 | 1796 | 234 | 3.82 |
| 12/3/20 | 28.87 | 33.70 | 2365 | 2052 | 8.01 | 452 | 1815 | 225 | 3.67 |
| 12/4/20 | 28.49 | 34.00 | 2381 | 2097 | 7.96 | 523 | 1878 | 206 | 3.35 |
| 2/17/21 | 26.64 | 35.75 | 2394 | 2069 | 8.03 | 426 | 1828 | 230 | 3.65 |
| 2/18/21 | 26.70 | 36.30 | 2383 | 2071 | 8.01 | 454 | 1838 | 221 | 3.50 |
| 2/19/21 | 26.00 | 36.29 | 2391 | 2075 | 8.02 | 436 | 1840 | 223 | 3.53 |
| 3/17/21 | 24.84 | 35.43 | 2425 | 2116 | 8.03 | 431 | 1882 | 221 | 3.50 |
| 3/18/21 | 25.07 | 35.66 | 2418 | 2113 | 8.02 | 444 | 1882 | 218 | 3.45 |
| 3/19/21 | 24.55 | 36.29 | 2416 | 2144 | 7.97 | 517 | 1933 | 196 | 3.07 |
| 5/14/21 | 25.89 | 35.08 | 2414 | 2095 | 8.04 | 422 | 1855 | 228 | 3.63 |
| 5/15/21 | 26.90 | 35.68 | 2414 | 2105 | 8.00 | 467 | 1871 | 221 | 3.52 |
| Season | Date | n | Temp (°C) | Salinity | AT(i) (µmol kg−1 SW) | AT(f) (µmol kg−1 SW) | Gnet (µmol CaCO3 m−2 h−1) | Dissolution (µmol CaCO3 m−2 h−1) |
|---|---|---|---|---|---|---|---|---|
| non-upwelling | 12/2/20 | 5 | 29.0 ± 0.4 | 33.3 ± 0.2 | 2369 | 2313 ± 34 | 27.7 ± 4.8 | ------.------ |
| 12/3/20 | 5 | 28.9 ± 0.2 | 33.4 ± 0.1 | 2364 | 2325 ± 42 | 19.9 ± 7.8 | ------.------ | |
| transition pre-upwelling | 2/17/21 | 5 | 26.6 ± 0.01 | 35.9 ± 0.1 | 2394 | 2304 ± 57 | 25.2 ± 5.4 | ------.------ |
| 2/19/21 | 5 | 26.1 ± 0.01 | 36.1 ± 0.2 | 2391 | 2385 ± 11 | ------.------ | 3.1 ± 4.6 | |
| upwelling | 3/17/21 | 5 | 24.8 ± 0.01 | 35.5 ± 0.4 | 2425 | 2270 ± 83 | 41.8 ± 11.6 | ------.------ |
| 3/19/21 | 5 | 25.1 ± 0.01 | 36.3 ± 0.1 | 2415 | 2429 ± 24 | ------.------ | −5.6 ± 10.7 | |
| transition post-upwelling | 5/14/21 | 5 | 26.1 ± 0.01 | 35.98 ± 0.22 | 2414 | 2311 ± 66 | 32.5 ± 6.9 | ------.------ |
| Source | Type-III Sum Squares | df | Mean Squares | F | Sig |
|---|---|---|---|---|---|
| Treatment | 1212.13 | 2 | 404.046 | 6.395 | 0.003 |
| Error | 1326.711 | 21 | 63.177 | ||
| Total | 24,170.51 | 25 | |||
| Post-Hoc Tukey HSD | |||||
| Treatment | non-upw | tran pre-upw | upw | tran post-upw | |
| non-upw | ----.---- | 0.989 | 0.003 | 0.223 | |
| tran pre-upw | 0.989 | ----.---- | 0.017 | 0.482 | |
| upw | 0.003 | 0.017 | ----.---- | 0.281 | |
| tran post-upw | 0.223 | 0.482 | 0.281 | ----.---- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, C.E.; Acosta-Chaparro, A.; Bernal, C.A.; Gómez-López, D.I.; Navas-Camacho, R.; Alonso, D. Seasonal Upwelling Conditions Modulate the Calcification Response of a Tropical Scleractinian Coral. Oceans 2023, 4, 170-184. https://doi.org/10.3390/oceans4020012
Gómez CE, Acosta-Chaparro A, Bernal CA, Gómez-López DI, Navas-Camacho R, Alonso D. Seasonal Upwelling Conditions Modulate the Calcification Response of a Tropical Scleractinian Coral. Oceans. 2023; 4(2):170-184. https://doi.org/10.3390/oceans4020012
Chicago/Turabian StyleGómez, Carlos E., Andrés Acosta-Chaparro, Cesar A. Bernal, Diana I. Gómez-López, Raúl Navas-Camacho, and David Alonso. 2023. "Seasonal Upwelling Conditions Modulate the Calcification Response of a Tropical Scleractinian Coral" Oceans 4, no. 2: 170-184. https://doi.org/10.3390/oceans4020012
APA StyleGómez, C. E., Acosta-Chaparro, A., Bernal, C. A., Gómez-López, D. I., Navas-Camacho, R., & Alonso, D. (2023). Seasonal Upwelling Conditions Modulate the Calcification Response of a Tropical Scleractinian Coral. Oceans, 4(2), 170-184. https://doi.org/10.3390/oceans4020012







