Use of Polyphosphates and Soluble Pyrophosphatase Activity in the Seaweed Ulva pseudorotundata
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Preculture Conditions
2.2. Experimental Design
2.3. Analyses
2.4. Statistics
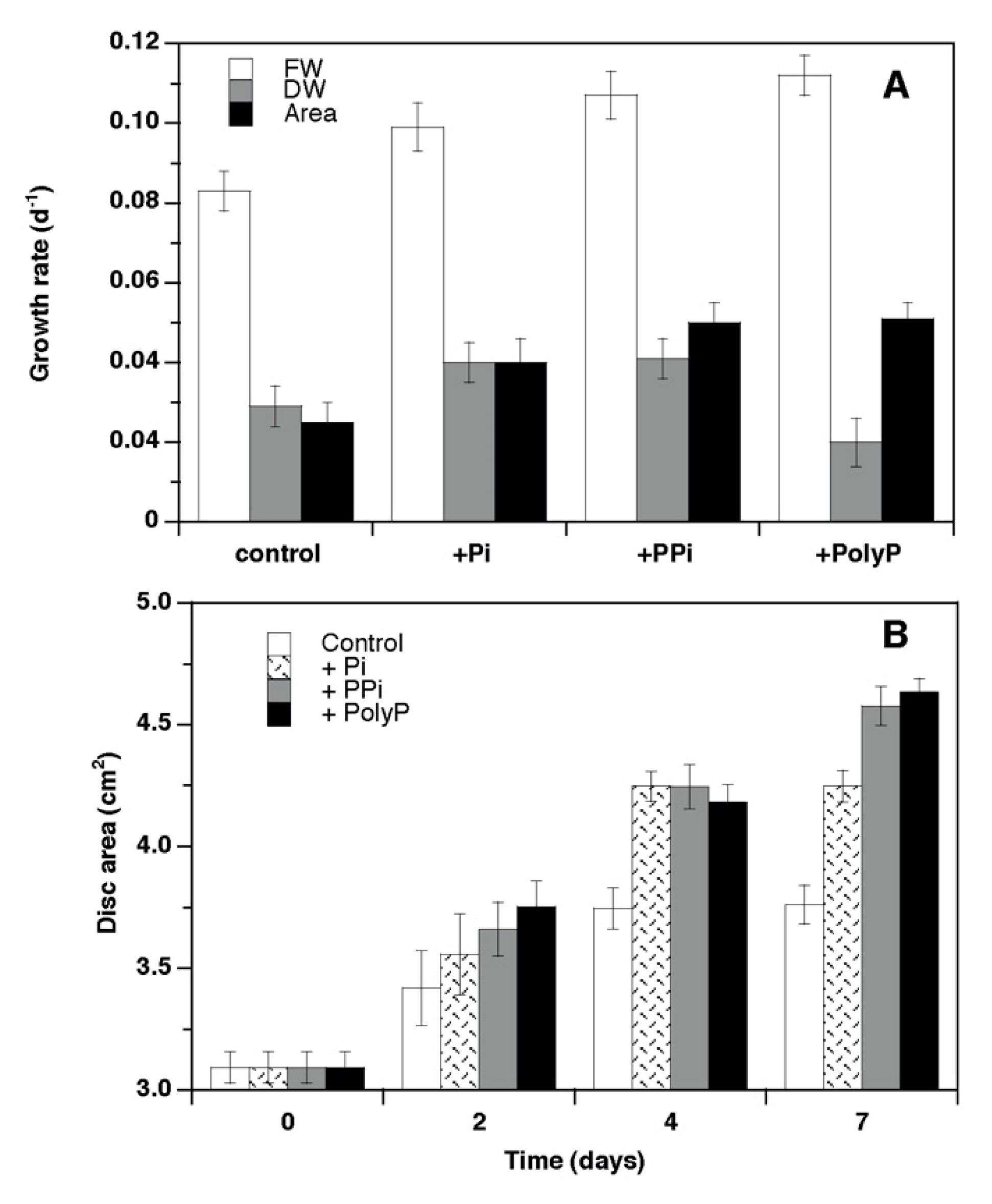
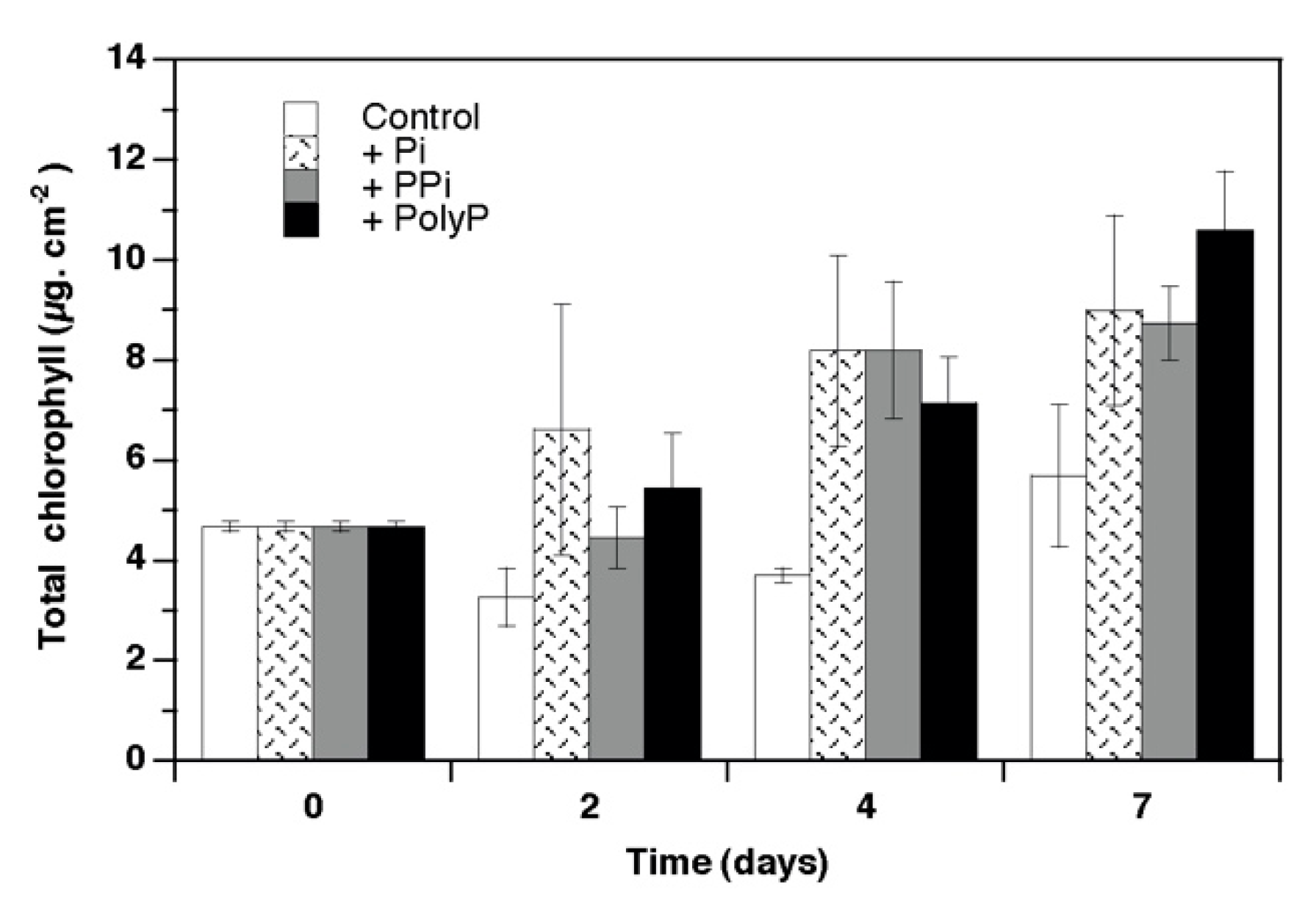
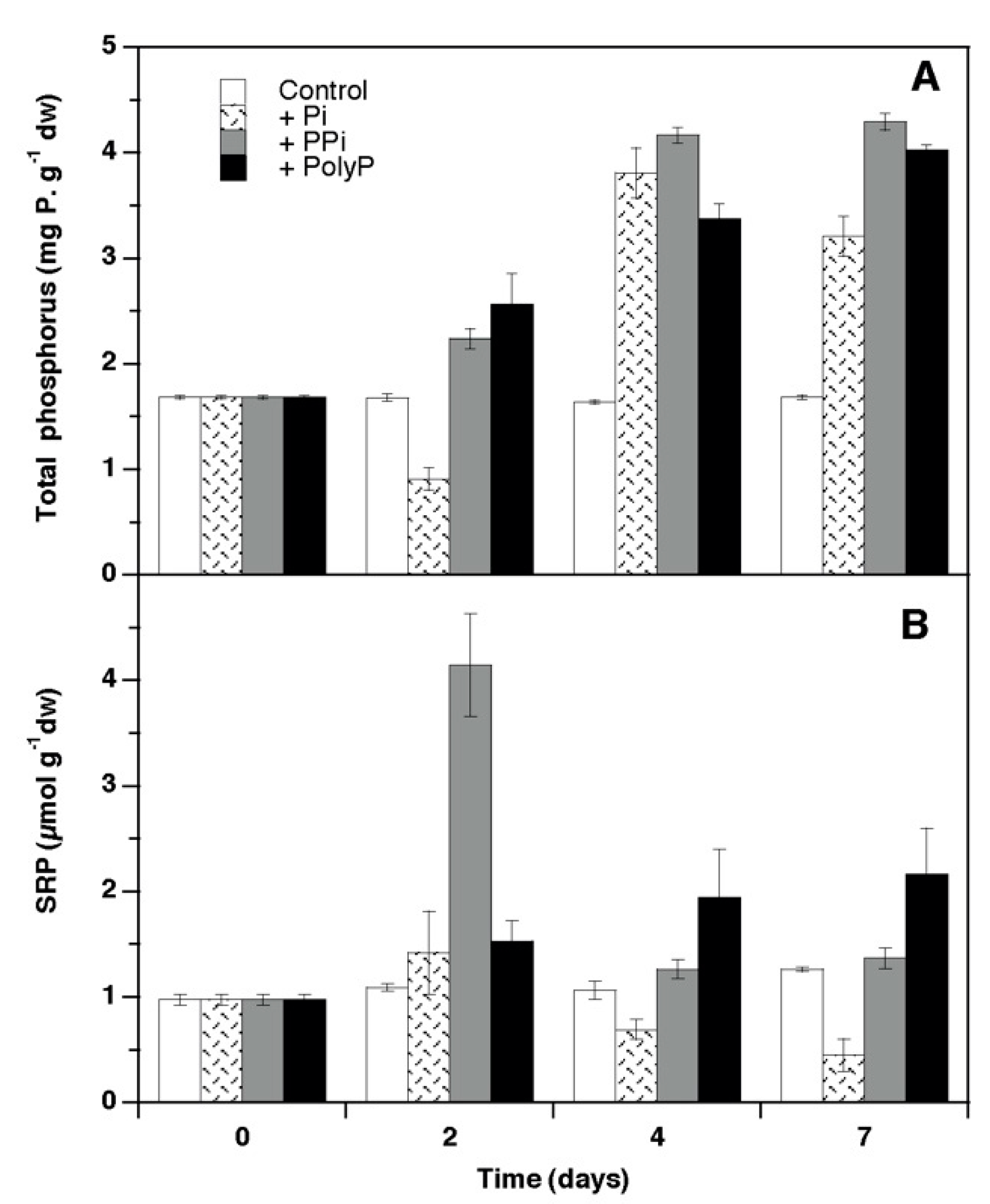
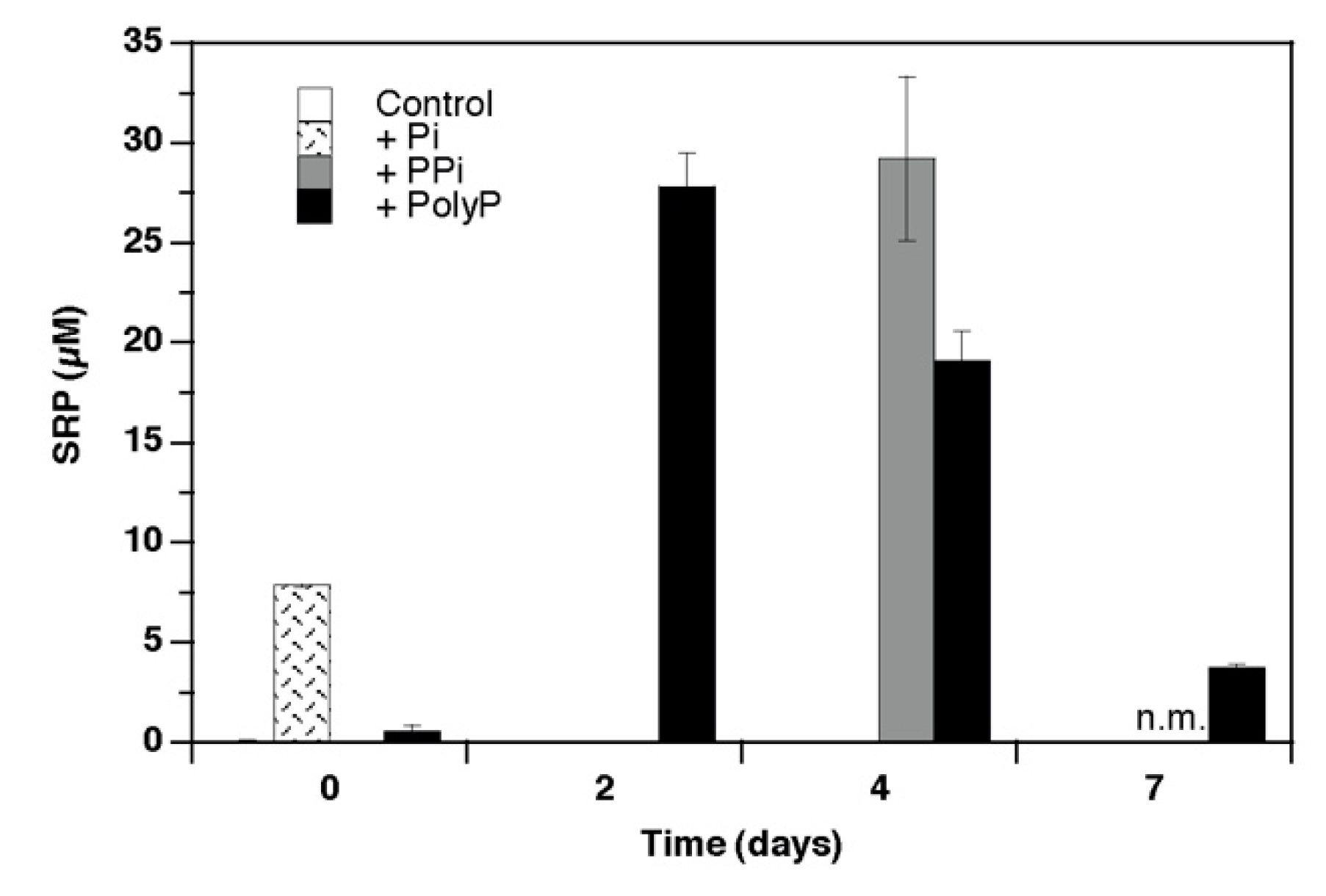
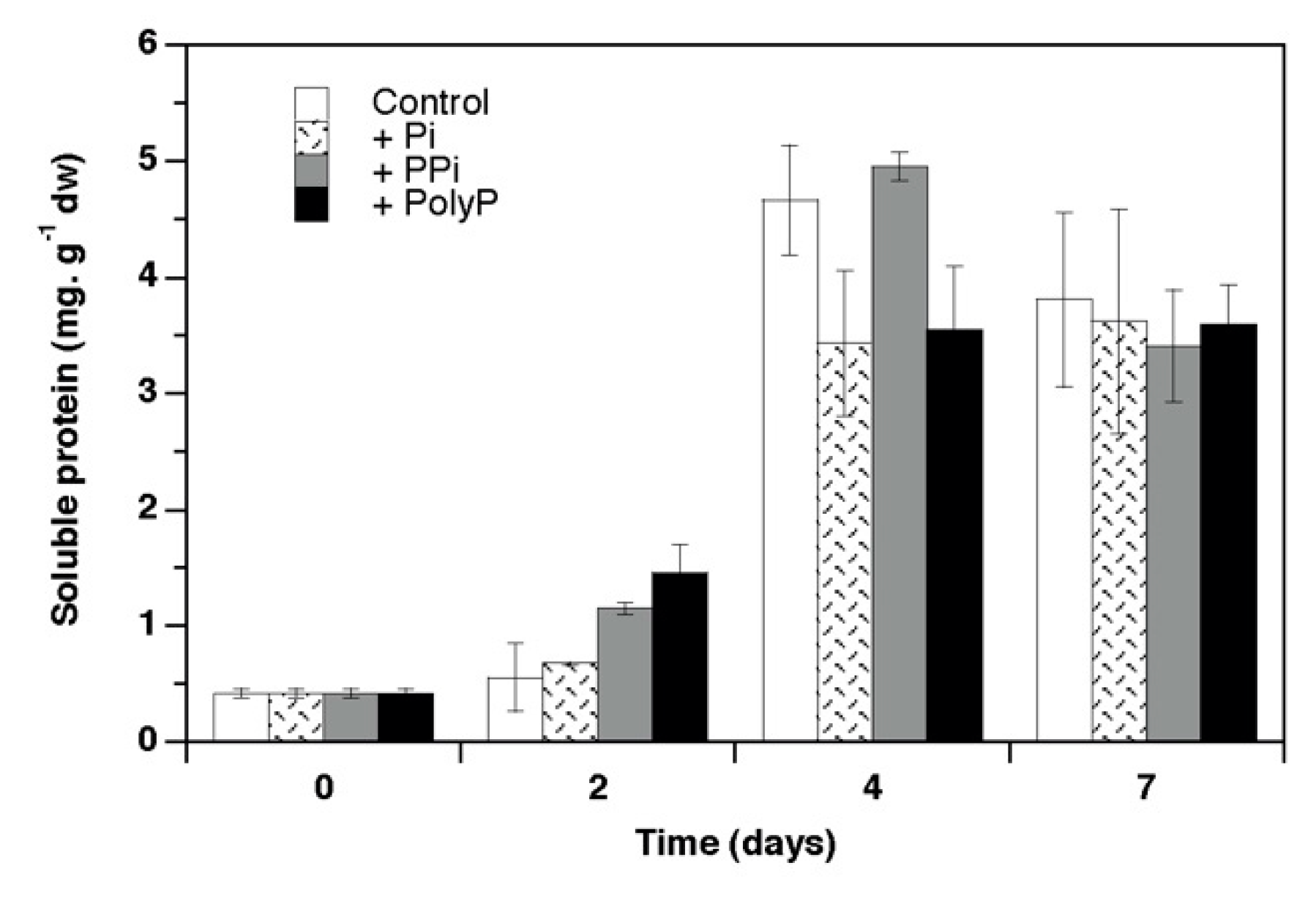
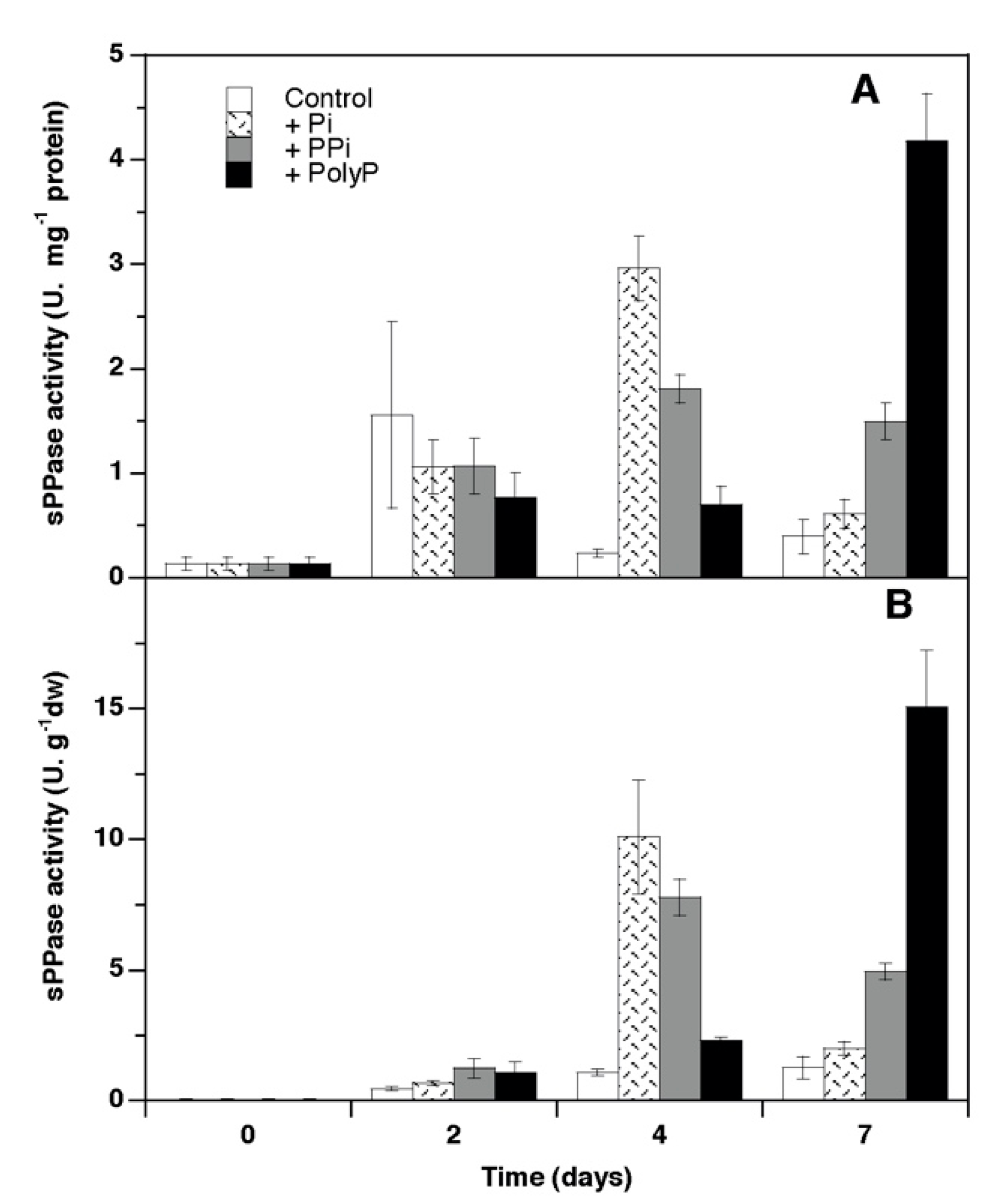
3. Results
4. Discussion
4.1. Growth and Phosphorus Use
4.2. Soluble Pyrophosphatase Activity
4.3. Ecological Implications
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Variable | Effect | df Treatment/ df Error | F Value, p |
|---|---|---|---|
| Disc area (cm2) | Time | 3/32 | 123.6 *** |
| Pi treatment | 3/32 | 18.4 *** | |
| Interaction | 9/32 | 4.8 *** | |
| Fresh weight: area (g FW m−2) | Time | 3/32 | 60.9 *** |
| Pi treatment | 3/32 | 3.6 * | |
| Interaction | 9/32 | 2.6 * | |
| Dry weight: fresh weight ratio | Time | 3/32 | 60.2 *** |
| Pi treatment | 3/32 | 2.6 n.s. | |
| Interaction | 9/32 | 2.2 n.s. | |
| Total Chlorophyll (µg cm−2) | Time | 3/32 | 10.9 *** |
| Pi treatment | 3/32 | 7.0 *** | |
| Interaction | 9/32 | 1.1 ns | |
| Total cell P (mg g−1DW) | Time | 3/32 | 177.8 *** |
| Pi treatment | 3/32 | 96.8 *** | |
| Interaction | 9/32 | 39.7 *** | |
| Cell Phosphate (µmol g−1DW) | Time | 3/32 | 10.2 *** |
| Pi treatment | 3/32 | 19.9 *** | |
| Interaction | 9/32 | 7.4 *** | |
| Soluble protein (mg g−1DW) | Time | 3/32 | 1223 *** |
| Pi treatment | 3/32 | 1.8 n.s. | |
| Interaction | 9/32 | 2.4 * | |
| sPPase activity (U g−1DW) | Time | 3/32 | 53.1 *** |
| Pi treatment | 3/32 | 16.3 *** | |
| Interaction | 9/32 | 24.1 *** | |
| sPPase activity (U mg−1protein) | Time | 3/32 | 21.0 *** |
| Pi treatment | 3/32 | 5.0 ** | |
| Interaction | 9/32 | 14.6 *** |
References
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis; Blackwell Science: Maiden, MA, USA, 1997; 375p. [Google Scholar]
- Lobban, C.S.; Harrison, P.G. Seaweed Ecology and Physiology; Cambridge University Press: New York, NY, USA, 1994; 366p. [Google Scholar] [CrossRef]
- Hurd, C.L.; Dring, M.J. Phosphate uptake by intertidal fucoid algae in relation to zonation and season. Mar. Biol. 1990, 107, 281–290. [Google Scholar] [CrossRef]
- Lavery, P.S.; McComb, A.J. The nutritional eco-physiology of Chaetomorpha linum and Ulva rigida in Peel Inlet, Western Australia. Bot. Mar. 1991, 34, 251–260. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus uptake by plants: From soil to cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Hernández, I.; Niell, F.X.; Whitton, B.A. Phosphatase activity of benthic marine algae. An overview. J. Appl. Phycol. 2002, 14, 475–487. [Google Scholar] [CrossRef]
- Lin, C.K. Accumulation of water soluble phosphorus and hydrolysis of polyphosphates by Cladophora glomerata (Chlorophyceae). J. Phycol. 1977, 13, 46–51. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Bhaya, D.; Grossman, A.R. Polyphosphate: A Multifunctional Metabolite in Cyanobacteria and Algae. Front. Plant Sci. 2020, 11, 938. [Google Scholar] [CrossRef]
- Sundareshwar, P.V.; Morris, J.T.; Pellechia, P.J.; Cohen, H.J.; Porter, D.E.; Jones, B.C. Occurrence and ecological implications of pyrophosphate in estuaries. Limnol. Oceanogr. 2001, 46, 1570–1577. [Google Scholar] [CrossRef]
- Ahlgreen, J.; Tranvik, L.; Gogoll, A.; Waldebäck, M.; Markides, K.; Rydin, E. Sediment depth attenuation of biogenic phosphorus compounds measured by 31P NMR. Environ. Sci. Technol. 2005, 39, 867–872. [Google Scholar] [CrossRef]
- Lundberg, P.I.; Weich, R.G.; Jensén, P.; Vogel, H.J. Phosphorus-31 and nitrogen-14 NMR studies of the uptake of phosphorus and nitrogen compounds in the marine macroalgae Ulva lactuca. Plant Physiol. 1989, 89, 1380–1387. [Google Scholar] [CrossRef]
- Chopin, T.; Morais, T.; Belyea, E.; Belfry, S. Polyphosphate and siliceous granules in the macroscopic gametophyte of the red alga Porphyra purpurea (Bangiophyceae, Rhodophyta). Bot. Mar. 2004, 47, 272–280. [Google Scholar] [CrossRef]
- Gómez-García, M.R.; Serrano, A. Expression studies of two paralogous ppa genes encoding distinct Family I pyrophosphatases in marine unicellular cyanobacteria reveal inactivation of the typical cyanobacterial gene. Biochem. Biophys. Res. Commun. 2002, 295, 890–897. [Google Scholar] [CrossRef]
- Gómez-García, M.R. Caracterización Molecular de la Pirofosfatasa Inorgánica Soluble de Microorganismos Fotosintéticos y Plástidos. Ph.D. Thesis, University of Seville, Sevilla, Spain, 2001; p. 266. [Google Scholar]
- Pérez-Castiñeira, J.R.; Gómez-García, R.; López-Marqués, L.; Losada, M.; Serrano, A. Enzymatic systems of inorganic pyrophosphate bioenergetics in photosynthetic and heterotrophic protists: Remnants or metabolic cornerstones? Int. Microbiol. 2001, 4, 135–142. [Google Scholar] [CrossRef]
- Lathi, R.; Pitkäranta, T.; Valve, E.; Ilta, I.; Kukko-Kalse, E.; Heinonen, J. Cloning and characterization of the gene encoding inorganic pyrophosphatase of Escherichia coli K-12. J. Bacteriol. 1988, 170, 5901–5907. [Google Scholar] [CrossRef]
- Baltscheffsky, M.; Nyrén, P. The Synthesis and Utilization of Inorganic Pyrophosphate. In Molecular Mechanisms in Bioenergetics; Ernster, L., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 187–206. [Google Scholar]
- Cormaci, M.; Furnari, G.; Alongi, G. Flora marina bentonica del Mediterraneo: Chlorophyta. Boll. dell’Accademia Gioenia Sci. Nat. Catania 2014, 47, 11–436. [Google Scholar]
- Pérez-Lloréns, J.L.; Vergara, J.J.; Pino, R.R.; Hernández, I.; Peralta, G.; Niell, F.X. The effect of photoacclimation on the photosynthetic physiology of Ulva curvata and U. rotundata (Ulvales, Chlorophyta). Eur. J. Phycol. 1996, 31, 349–359. [Google Scholar] [CrossRef][Green Version]
- Lewin, J. Silicon metabolism in diatoms. V. Germanium dioxide, a specific inhibitor of diatom growth. Phycologia 1966, 6, 1–12. [Google Scholar] [CrossRef]
- Wheeler, P.A.; Björnsater, R. Seasonal fluctuations in tissue nitrogen, phosphorus, and N:P for five macroalgal species common to the Pacific Northwest coast. J. Phycol. 1992, 28, 1–6. [Google Scholar] [CrossRef]
- Woelkerling, W.J.; Spencer, K.G.; West, J.A. Studies on selected Corallinaceae (Rhodophyta) and other algae in a defined marine culture medium. J. Exp. Mar. Biol. Ecol. 1983, 67, 61–77. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 26, 31–36. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ames, B.N. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 1966, 8, 115–118. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1984; 718p. [Google Scholar]
- Lyngby, J.E.; Mortensen, S.; Ahrensberg, N. Bioassessment techniques for monitoring of eutrophication and nutrient limitation in coastal ecosystems. Mar. Poll. Bull. 1999, 39, 212–223. [Google Scholar] [CrossRef]
- Hernández, I.; Peralta, G.; Pérez-Lloréns, J.L.; Vergara, J.J.; Niell, F.X. Biomass and dynamic of growth of Ulva species in Palmones River estuary. J. Phycol. 1997, 33, 764–772. [Google Scholar] [CrossRef]
- Droop, M.R. 25 years of algal growth kinetics. A personal view. Bot. Mar. 1983, 26, 99–112. [Google Scholar] [CrossRef]
- McComb, R.B.; Bowers, G.N., Jr.; Posen, S. Alkaline Phosphatase; Plenum Press: New York, NY, USA, 1979; 986p. [Google Scholar]
- Gómez-García, M.R.; Losada, M.; Serrano, A. Concurrent transcriptional activation of ppa and ppx genes by phosphate deprivation in the cyanobacterium Synechocystis sp. strain PCC 6803. Biochem. Biophys. Res. Commun. 2003, 302, 601–609. [Google Scholar] [CrossRef]
- Vergara, J.J.; Pérez-Lloréns, H.J.L.; Peralta, G.; Hernández, I.; Niell, F.X. Photosynthetic performance and light attenuation in Ulva canopies from Palmones river estuary. J. Phycol. 1997, 33, 773–779. [Google Scholar] [CrossRef]
- Palomo, L.; Clavero, V.; Izquierdo, J.J.; Avilés, A.; Becerra, J.; Niell, F.X. Influence of macrophytes on sediment phosphorus accumulation in a eutrophic estuary (Palmones river, Southern Spain). Aquat. Bot. 2004, 80, 103–113. [Google Scholar] [CrossRef]

| Substrate | Enzyme Activity (U·mg prot−1) |
|---|---|
| Pyrophosphate | 4.71 ± 0.10 a |
| Tripoliphosphate | 4.87 ± 0.10 a |
| Trimetaphosphate | 2.12 ± 0.01 b |
| Polyphosphate | 16.94 ± 0.11 c |
| Initial | Control | Phosphate | Pyrophosphate | Polyphosphate | |
|---|---|---|---|---|---|
| Carbon (%DW) | 22.7 ± 0.9 a | 25.2 ± 0.4 b | 27.1 ± 0.1 b | 27.6 ± 0.3 b | 27.3 ± 0.3 b |
| Nitrogen (%DW) | 1.16 ± 0.03 a | 3.65 ± 0.09 b | 4.57 ± 0.10 c | 4.81 ± 0.13 c | 4.04 ± 0.12 b |
| Phosphorus (%DW) | 0.17 ± 0.01 a | 0.17 ± 0.01 a | 0.32 ± 0.02 b | 0.43 ± 0.01 c | 0.40 ± 0.01 c |
| C:N (by atoms) | 22.8 ± 0.5 a | 8.08 ± 0.32 b | 6.94 ± 0.13 b | 6.68 ± 0.12 b | 7.91 ± 0.13 b |
| C:P (by atoms) | 349 ± 13 a | 387 ± 9 a | 220 ± 14 b | 166 ± 7 b | 176 ± 4 b |
| N:P (by atoms) | 15.3 ± 0.5 a | 48.0 ± 1.3 b | 31.8 ± 2.2 b | 25.0 ± 1.5 bc | 22.2 ± 0.8 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergara, J.J.; Herrera-Pérez, P.; Brun, F.G.; Pérez-Lloréns, J.L. Use of Polyphosphates and Soluble Pyrophosphatase Activity in the Seaweed Ulva pseudorotundata. Oceans 2020, 1, 343-354. https://doi.org/10.3390/oceans1040023
Vergara JJ, Herrera-Pérez P, Brun FG, Pérez-Lloréns JL. Use of Polyphosphates and Soluble Pyrophosphatase Activity in the Seaweed Ulva pseudorotundata. Oceans. 2020; 1(4):343-354. https://doi.org/10.3390/oceans1040023
Chicago/Turabian StyleVergara, Juan J., Patricia Herrera-Pérez, Fernando G. Brun, and José Lucas Pérez-Lloréns. 2020. "Use of Polyphosphates and Soluble Pyrophosphatase Activity in the Seaweed Ulva pseudorotundata" Oceans 1, no. 4: 343-354. https://doi.org/10.3390/oceans1040023
APA StyleVergara, J. J., Herrera-Pérez, P., Brun, F. G., & Pérez-Lloréns, J. L. (2020). Use of Polyphosphates and Soluble Pyrophosphatase Activity in the Seaweed Ulva pseudorotundata. Oceans, 1(4), 343-354. https://doi.org/10.3390/oceans1040023







