Posidonia oceanica (L.) Delile at Its Westernmost Biogeographical Limit (Northwestern Alboran Sea): Meadow Features and Plant Phenology
Abstract
1. Introduction
2. Materials and Methods
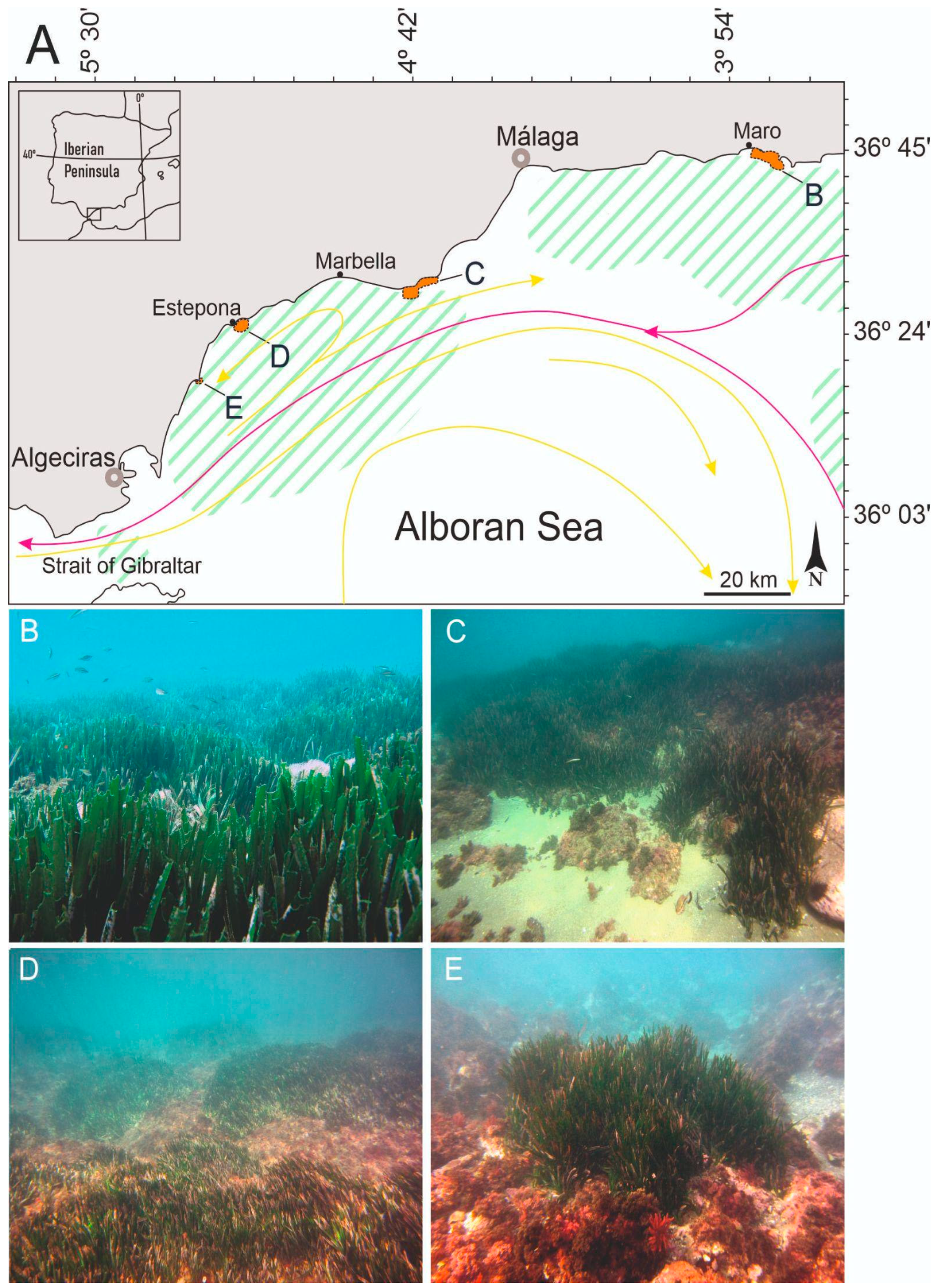
2.1. Study Area
2.2. Data Collection
2.3. Statistical Analyses
3. Results
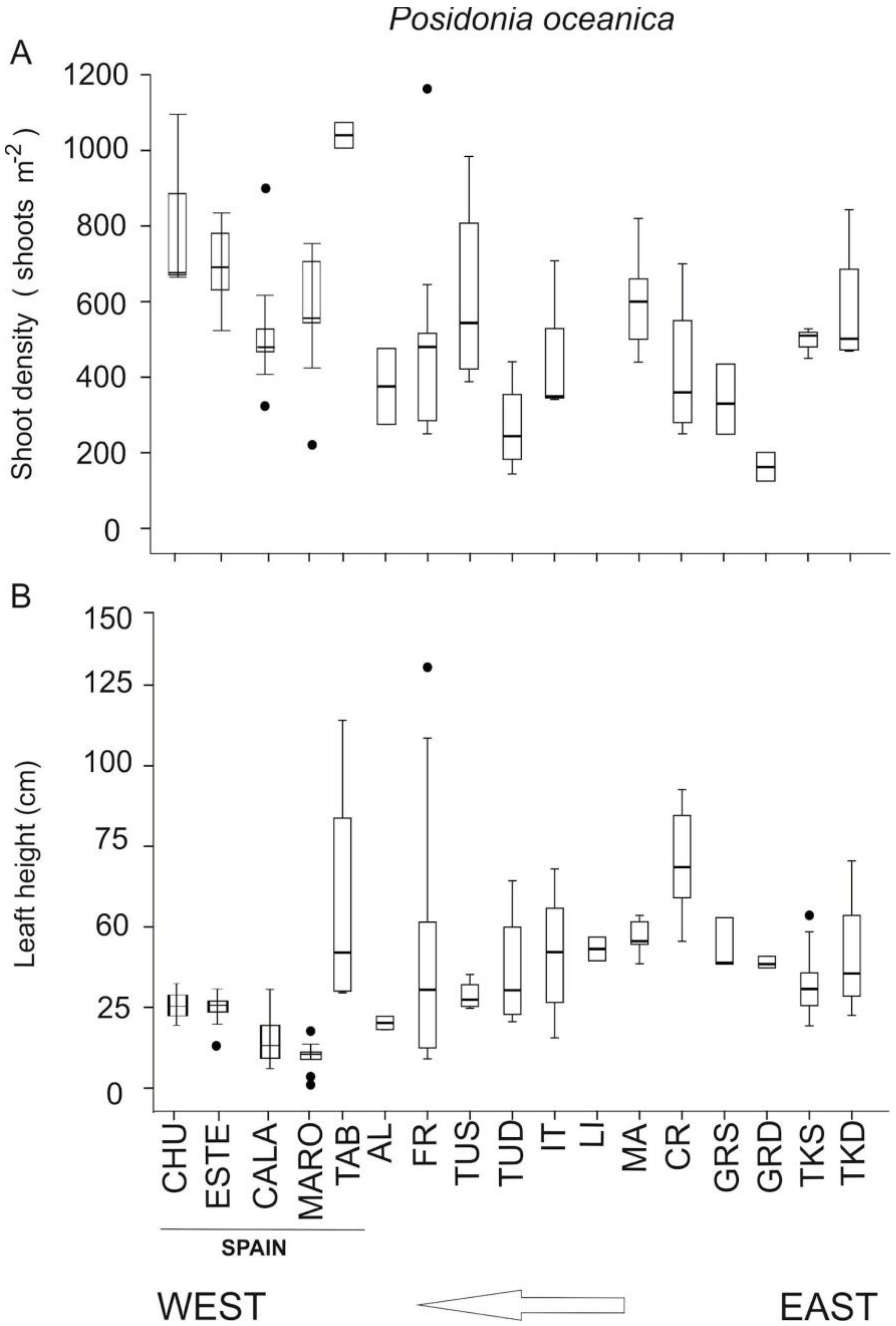
3.1. Spatial Variability along the Northwestern Alboran Sea
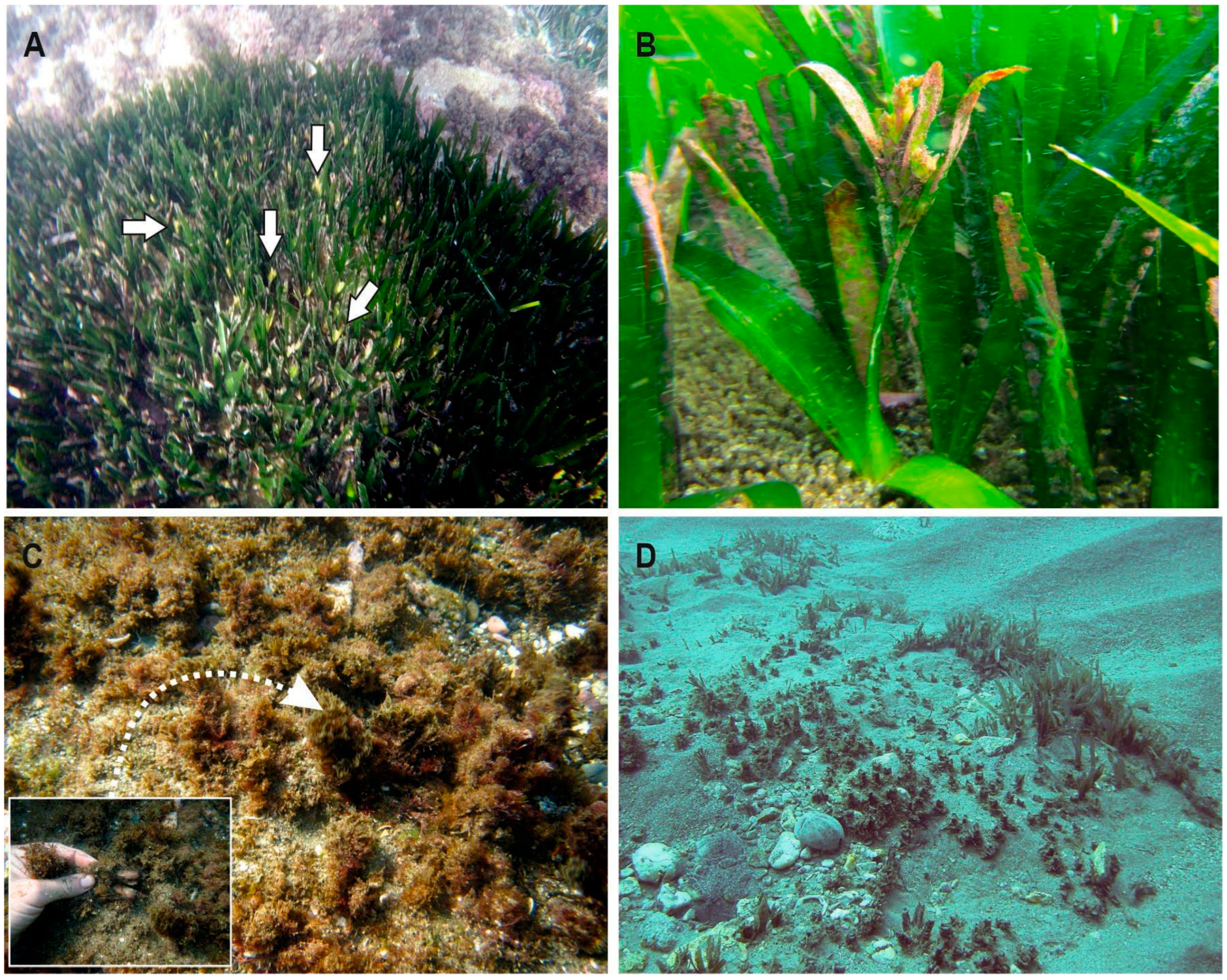
3.2. Flowering
3.3. Seasonal and Interannual Variability
3.4. Relationships between Phenological, Sediment and Environmental Variables
3.5. Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Mazzella, L.; Buia, M.C.; Gambi, M.C.; Lorenti, M.; Russo, G.F.; Scipione, M.B.; Zupo, V. Plant-animal trophic relationships in the Posidonia oceanica ecosystem of the Mediterranean Sea: A review. In Plant-Animal Interactions in the Marine Benthos; John, D.M., Howkins, S.J., Price, J.H., Eds.; Clarendon Press: Oxford, UK, 1992; pp. 165–187. [Google Scholar]
- Boudouresque, C.F.; Bernard, G.; Bonhomme, P.; Charbonnel, E.; Diviacco, G.; Meinesz, A.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Tunesi, L. Protection and Conservation of Posidonia oceanica Meadows; RAMOGE and RAC/SPA: Tunis, Tunisia, 2012; p. 202. ISBN 2905540311. [Google Scholar]
- Pasqualini, V.; Pergent-Martini, C.; Clabaut, P.; Pergent, G. Mapping of Posidonia oceanica using Aerial Photographs and Side Scan Sonar: Application off the Island of Corsica (France). Estuar. Coast. Shelf Sci. 1998, 47, 359–367. [Google Scholar] [CrossRef]
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar] [CrossRef]
- Luque, Á.A.; Templado, J. Praderas y Bosques Marinos de Andalucía; Consejería de Medio Ambiente Junta de Andalucía: Sevilla, Spain, 2004; p. 334. ISBN 9788496329225. [Google Scholar]
- Pergent-Martini, C.; Pergent, G.; Monnier, B.; Boudouresque, C.-F.; Mori, C.; Valette-Sansevin, A. Contribution of Posidonia oceanica meadows in the context of climate change mitigation in the Mediterranean Sea. Mar. Environ. Res. 2020, 165, 105236. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M. The future of seagrass meadows. Environ. Conserv. 2002, 29, 192–206. [Google Scholar] [CrossRef]
- Marbà, N.; Duarte, C.; Cebrián, J.; Gallegos, M.; Olesen, B.; Sand-Jensen, K. Growth and population dynamics of Posidonia oceanica on the Spanish Mediterranean coast: Elucidating seagrass decline. Mar. Ecol. Prog. Ser. 1996, 137, 203–213. [Google Scholar] [CrossRef]
- Marbà, N.; Duarte, C.M. Mediterranean warming triggers seagrass (Posidonia oceanica) shoot mortality. Glob. Chang. Biol. 2009, 16, 2366–2375. [Google Scholar] [CrossRef]
- Telesca, L.; Belluscio, A.; Criscoli, A.; Ardizzone, G.; Apostolaki, E.T.; Fraschetti, S.; Gristina, M.; Knittweis, L.; Martin, C.S.; Pergent, G. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 2015, 5, 12505. [Google Scholar] [CrossRef]
- Arroyo, M.C.; Barrajón, A.; Brun, F.; del Castillo, F.; de la Rosa, J.; Díaz-Almela, E.; Fernández-Casado, M.; Hernández, I.; Moreno, D.; Pérez-Lloréns, J.L.; et al. Praderas de angiospermas marinas de Andalucía. In Atlas de las Praderas Marinas de España; Ruiz, J.M., Guillén, J.E., Ramos-Segura, A., Otero, M.M., Eds.; IEO/IEL/UICN: Murcia-Alicante-Málaga, Spain, 2015; pp. 313–398. [Google Scholar]
- Celebi, B. A study on Posidonia oceanica (L.) Delile, 1813, seagrass meadows in the Levantine Sea. Master’s Thesis, METU Institute of Marine Science, Mersin, Turkey, 2007; p. 124. [Google Scholar]
- Pérez-Lloréns, J.L.; Vergara, J.J.; Olivé, I.; Mercado, J.M.; Conde-Álvarez, R.; Pérez-Ruzafa, Á.; Figueroa, F.L. Autochthonous seagrasses. In The Mediterranean Sea: Its history and Present Challenges; Goffredo, S., Dubinsky, Z., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 137–158. [Google Scholar] [CrossRef]
- Urra, J.; Gofas, S.; Rueda, J.L.; Marina, P. Molluscan assemblages in littoral soft bottoms of the Alboran Sea (Western Mediterranean Sea). Mar. Biol. Res. 2010, 7, 27–42. [Google Scholar] [CrossRef]
- Mateo-Ramírez, Á.; Urra, J.; Marina, P.; Rueda, J.L.; García Raso, J.E. Crustacean decapod assemblages associated with fragmented Posidonia oceanica meadows in the Alboran Sea (Western Mediterranean Sea): Composition, temporal dynamics and influence of meadow structure. Mar. Ecol. 2016, 37, 344–358. [Google Scholar] [CrossRef]
- Vargas-Yáñez, M.; García-Martínez, M.; Moya, F.; Balbín, R.; López-Jurado, J.; Serra, M.; Zunino, P.; Pascual, J.; Salat, J. Updating temperature and salinity mean values and trends in the Western Mediterranean: The RADMED project. Prog. Oceanogr. 2017, 157, 27–46. [Google Scholar] [CrossRef]
- Kinder, T.H.; Parrilla, G. Yes, some of the Mediterranean outflow does come from great depth. J. Geophys. Res. Earth Surf. 1987, 92, 2901. [Google Scholar] [CrossRef]
- Lafuente, J.G.; Vargas, J.M.; Sarhan, T.; Candela, J.; Bascheck, B.; Plaza, F. Tide at the eastern section of the Strait of Gibraltar. J. Geophys. Res. Earth Surf. 2000, 105, 14197–14213. [Google Scholar] [CrossRef]
- Rodríguez, J. Las reservas marinas en el marco ecológico y oceanográfico del Mediterráneo Occidental. In La gestión de los Espacios Marinos en el Mediterráneo Occidental; Guirado, J., Ed.; Instituto de Estudios Almerienses: Almería, Spain, 1995; pp. 13–28. Available online: https://isni.org/isni/000000005921782X (accessed on 27 December 2022).
- Sarhan, T. Upwelling mechanisms in the northwestern Alboran Sea. J. Mar. Syst. 2000, 23, 317–331. [Google Scholar] [CrossRef]
- Templado, J. La diversidad marina en España. In Biodiversidad: Aproximación a la Diversidad Botánica y Zoológica en España, Segunda época, Tomo IX; Viejo, J.L., Ed.; Memorias de la Real Sociedad Española de Historia Natural: Madrid, Spain, 2011; pp. 343–362. [Google Scholar]
- Rueda, J.L.; Gofas, S.; Aguilar, R.; de la Torriente, A.; Raso, J.E.G.; Iacono, C.L.; Luque, A.; Marina, P.; Mateo-Ramírez; Moya-Urbano, E.; et al. Benthic Fauna of Littoral and Deep-Sea Habitats of the Alboran Sea: A Hotspot of Biodiversity. In Alboran Sea—Ecosystems and Marine Resources; Báez, J.C., Vázquez, J.T., Camiñas, J.A., Malouli-Idrissi, M., Eds.; Springer Nature: Geneva, Switzerland, 2021; pp. 285–358. ISBN 9783030655167. [Google Scholar] [CrossRef]
- Agostini, V.N.; Bakun, A. Ocean triads’ in the Mediterranean Sea: Physical mechanisms potentially structuring reproductive habitat suitability (with example application to European anchovy, Engraulis encrasicolus). Fish. Oceanogr. 2002, 11, 129–142. [Google Scholar] [CrossRef]
- García, A. Post-larval research of small pelagic species in the Alboran Sea. A call for safeguarding fry concentration sites. In Proceedings of the CGPM—SAC Subcommittee of Marine Environment and Ecosystems (GFCM:SAC13/2011/Inf.5.), Malta, 26 November 29–December 2010. [Google Scholar]
- Quintanilla, J.M.; Laiz-Carrión, R.; García, A.; Quintanilla, L.F.; Cortés, D.; Gómez-Jakobsen, F.; Yebra, L.; Salles, S.; Putzeys, S.; León, P.; et al. Early life trophodynamic influence on daily growth patterns of the Alboran Sea sardine (Sardina pilchardus) from two distinct nursery habitats (bays of málaga and almería) in the western Mediterranean Sea. Mar. Environ. Res. 2020, 162, 105195. [Google Scholar] [CrossRef]
- Baro, J.; García, T.; Serna-Quintero, J.M. Description of artisanal fisheries in northern Alboran Sea. In Alboran Sea—Ecosystems and Marine Resources; Báez, J.C., Vázquez, J.T., Camiñas, J.A., Malouli-Idrissi, M., Eds.; Springer Nature: Geneva, Switzerland, 2021; pp. 521–542. ISBN 9783030655167. [Google Scholar] [CrossRef]
- Rueda, J.L.; Marina, P.; Urra, J.; Salas, C. Changes in the composition and structure of a molluscan assemblage due to eelgrass loss in southern Spain (Alboran Sea). J. Mar. Biol. Assoc. United Kingd. 2009, 89, 1319–1330. [Google Scholar] [CrossRef]
- Rueda, J.L.; Marina, P.; Urra, J.; Salas, C. Effects of eelgrass loss on coastal biodiversity. JMBA Glob. Mar. Environ. 2009, 10, 12–13. [Google Scholar]
- Bay, D. A field study of the growth dynamics and productivity of Posidonia oceanica (L.) delile in Calvi Bay, Corsica. Aquat. Bot. 1984, 20, 43–64. [Google Scholar] [CrossRef]
- Pergent, G.; Pergent Martini, C. Phénologie de Posidonia oceanica (L.) Delile dans le bassin Méditerranéen. Ann. De L’institut Océanograph. 1988, 64, 79–100. [Google Scholar]
- Buia, M.C.; Zupo, V.; Mazzella, L. Primary Production and Growth Dynamics in Posidonia oceanica. Mar. Ecol. 1992, 13, 2–16. [Google Scholar] [CrossRef]
- Sánchez Lizaso, J.L. Estudio de la Pradera de Posidonia oceanica (L.) Delile de la Reserva Marina de Tabarca (Alicante): Fenología y Producción Primaria. Ph.D. Thesis, University of Alicante, Alicante, Spain, 1993; p. 121. [Google Scholar]
- Guidetti, P.; Lorenti, M.; Buia, M.C.; Mazzella, L. Temporal Dynamics and Biomass Partitioning in Three Adriatic Seagrass Species: Posidonia oceanica, Cymodocea nodosa, Zostera marina. Mar. Ecol. 2002, 23, 51–67. [Google Scholar] [CrossRef]
- Marbà, N.; Duarte, C.M. Interannual changes in seagrass (Posidonia oceanica) growth and environmental change in the Spanish Mediterranean littoral zone. Limnol. Oceanogr. 1997, 42, 800–810. [Google Scholar] [CrossRef]
- Gobert, S. Variations Spatiale et Temporelle de L’herbier à Posidonia oceanica (L.) Delile. (Baie de La Revellata, Calvi, Corse). Ph.D. Thesis, University of Liège, Liege, Belgium, 2002; p. 207. [Google Scholar]
- Peirano, A.; Cocito, S.; Banfi, V.; Cupido, R.; Damasso, V.; Farina, G.; Lombardi, C.; Mauro, R.; Morri, C.; Roncarolo, I.; et al. Phenology of the Mediterranean seagrass Posidonia oceanica (L.) Delile: Medium and long-term cycles and climate inferences. Aquat. Bot. 2011, 94, 77–92. [Google Scholar] [CrossRef]
- Lesica, P.; Allendorf, F.W. When Are Peripheral Populations Valuable for Conservation? Conserv. Biol. 1995, 9, 753–760. [Google Scholar] [CrossRef]
- Gibson, S.Y.; Van Der Marel, R.C.; Starzomski, B.M. Climate Change and Conservation of Leading-Edge Peripheral Populations. Conserv. Biol. 2009, 23, 1369–1373. [Google Scholar] [CrossRef]
- Eckert, C.G.; Samis, K.E.; Lougheed, S.C. Genetic variation across species’ geographical ranges: The central–marginal hypothesis and beyond. Mol. Ecol. 2008, 17, 1170–1188. [Google Scholar] [CrossRef]
- Bennett, S.; Alcoverro, T.; Kletou, D.; Antoniou, C.; Boada, J.; Buñuel, X.; Cucala, L.; Jorda, G.; Kleitou, P.; Roca, G.; et al. Resilience of seagrass populations to thermal stress does not reflect regional differences in ocean climate. New Phytol. 2021, 233, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.F.; Engelen, A.H.; Serrao, E.A.; Coelho, M.A.G.; Marbà, N.; Krause-Jensen, D.; Gareth, A.P. Differentiation in fitness-related traits in response to elevated temperatures between leading and trailing edge populations of marine macrophytes. PLoS ONE 2018, 13, e0203666. [Google Scholar] [CrossRef] [PubMed]
- King, N.G.; McKeown, N.J.; Smale, D.A.; Wilcockson, D.C.; Hoelters, L.; Groves, E.A.; Stamp, T.; Moore, P.J. Evidence for different thermal ecotypes in range centre and trailing edge kelp populations. J. Exp. Mar. Biol. Ecol. 2019, 514–515, 10–17. [Google Scholar] [CrossRef]
- Mateo-Ramírez; Marina, P.; Moreno, D.; Valero, A.F.A.; Aguilar, R.; Báez, J.C.; Bárcenas, P.; Baro, J.; Caballero-Herrera, J.A.; Camiñas, J.A.; et al. Marine Protected Areas and Key Biodiversity Areas of the Alboran Sea and Adjacent Areas. In Alboran Sea, Ecosystems and Marine Resources; Báez, J.C., Vázquez, J.T., Camiñas, J.A., Malouli-Idrissi, M., Eds.; Springer Nature: Geneva, Switzerland, 2021; pp. 819–923. ISBN 9783030655167. [Google Scholar] [CrossRef]
- Ruiz, J.M.; Guillén, E.; Ramos-Segura, A.; Otero, M. Atlas de las Praderas Marinas de España; IEO/IEL/UICN: Murcia-Alicante-Málaga, Spain, 2015; p. 681. [Google Scholar]
- Ramírez, T.; Cortés, D.; Mercado, J.; Vargas-Yañez, M.; Sebastián, M.; Liger, E. Seasonal dynamics of inorganic nutrients and phytoplankton biomass in the NW Alboran Sea. Estuarine, Coast. Shelf Sci. 2005, 65, 654–670. [Google Scholar] [CrossRef]
- García-Gómez, C.; Yebra, L.; Cortés, D.; Sánchez, A.; Alonso, A.; Valcárcel-Pérez, N.; Gómez-Jakobsen, F.; Herrera, I.; Johnstone, C.; Mercado, J.M. Shifts in the protist community associated with an anticyclonic gyre in the Alboran Sea (Mediterranean Sea). FEMS Microbiol. Ecol. 2020, 96, fiaa197. [Google Scholar] [CrossRef]
- Junta de Andalucía. Red de Información Ambiental de Andalicía. REDIAM, pagina de Descargas. Available online: https://portalrediam.cica.es/descargas (accessed on 4 June 2022).
- Ministerio de Transporte, Movilidad y Agencia Urbana, Gobierno de España. Datos Oceanográficos. Available online: https://www.puertos.es/es-es/oceanografia/Paginas/portus.aspx (accessed on 15 February 2022).
- Amoutzpoulou-Schina, H.; Haritonidis, S. Distribution and phenology of the marine phanerogam Posidonia oceanica in the Pagassitikos Gulf, Greece. J. Biol. Res. 2005, 4, 203–211. [Google Scholar]
- Kruzic, P. Variations in Posidonia oceanica meadow structure along the coast of the Dugi Otok Island (eastern Adriatic Sea). J. Mar. Biol. Assoc. 2008, 88, 883–892. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Leoni, V.; Pasqualini, V.; Ardizzone, G.D.; Balestri, E.; Bedini, R.; Belluscio, A.; Belsher, T.; Borg, J.; Boudouresque, C.F.; et al. Descriptors of Posidonia oceanica meadows: Use and application. Ecol. Indic. 2005, 5, 213–230. [Google Scholar] [CrossRef]
- Conde, F.; Seoane, J.A. Corología de las especie de algas en relación a ciertos factores ecológicos en el litoral malagueño. Collect. Bot. 1982, 13, 783–802. [Google Scholar]
- Flores-Moya, A.; Vera-Gonzalez, J.J.; Conde, F. Contribución a la corología de las macroalgas marinas bentónicas del litoral malagueño. Acta Bot. Malac. 1989, 14, 199–201. [Google Scholar] [CrossRef]
- Jeffrey, S.; Humphrey, G.T. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and phytoplankton. Biochem. Physiol. Pflanzen 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Lopez y Royo, C.; Casazza, G.; Pergent-Martini, C.; Pergent, G. A biotic index using the seagrass Posidonia oceanica (BiPo), to evaluate ecological status of coastal waters. Ecol. Indic. 2010, 1, 380–389. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Moreno, D.; Guirado, J. Nuevos datos sobre la distribución de las fanerógamas marinas en las provincias de Almería y Granada (SE España). Acta Bot. Malacit. 2003, 28, 105–120. [Google Scholar] [CrossRef]
- Báez, J.C.; Vázquez, J.T.; Camiñas, J.A.; Malouli, M. Alboran Sea—Ecosystems and Marine Resources; Springer Nature: Geneva, Switzerland, 2021; p. 939. ISBN 9783030655167. [Google Scholar] [CrossRef]
- García Raso, J.E.; Gofas, S.; Salas Casanova, C.; Manjón-Cabeza, E.; Urra, J.; García Muñóz, J.E. El Mar Más rico de Europa: Biodiversidad del Litoral Occidental de Málaga Entre Calaburras y Calahonda; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2010; p. 138. ISBN 9788492807499. [Google Scholar]
- Urra, J.; Rueda, J.L.; Gofas, S.; Marina, P.; Salas, C. A species-rich molluscan assemblage in a coralligenous bottom of the Alboran Sea (south-western Mediterranean): Intra-annual changes and ecological considerations. J. Mar. Biol. Assoc. United Kingd. 2011, 92, 665–677. [Google Scholar] [CrossRef]
- Lazzari, P.; Solidoro, C.; Ibello, V.; Salon, S.; Teruzzi, A.; Béranger, K.; Colella, S.; Crise, A. Seasonal and inter-annual variability of plankton chlorophyll and primary production in the Mediterranean Sea: A modelling approach. Biogeosciences 2012, 9, 217–233. [Google Scholar] [CrossRef]
- Ramírez, T.; Muñoz, M.; Reul, A.; García-Martínez, M.C.; Moya, F.; Vargas-Yáñez, M.; Bautista, B. The Biogeochemical Context of Marine Planktonic Ecosystems. In Alboran Sea, Ecosystems and Marine Resources; Báez, J.C., Vázquez, J.T., Camiñas, J.A., Malouli-Idrissi, M., Eds.; Springer Nature: Geneva, Switzerland, 2021; pp. 207–246. ISBN 9783030655167. [Google Scholar] [CrossRef]
- Sand-Jensen, K. Biomass, net production and growth dynamics in an eelgrass (Zostera marina L.) population in Vellerup Vig, Denmark. Ophelia 1975, 14, 185–201. [Google Scholar] [CrossRef]
- Fonseca, M.S.; Zieman, J.C.; Thayer, G.W.; Fisher, J.S. The role of current velocity in structuring eelgrass (Zostera marina) meadows. Estuar. Coast. Shelf Sci. 1983, 17, 367–380. [Google Scholar] [CrossRef]
- Reyes, J.; Sansón, M.; Carrillo, A. Leaf phenology, growth and production of the seagrass Cymodocea nodosa at El Médano (South of Tenerife, Canary Islands). Bot. Mar. 1995, 38, 457–465. [Google Scholar] [CrossRef]
- Turk, R.; Vukovic, A. Phenology of Posidonia oceanica (L.) Delile in the Gulf of Koper (Gulf of Trieste), North Adriatic. Rapp. Comm. Int. Pour L’exploration Sci. De La Mer Méditerranée 1998, 35, 592–593. [Google Scholar]
- Ballesta, L.; Pergent, G.; Pasqualini, V.; Pergent-Martini, C. Distribution and Dynamics of Posidonia oceanica Beds along the Albères Coastline; Elsevier: Amsterdam, The Netherlands, 2000; Volume 323, pp. 407–414. [Google Scholar] [CrossRef]
- Rubegni, F.; Franchi, E.; Lenzi, M. Relationship between wind and seagrass meadows in a non-tidal eutrophic lagoon studied by a Wave Exposure Model (WEMo). Mar. Pollut. Bull. 2013, 70, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Pace, M.; Borg, J.A.; Galdies, C.; Malhotra, A. Influence of wave climate on architecture and landscape characteristics of Posidonia oceanica meadows. Mar. Ecol. 2016, 38, e12387. [Google Scholar] [CrossRef]
- Mabrouk, L.; Hamza, A.; Sahraoui, H.; Bradai, M.N. Donnees sur les caracteristiques et la phenology de l’herbier de Posidonia oceanica (L.) Delile sur les côtes de mahdia (región est de la Tunisie). Bull. Inst. Natl. Sci. Tech. Oceanogr. Peche Salammbo 2009, 36, 139–147. [Google Scholar]
- Abbate, M.; Peirano, A.; Ugolini, U. Structural changes in Posidonia oceanica leaves along the coast of Liguria (Italy): Response to environmental stress? Biol. Mar. Medit. 2000, 7, 320–323. [Google Scholar]
- Giovannetti, E.; Lasagna, R.; Montefalcone, M.; Bianchi, C.; Albertelli, G.; Morri, C. Inconsistent responses to substratum nature in Posidonia oceanica meadows: An integration through complexity levels? Chem. Ecol. 2008, 24, 83–91. [Google Scholar] [CrossRef]
- Sghaier, Y.R.; Zakhama, R.S.; Charfi, F.C. Status of Posidonia oceanica meadows along the Eastern coast of Tunisia. Biol. Mar. Medit. 2006, 13, 85–91. [Google Scholar]
- Pergent, G.; Gucu, A.C.; Sakinan, S.; Pergent-Martini, C. Cartographie et surveillance de l´herbier de Mersin (Turquie). Programme MedPosidonia /SPA/RAC, Fondation d´entrepise. TOTAL pour La Biodiversité Et La Mer, Memorandun dáccord 01/SPA/CAR, MedPosidonia. 2008; 1–16. [Google Scholar]
- Dural, B. Phenological observations on Posidonia oceanica (L.) Delile medows along the coast of Akkum (Sıgacık Bay, Aegean Sea, Turkey). J. Black Sea/Medit. Environ. 2010, 16, 133–144. [Google Scholar]
- Peduzzi, P.; Vukovic, A. Primary production of Cymodocea nodosa in the Gulf of Trieste (Northern Adriatic Sea): A comparison of methods. Mar. Ecol. Prog. Ser. 1990, 64, 197–207. [Google Scholar] [CrossRef]
- Semroud, R.; Benkorteby, N.; Tamouza, F.Z. Phenologie de Posidonia oceanica dans la region d´Alger (Algerie): Donnes preliminaires sur la biometriefoliare. Rapp. Comm. Int. Pour L’exploration Sci. De La Méditerranée 1990, 32, B-I16: 10-11. [Google Scholar]
- Pergent, G.; Djellouli, A.; Hamza, A.A.; Ettayeb, K.S.; El Mansouri, A.A.; Talha, F.M.; Hamza, M.A.A.; Pergent-Martini, C.; Platini, F. Characterization of the benthic vegetation in the FarwàLaggon (Libya). J. Coast. Conser. 2002, 8, 119–126. [Google Scholar] [CrossRef]
- Borg, J.A.; Attrill, M.J.; Rowden, A.A.; Schembri, P.J.; Jones, M.B. Architectural characteristics of two bed types of the seagrass Posidonia oceanica over different spatial scales. Estuar. Coast. Shelf Sci. 2005, 62, 667–678. [Google Scholar] [CrossRef]
- Urra, J.; Mateo; Marina, P.; Rueda, J.L.; Raso, J.E.G. First records of Posidonia oceanica flowering at its westernmost distributional limit (Málaga, Alboran Sea). Bot. Mar. 2011, 54, 101–104. [Google Scholar] [CrossRef]
- Balestri, E. Flowering of the seagrass Posidonia oceanica in a north-western Mediterranean coastal area: Temporal and spatial variations. Mar. Biol. 2004, 145, 61–68. [Google Scholar] [CrossRef]
- Montefalcone, M.; Giovannetti, E.; Morri, C.; Peirano, A.; Bianchi, C.N. Flowering of the seagrass Posidonia oceanica in NW Mediterranean: Is there a link with solar activity? Mediterr. Mar. Sci. 2013, 14, 416. [Google Scholar] [CrossRef]
- Balestri, E.; Vallerini, F.; Lardicci, C. Recruitment and patch establishment by seed in the seagrass Posidonia oceanica: Importance and conservation implications. Front Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Almela, E.; Marbà, N.; Duarte, C.M. Consequences of Mediterranean warming events in seagrass (Posidonia oceanica) flowering records. Glob. Chang. Biol. 2006, 13, 224–235. [Google Scholar] [CrossRef]
- Ruiz, J.; Marín-Guirao, L.; García-Muñoz, R.; Ramos-Segura, A.; Bernardeau-Esteller, J.; Pérez, M.; Sanmartí, N.; Ontoria, Y.; Romero, J.; Arthur, R.; et al. Experimental evidence of warming-induced flowering in the Mediterranean seagrass Posidonia oceanica. Mar. Pollut. Bull. 2017, 134, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Balestri, E.; Cinelli, F. Sexual reproductive success in Posidonia oceanica. Aquat. Bot. 2003, 75, 21–32. [Google Scholar] [CrossRef]
- Balestri, E.; Vallerini, F.; Lardicci, C. On the unusual flowering of plagiotropic shoots in the seagrass Posidonia oceanica. Aquat. Bot. 2005, 82, 82–88. [Google Scholar] [CrossRef]
- Cabaço, S.; Santos, R. Seagrass reproductive effort as an ecological indicator of disturbance. Ecol. Indic. 2012, 23, 116–122. [Google Scholar] [CrossRef]
- Consejería de Agricultura, Ganadería, Pesca y Desarrollo Sostenible, Junta de Andalucía. Informe Final de Resultados del Programa de Gestión Sostenible del Medio Marino Andaluz, 2020. p. 168. Available online: https://www.juntadeandalucia.es/medioambiente/portal/landing-page-documento/-/asset_publisher/jXKpcWryrKar/content/informes-regionales-sobre-gesti-c3-b3n-sostenible-del-medio-marino-andaluz-2008-2018-/20151 (accessed on 14 April 2022).
- Urra, J.; Marina, P.; Rueda, J.L.S.O.S. de las praderas de fanerógamas marinas en la Costa del Sol. Quercus 2008, 270, 28–37. [Google Scholar]



| Source of Variation | df | MS | F | p |
|---|---|---|---|---|
| Shoot density | ||||
| Site | 3 | 77,026 | 5.7217 | ** |
| Patch size | 2 | 9290.4 | 0.69011 | 0.5228 |
| Site × Patch size | 5 | 30,653 | 2.2769 | 0.0767 |
| Residual | 21 | 13,462 | ||
| Total | 31 | |||
| Leaf height | ||||
| Site | 3 | 1215.5 | 66.207 | *** |
| Patch size | 2 | 397.53 | 21.653 | *** |
| Site × Patch size | 5 | 143.97 | 7.8415 | *** |
| Residual | 309 | 18.359 | ||
| Total | 319 | |||
| Number of leaves | ||||
| Site | 3 | 8.7974 | 13.739 | *** |
| Patch size | 2 | 3.1323 | 4.8916 | ** |
| Site × Patch size | 5 | 2.4972 | 38,998 | ** |
| Residual | 309 | 0.64035 | ||
| Total | 319 | |||
| Inflorescences density | ||||
| Patch size | 2 | 15,900 | 2.1032 | 0.2107 |
| Residual | 6 | 7559.8 | ||
| Total | 8 |
| Source of Variation | Surface Seawater Temperature | Solar Irradiance | Chlorophyll a | Percentage of Organic Matter | |
|---|---|---|---|---|---|
| Calaburras | Year | F = 4.033 * | -- | F = 2.896 | F = 0.898 |
| p = 0.068 | p = 0.414 | ||||
| Season | F = 75.677 *** | F = 2.075 | F = 5.324 ** | F = 6.474 *** | |
| p = 0.164 | |||||
| Year × Season | F = 19.292 *** | -- | F = 4.971 ** | F = 1.315 | |
| p = 0.263 | |||||
| Calahonda | Year | F = 1.172 | -- | F = 6.575 ** | F = 1.822 |
| p = 0.344 | p = 0.171 | ||||
| Season | F = 8.872 ** | F = 1.410 | F = 4.410 * | F = 10.323 *** | |
| p = 0.307 | |||||
| Year × Season | F = 0.646 | -- | F = 3.947 * | F = 2.324 * | |
| p = 0.639 | |||||
| Among sampling stations | F = 0.195 | F = 3.410 | F = 7.136 ** | F = 4.498 * | |
| p = 0.657 | p = 0.078 | ||||
| Source of Variation | Calaburras | Calahonda | ||||||
|---|---|---|---|---|---|---|---|---|
| df | MS | F | p | df | MS | F | p | |
| Shoot density | ||||||||
| Year | 2 | 12,801 | 1.178 | 0.324 | 2 | 14,726 | 1.616 | 0.206 |
| Season | 3 | 11,404 | 1.05 | 0.382 | 3 | 162,810 | 17.867 | *** |
| Year × Season | 5 | 23,106 | 2.127 | 0.080 | 5 | 50,290 | 5.519 | *** |
| Residual | 43 | 10,865 | 43 | 9112.4 | ||||
| Total | 53 | 53 | ||||||
| Leaf height | ||||||||
| Year | 2 | 136.59 | 8.005 | * | 2 | 214.8 | 20.952 | *** |
| Season | 3 | 1449 | 84.921 | *** | 3 | 1052.8 | 102.7 | *** |
| Year × Season | 5 | 97.508 | 5.715 | *** | 5 | 32.055 | 3.1267 | * |
| Residual | 43 | 17.062 | 43 | 10.252 | ||||
| Total | 53 | 53 | ||||||
| Number of leaves | ||||||||
| Year | 2 | 0.361 | 2.583 | 0.084 | 2 | 0.098 | 0.751 | 0.473 |
| Season | 3 | 1.39 | 9.943 | *** | 3 | 1.713 | 13.176 | *** |
| Year × Season | 5 | 0.232 | 1.663 | 0.167 | 5 | 0.175 | 1.349 | 0.267 |
| Residual | 43 | 0.14 | 43 | 0.13 | ||||
| Total | 53 | 53 | ||||||
| Coefficient | SE | F Ratio | R2 | p | |
|---|---|---|---|---|---|
| Number of leaves | |||||
| Constant | 6.308 | 0.311 | |||
| Temperature | −0.037 | 0.17 | 4.573 | ||
| Number of leaves | 0.479 | * | |||
| Constant | 10.635 | 1.929 | |||
| Irradiance | −1.319 | 0.52 | 6.424 | ||
| Shoot density | 0.594 | * | |||
| Constant | −571.8 | 431.4 | |||
| Irradiance | 372.4 | 116.3 | 10.255 |
| Site-Sampling Station (Year) | Lower Limit Depth (m) | Lower Limit Type | Shoot Density (Shoots m−2) | Leaf Height (mm) | EQR | Class | |
|---|---|---|---|---|---|---|---|
| Chullera | (2015) | 2 | Sharp | 753 | 258 | 0.63 | Good |
| Estepona SAC | (2015) | 4 | Sharp | 662 | 253 | 0.61 | Good |
| Calahonda SAC | Calaburrras (2007–2010) | 5 | Sharp | 849 | 250 | 0.61 | Good |
| Calahonda (2007–2010) | 5 | Sharp | 945 | 237 | 0.64 | Good | |
| Calahonda (2015) | 5 | Sharp | 514 | 221 | 0.57 | Good | |
| Maro MPA | (2015) | 12 | Sparse | 550 | 176 | 0.52 | Moderate |
| Studied Area | Country | Reference | Shoot Density | Leaf Height | Number of Leaves |
|---|---|---|---|---|---|
| Calaburras | Spain | Present study | 13.1 | 38.7 | 8.5 |
| Calahonda | Spain | Present study | 17.4 | 34.7 | 8.3 |
| Tabarca | Spain | [31] | 58.4 | 10.7 | |
| Banyuls-sur-Mer | France | [29] | 23.5 | 6.7 | |
| Port-Cros | France | [29] | 39.6 | 19.4 | |
| La Revellata Bay (Corsica) | France | [34] | 20.6 | 30 | 18.4 |
| Monterosso al Mare | Italy | [35] | 42.4 | 43.7 | 20.6 |
| Lacco Ameno (Ischia Island) | Italy | [1] | 32.3 | 10.4 | |
| Otranto (Apulia) | Italy | [32] | 41.2 | 34.5 | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateo-Ramírez, Á.; Marina, P.; Martín-Arjona, A.; Bañares-España, E.; García Raso, J.E.; Rueda, J.L.; Urra, J. Posidonia oceanica (L.) Delile at Its Westernmost Biogeographical Limit (Northwestern Alboran Sea): Meadow Features and Plant Phenology. Oceans 2023, 4, 27-48. https://doi.org/10.3390/oceans4010003
Mateo-Ramírez Á, Marina P, Martín-Arjona A, Bañares-España E, García Raso JE, Rueda JL, Urra J. Posidonia oceanica (L.) Delile at Its Westernmost Biogeographical Limit (Northwestern Alboran Sea): Meadow Features and Plant Phenology. Oceans. 2023; 4(1):27-48. https://doi.org/10.3390/oceans4010003
Chicago/Turabian StyleMateo-Ramírez, Ángel, Pablo Marina, Alejandro Martín-Arjona, Elena Bañares-España, José E. García Raso, José L. Rueda, and Javier Urra. 2023. "Posidonia oceanica (L.) Delile at Its Westernmost Biogeographical Limit (Northwestern Alboran Sea): Meadow Features and Plant Phenology" Oceans 4, no. 1: 27-48. https://doi.org/10.3390/oceans4010003
APA StyleMateo-Ramírez, Á., Marina, P., Martín-Arjona, A., Bañares-España, E., García Raso, J. E., Rueda, J. L., & Urra, J. (2023). Posidonia oceanica (L.) Delile at Its Westernmost Biogeographical Limit (Northwestern Alboran Sea): Meadow Features and Plant Phenology. Oceans, 4(1), 27-48. https://doi.org/10.3390/oceans4010003






