Effect of Temperature on the Daily Increment Deposition in the Otoliths of European Sardine Sardina pilchardus (Walbaum, 1792) Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Rearing Experiment
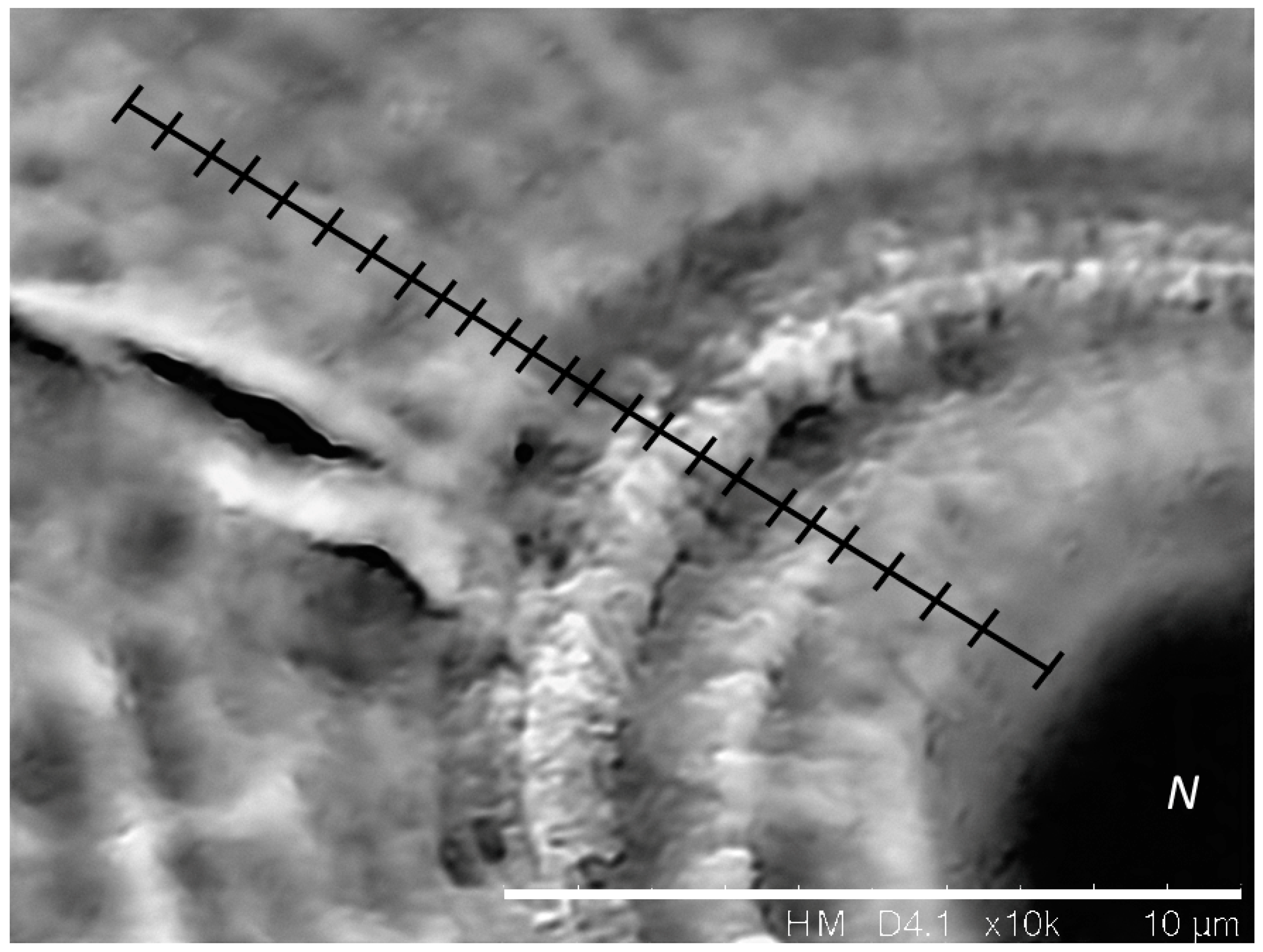
2.2. Otoliths Extraction, Preparation, and Analysis
2.3. Data Analysis
3. Results
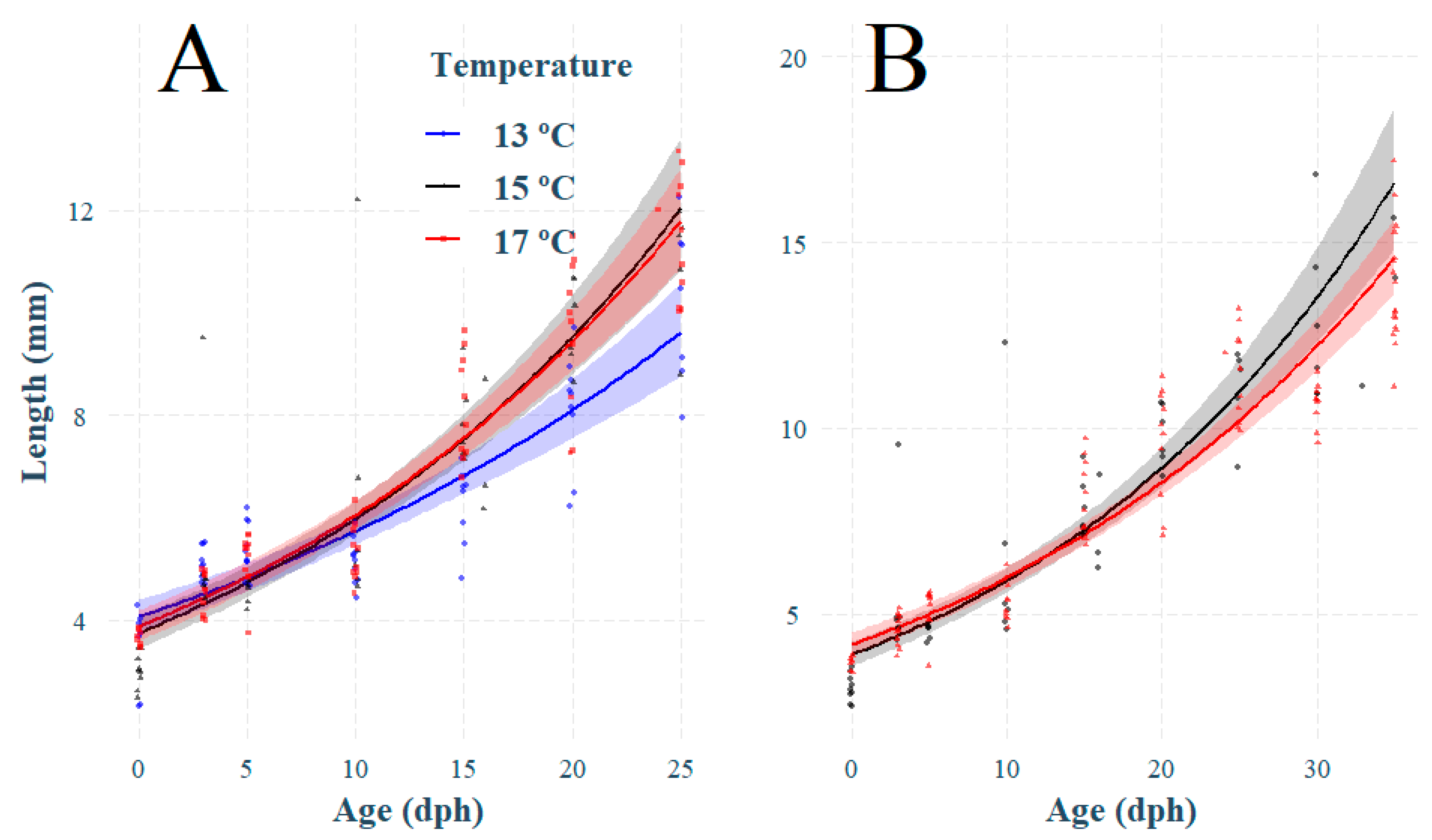
3.1. Effect of Temperature on Larval Growth
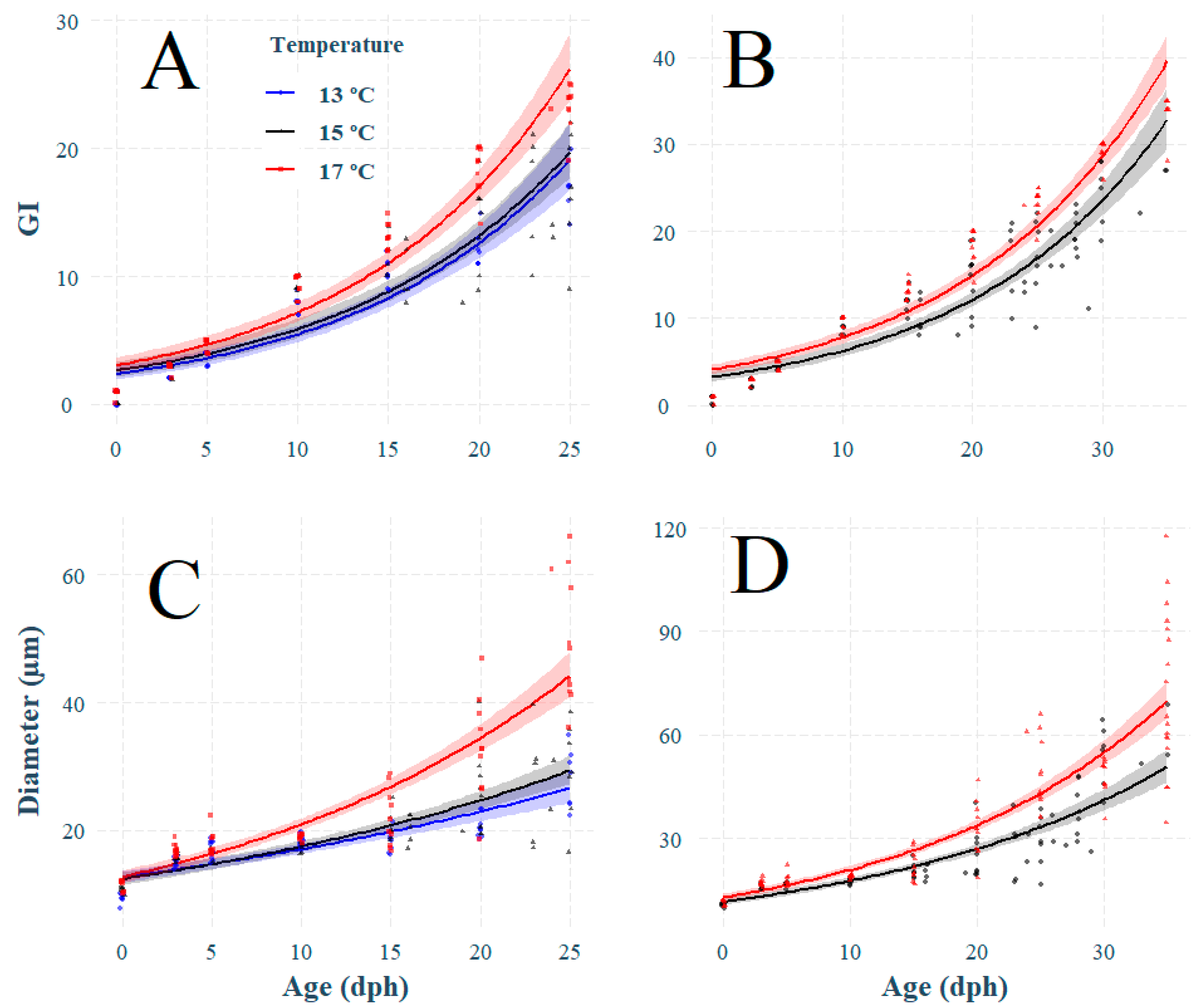
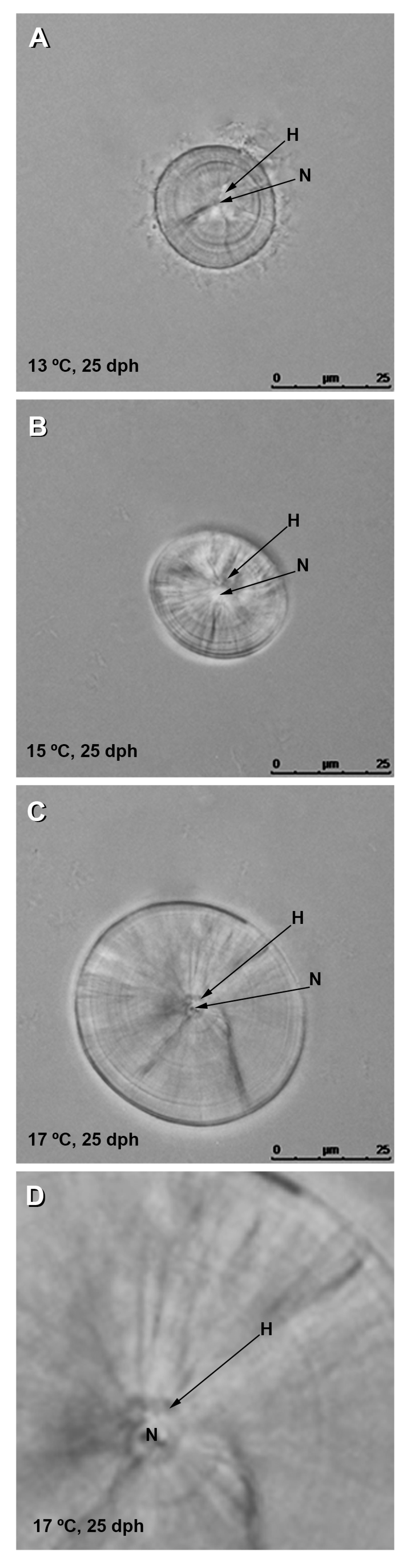
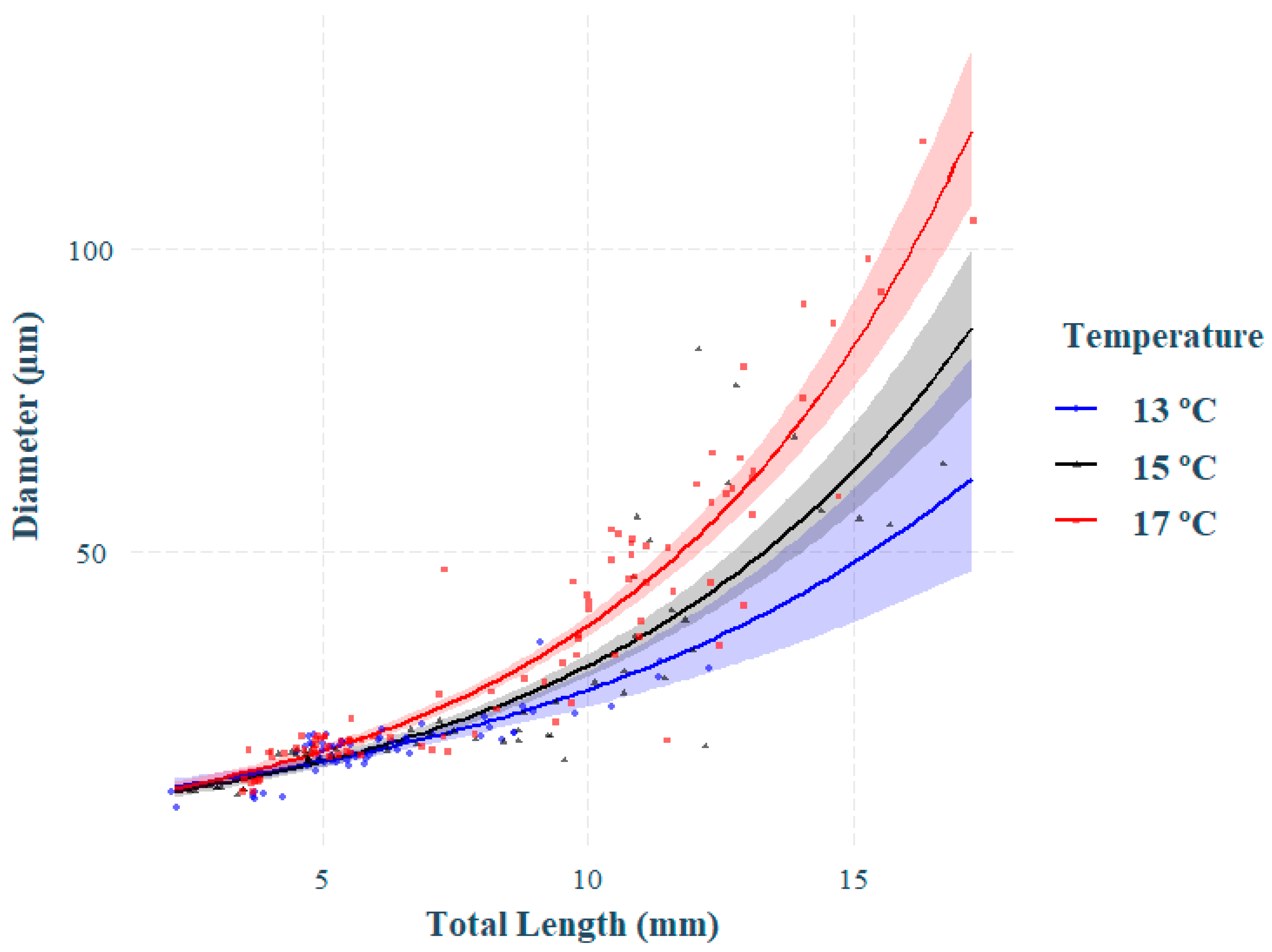
3.2. Effect of Temperature on Otolith Growth and Microstructure
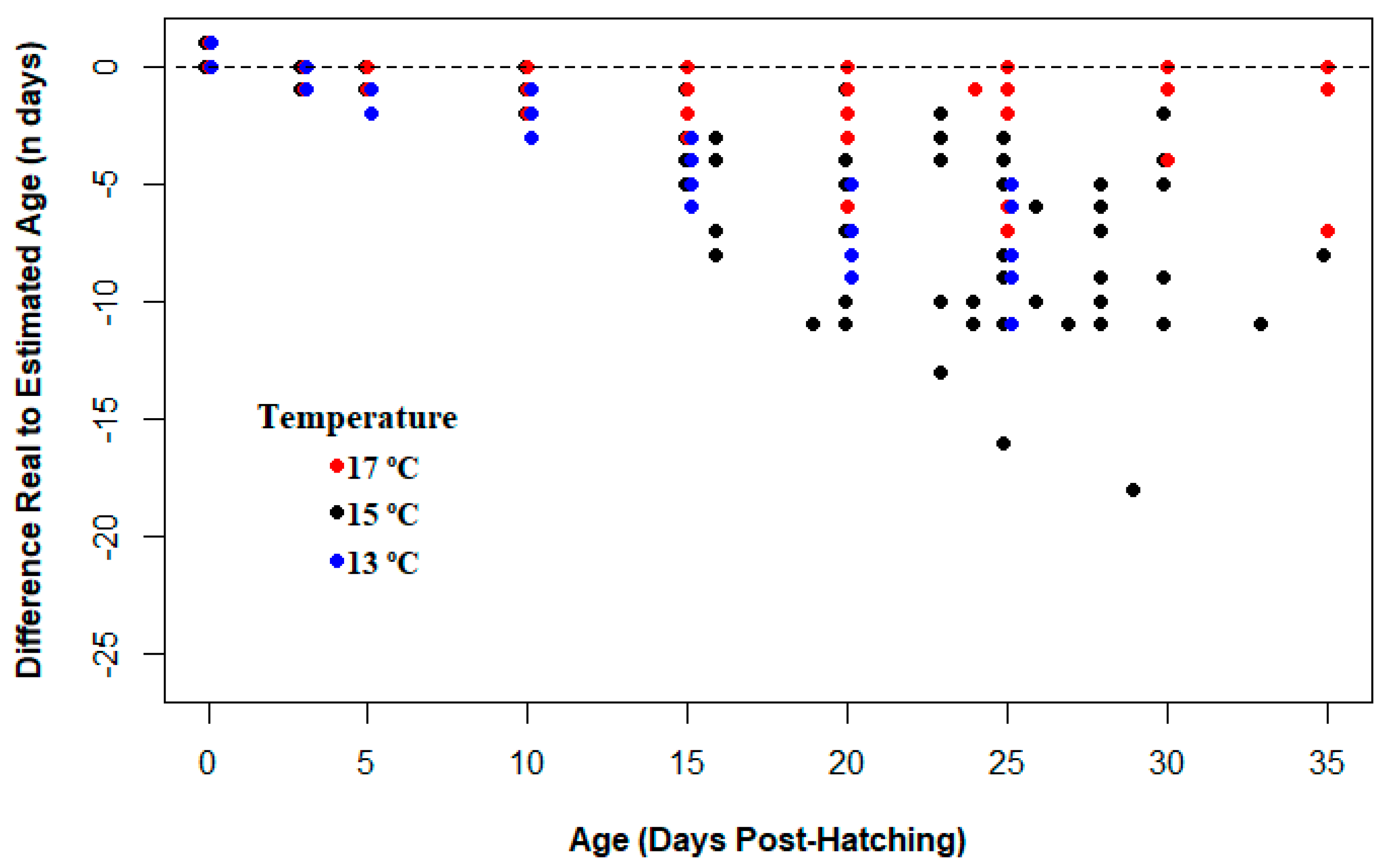
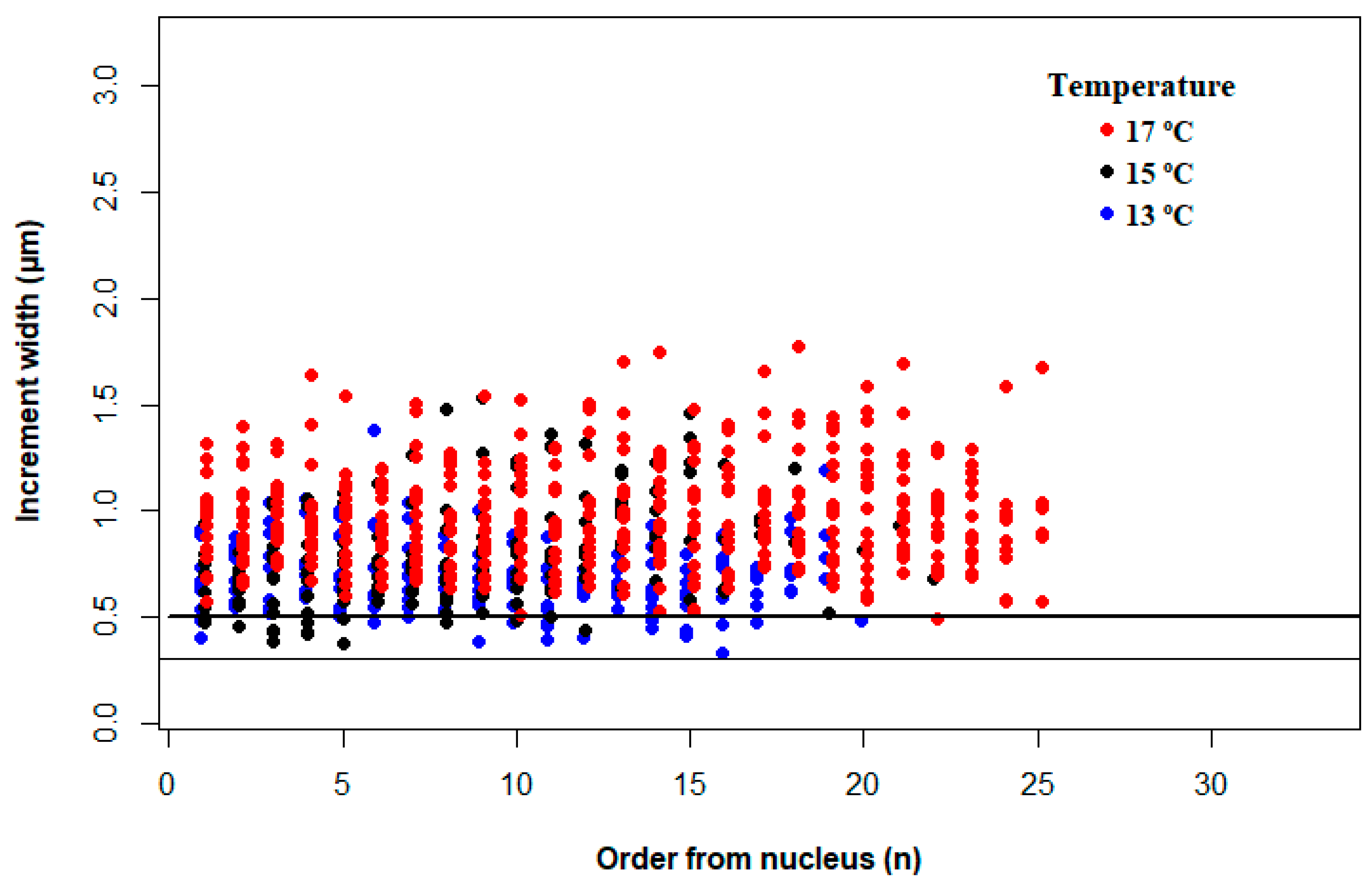
3.3. Effect of Temperature on Otolith Increment Deposition
4. Discussion
4.1. Effect of Temperature on Growth, Survival, and Otolith Development
4.2. Effect of Temperature on Increment Growth Formation
4.3. Implications for Stock Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlson, A.K.; Phelps, Q.E.; Graeb, D.S. Chemistry to conservation: Using otoliths to advance recreational and commercial fisheries management. J. Fish Biol. 2016, 90, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Campana, S.E. Measurement and interpretation of the microstructure of fish otoliths. In Otolith Microstructure Examination and Analysis; Stevenson, D.K., Campana, S.E., Eds.; Canadian Special Publication of Fisheries and Aquatic Sciences: Ottawa, ON, Canada, 1992; pp. 59–71. [Google Scholar]
- Baumann, H.; Hinrichsen, H.-H.; Voss, R.; Stepputtis, D.; Grygiel, W.; Clausen, L.W.; Temming, A. Linking growth to environmental histories in central Baltic young-of-the-year sprat, Sprattus sprattus: An approach based on otolith microstructure analysis and hydrodynamic modelling. Fish. Oceanogr. 2006, 15, 465–476. [Google Scholar] [CrossRef]
- Folkvord, A.; Mosegaard, H.V. Some uses of individual age data. A. Age and growth analysis. In Manual of Fish Sclerochronology; Panfili, J., Troadec, H., de Pontual, H., Wright, P.J., Eds.; Ifremer–IRD co-edition: Brest, France, 2002; pp. 146–166. [Google Scholar]
- Mosegaard, H.; Folkvord, A.; Wright, P.J.V. Some uses of individual age data. B. Ecological applications. In Manual of Fish Sclerochronology; Panfili, J., Troadec, H., de Pontual, H., Wright, P.J., Eds.; Ifremer–IRD co-edition: Brest, France, 2002; pp. 167–178. [Google Scholar]
- Álvarez, F.; Alemany, F. Regional growth differences in sardine (Sardina pilchardus Walb.) larvae from Catabrian and Galician coasts. ICES. CM 1992, H:9, 10p. [Google Scholar]
- Geffen, A.J.; Otolith Microstructure Examination and Analysis. CHAPTER 6: Validation of Otolith Increment Deposition Rate. In Otolith Microstructure Examination and Analysis; Stevenson, D.K., Campana, S.E., Eds.; Canadian Special Publication of Fisheries and Aquatic Sciences: Ottawa, Canada, 1992; Volume 117, pp. 101–113. [Google Scholar]
- Ré, P.; Gonçalves, E. Growth of sprat Sprattus sprattus larvae in the German Bight (North Sea) as inferred by otolith microstructure. Mar. Ecol. Prog. Ser. 1993, 96, 139–145. [Google Scholar] [CrossRef]
- Feet, P.Ø.; Ugland, K.I.; Moksness, E. Accuracy of age estimates in spring spawning herring (Clupea harengus L.) reared under different prey densities. Fish. Res. 2002, 56, 59–67. [Google Scholar] [CrossRef]
- Stenevik, E.K.; Fossum, P.; Johannessen, A.; Folkvord, A. Identification of Norwegian spring spawning herring (Clupea harengus L.) larvae from spawning grounds off western Norway applying otolith microstructure analysis. Sarsia 1996, 80, 285–292. [Google Scholar] [CrossRef]
- Allain, G.; Petitgas, P.; Lazure, P.; Grellier, P. Biophysical modelling of larval drift, growth and survival for the prediction of anchovy (Engraulis encrasicolus) recruitment in the Bay of Biscay (NE Atlantic). Fish. Oceanogr. 2007, 16, 489–505. [Google Scholar] [CrossRef]
- Folkvord, A.; Johannessen, A.; Moksness, E. Temperature-dependent otolith growth in Norwegian spring-spawning herring (Clupea harengus L.) larvae. Sarsia 2004, 89, 297–310. [Google Scholar] [CrossRef]
- Fitzhugh, G.R.; Nixon, S.W.; Ahrenholz, D.W.; Rice, J.A. Temperature Effects on Otolith Microstructure and Birth Month Estimation from Otolith Increment Patterns in Atlantic Menhaden. Trans. Am. Fish. Soc. 1997, 126, 579–593. [Google Scholar] [CrossRef]
- Folkvord, A.; Blom, G.; Johannessen, A.; Moksness, E. Growth-dependent age estimation in herring (Clupea harengus L.) larvae. Fish. Res. 2000, 46, 91–103. [Google Scholar] [CrossRef]
- Fey, D.P. The effect of temperature and somatic growth on otolith growth: The discrepancy between two clupeid species from a similar environment. J. Fish Biol. 2006, 69, 794–806. [Google Scholar] [CrossRef]
- Payne, M.R.; Ross, S.D.; Chausen, L.W.; Munk, P.; Mosegaard, H.; Nash, R.D.M. Recruitment decline in North Sea herring is accompanied by reduced larval growth rates. Mar. Ecol. Prog. Ser. 2013, 489, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Moksness, E. Validation of daily increments in the otolith microstructure of Norwegian spring-spawning herring (Clupea harengus L.). ICES J. Mar. Sci. J. Du Cons. 1992, 49, 231–235. [Google Scholar] [CrossRef]
- Cieri, M.D.; McCleave, J.D. Validation of daily otolith increments in glass-phase American eels Anguilla rostrate (Lesueur) during estuarine residency. J. Exp. Mar. Biol. Ecol. 2001, 257, 219–227. [Google Scholar] [CrossRef]
- Kano, Y.; Takatsu, T.; Hashimoto, Y.; Inagaki, Y.; Nakatani, T. Annual variation in otolith increment widths of walleye pollock (Gadus chalcogrammus) larvae in Funka Bay, Hokkaido, Japan. Fish. Oceanogr. 2015, 24, 325–334. [Google Scholar] [CrossRef]
- Bakun, A. Wasp-waist populations and marine ecosystem dynamics: Navigating the ‘‘predator pit’’ topographies. Progr. Oceanogr. 2006, 68, 271–288. [Google Scholar] [CrossRef]
- Peck, M.A.; Reglero, P.; Takahashi, M.; Catalán, I.A. Life cycle ecophysiology of small pelagic fish and climate-driven changes in populations. Prog. Oceanogr. 2013, 116, 220–245. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, T. Long term variability of pelagic fish populations. In Long-Term Variability of Pelagic Fish Populations and Their Enviroment. Proceedings of the International Symposium, Sendai, Japan, 14–18 November 1989; Kawasaki, T., Tanaka, S., Toba, Y., Taniguchi, A., Eds.; Pergamon Press: Oxford, UK, 1991; pp. 47–60. [Google Scholar]
- Petigas, P.; Secor, D.H.; McQuinn, I.; Huse, G.; Lo, N. Stock collapses and their recovery: Mechanisms that establish and maintain life-cycle closure in space and time. ICES J. Mar. Sci. 2010, 67, 1841–1848. [Google Scholar] [CrossRef] [Green Version]
- Alheit, J.; Bakun, A. Population synchronies within and between ocean basins: Apparent teleconnections and implications as to physical–biological linkage mechanisms. J. Mar. Syst. 2010, 79, 267–285. [Google Scholar] [CrossRef]
- Alemany, F.; Álvarez, F. Growth differences among sardine (Sardina pilchardus Walb.) population in Western Mediterranean. Sci. Mar. 1993, 57, 229–239. [Google Scholar]
- Garrido, S.; Santos, A.M.P.; Santos, A.; Ré, P. Spatial distribution and vertical migrations of fish larvae communities off Northwestern Iberia sampled with LHPR and Bongo nets. Estuar. Coast. Shelf Sci. 2009, 84, 463–475. [Google Scholar] [CrossRef]
- Correia, A.T.; Hamer, P.; Carocinho, B.; Silva, A. Evidence for meta-population structure of Sardina pilchardus in the Atlantic Iberian waters from otolith elemental signatures of a strong cohort. Fish. Res. 2014, 149, 76–85. [Google Scholar] [CrossRef]
- Feijó, D.; Marçalo, A.; Wise, L.; Bento, T.; Barra, J.; Correia, M.; Pinto, D.; Lechuga, R.; Felício, M.; Dinis, D.; et al. The Portuguese purse seine fishery (2006–2016): What has changed in 10 years? FiSHMED 2018, 11. [Google Scholar]
- ICES. Management plan evaluation for sardine in Divisions VIIIc and IXa. In Report of the ICES Advisory Committee; Book 7, Section 7.3.5.1; ICES Advice: Copenhagen, Denmark, 2013; pp. 8–15. [Google Scholar]
- Bode, A.; Álvarez-Ossorio, M.T.; Carrera, P.; Lorenzo, J. Reconstrution of trophic pathways between plankton and the North Iberian sardina (Sardina pilchardus) using stable isotopes. Sci. Mar. 2004, 68, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Alemany, F.; Álvarez, I.; García, A.; Cortés, D.; Ramírez, T.; Quintanilla, J.; Álvarez, F.; Rodríguez, J.M. Postflexion larvae and juvenile daily growth patterns of the Alborán Sea sardine (Sardina pilchardus Walb.): Influence of wind. Sci. Mar. 2006, 70, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Sponaugle, S.; Boulay, J.N.; Rankin, T.L. Growth- and size-selective mortality in pelagic larvae of a common reef fish. Aquat. Biol. 2011, 13, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Takasuka, A.; Aoki, I.; Mitani, I. Evidence of growth-selective predation on larval Japanese anchovy Engraulis japonicus in Sagami Bay. Mar. Ecol. Prog. Ser. 2003, 252, 223–238. [Google Scholar] [CrossRef]
- Aldanondo, N.; Cotano, U.; Etxebeste, E.; Irigoien, X.; Alvarez, P.; Martínez de Murguía, A.; Herrero, D.L. Validation of daily increments deposition in the otoliths of European anchovy larvae (Engraulis encrasicolus L.) reared under different temperature conditions. Fish. Res. 2008, 93, 257–264. [Google Scholar] [CrossRef]
- Garrido, S.; Cristóvão, A.; Caldeira, C.; Ben-Hamadou, R.; Baylina, N.; Batista, H.; Saiz, E.; Peck, M.A.; Ré, P.; Santos, A.M.P. Effect of temperature on the growth, survival, development and foraging behaviour of Sardina pilchardus larvae. Mar. Ecol. Prog. Ser. 2016, 559, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.; Faria, A.M.; Teodósio, M.A.; Garrido, S. Ontogeny of swimming behaviour in sardine Sardina pilchardus larvae and effect of larval nutritional condition on critical speed. Mar. Ecol. Prog. Ser. 2014, 504, 287–300. [Google Scholar] [CrossRef]
- Caldeira, C.; Santos, A.M.P.; Ré, P.; Peck, M.A.; Saiz, E.; Garrido, S. Effects of prey concentration on ingestion rates of European sardine (Sardina pilchardus) larvae in the laboratory. Mar. Ecol. Prog. Ser. 2014, 517, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Ré, P. Otolith microstructure and detection of life history events in sardine and anchovy larvae. Ciênc. Biol. Ecol. Syst. 1986, 6, 9–17. [Google Scholar]
- Chícharo, M.A.; Chícharo, L.; Valdés, L.; Lopez-Jamar, E.; Ré, P. Does the nutritional condition limit survival potential of sardine Sardina pilchardus (Walbaum, 1792) larvae off the north coast of Spain? RNA/DNA ratios and their variability. Fish. Res. 1998, 39, 43–54. [Google Scholar] [CrossRef]
- Garrido, S.; Silva, A.; Marques, V.; Figueiredo, I.; Bryère, P.; Mangin, A.; Santos, A.M.P. Temperature and food-mediated variability of European Atlantic sardine recruitment. Prog. Oceanogr. 2017, 159, 267–275. [Google Scholar] [CrossRef]
- Ottersen, G.; Stige, L.C.; Durant, J.M.; Chan, K.-S.; Rouyer, T.A.; Drinkwater, K.F.; Stenseth, N.C. Temporal Shifts in recruitment dynamics of North Atlantic fish stocks: Effects of spawning stock and temperature. Mar. Ecol. Progr. Ser. 2013, 480, 205–225. [Google Scholar] [CrossRef] [Green Version]
- Ré, P.; Cabral e Silva, R.; Cunha, E.; Farinha, A.; Meneses, I.; Moita, T. Sardine spawning off Portugal. Boletim do Instituto Nacional de Investigação das Pescas 1990, 15, 31–44. [Google Scholar]
- Stratoudakis, Y.; Coombs, S.; de Lanzós, A.L.; Halliday, N.; Costas, G.; Caneco, B.; Franco, C.; Conway, D.; Santos, M.B.; Silva, A.; et al. Sardine (Sardina pilchardus) spawning seasonality in European waters of the northeast Atlantic. Mar. Biol. 2007, 152, 201–212. [Google Scholar] [CrossRef]
- Bernal, M.; Ibaibarriaga, L.; Lago de Lanzós, A.; Lonergan, M.E.; Hernández, C.; Franco, C.; Rasines, I.; Valdés, L.; Borchers, D.L. Using multinomial models to analyse data from Iberian sardine egg incubation experiments: A comparison with traditional techniques. ICES J. Mar. Sci. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Miranda, A.; Cal, R.M.; Iglesias, J. Effect of temperature on the development of eggs and larvae of Sardina pilchardus Walbaum in captivity. J. Exp. Mar. Biol. Ecol. 1990, 140, 69–77. [Google Scholar] [CrossRef]
- Zwolinski, J.; Mason, E.; Oliveira, P.B.; Stratoudakis, Y. Fine-scale distribution of sardine (Sardina pilchardus) eggs and adults during a spawning event. J. Sea Res. 2006, 56, 294–304. [Google Scholar] [CrossRef]
- Coombs, S.H.; Boyra, G.; Rueda, L.D.; Uriarte, A. Buoyancy measurements and vertical distribution of eggs of sardine (Sardina pilchardus) and anchovy (Engraulis encrasicolus). Mar. Biol. 2004, 145, 959–970. [Google Scholar] [CrossRef]
- Palmer, P.; Burke, M.J.; Palmer, C.J.; Burke, J.B. Deveolpments in controlled green-water larval culture technologies for estuarine fishes in Queensland, Australia and elsewhere. Aquaculture 2007, 272, 1–21. [Google Scholar] [CrossRef]
- Hedges, K.J.; Ludsin, S.A.; Fryer, B.J. Effects of ethanol preservation on otolith microchemistry. J. Fish. Biol. 2004, 64, 923–937. [Google Scholar] [CrossRef] [Green Version]
- Campana, S.; Cagne, J.; Munro, J. Otolith microstructure of larval herring (Clupea harengus): Image or reality? Can. J. Fish. Aquat. Sci. 1987, 44, 1922–1929. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; Version 4.0.2.; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 12 September 2021).
- Long, J.A. Interactions: Comprehensive, User-Friendly Toolkit for Probing Interactions, R Package Version 1.1.0. 2019. Available online: https://cran.r-project.org/package=interactions (accessed on 12 September 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Pepin, P. Effect of temperature and size on development, mortality, and survival rates of the pelagic early life history stages of marine fish. Can. J. Fish. Aquat. Sci. 1991, 48, 503–518. [Google Scholar] [CrossRef]
- Takasuka, A.; Oozeki, Y.; Aoki, I. Optimal growth temperature hypothesis: Why do anchovy flourish and sardine collapse or vice versa under the same ocean regime? Can. J. Fish. Aquat. Sci. 2007, 64, 768–776. [Google Scholar] [CrossRef]
- Fuiman, L.A.; Batty, R.S. What a drag it is getting cold: Partitioning the physical and physiological effects of temperature on fish swimming. J. Exp. Biol. 1997, 200, 1745–1755. [Google Scholar] [CrossRef]
- Garrido, S.; Ben-Hamadou, R.; Santos, A.M.P.; Ferreira, S.; Teodósio, M.A.; Cotano, U.; Irigoien, X.; Peck, M.A.; Saiz, E.; Ré, P. Born small, die young: Intrinsic, size-selective mortality in marine larval fish. Sci. Rep. 2015, 5, 17065. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Ueda, H.; Higashi, S. Correlation among dominance status, metabolic rate and otolith size in masu salmon. J. Fish Biol. 1998, 52, 281–290. [Google Scholar] [CrossRef]
- Bochdansky, A.B.; Grønkjær, P.; Herra, T.P.; Leggett, W.C. Experimental evidence for selection against larvae with high metabolic rates in a food limited environment. Mar. Biol. 2005, 147, 1413–1417. [Google Scholar] [CrossRef]
- Geffen, A.J. Otolith ring deposition in relation to growth rate in herring (Clupea harengus) and turbot (Scophthalmus maximus) larvae. Mar. Biol. 1982, 71, 317–326. [Google Scholar] [CrossRef]
- Fox, C.J.; Folkvord, A.; Geffen, A.J. Otolith microincrement formation in herring Clupea harengus larvae in relation to growth rate. Mar. Ecol. Prog. Ser. 2003, 264, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Wright, P.J. The influence of metabolic rate on otolith increment width in Atlantic salmon parr, Salmo salar L. J. Fish Biol. 1991, 38, 929–933. [Google Scholar] [CrossRef]
- Morales-Nin, B. Review of the growth regulation processes of otolith daily increment formation. Fish. Res. 2000, 46, 53–67. [Google Scholar] [CrossRef]
- Garrido, S.; Ferreira, S.; Soares, C.; Meneses, I.; Baylina, N.; Teodósio, M.A.; Santos, A.M.P.; Ré, P. Validation of daily increments in the otoliths European sardine larvae (Sardina pilchardus Walbaum, 1972) reared with different diets. J. Biol. Assoc. UK 2021, 101, 609–619. [Google Scholar] [CrossRef]
- Alemany, F.; Álvarez, F. Formation of initial daily increments in sagittal otoliths of reared and wild Sardina pilchardus yolk-sac larvae. Mar. Biol. 1994, 121, 35–39. [Google Scholar] [CrossRef]
- Dulčić, J. Estimation of age and growth of sardine, Sardina pilchardus (Walbaum, 1792), larvae by reading daily otolith increments. Fish. Res. 1995, 22, 265–277. [Google Scholar] [CrossRef]
- Ré, P. Evidence of daily and hourly growth in pilchard larvae based on otolith growth increments Sardina pilchardus (Walbaum, 1792). Cybium 1984, 8, 33–38. [Google Scholar]
- Geffen, A.J. The deposition of otolith rings in Atlantic salmon, Salmo salar L., embryos. J. Fish Biol. 1983, 23, 467–474. [Google Scholar] [CrossRef]
- Mendiola, D.; Álvares, P. Validation of daily increments in the otolith microstructure of Northeast Atlantic mackerel fish larvae. Fish. Res. 2008, 89, 300–304. [Google Scholar] [CrossRef]
- Namiki, S.; Tanaka, H.; Katayama, S.; Funaki, O.; Aoki, I.; Oozeki, Y. Validation of daily increment formation in otoliths of immature and adult Japanese anchovy Engraulis Japonicus. Fish. Sci. 2010, 76, 951–959. [Google Scholar] [CrossRef]
- Santos, A.M.P.; Chícharo, A.; Dos Santos, A.; Moita, T.; Oliveira, P.O.; Peliz, A.; Ré, P. Physical–biological interactions in the life history of small pelagic fish in the Western Iberia Upwelling Ecosystem. Prog. Oceanogr. 2007, 74, 192–209. [Google Scholar] [CrossRef]
- Rosa, H.C.; Ré, P. Incfluence of exogenous factors on the formation of Daily microgrowth increments in otoliths of Tilapia mariae (Boulenger, 1899) juveniles. Cybium 1985, 9, 341–357. [Google Scholar]
- Jones, C.M. Determining age of larval fish with the otolith increment technique. Fish. Bull. 1986, 84, 91–103. [Google Scholar]
- Álvarez, F.; Alemany, F. Birthdate analysis and its application to the study of recruitment of the Atlanto-Iberian sardine Sardina Pilchardus. Fish. Bull. 1997, 95, 187–194. [Google Scholar]
- Brophy, D.; Danilowicz, B.S. Tracing populations of Atlantic herring (Clupea harengus L.) in the Irish and Celtic Seas using otolith microstructure. ICES J. Mar. Sci. 2002, 59, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- ICES. Report of the Working Group on Southern Horse Mackerel, Anchovy and Sardine (WGHANSA). 24–29 June 2015, Lisbon, Portugal; ICES CM 2015/ACOM 16; ICES/CIEM: Copenhagen, Denmark, 2015; 612p. [Google Scholar]
- Checkley, D.M., Jr.; Ayón, P.; Baumgartner, T.R.; Bernal, M.; Coetzee, J.C.; Emmett, R.; Guevara-Carrasco, R.; Hutchings, L.; Ibaibarriaga, L.; Nakata, H.; et al. Habitats. In Climate Changers and Small Pelagic Fish; Checkley, D., Alheit, J., Oozeki, Y., Roy, C., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 12–44. [Google Scholar]
| Model | Age Range (dph) | Parameters | Estimate | Std. Error | t Value | Pr(>t) | AIC |
|---|---|---|---|---|---|---|---|
| TL~AGE × TEMP | 0–25 | Intercept13 | 1.403 | 0.039 | 36.28 | <0.0001 | 540.9 |
| Slope13 | 0.034 | 0.003 | 11.94 | <0.0001 | |||
| Intercept15 | −0.080 | 0.058 | −1.39 | 0.165 | |||
| Slope15 | −0.046 | 0.055 | −0.85 | 0.392 | |||
| Intercept17 | 0.012 | 0.004 | 2.82 | 0.0053 | |||
| Slope17 | 0.010 | 0.004 | 2.55 | 0.011 | |||
| 0–35 | Intercept15 | 1.362 | 0.042 | 31.99 | <0.0001 | 529.4 | |
| Slope15 | 0.041 | 0.002 | 17.09 | <0.0001 | |||
| Intercept17 | 0.068 | 0.056 | 1.22 | 0.224 | |||
| Slope17 | −0.005 | 0.003 | −1.91 | 0.057 | |||
| GI~AGE × TEMP | 0–25 | Intercept13 | 0.864 | 0.106 | 8.11 | <0.0001 | 885.3 |
| Slope13 | 0.083 | 0.006 | 14.24 | <0.0001 | |||
| Intercept15 | 0.104 | 0.156 | 0.66 | 0.504 | |||
| Slope15 | −0.002 | 0.008 | −0.35 | 0.072 | |||
| Intercept17 | 0.250 | 0.141 | 1.77 | 0.075 | |||
| Slope17 | 0.002 | 0.007 | 0.28 | 0.778 | |||
| 0–35 | Intercept15 | 1.170 | 0.091 | 12.80 | <0.0001 | 880.3 | |
| Slope15 | 0.066 | 0.004 | 17.81 | <0.0001 | |||
| Intercept17 | 0.229 | 0.115 | 1.99 | 0.043 | |||
| Slope17 | −0.001 | 0.004 | −0.28 | 0.778 | |||
| GI~TL × TEMP | 0–25 | Intercept13 | 0.454 | 0.14 | 3.19 | 0.001 | 980.3 |
| Slope13 | 0.229 | 0.018 | 12.59 | <0.0001 | |||
| Intercept15 | −0.046 | 0.214 | −0.21 | 0.826 | |||
| Slope15 | −0.006 | 0.025 | −0.26 | 0.790 | |||
| Intercept17 | 0.251 | 0.183 | 1.36 | 0.172 | |||
| Slope17 | −0.017 | 0.022 | −0.80 | 0.421 | |||
| 0–35 | Intercept15 | 0.749 | 0.115 | 6.48 | <0.0001 | 858.6 | |
| Slope15 | 0.181 | 0.010 | 17.24 | <0.0001 | |||
| Intercept17 | −0.000 | 0.152 | −0.00 | 0.997 | |||
| Slope17 | 0.034 | 0.014 | 2.48 | 0.013 | |||
| DIAM~AGE × TEMP | 0–25 | Intercept13 | 2.538 | 0.037 | 67.65 | <0.0001 | 1056.3 |
| Slope13 | 0.029 | 0.003 | 10.61 | <0.0001 | |||
| Intercept15 | −0.024 | 0.056 | −0.43 | 0.665 | |||
| Slope15 | 0.003 | 0.053 | 0.07 | 0.944 | |||
| Intercept17 | 0.004 | 0.004 | 1.28 | 0.199 | |||
| Slope17 | 0.020 | 0.004 | 5.29 | <0.0001 | |||
| 0–35 | Intercept15 | 2.447 | 0.046 | 53.18 | <0.0001 | 1140.8 | |
| Slope15 | 0.042 | 0.002 | 18.33 | <0.0001 | |||
| Intercept17 | 0.103 | 0.061 | 1.69 | 0.092 | |||
| Slope17 | 0.006 | 0.003 | 2.11 | 0.036 | |||
| DIAM~TL × TEMP | 0–25 | Intercept13 | 2.155 | 0.070 | 30.37 | <0.0001 | 967.4 |
| Slope13 | 0.114 | 0.010 | 10.41 | <0.0001 | |||
| Intercept15 | 0.050 | 0.096 | 0.52 | 0.601 | |||
| Slope15 | −0.011 | 0.014 | −0.81 | 0.419 | |||
| Intercept17 | −0.061 | 0.092 | −0.66 | 0.507 | |||
| Slope17 | 0.033 | 0.013 | 2.45 | 0.014 | |||
| 0–35 | Intercept15 | 2.447 | 0.046 | 53.18 | <0.0001 | 1140.8 | |
| Slope15 | 0.042 | 0.002 | 18.33 | <0.0001 | |||
| Intercept17 | 0.103 | 0.061 | 1.69 | 0.092 | |||
| Slope17 | 0.006 | 0.002 | 2.11 | 0.036 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, C.; Ferreira, S.; Ré, P.; Teodósio, M.A.; Santos, A.M.; Batista, H.; Baylina, N.; Garrido, S. Effect of Temperature on the Daily Increment Deposition in the Otoliths of European Sardine Sardina pilchardus (Walbaum, 1792) Larvae. Oceans 2021, 2, 723-737. https://doi.org/10.3390/oceans2040041
Soares C, Ferreira S, Ré P, Teodósio MA, Santos AM, Batista H, Baylina N, Garrido S. Effect of Temperature on the Daily Increment Deposition in the Otoliths of European Sardine Sardina pilchardus (Walbaum, 1792) Larvae. Oceans. 2021; 2(4):723-737. https://doi.org/10.3390/oceans2040041
Chicago/Turabian StyleSoares, Claudia, Susana Ferreira, Pedro Ré, Maria Alexandra Teodósio, António Miguel Santos, Hugo Batista, Nuria Baylina, and Susana Garrido. 2021. "Effect of Temperature on the Daily Increment Deposition in the Otoliths of European Sardine Sardina pilchardus (Walbaum, 1792) Larvae" Oceans 2, no. 4: 723-737. https://doi.org/10.3390/oceans2040041
APA StyleSoares, C., Ferreira, S., Ré, P., Teodósio, M. A., Santos, A. M., Batista, H., Baylina, N., & Garrido, S. (2021). Effect of Temperature on the Daily Increment Deposition in the Otoliths of European Sardine Sardina pilchardus (Walbaum, 1792) Larvae. Oceans, 2(4), 723-737. https://doi.org/10.3390/oceans2040041








