Divergent Proteomic Responses Offer Insights into Resistant Physiological Responses of a Reef-Foraminifera to Climate Change Scenarios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Experimental Design
2.3. Physiological Assessment
2.3.1. Microelectrode Measurements
2.3.2. Holobiont Mortality and Growth
2.3.3. Color and Chlorophyll a
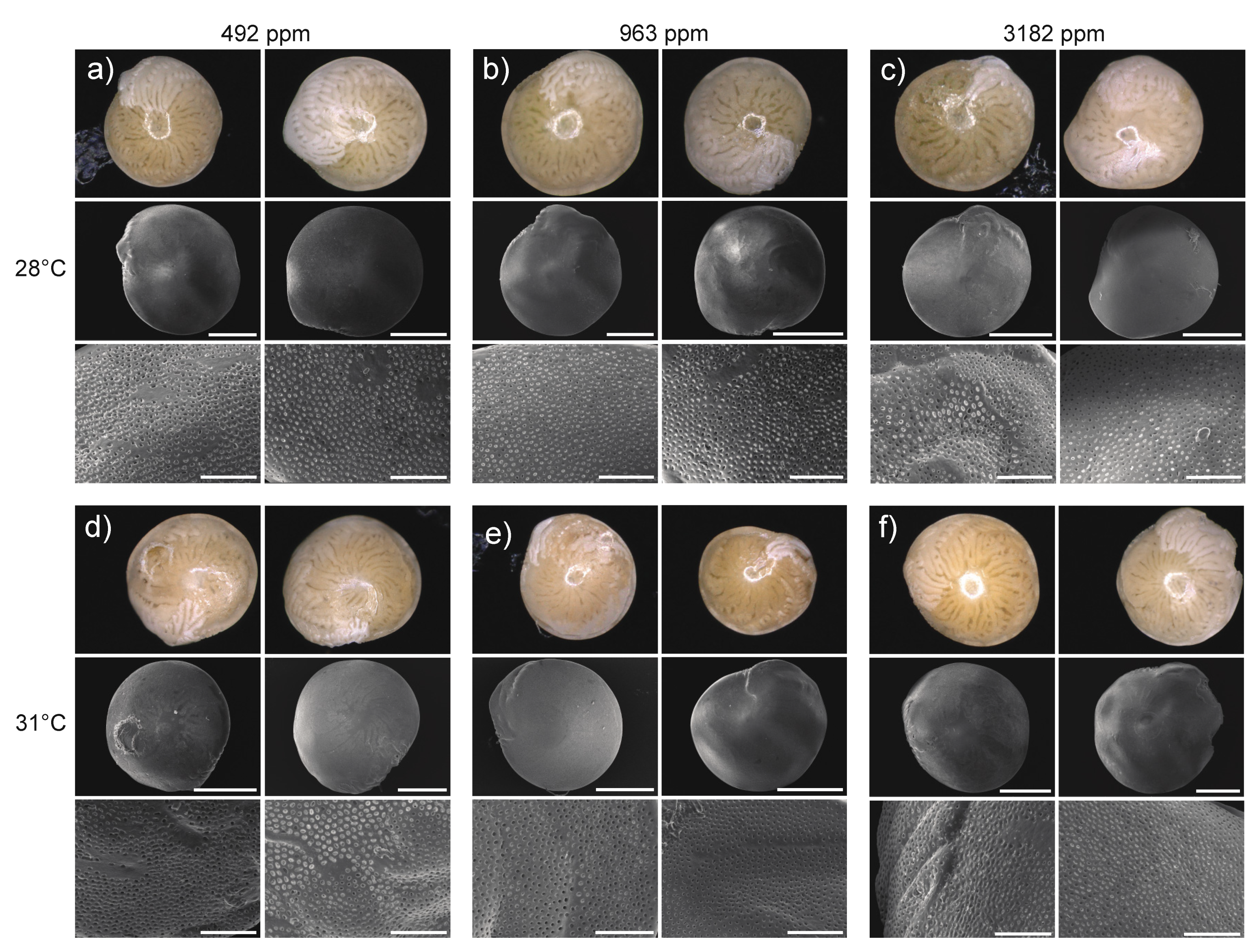
2.3.4. Test Surface, Pore Size and Breakage
2.3.5. Statistics
2.4. Identification of Photosymbionts
2.5. Proteomics
2.5.1. Label-Free Peptide Quantification and Protein Identification
2.5.2. Differential Protein Abundance and Functional Annotations
3. Results
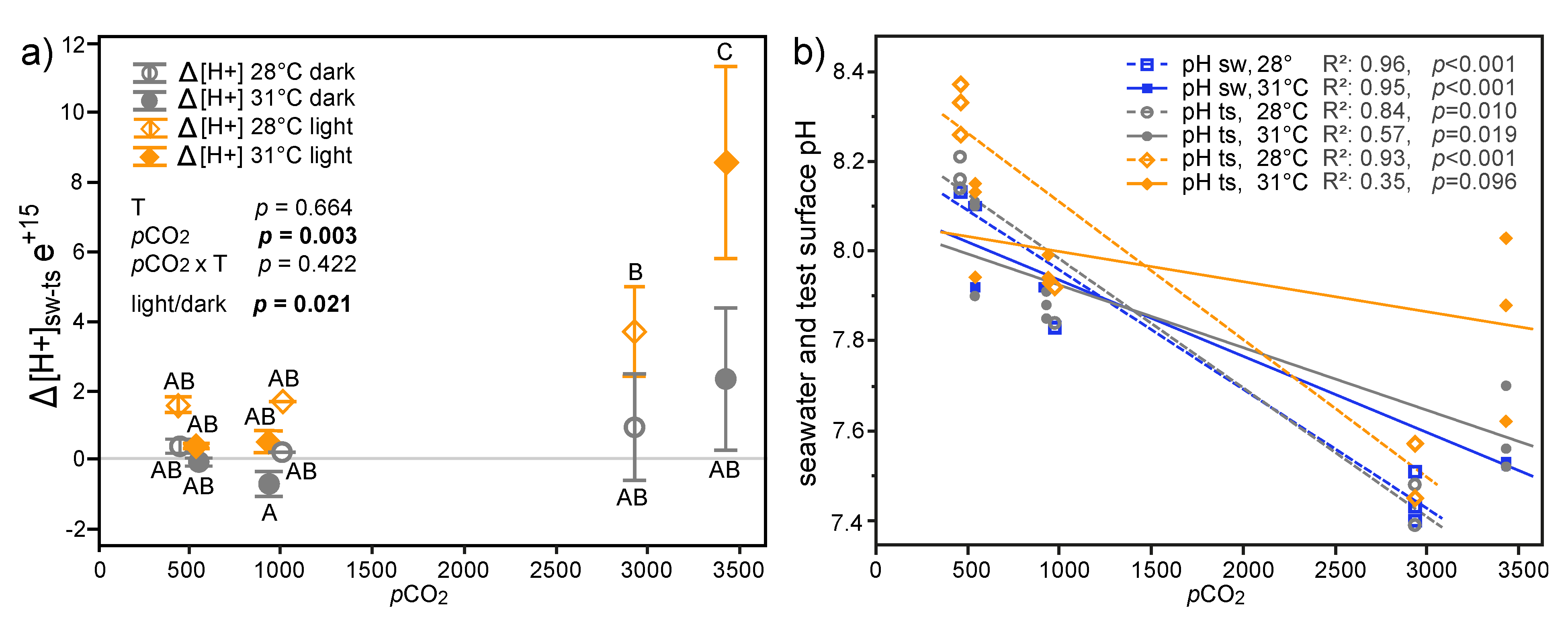
3.1. Physicochemical Parameters of Seawater Treatments
3.2. Identification of Photosymbionts
3.3. Physiological Assessments of Holobiont and Photosymbiont Condition
3.3.1. Microelectrode Measurements
3.3.2. Color and Chlorophyll a
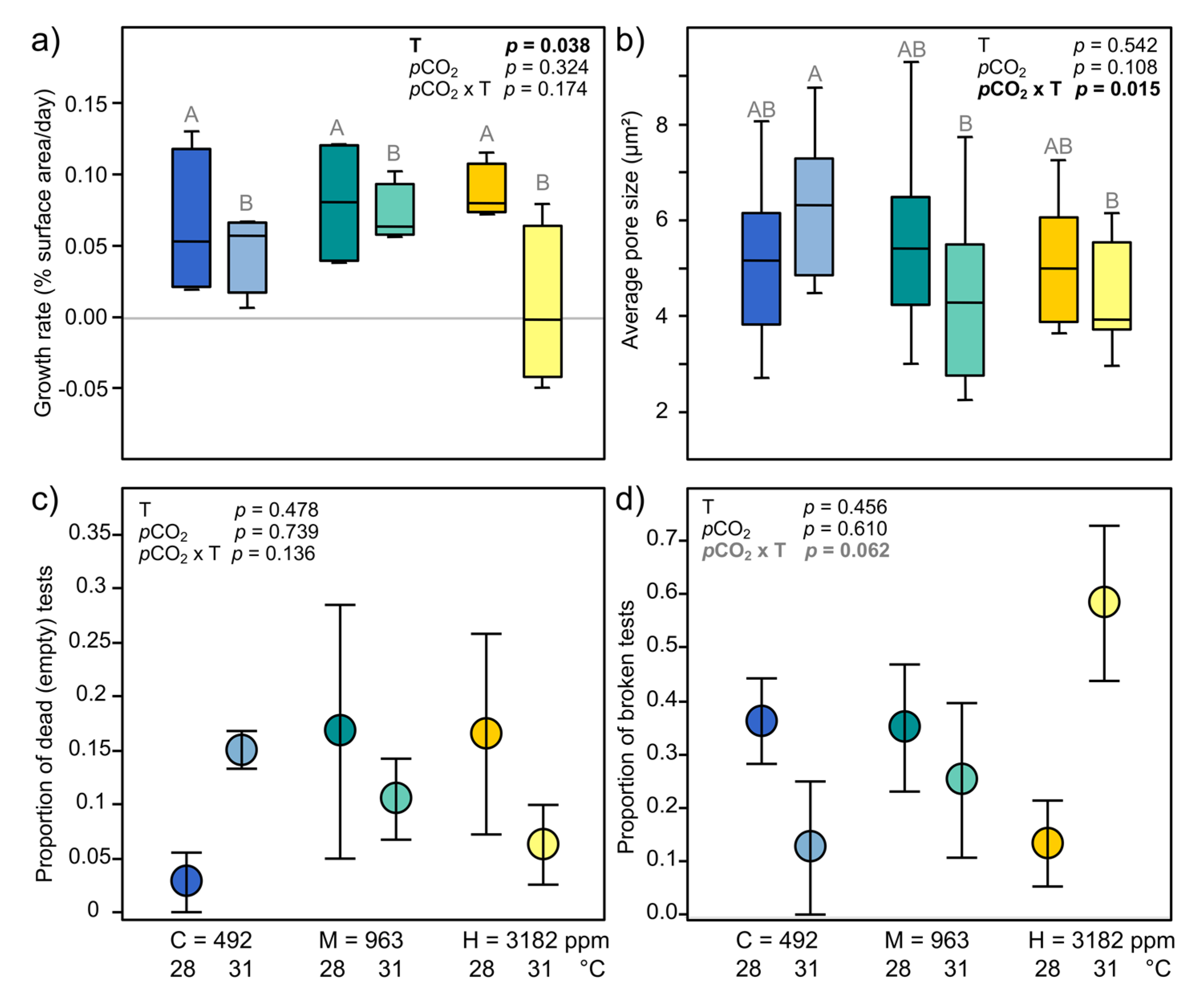
3.3.3. Holobiont Mortality and Growth
3.3.4. Test Surface, Pore Size and Breakage
3.4. Proteomics
3.4.1. Label-Free Peptide Quantification and Protein Identification
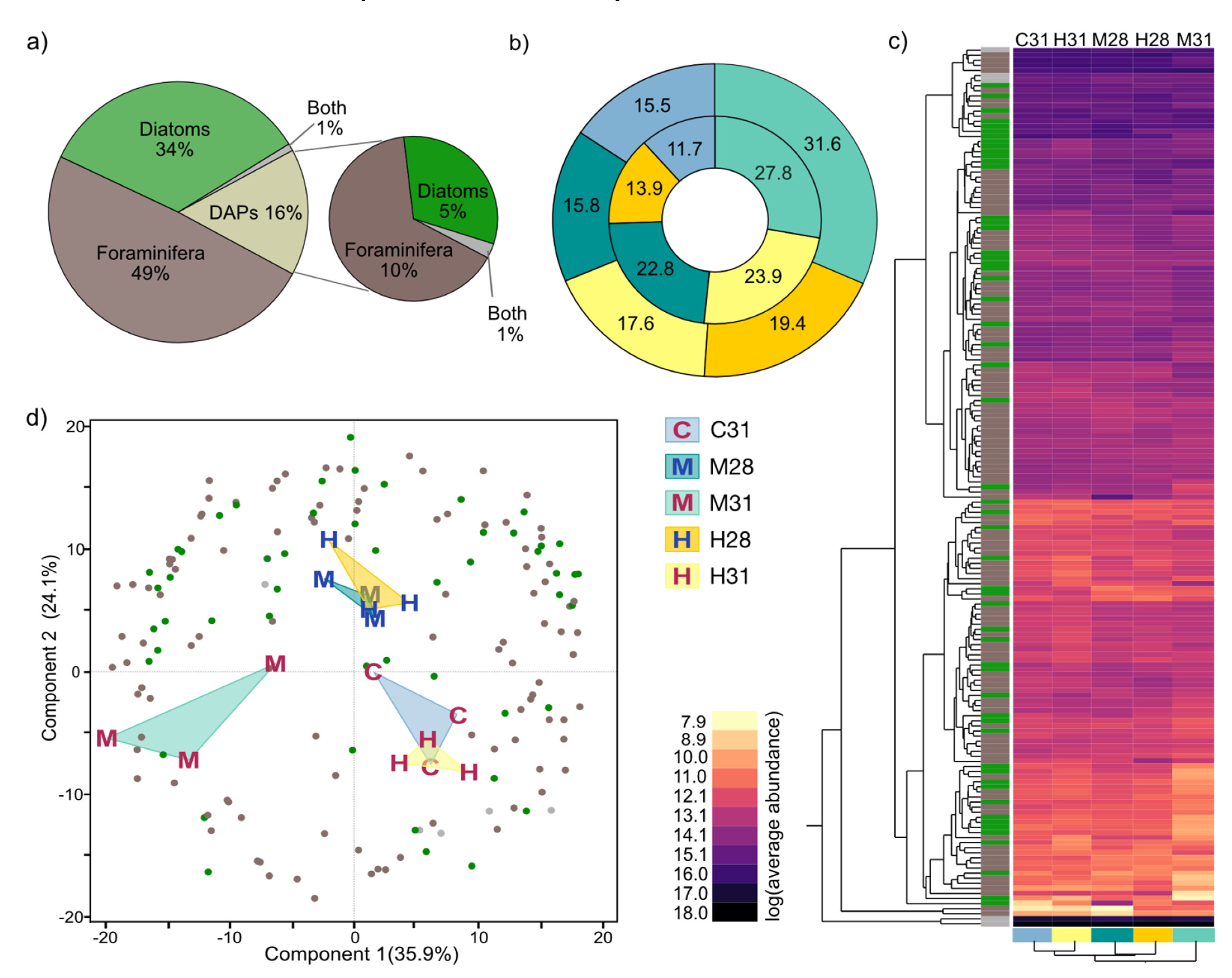
3.4.2. Differential Abundant Protein (DAP) Distribution in Treatments and Compartments
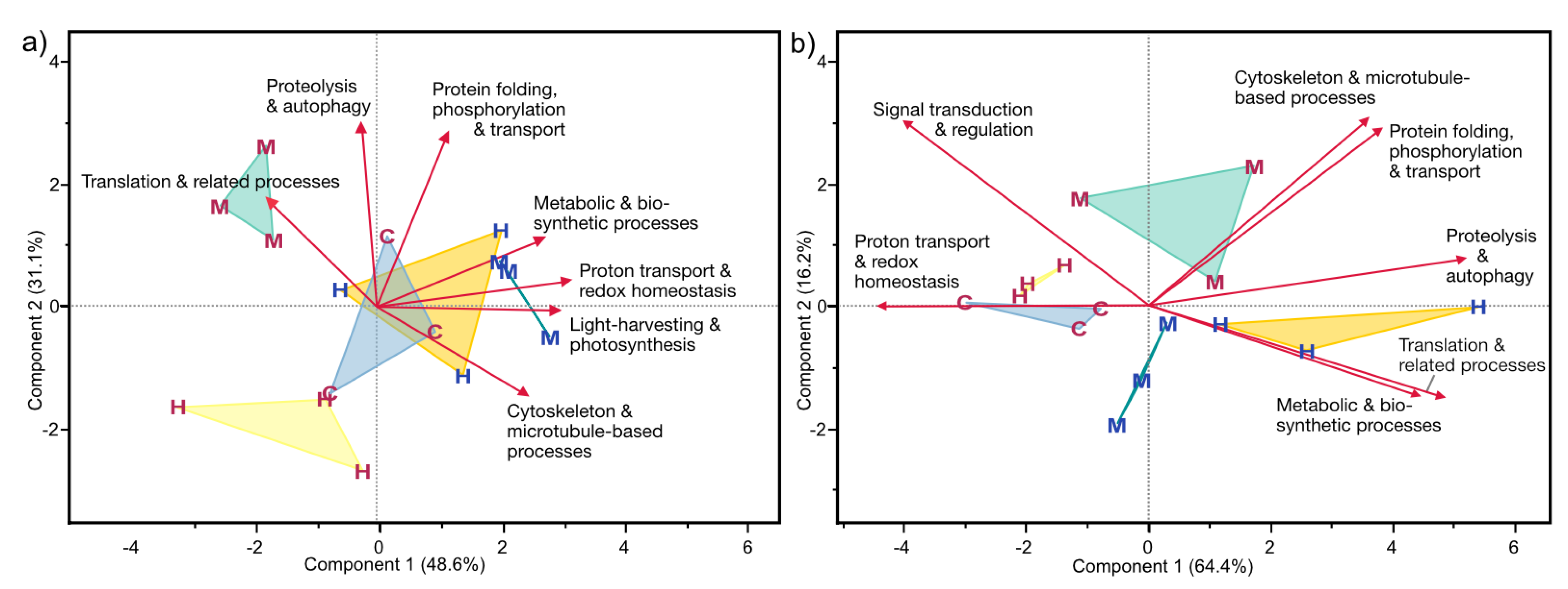
3.4.3. Functional Categories of Photosymbiont- and Host-Associated DAPs
4. Discussion
4.1. Climate Change Impacts on Photosymbiont and Holobiont Performance
4.1.1. Bleaching and Mortality
4.1.2. Growth
4.2. Buffering of OA in the LBF Microenvironment
4.3. Non-Linear Responses to Multiple Climate Change Stressors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Proteomics Analysis
References
- Field, C.B.; Barros, V.R. (Eds.) Climate Change 2014–Impacts, Adaptation and Vulnerability: Regional Aspects; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Caldeira, K.; Wickett, M.E. Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef]
- Feely, R.A.; Alin, S.R.; Newton, J.; Sabine, C.L.; Warner, M.; Devol, A.; Krembs, C.; Maloy, C. The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuar. Coast. Shelf Sci. 2010, 88, 442–449. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681. [Google Scholar] [CrossRef]
- Pandolfi, J.M.; Connolly, S.R.; Marshall, D.J.; Cohen, A.L. Projecting coral reef futures under global warming and ocean acidification. Science 2011, 333, 418–422. [Google Scholar] [CrossRef] [Green Version]
- Ries, J.B. A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim. Cosmochim. Acta 2011, 75, 4053–4064. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Duarte, C.M.; Gattuso, J.P. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, K.D.; Ries, J.B.; Bruno, J.F.; Westfield, I.T. The reef-building coral Siderastrea siderea exhibits parabolic responses to ocean acidification and warming. Proc. R. Soc. B Biol. Sci. 2014, 281. [Google Scholar] [CrossRef] [Green Version]
- Ries, J.B.; Cohen, A.L.; McCorkle, D.C. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 2009, 37, 1131–1134. [Google Scholar] [CrossRef]
- Ries, J.B.; Cohen, A.L.; McCorkle, D.C. A nonlinear calcification response to CO2-induced ocean acidification by the coral Oculina arbuscula. Coral Reefs 2010, 29, 661–674. [Google Scholar] [CrossRef]
- Glas, M.S.; Fabricius, K.E.; de Beer, D.; Uthicke, S. The O2, pH and Ca2+ microenvironment of benthic foraminifera in a high CO2 world. PLoS ONE 2012, 7, e50010. [Google Scholar] [CrossRef] [Green Version]
- De Nooijer, L.J.; Toyofuku, T.; Kitazato, H. Foraminifera promote calcification by elevating their intracellular pH. PNAS 2009, 106, 15374–15378. [Google Scholar] [CrossRef] [Green Version]
- Toyofuku, T.; Matsuo, M.Y.; de Nooijer, L.J.; Nagai, Y.; Kawada, S.; Fujita, K.; Reichart, G.-J.; Nomaki, H.; Tsuchiya, M.; Sakaguchi, H.; et al. Proton pumping accompanies calcification in foraminifera. Nat. Commun. 2017, 8, 14145. [Google Scholar] [CrossRef]
- Guillermic, M.; Cameron, L.; De Corte, I.; Misra, S.; Bijma, J.; De Beer, D.; Reymond, C.; Westphal, H.; Ries, J.B.; Eagle, R. Thermal stress reduces Pocilloporid coral resilience to ocean acidification by impairing control over calcifying fluid chemistry. Sci. Adv. 2021, 7, eaba9958. [Google Scholar] [CrossRef]
- Hallock, P. Symbiont-bearing foraminifera: Harbingers of global change? Micropaleontology 2000, 46, 95–104. [Google Scholar]
- Lee, J.J.; Saks, N.M.; Kapiotou, F.; Wilen, S.H.; Shilo, M. Effects of host cell extracts on cultures of endosymbiotic diatoms from larger foraminifera. Mar. Biol. 1984, 82, 113–120. [Google Scholar] [CrossRef]
- Langer, M.R. Assessing the contribution of foraminiferan protists to global ocean carbonate production. J. Eukaryot. Microbiol. 2008, 55, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Narayan, G.R.; Reymond, C.E.; Stuhr, M.; Doo, S.; Schmidt, C.; Mann, T.; Westphal, H. Response of large benthic foraminifera to climate and local changes: Implications for future carbonate production. Sedimentology 2021. [Google Scholar] [CrossRef]
- Hallock, P. Why are larger Foraminifera large? Paleobiology 1985, 11, 195–208. [Google Scholar] [CrossRef]
- Engel, B.E.; Hallock, P.; Price, R.E.; Pichler, T. Shell dissolution in larger benthic foraminifers exposed to pH and temperature extremes: Results from an in situ experiment. J. Foraminifer. Res. 2015, 45, 190–203. [Google Scholar] [CrossRef]
- Schmidt, C.; Morard, R.; Prazeres, M.; Barak, H.; Kucera, M. Retention of high thermal tolerance in the invasive foraminifera Amphistegina lobifera from the Eastern Mediterranean and the Gulf of Aqaba. Mar. Biol. 2016, 163, 228. [Google Scholar] [CrossRef]
- Stuhr, M.; Reymond, C.E.; Rieder, V.; Hallock, P.; Westphal, H.; Kucera, M. Reef calcifiers are adapted to episodic heat stress but vulnerable to sustained warming. PLoS ONE 2017, 12, e0179753. [Google Scholar] [CrossRef]
- Pomar, L.; Baceta, J.I.; Hallock, P.; Mateu-Vicens, G.; Basso, D. Reef building and carbonate production modes in the west-central Tethys during the Cenozoic. Mar. Pet. Geol. 2017, 83, 261–304. [Google Scholar] [CrossRef]
- Hallock, P.; Silva, I.P.; Boersma, A. Similarities between planktonic and larger foraminiferal evolutionary trends through Paleogene paleoceanographic changes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1991, 83, 49–64. [Google Scholar] [CrossRef]
- Pearson, P.; Palmer, M. Middle eocene seawater pH and atmospheric carbon dioxide concentrations. Science 1999, 284, 1824–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prazeres, M.; Morard, R.; Roberts, T.E.; Doo, S.; Schmidt, C.; Stuhr, M.; Renema, W.; Kucera, M. High dispersal capacity and biogeographic breaks shape the genetic diversity of a globally-distributed reef-dwelling calcifier. Ecol. Evol. 2020, 10, 5976–5989. [Google Scholar] [CrossRef]
- Langer, M.R.; Hottinger, L. Biogeography of selected “larger” foraminifera. Micropaleontology 2000, 46, 105–126. [Google Scholar]
- Langer, M.R.; Weinmann, A.E.; Lötters, S.; Bernhard, J.M.; Rödder, D. Climate-driven range extension of Amphistegina (Protista, Foraminiferida): Models of current and predicted future ranges. PLoS ONE 2013, 8, e54443. [Google Scholar] [CrossRef] [Green Version]
- Doo, S.S.; Hamylton, S.; Finfer, J.; Byrne, M. Spatial and temporal variation in reef-scale carbonate storage of large benthic foraminifera: A case study on One Tree Reef. Coral Reefs 2016, 36, 293–303. [Google Scholar] [CrossRef]
- Kawahata, H.; Fujita, K.; Iguchi, A.; Inoue, M.; Iwasaki, S.; Kuroyanagi, A.; Maeda, A.; Manaka, T.; Moriya, K.; Takagi, H.; et al. Perspective on the response of marine calcifiers to global warming and ocean acidification—Behavior of corals and foraminifera in a high CO2 world “hot house”. Prog. Earth Planet. Sci. 2019, 6, 37. [Google Scholar] [CrossRef]
- Schmidt, C.; Heinz, P.; Kucera, M.; Uthicke, S. Temperature-induced stress leads to bleaching in larger benthic foraminifera hosting endosymbiotic diatoms. Limnol. Oceanogr. 2011, 56, 1587–1602. [Google Scholar] [CrossRef]
- Talge, H.K.; Hallock, P. Ultrastructural responses in field-bleached and experimentally stressed Amphistegina gibbosa (Class Foraminifera). J. Eukaryot. Microbiol. 2003, 50, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Okai, T.; Hosono, T. Oxygen metabolic responses of three species of large benthic foraminifers with algal symbionts to temperature stress. PLoS ONE 2014, 9, e90304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuhr, M.; Meyer, A.; Reymond, C.E.; Narayan, G.R.; Rieder, V.; Rahnenführer, J.; Kucera, M.; Westphal, H.; Muhando, C.A.; Hallock, P. Variable thermal stress tolerance of the reef-associated symbiont-bearing foraminifera Amphistegina linked to differences in symbiont type. Coral Reefs 2018, 37, 811–824. [Google Scholar] [CrossRef]
- Hallock, P.; Williams, D.; Toler, S.; Fisher, E.M.; Talge, H.K. Bleaching in reef-dwelling foraminifers: Implications for reef decline. Int. Coral Reef Symp. 2006, 737, 729–737. [Google Scholar]
- Fine, M.; Gildor, H.; Genin, A. A coral reef refuge in the Red Sea. Glob. Chang. Biol. 2013, 19, 3640–3647. [Google Scholar] [CrossRef]
- Osman, E.O.; Smith, D.J.; Ziegler, M.; Kürten, B.; Conrad, C.; El-Haddad, K.M.; Voolstra, C.R.; Suggett, D.J. Thermal refugia against coral bleaching throughout the northern Red Sea. Glob. Chang. Biol. 2018, 24, 474–484. [Google Scholar] [CrossRef]
- Uthicke, S.; Momigliano, P.; Fabricius, K.E. High risk of extinction of benthic foraminifera in this century due to ocean acidification. Sci. Rep. 2013, 3, 1769. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.; Hikami, M.; Suzuki, A.; Kuroyanagi, A.; Kawahata, H. Effects of ocean acidification on calcification of symbiont-bearing reef foraminifera. Biogeosci. Discuss. 2011, 8, 1809–1829. [Google Scholar]
- Schmidt, C.; Kucera, M.; Uthicke, S. Combined effects of warming and ocean acidification on coral reef foraminifera Marginopora vertebralis and Heterostegina depressa. Coral Reefs 2014, 33, 805–818. [Google Scholar] [CrossRef]
- Uthicke, S.; Fabricius, K.E. Productivity gains do not compensate for reduced calcification under near-future ocean acidification in the photosynthetic benthic foraminifer species Marginopora vertebralis. Glob. Chang. Biol. 2012, 18, 2781–2791. [Google Scholar] [CrossRef]
- McIntyre-Wressnig, A.; Bernhard, J.M.; McCorkle, D.C.; Hallock, P. Non-lethal effects of ocean acidification on the symbiont-bearing benthic foraminifer Amphistegina gibbosa. Mar. Ecol. Prog. Ser. 2013, 472, 45–60. [Google Scholar] [CrossRef]
- Reymond, C.E.; Lloyd, A.; Kline, D.I.; Dove, S.G.; Pandolfi, J.M. Decline in growth of foraminifer Marginopora rossi under eutrophication and ocean acidification scenarios. Glob. Chang. Biol. 2013, 19, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Vogel, N.; Uthicke, S. Calcification and photobiology in symbiont-bearing benthic foraminifera and responses to a high CO2 environment. J. Exp. Mar. Bio. Ecol. 2012, 424–425, 15–24. [Google Scholar] [CrossRef]
- Marques, J.A.; de Barros Marangoni, L.F.; Bianchini, A. Combined effects of sea water acidification and copper exposure on the symbiont-bearing foraminifer Amphistegina gibbosa. Coral Reefs 2017, 36, 489–501. [Google Scholar] [CrossRef]
- Prazeres, M.; Uthicke, S.; Pandolfi, J.M. Ocean acidification induces biochemical and morphological changes in the calcification process of large benthic foraminifera. Proc. R. Soc. B Biol. Sci. 2015, 282, 8–11. [Google Scholar] [CrossRef] [Green Version]
- Sinutok, S.; Hill, R.; Doblin, M.; Wuhrer, R.; Ralph, P. Warmer more acidic conditions cause decreased productivity and calcification in subtropical coral reef sediment-dwelling calcifiers. Limnol. Oceanogr. 2011, 56, 1200–1212. [Google Scholar] [CrossRef]
- Stuhr, M.; Blank-Landeshammer, B.; Reymond, C.E.; Kollipara, L.; Sickmann, A.; Kucera, M.; Westphal, H. Disentangling thermal stress responses in a reef-calcifier and its photosymbionts by shotgun proteomics. Sci. Rep. 2018, 8, 3524. [Google Scholar] [CrossRef]
- Lee, J.J. Algal symbiosis in larger foraminifera. Symbiosis 2006, 42, 63–75. [Google Scholar]
- Lee, J.J.; McEnery, M.E.; ter Kuile, B.; Erez, J.; Röttger, R.; Rockwell, R.F.; Faber, W., Jr.; Lagziel, A.; Faber, W., Jr.; Lagziel, A. Identification and distribution of endosymbiotic diatoms in larger foraminifera. Micropaleontology 1989, 35, 353–366. [Google Scholar] [CrossRef]
- Prazeres, M.; Roberts, T.E.; Ramadhani, S.F.; Doo, S.S.; Schmidt, C.; Stuhr, M.; Renema, W. Diversity and flexibility of algal symbiont community in globally distributed larger benthic foraminifera of the genus Amphistegina. BMC Microbiol. 2021. in review. [Google Scholar]
- Hallock, P. Production of carbonate sediments by selected large benthic foraminifera on two Pacific coral reefs. J. Sediment. Petrol. 1981, 51, 467–474. [Google Scholar]
- ter Kuile, B.; Erez, J. In situ growth rate experiments on the symbiont-bearing foraminifera Amphistegina lobifera and Amphisorus hemprechii. J. Foraminifer. Res. 1984, 14, 262–276. [Google Scholar] [CrossRef]
- Segev, E.; Erez, J. Effect of Mg/Ca ratio in seawater on shell composition in shallow benthic foraminifera. Geochem. Geophys. Geosyst. 2006, 7, Q02P09. [Google Scholar] [CrossRef]
- Hallock, P. Distribution of selected species of living algal symbiont-bearing foraminifera on two Pacific coral reefs. J. Foraminifer. Res. 1984, 14, 250–261. [Google Scholar] [CrossRef]
- Renema, W. Terrestrial influence as a key driver of spatial variability in large benthic foraminiferal assemblage composition in the Central Indo-Pacific. Earth Sci. Rev. 2018, 177, 514–544. [Google Scholar] [CrossRef]
- Hallock, P.; Forward, L.B.; Hansen, H.J. Influence of environment on the test shape of Amphistegina. J. Foraminifer. Res. 1986, 16, 224–231. [Google Scholar] [CrossRef]
- Hansen, H.J.; Buchard, B. Depth distribution of Amphistegina in the Gulf of Elat, Israel. In Depth-Relationships of Recent Larger Foraminifera in the Gulf of Aqaba-Elat; Reiss, Z., Leutenegger, S., Hottinger, L., Fermont, W.J.J., Meulenkamp, J.E., Thomas, E., Hansen, H.J., Buchardt, B., Larsen, A.R., Drooger, C.W., Eds.; Micropaleontological Bulletins: Utrecht, The Netherlands, 1977; pp. 205–224. [Google Scholar]
- Cameron, L.P.; Reymond, C.E.; Bijma, J.; Büscher, J.V.; de Beer, D.; Eagle, R.T.; Gunnell, J.; Müller-Lundin, F.; Schmidt-Grieb, G.M.; Westfield, I.; et al. Impacts of warming and acidification on corals linked to symbiont abundance and pH-regulation. Preprints 2021. [Google Scholar] [CrossRef]
- Lee, J.; Erez, J.; ter Kuile, B.; Lagziel, A.; Burgos, S. Feeding rates of two species of larger foraminifera Amphistegina lobifera and Amphisorus hemprichii, from the Gulf of Eilat (Red Sea). Symbiosis 1988, 5, 61–102. [Google Scholar]
- Lee, J.J.; Sang, K.; ter Kuile, B.; Strauss, E.; Lee, P.J.; Faber, W.W. Nutritional and related experiments on laboratory maintenance of three species of symbiont-bearing, large foraminifera. Mar. Biol. 1991, 109, 417–425. [Google Scholar] [CrossRef]
- Lewis, E.; Wallace, D. Program Developed for CO2 System Calculations; Environmental Sciences Division publication: Oak Ridge, TN, USA, 1998. [Google Scholar]
- Roy, R.N.; Roy, L.N.; Vogel, K.M.; Portermoore, C.; Pearson, T.; Good, C.E.; Millero, F.J.; Campbell, D.M. The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperatures 0 to 45 °C. Mar. Chem. 1993, 44, 249–267. [Google Scholar] [CrossRef]
- Mucci, A. The solubility of calcite and aragonite in seawater at various salinities, temperatures and one atmosphere total pressure. Am. J. Sci. 1983, 283, 780–799. [Google Scholar] [CrossRef]
- Cai, W.J.; Ma, Y.; Hopkinson, B.M.; Grottoli, A.G.; Warner, M.E.; Ding, Q.; Hu, X.; Yuan, X.; Schoepf, V.; Xu, H.; et al. Microelectrode characterization of coral daytime interior pH and carbonate chemistry. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhler-Rink, S.; Kühl, M. Microsensor studies of photosynthesis and respiration in larger symbiotic foraminifera. I The physico-chemical microenvironment of Marginopora vertebralis, Amphistegina lobifera and Amphisorus hemprichii. Mar. Biol. 2000, 137, 473–486. [Google Scholar] [CrossRef]
- de Beer, D.; Schramm, A.; Santegoeds, C.M.; Kühl, M. A nitrite microsensor for profiling environmental biofilms. Appl. Environ. Microbiol. 1997, 63, 973–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhard, J.M. Distinguishing live from dead foraminifera: Methods review and proper applications. Micropaleontology 2000, 46, 38–46. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Hosono, T.; Fujita, K.; Kayanne, H. Estimating photophysiological condition of endosymbiont-bearing Baculogypsina sphaerulata based on the holobiont color represented in CIE L*a*b* color space. Mar. Biol. 2012, 159, 2663–2673. [Google Scholar] [CrossRef]
- Schwartzwald, D. Color Space Converter Plugin for Imagej. Available online: https://imagej.nih.gov/ij/plugins/color-space-converter.html (accessed on January 2017).
- Petersen, J.; Riedel, B.; Barras, C.; Pays, O.; Guihéneuf, A.; Mabilleau, G.; Schweizer, M.; Meysman, F.J.R.; Jorissen, F.J. Improved methodology for measuring pore patterns in the benthic foraminiferal genus Ammonia. Mar. Micropaleontol. 2016, 128, 1–13. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T.A. BioEdit: A user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Glez-Peña, D.; Gómez-Blanco, D.; Reboiro-Jato, M.; Fdez-Riverola, F.; Posada, D. ALTER: Program-oriented conversion of DNA and protein alignments. Nucleic Acids Res. 2010, 38, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizcaíno, J.A.; Csordas, A.; Del-Toro, N.; Dianes, J.A.; Griss, J.; Lavidas, I.; Mayer, G.; Perez-Riverol, Y.; Reisinger, F.; Ternent, T.; et al. Update of the PRIDE database and its related tools. Nucleic Acids Res. 2016, 44, D447–D456. [Google Scholar] [CrossRef] [PubMed]
- Woehle, C.; Roy, A.-S.; Glock, N.; Wein, T.; Weissenbach, J.; Rosenstiel, P.; Hiebenthal, C.; Michels, J.; Schönfeld, J.; Dagan, T. A novel eukaryotic denitrification pathway in foraminifera. Curr. Biol. 2018, 28, 2536–2543. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernández-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J.; et al. EggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019, 47, 309–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huerta-Cepas, J.; Forslund, K.; Coelho, L.P.; Szklarczyk, D.; Jensen, L.J.; Von Mering, C.; Bork, P. Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Mol. Biol. Evol. 2017, 34, 2115–2122. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2016. [Google Scholar]
- Oakley, C.A.; Durand, E.; Wilkinson, S.P.; Peng, L.; Weis, V.M.; Grossman, A.R.; Davy, S.K. Thermal shock induces host proteostasis disruption and endoplasmic reticulum stress in the model symbiotic cnidarian Aiptasia. J. Proteome Res. 2017, 16, 2121–2134. [Google Scholar] [CrossRef]
- Sproles, A.E.; Oakley, C.A.; Matthews, J.L.; Peng, L.; Owen, J.G.; Grossman, A.R.; Weis, V.M.; Davy, S.K. Proteomics quantifies protein expression changes in a model cnidarian colonised by a thermally tolerant but suboptimal symbiont. ISME J. 2019, 13, 2334–2345. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. Available online: https://cran.r-project.org/web/packages/vegan (accessed on May 2020).
- Hervé, M. Aide-Mémoire de Statistique Appliquée à la Biologie—Construire son Étude et Analyser les Résultats à L’aide du Logiciel R. Available online: https://cran.r-project.org/doc/contrib/Herve-Aide-memoire-statistique.pdf (accessed on January 2021).
- Barnes, K.H. Diversity and distribution of diatom endosymbionts in Amphistegina spp. (Foraminifera) based on molecular and morphological techniques; University of South Florida: Tampa, FL, USA, 2016. [Google Scholar]
- Doo, S.S.; Fujita, K.; Byrne, M.; Uthicke, S. Fate of calcifying tropical symbiont-bearing large benthic foraminifera: Living sands in a changing ocean. Biol. Bull. 2014, 226, 169–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntyre-Wressnig, A.; Bernhard, J.M.; McCorkle, D.C.; Hallock, P. Non-lethal effects of ocean acidification on two symbiont-bearing benthic foraminiferal species. Biogeosci. Discuss. 2011, 8, 9165–9200. [Google Scholar]
- Davies, S.W.; Ries, J.B.; Marchetti, A.; Castillo, K.D. Symbiodinium functional diversity in the coral Siderastrea siderea is influenced by thermal stress and reef environment, but not ocean acidification. Front. Mar. Sci. 2018, 5, 150. [Google Scholar] [CrossRef]
- González-Pech, R.A.; Vargas, S.; Francis, W.R.; Wörheide, G. Transcriptomic resilience of the Montipora digitata holobiont to Low pH. Front. Mar. Sci. 2017, 4, 403. [Google Scholar] [CrossRef] [Green Version]
- Tomanek, L. Proteomic responses to environmentally induced oxidative stress. J. Exp. Biol. 2015, 218, 1867–1879. [Google Scholar] [CrossRef] [Green Version]
- Cleves, P.A.; Shumaker, A.; Lee, J.M.; Putnam, H.M.; Bhattacharya, D. Unknown to known: Advancing knowledge of coral gene function. Trends Genet. 2020, 36, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Mollica, N.R.; Guo, W.; Cohen, A.L.; Huang, K.F.; Foster, G.L.; Donald, H.K.; Solow, A.R. Ocean acidification affects coral growth by reducing skeletal density. Proc. Natl. Acad. Sci. USA 2018, 115, 1754–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leutenegger, S.; Hansen, H.J. Ultrastructural and radiotracer studies of pore function in foraminifera. Mar. Biol. 1979, 54, 11–16. [Google Scholar] [CrossRef]
- Kleypas, J.A.; Buddemeier, R.W.; Archer, D.; Gattuso, J.P.; Langdon, C.; Opdyke, B.N. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 1999, 284, 118–120. [Google Scholar] [CrossRef]
- Ries, J.B.; Ghazaleh, M.N.; Connolly, B.; Westfield, I.; Castillo, K.D. Impacts of seawater saturation state (ΩA = 0.4–4.6) and temperature (10, 25 °C) on the dissolution kinetics of whole-shell biogenic carbonates. Geochim. Cosmochim. Acta 2016, 192, 318–337. [Google Scholar] [CrossRef] [Green Version]
- Travis, J.; Bowser, S. The motility of foraminifera. In Biology of Foraminifera; Academic Press: London, UK, 1991; pp. 91–155. [Google Scholar]
- Ter Kuile, B.; Erez, J.; Padan, E. Mechanisms for the uptake of inorganic carbon by two species of symbiont-bearing foraminifera. Mar. Biol. 1989, 103, 241–251. [Google Scholar] [CrossRef]
- Bentov, S.; Brownlee, C.; Erez, J. The role of seawater endocytosis in the biomineralization process in calcareous foraminifera. Proc. Natl. Acad. Sci. USA 2009, 106, 21500–21504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erez, J. The source of ions for biomineralization in foraminifera and their implications for paleoceanographic proxies (Review). Rev. Miner. Geochem. 2003, 54, 115. [Google Scholar] [CrossRef] [Green Version]
- Sutton, J.N.; Liu, Y.W.; Ries, J.B.; Guillermic, M.; Ponzevera, E.; Eagle, R.A. δ11B as monitor of calcification site pH in divergent marine calcifying organisms. Biogeosciences 2018, 15, 1447–1467. [Google Scholar] [CrossRef] [Green Version]
- Moya, A.; Huisman, L.; Ball, E.E.; Hayward, D.C.; Grasso, L.C.; Chua, C.M.; Woo, H.N.; Gattuso, J.P.; Forêt, S.; Miller, D.J. Whole transcriptome analysis of the coral Acropora millepora reveals complex responses to CO2-driven acidification during the initiation of calcification. Mol. Ecol. 2012, 21, 2440–2454. [Google Scholar] [CrossRef] [Green Version]
- Rocker, M.M.; Noonan, S.; Humphrey, C.; Moya, A.; Willis, B.L.; Bay, L.K. Expression of calcification and metabolism-related genes in response to elevated pCO2 and temperature in the reef-building coral Acropora millepora. Mar. Genom. 2015, 24, 313–318. [Google Scholar] [CrossRef]
- Kenkel, C.D.; Moya, A.; Strahl, J.; Humphrey, C.; Bay, L.K. Functional genomic analysis of corals from natural CO2-seeps reveals core molecular responses involved in acclimatization to ocean acidification. Glob. Chang. Biol. 2018, 24, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Bellworthy, J.; Fine, M. Beyond peak summer temperatures, branching corals in the Gulf of Aqaba are resilient to thermal stress but sensitive to high light. Coral Reefs 2017, 36, 1071–1082. [Google Scholar] [CrossRef]
- Krueger, T.; Horwitz, N.; Bodin, J.; Giovani, M.-E.; Escrig, S.; Meibom, A.; Fine, M. Common reef-building coral in the northern Red Sea resistant to elevated temperature and acidification. R. Soc. Open Sci. 2017, 4, 170038. [Google Scholar] [CrossRef] [Green Version]
- Ries, J.B. Skeletal mineralogy in a high-CO2 world. J. Exp. Mar. Bio. Ecol. 2011, 403, 54–64. [Google Scholar] [CrossRef]
- Robbins, L.L.; Knorr, P.O.; Wynn, J.G.; Hallock, P.; Harries, P.J. Interpreting the role of pH on stable isotopes in large benthic foraminifera. ICES J. Mar. Sci. 2017, 74, 955–964. [Google Scholar] [CrossRef] [Green Version]
- Purton, L.M.A.; Brasier, M.D. Giant protist Nummulites and its Eocene environment: Life span and habitat insights from δ18O and δ13C data from Nummulites and Venericardia, Hampshire basin, UK. Geology 1999, 27, 711–714. [Google Scholar] [CrossRef]
- Weinmann, A.E.; Rödder, D.; Lötters, S.; Langer, M.R. Heading for new shores: Projecting marine distribution ranges of selected larger foraminifera. PLoS ONE 2013, 8, 14. [Google Scholar] [CrossRef]
- Kleinhaus, K.; Al-Sawalmih, A.; Barshis, D.J.; Genin, A.; Grace, L.N.; Hoegh-Guldberg, O.; Loya, Y.; Meibom, A.; Osman, E.O.; Ruch, J.-D.; et al. Science, diplomacy, and the Red Sea’s unique coral reef: It’s time for action. Front. Mar. Sci. 2020, 7, 00090. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.A.; Michaud, D.P. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal. Biochem. 1993, 211, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Shindo, N.; Nojima, S.; Fujimura, T.; Taka, H.; Mineki, R.; Murayama, K. Separation of 18 6-aminoquinolyl-carbamyl-amino acids by ion-pair chromatography. Anal. Biochem. 1997, 249, 79–82. [Google Scholar] [CrossRef]
- Manza, L.L.; Stamer, S.L.; Ham, A.-J.L.; Codreanu, S.G.; Liebler, D.C. Sample preparation and digestion for proteomic analyses using spin filters. Proteomics 2005, 5, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Kollipara, L.; Zahedi, R.P. Protein carbamylation: In vivo modification or in vitro artefact? Proteomics 2013, 13, 941–944. [Google Scholar] [CrossRef]
- Burkhart, J.M.; Schumbrutzki, C.; Wortelkamp, S.; Sickmann, A.; Zahedi, R.P. Systematic and quantitative comparison of digest efficiency and specificity reveals the impact of trypsin quality on MS-based proteomics. J. Proteom. 2012, 75, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.V.; de Godoy, L.M.; Li, G.; Macek, B.; Mortensen, P.; Pesch, R.; Makarov, A.; Lange, O.; Horning, S.; Mann, M. Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell. Proteom. 2005, 4, 2010–2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Xin, L.; Shan, B.; Chen, W.; Xie, M.; Yuen, D.; Zhang, W.; Zhang, Z.; Lajoie, G.A.; Ma, B. PEAKS DB: De novo sequencing assisted database search for sensitive and accurate peptide identification. Mol. Cell. Proteom. 2012, 11, 010587. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Niu, B.; Gao, Y.; Fu, L.; Li, W. CD-HIT Suite: A web server for clustering and comparing biological sequences. Bioinformatics 2010, 26, 680–682. [Google Scholar] [CrossRef] [PubMed]

| Treatment | T (°C) | DIC (µM) | TA (µM) | pHSW | pCO2 (ppm) | ΩC | ΩA |
|---|---|---|---|---|---|---|---|
| C28 | 28.2 ± 0.2 | 2562 ± 288 | 2928 ± 280 | 8.21 ± 0.10 | 483 ± 86 | 8.5 ± 1.2 | 5.5 ± 1.2 |
| C31 | 31.0 ± 0.6 | 2375 ± 283 | 2738 ± 278 | 8.11 ± 0.13 | 500 ± 74 | 8.1 ± 1.3 | 4.8 ± 1.2 |
| M28 | 28.0 ± 0.1 | 2686 ± 285 | 2937 ± 258 | 7.95 ± 0.07 | 973 ± 138 | 5.9 ± 2.6 | 3.7 ± 1.9 |
| M31 | 30.9 ± 0.2 | 2806 ± 254 | 3088 ± 233 | 7.98 ± 0.07 | 953 ± 131 | 6.6 ± 0.5 | 4.2 ± 0.6 |
| H28 | 28.0 ± 0.2 | 2858 ± 430 | 2908 ± 456 | 7.49 ± 0.11 | 2934 ± 898 | 2.3 ± 0.4 | 1.4 ± 0.4 |
| H31 | 30.9 ± 0.1 | 3231 ± 281 | 3294 ± 324 | 7.50 ± 0.10 | 3431 ± 810 | 2.9 ± 0.4 | 1.8 ± 0.5 |
| Proteins | DF | MS | F | Pr (>F) | Post-Hoc Test Summary |
|---|---|---|---|---|---|
| Holobiont | |||||
| pCO2 | 2 | 0.0003 | 1.818 | 0.028 | H:C 0.480, M:C 0.290, H:M 0.130 |
| Temp. | 1 | 0.0004 | 2.335 | 0.013 | 31 °C:28 °C 0.080 |
| pCO2×Temp. | 1 | 0.0003 | 1.719 | 0.075 | |
| Foraminifera | |||||
| pCO2 | 2 | 0.0003 | 1.854 | 0.030 | H:C 0.390 M:C 0.270, H:M 0.170 |
| Temp. | 1 | 0.0004 | 2.358 | 0.018 | 31 °C:28 °C 0.079 |
| pCO2×Temp. | 1 | 0.0003 | 1.697 | 0.091 | |
| Photosymbiont | |||||
| pCO2 | 2 | 0.0004 | 1.775 | 0.030 | H:C 0.607, M:C 0.321, H:M 0.091 |
| Temp. | 1 | 0.0005 | 2.324 | 0.011 | 31 °C:28 °C 0.089 |
| pCO2×Temp. | 1 | 0.0004 | 1.734 | 0.067 | |
| Protein Description | Log2(Fold-Change) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M31/ M28 | H31/ H28 | H28/ M28 | H31/ M31 | M31/ C31 | H31/ C31 | M31/ H28 | C31/ M28 | H31/ M28 | C31/ H28 | |
| Light-harvesting and photosynthesis | ||||||||||
| protein fucoxanthin chlorophyll a/c | −2.34 | −0.27 | 0.05 | 2.11 | −2.63 | −0.51 | −2.38 | 0.29 | −0.22 | 0.24 |
| protein fucoxanthin chlorophyll a/c | −1.90 | 0.15 | −0.19 | 1.86 | −2.12 | −0.25 | −1.71 | 0.21 | −0.04 | 0.40 |
| divinyl chlorophyllidea 8-vinyl-reduct. | −2.60 | 0.81 | −0.48 | 2.93 | −2.56 | 0.37 | −2.12 | −0.04 | 0.33 | 0.44 |
| thylakoid lumenal 15.0 kDa protein 2 | −1.03 | −0.09 | −0.16 | 0.78 | −0.76 | 0.02 | −0.87 | −0.28 | −0.25 | −0.12 |
| cytochrome b6/f complex subunit IV | −3.18 | −0.17 | −4.64 | −1.64 | 4.27 | 2.63 | 1.46 | −7.45 | −4.81 | −2.81 |
| Metabolic and biosynthetic processes | ||||||||||
| uroporphyrinogen decarboxylase | −4.78 | −2.04 | −0.75 | 1.99 | −3.61 | −1.62 | −4.03 | −1.16 | −2.79 | −0.41 |
| fructose-bisphosphate aldolase | −2.03 | −0.51 | 0.11 | 1.63 | −2.28 | −0.65 | −2.14 | 0.25 | −0.40 | 0.14 |
| demethylmenaquinone methyltransfer. | −2.36 | −0.71 | 0.11 | 1.75 | −2.24 | −0.49 | −2.46 | −0.12 | −0.60 | −0.22 |
| 3-isopropylmalate dehydrogenase | −2.94 | −0.52 | −0.51 | 1.90 | −1.56 | 0.34 | −2.43 | −1.38 | −1.04 | −0.87 |
| glucose-6-phosphate isomerase | −1.14 | 0.47 | −0.09 | 1.53 | −1.08 | 0.44 | −1.05 | −0.05 | 0.39 | 0.03 |
| cysteine desulfurase | −0.26 | 0.54 | 0.65 | 1.46 | −0.58 | 0.88 | −0.91 | 0.32 | 1.19 | −0.34 |
| taurine catabolism dioxygenase TauD | −0.75 | −0.88 | −0.51 | −0.63 | −1.58 | −2.21 | −0.25 | 0.82 | −1.39 | 1.33 |
| alanine dehydrogenase 2 | −1.00 | −0.43 | 0.03 | 0.60 | −0.47 | 0.12 | −1.03 | −0.52 | −0.40 | −0.55 |
| inorganic pyrophosphatase | −0.72 | −0.44 | 0.30 | 0.58 | −0.61 | −0.03 | −1.02 | −0.12 | −0.14 | −0.41 |
| inorganic pyrophosphatase | −0.79 | −0.63 | −0.03 | 0.13 | 0.00 | 0.13 | −0.76 | −0.79 | −0.66 | −0.76 |
| phosphoadenosine-phosphosulphate red. | −0.36 | −1.08 | 0.27 | −0.45 | 0.11 | −0.33 | −0.63 | −0.47 | −0.81 | −0.75 |
| S-adenosylmethionine synthase 2 | 0.65 | −0.25 | 0.20 | −0.70 | 1.03 | 0.33 | 0.45 | −0.38 | −0.05 | −0.58 |
| geranylgeranyl reductase | 0.57 | −0.41 | −0.13 | −1.11 | 1.04 | −0.07 | 0.70 | −0.47 | −0.53 | −0.34 |
| carbamoyl-phosphate synthase | −0.62 | −0.75 | −0.41 | −0.53 | −0.45 | −0.98 | −0.22 | −0.17 | −1.15 | 0.24 |
| acetyl-coa carboxylase | −0.46 | −1.05 | −0.11 | −0.71 | −0.25 | −0.96 | −0.34 | −0.21 | −1.17 | −0.09 |
| acetyl-coa carboxylase | −0.20 | −1.04 | −0.09 | −0.93 | −0.15 | −1.08 | −0.11 | −0.05 | −1.13 | 0.04 |
| ALA dehydratase | −0.55 | −0.95 | −0.72 | −1.12 | 0.30 | −0.82 | 0.17 | −0.85 | −1.67 | −0.13 |
| agmatinase | −1.54 | 0.40 | −1.19 | 0.75 | −0.10 | 0.65 | −0.35 | −1.44 | −0.79 | −0.24 |
| glutamate synthase 2 [NADH] | −0.65 | −1.42 | −0.97 | −1.74 | 0.87 | −0.87 | 0.32 | −1.52 | −2.40 | −0.55 |
| aspartate--ammonia ligase | 1.66 | 0.16 | 0.30 | −1.20 | 1.74 | 0.54 | 1.36 | −0.09 | 0.45 | −0.38 |
| Proton transmembrane transport, cell redox homeostasis and cellular detoxification | ||||||||||
| thioredoxin | −1.08 | 0.38 | −0.28 | 1.18 | −1.65 | −0.47 | −0.80 | 0.56 | 0.10 | 0.85 |
| L-ascorbate peroxidase | −0.17 | −1.11 | 0.45 | −0.50 | 0.16 | −0.34 | −0.62 | −0.32 | −0.66 | −0.77 |
| Protein folding, phosphorylation and transport | ||||||||||
| translocation protein sec62 | −2.44 | 0.03 | −0.18 | 2.28 | −1.74 | 0.54 | −2.25 | −0.69 | −0.16 | −0.51 |
| T-complex protein 1 subunit eta | −0.77 | 1.25 | 0.75 | 2.77 | −1.53 | 1.24 | −1.52 | 0.76 | 2.00 | 0.01 |
| leucine-rich repeat-containing protein | 1.32 | 1.07 | 1.13 | 0.88 | −1.27 | −0.39 | 0.19 | 2.59 | 2.20 | 1.46 |
| ATP-dependent chaperone ClpB | 1.29 | 0.12 | 0.34 | −0.83 | 0.84 | 0.00 | 0.95 | 0.46 | 0.46 | 0.11 |
| luminal-binding protein 5 | 0.22 | −0.56 | −0.24 | −1.03 | 0.67 | −0.36 | 0.46 | −0.45 | −0.81 | −0.21 |
| Proteolysis and autophagy | ||||||||||
| Clp protease ATP binding subunit | 0.37 | −0.39 | −0.27 | −1.04 | 0.42 | −0.62 | 0.64 | −0.05 | −0.67 | 0.23 |
| Translation and related process | ||||||||||
| 40S ribosomal protein | 0.24 | −0.72 | −0.52 | −1.49 | 0.76 | −0.73 | 0.77 | −0.51 | −1.24 | 0.01 |
| Cytoskeleton and microtubule-based processes | ||||||||||
| cell division protein FtsZ | −2.18 | 0.06 | −0.86 | 1.38 | −1.92 | −0.54 | −1.32 | −0.26 | −0.80 | 0.60 |
| tubulin-specific chaperone d | −2.15 | −0.40 | −0.34 | 1.42 | −1.78 | −0.37 | −1.82 | −0.37 | −0.74 | −0.04 |
| Unknown functions | ||||||||||
| hypothetical protein | −1.54 | 0.78 | −1.74 | 0.57 | −0.64 | −0.06 | 0.21 | −0.90 | −0.96 | 0.84 |
| Protein Description | Log2(Fold-Change) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M31/ M28 | H31/ H28 | H28/ M28 | H31/ M31 | M31/ C31 | H31/ C31 | M31/ H28 | C31/ M28 | H31/ M28 | C31/ H28 | |
| Signal transduction and regulation | ||||||||||
| NEDD8-activating enzyme E1 regulat. | −2.34 | 0.42 | 0.35 | 3.11 | −3.08 | 0.03 | −2.69 | 0.74 | 0.77 | 0.39 |
| cullin-associated NEDD8-dissociated 1 | −1.17 | 0.28 | 0.09 | 1.53 | −2.20 | −0.66 | −1.26 | 1.03 | 0.37 | 0.94 |
| Ras-related protein Rab-21 | −1.08 | 0.55 | −0.15 | 1.48 | −1.76 | −0.27 | −0.93 | 0.68 | 0.40 | 0.83 |
| GTPase HRas | −0.69 | 1.33 | 0.14 | 2.15 | −1.84 | 0.31 | −0.82 | 1.16 | 1.46 | 1.02 |
| Ras-related protein Rab-7a-like | −1.37 | 0.74 | −0.83 | 1.28 | −1.34 | −0.05 | −0.55 | −0.04 | −0.09 | 0.79 |
| cytochrome c oxidase assembly (SCO) | −1.12 | 0.32 | −0.28 | 1.15 | −0.92 | 0.23 | −0.84 | −0.20 | 0.03 | 0.09 |
| guanine nucleotide-binding protein G | −1.11 | 0.35 | −0.96 | 0.50 | −0.79 | −0.29 | −0.15 | −0.32 | −0.61 | 0.63 |
| Metabolic and biosynthetic processes | ||||||||||
| long-chain-fatty-acid--CoA ligase | −5.28 | −0.68 | 1.28 | 5.88 | −5.41 | 0.47 | −6.56 | 0.13 | 0.60 | −1.15 |
| aldo/keto reductase | −2.87 | −0.39 | −0.05 | 2.43 | −2.96 | −0.54 | −2.82 | 0.09 | −0.44 | 0.15 |
| fructose-bisphosphate aldolase 6 | −1.47 | −0.19 | 0.19 | 1.47 | −1.65 | −0.18 | −1.66 | 0.18 | 0.00 | −0.01 |
| glutathione S-transferase | −1.34 | 0.17 | −0.24 | 1.27 | −1.60 | −0.33 | −1.10 | 0.26 | −0.07 | 0.50 |
| methylglutaconyl-CoA hydratase | −1.12 | −0.21 | −0.14 | 0.77 | −1.38 | −0.61 | −0.98 | 0.26 | −0.34 | 0.40 |
| oligosaccharyltransferase complex | −1.33 | 0.39 | −0.12 | 1.59 | −1.01 | 0.58 | −1.20 | −0.32 | 0.27 | −0.19 |
| putative glyoxalase I | −2.13 | −0.47 | −0.48 | 1.18 | −1.56 | −0.38 | −1.65 | −0.57 | −0.95 | −0.09 |
| UDP-glucose 4-epimerase | −1.15 | −0.59 | 0.08 | 0.64 | −0.89 | −0.25 | −1.23 | −0.26 | −0.51 | −0.34 |
| asparagine synthetase | −0.69 | 0.29 | −0.31 | 0.67 | −1.42 | −0.75 | −0.38 | 0.73 | −0.02 | 1.05 |
| isocitrate dehydrogenase [NADP] | 0.08 | −0.65 | 0.72 | −0.02 | 0.39 | 0.37 | −0.64 | −0.31 | 0.06 | −1.02 |
| cytochrome b-c1 complex subu. Rieske | −0.08 | −1.57 | 0.95 | −0.54 | 0.81 | 0.27 | −1.03 | −0.89 | −0.62 | −1.84 |
| cytochrome c oxidase subunit 5b-1 | −0.17 | −1.25 | 0.43 | −0.65 | 0.57 | −0.08 | −0.60 | −0.74 | −0.82 | −1.17 |
| 2-oxoglutarate dehydrogenase | 0.17 | −1.08 | 0.35 | −0.90 | 0.70 | −0.20 | −0.18 | −0.53 | −0.73 | −0.88 |
| adenylyl-sulfate kinase | 0.16 | −0.75 | −0.34 | −1.26 | 0.72 | −0.53 | 0.51 | −0.56 | −1.09 | −0.22 |
| NADH dehydrogenase flavoprotein 1 | 0.89 | −0.80 | 0.72 | −0.97 | 1.09 | 0.12 | 0.17 | −0.20 | −0.08 | −0.92 |
| phytanoyl-CoA dioxygenase | 1.12 | −0.40 | −0.10 | −1.61 | 1.37 | −0.24 | 1.22 | −0.25 | −0.49 | −0.15 |
| fatty acyl-CoA synthetase | 0.97 | −0.34 | −0.23 | −1.55 | 1.57 | 0.02 | 1.20 | −0.60 | −0.58 | −0.37 |
| UDP-glucose 4-epimerase GalE | 2.33 | −0.99 | 2.50 | −0.82 | 0.62 | −0.20 | −0.17 | 1.71 | 1.51 | −0.79 |
| Proton transmembrane transport, cell redox homeostasis and cellular detoxification | ||||||||||
| peroxiredoxin-1 | −1.28 | 0.57 | −0.73 | 1.13 | −0.40 | 0.73 | −0.56 | −0.88 | −0.15 | −0.15 |
| Protein folding, phosphorylation and transport | ||||||||||
| phage shock protein A homolog | −1.65 | 0.64 | −0.48 | 1.82 | −1.44 | 0.37 | −1.17 | −0.21 | 0.16 | 0.27 |
| synaptobrevin domain-containing prot. | −0.09 | 0.89 | −0.78 | 0.20 | −0.62 | −0.42 | 0.69 | 0.54 | 0.12 | 1.31 |
| CAMK/CDPK protein kinase | −0.07 | −0.13 | −1.20 | −1.27 | 0.51 | −0.76 | 1.13 | −0.58 | −1.33 | 0.62 |
| sorting nexin 1 | 1.19 | −0.49 | 0.05 | −1.63 | 0.88 | −0.75 | 1.14 | 0.32 | −0.44 | 0.26 |
| chaperone protein | 1.51 | 0.29 | 0.24 | −0.98 | 1.46 | 0.49 | 1.27 | 0.04 | 0.53 | −0.19 |
| mitochondrial carrier family | −0.24 | −1.94 | 1.53 | −0.18 | −0.08 | −0.25 | −1.76 | −0.16 | −0.42 | −1.69 |
| Proteolysis and autophagy | ||||||||||
| oligopeptidase B | −2.93 | −2.72 | 0.02 | 0.23 | −1.85 | −1.62 | −2.95 | −1.08 | −2.70 | −1.10 |
| ubiquitin carboxyl-terminal hydrolase | −0.42 | −0.41 | 0.77 | 0.77 | −0.80 | −0.02 | −1.19 | 0.38 | 0.35 | −0.39 |
| mitochondrial-processing peptidase | 0.00 | −0.81 | 0.79 | −0.02 | 0.38 | 0.36 | −0.79 | −0.39 | −0.03 | −1.18 |
| mitochondrial-processing peptidase | 0.22 | −0.92 | 0.75 | −0.40 | 0.69 | 0.29 | −0.53 | −0.47 | −0.18 | −1.22 |
| ubiquitin carboxyl-terminal hydrolase | 0.66 | −0.50 | −0.17 | −1.32 | 0.68 | −0.65 | 0.83 | −0.02 | −0.66 | 0.15 |
| guanine nucleotide-binding protein | 0.50 | −1.43 | 0.27 | −1.65 | 0.97 | −0.68 | 0.23 | −0.47 | −1.16 | −0.74 |
| autophagy-related protein 3 | 1.01 | 0.32 | −0.21 | −0.90 | 1.34 | 0.44 | 1.22 | −0.33 | 0.11 | −0.12 |
| COP9 signalosome complex subunit 5 | −0.52 | −0.53 | −1.26 | −1.28 | 0.40 | −0.87 | 0.74 | −0.92 | −1.80 | 0.34 |
| Translation and related process | ||||||||||
| 40S ribosomal protein S20 | −1.57 | −1.73 | 0.00 | −0.17 | −0.69 | −0.86 | −1.56 | −0.88 | −1.74 | −0.88 |
| 60S ribosomal protein L9-like | −1.07 | −0.95 | 0.30 | 0.42 | −0.46 | −0.04 | −1.37 | −0.61 | −0.65 | −0.91 |
| 60S ribosomal protein L12 | −0.64 | −0.47 | 0.40 | 0.56 | −0.33 | 0.23 | −1.04 | −0.30 | −0.07 | −0.71 |
| 60S acidic ribosomal protein P0-like | 0.07 | −1.34 | 0.70 | −0.70 | 0.48 | −0.22 | −0.64 | −0.41 | −0.63 | −1.12 |
| ribonuclease P protein subunit p25-like | −0.28 | −0.93 | −0.25 | −0.90 | 0.75 | −0.15 | −0.03 | −1.03 | −1.18 | −0.78 |
| ribonucleoside-diphosphate reductase | 0.59 | −2.64 | 2.08 | −1.15 | 0.76 | −0.39 | −1.49 | −0.17 | −0.56 | −2.24 |
| 60S ribosomal protein L5 | 0.98 | −1.93 | 0.52 | −2.38 | 1.23 | −1.15 | 0.45 | −0.25 | −1.40 | −0.78 |
| guanine nucleotide-binding protein | 1.01 | −1.33 | −0.13 | −2.46 | 1.78 | −0.68 | 1.13 | −0.77 | −1.46 | −0.65 |
| ATP-dependent RNA helicase ddx6 | 2.86 | −0.74 | 0.15 | −3.44 | 1.48 | −1.96 | 2.70 | 1.37 | −0.58 | 1.22 |
| Cytoskeleton and microtubule-based processes | ||||||||||
| tubulin alpha-3 chain | −0.02 | −0.76 | 0.72 | −0.02 | 0.28 | 0.26 | −0.74 | −0.30 | −0.04 | −1.02 |
| villin-2 | 1.25 | 0.41 | −0.57 | −1.41 | 1.08 | −0.33 | 1.82 | 0.17 | −0.15 | 0.74 |
| dynein heavy chain | 1.50 | 0.39 | −0.28 | −1.38 | 1.66 | 0.28 | 1.77 | −0.16 | 0.12 | 0.12 |
| clathrin light chain-like | 0.48 | −1.01 | −0.52 | −2.02 | 1.36 | −0.66 | 1.01 | −0.88 | −1.53 | −0.35 |
| dynein intermediate chain 3, ciliary | 4.61 | −3.84 | 4.99 | −3.46 | 4.11 | 0.65 | −0.38 | 0.50 | 1.15 | −4.49 |
| Unknown functions | ||||||||||
| tartrate-resistant acid phosphatase ty. 5 | −2.28 | −1.51 | 1.19 | 1.95 | −2.80 | −0.85 | −3.47 | 0.52 | −0.33 | −0.66 |
| hypothetical protein | −1.08 | 0.40 | −0.11 | 1.37 | −1.53 | −0.16 | −0.97 | 0.45 | 0.29 | 0.56 |
| hypothetical protein | −1.62 | −0.57 | −0.03 | 1.02 | −1.51 | −0.49 | −1.59 | −0.11 | −0.60 | −0.08 |
| hypothetical protein RFI_01644, partial | −0.32 | 0.62 | 0.13 | 1.07 | −0.91 | 0.16 | −0.45 | 0.59 | 0.75 | 0.46 |
| hypothetical protein | −0.92 | 0.56 | −0.36 | 1.12 | −0.88 | 0.24 | −0.56 | −0.05 | 0.19 | 0.31 |
| RING zinc finger-containing protein | 0.83 | −0.46 | −0.14 | −1.43 | 1.19 | −0.24 | 0.97 | −0.36 | −0.60 | −0.22 |
| hypothetical protein RFI_36204, partial | −3.22 | −0.89 | −2.23 | 0.10 | 0.16 | 0.26 | −0.99 | −3.38 | −3.12 | −1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuhr, M.; Cameron, L.P.; Blank-Landeshammer, B.; Reymond, C.E.; Doo, S.S.; Westphal, H.; Sickmann, A.; Ries, J.B. Divergent Proteomic Responses Offer Insights into Resistant Physiological Responses of a Reef-Foraminifera to Climate Change Scenarios. Oceans 2021, 2, 281-314. https://doi.org/10.3390/oceans2020017
Stuhr M, Cameron LP, Blank-Landeshammer B, Reymond CE, Doo SS, Westphal H, Sickmann A, Ries JB. Divergent Proteomic Responses Offer Insights into Resistant Physiological Responses of a Reef-Foraminifera to Climate Change Scenarios. Oceans. 2021; 2(2):281-314. https://doi.org/10.3390/oceans2020017
Chicago/Turabian StyleStuhr, Marleen, Louise P. Cameron, Bernhard Blank-Landeshammer, Claire E. Reymond, Steve S. Doo, Hildegard Westphal, Albert Sickmann, and Justin B. Ries. 2021. "Divergent Proteomic Responses Offer Insights into Resistant Physiological Responses of a Reef-Foraminifera to Climate Change Scenarios" Oceans 2, no. 2: 281-314. https://doi.org/10.3390/oceans2020017
APA StyleStuhr, M., Cameron, L. P., Blank-Landeshammer, B., Reymond, C. E., Doo, S. S., Westphal, H., Sickmann, A., & Ries, J. B. (2021). Divergent Proteomic Responses Offer Insights into Resistant Physiological Responses of a Reef-Foraminifera to Climate Change Scenarios. Oceans, 2(2), 281-314. https://doi.org/10.3390/oceans2020017











