1. Introduction
Globally, many species of shark have suffered a dramatic decline in abundance over the past 30 years [
1,
2,
3], principally as a result of by-catch and finning in response to the demand for shark fin by the Far-East restaurant trade [
4,
5]. As a consequence, 11 shark species are now considered Critically Endangered, 15 Endangered and 48 Vulnerable [
6]. Most large-bodied sharks are threatened or endangered [
2,
7], while even once common reef species have become scarce in many areas with, for example, the long-established shark fishery in the Seychelles recording a significant decrease in catch per effort from 16 to 6 kg day
−1 between 1992 and 2016 [
8].
As a result, monitoring the population abundance of sharks is much needed [
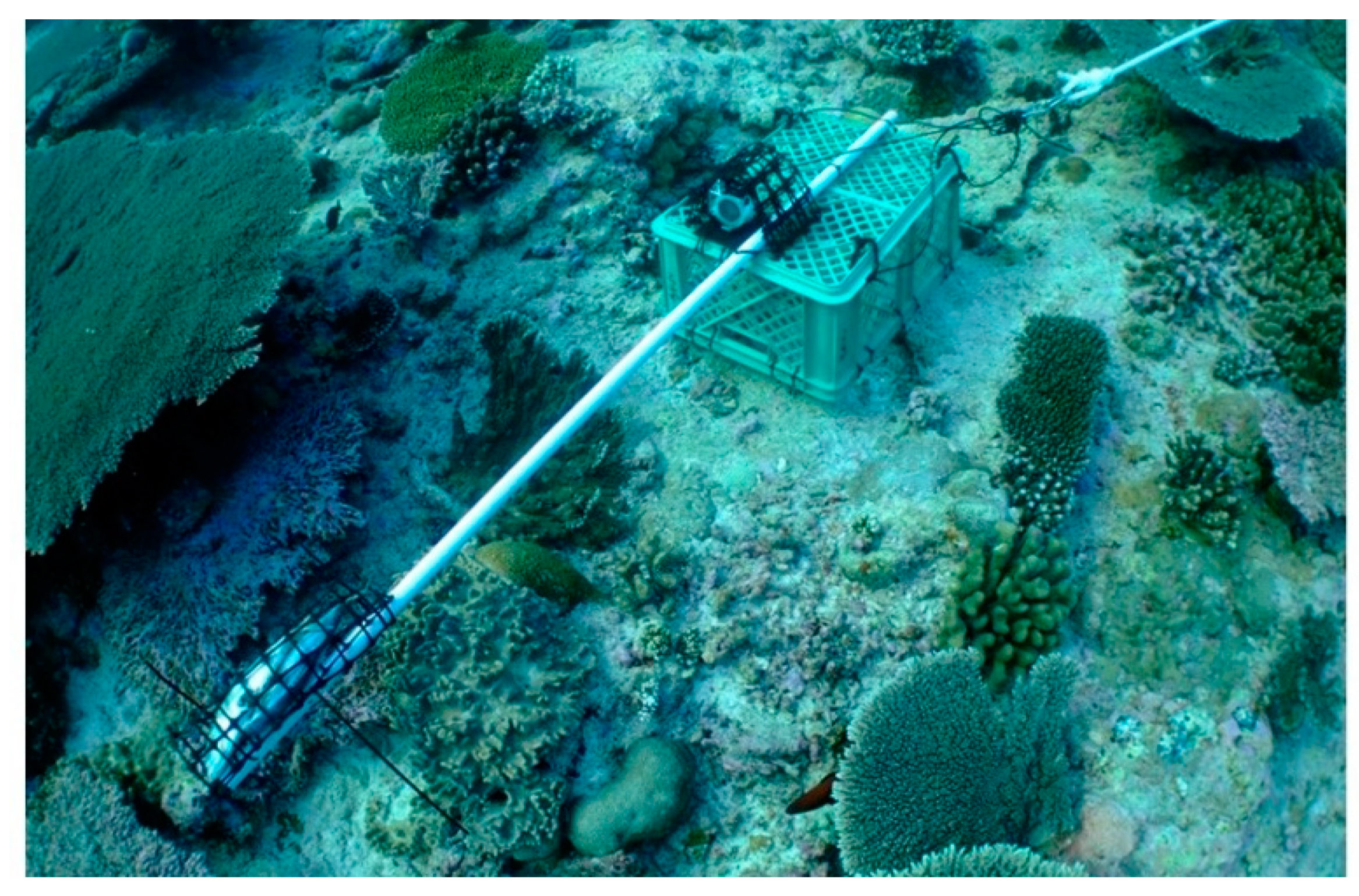
9]. Baited Remote Underwater Video Stations (BRUVS), underwater video cameras set to record the fishes attracted to bait, is now the method most frequently used for surveying and monitoring sharks in particular [
10,
11,
12]. Stereo-BRUVS, in which two cameras are focused towards the bait, can provide further data by enabling both the distance to a fish and its length to be determined [
13], although the associated equipment costs are necessarily higher if similar numbers of units are to be deployed. Cappo et al. [
14] have reviewed BRUVS techniques for camera, bait, and orientation of bait and camera, as well as potential auditory, olfactory and behavioural cues, while Harvey et al. [
15] concluded that BRUVS provide a statistically robust and cost-effective method of monitoring diverse assemblages of fishes in a number of habitat types.
BRUVS are not the only visually-based method regularly employed for monitoring fishes. Several forms of Underwater Visual Census (UVC) are widely used by divers [
16,
17,
18]. However, remote video has the advantage of being practical in conditions where divers cannot easily operate, as in very deep water or rough conditions, and of being able to detect species, such as large sharks, which are often too scarce or too wary to be regularly sighted by divers [
13]. Video methods are only fully effective in reasonably clear water, and baited video will only detect species that either are resident close to the BRUVS, or passing through, such as roaming schools of carangids, or like sharks have a well-developed olfactory sense enabling them to home-in on the bait [
19].
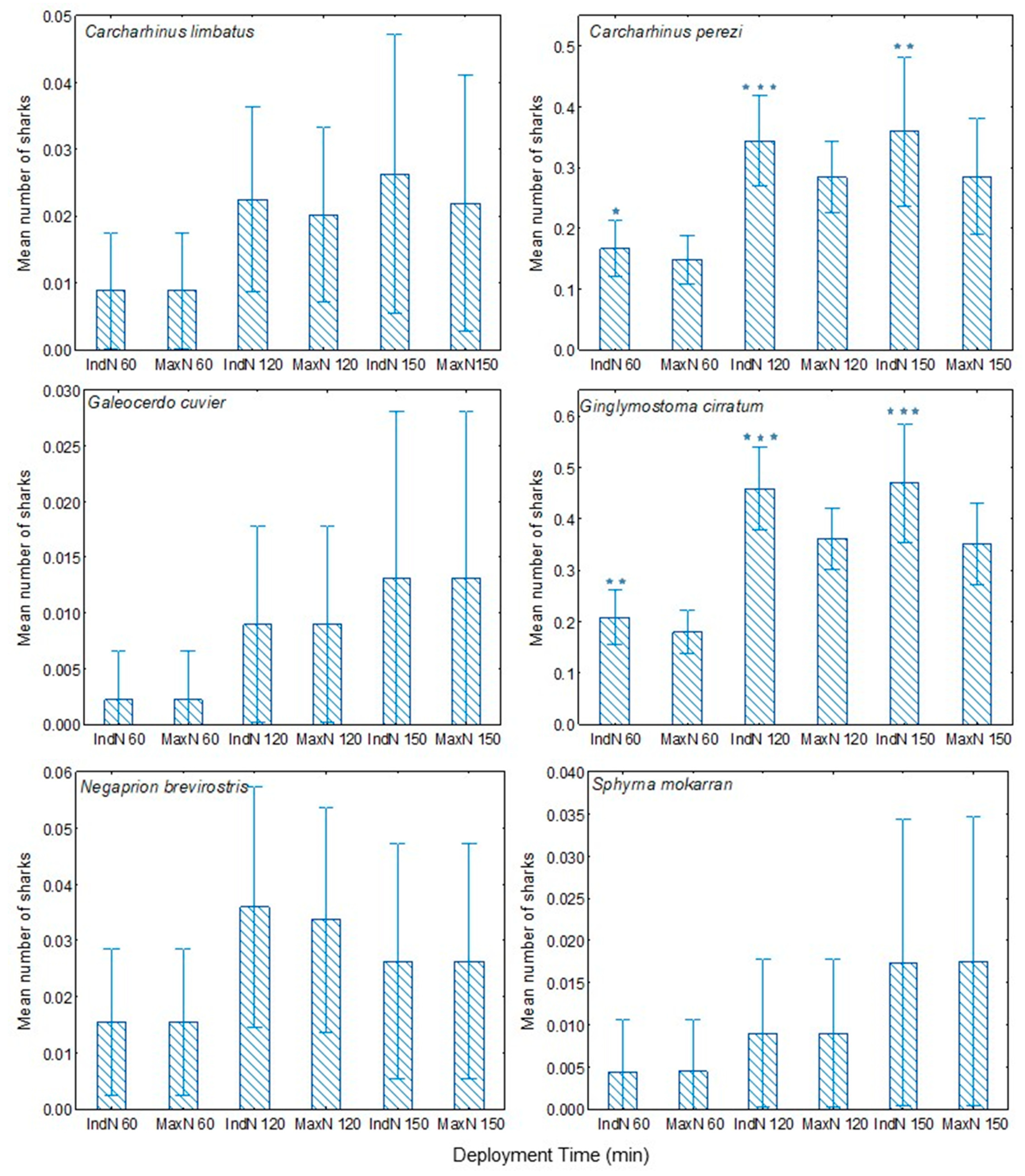
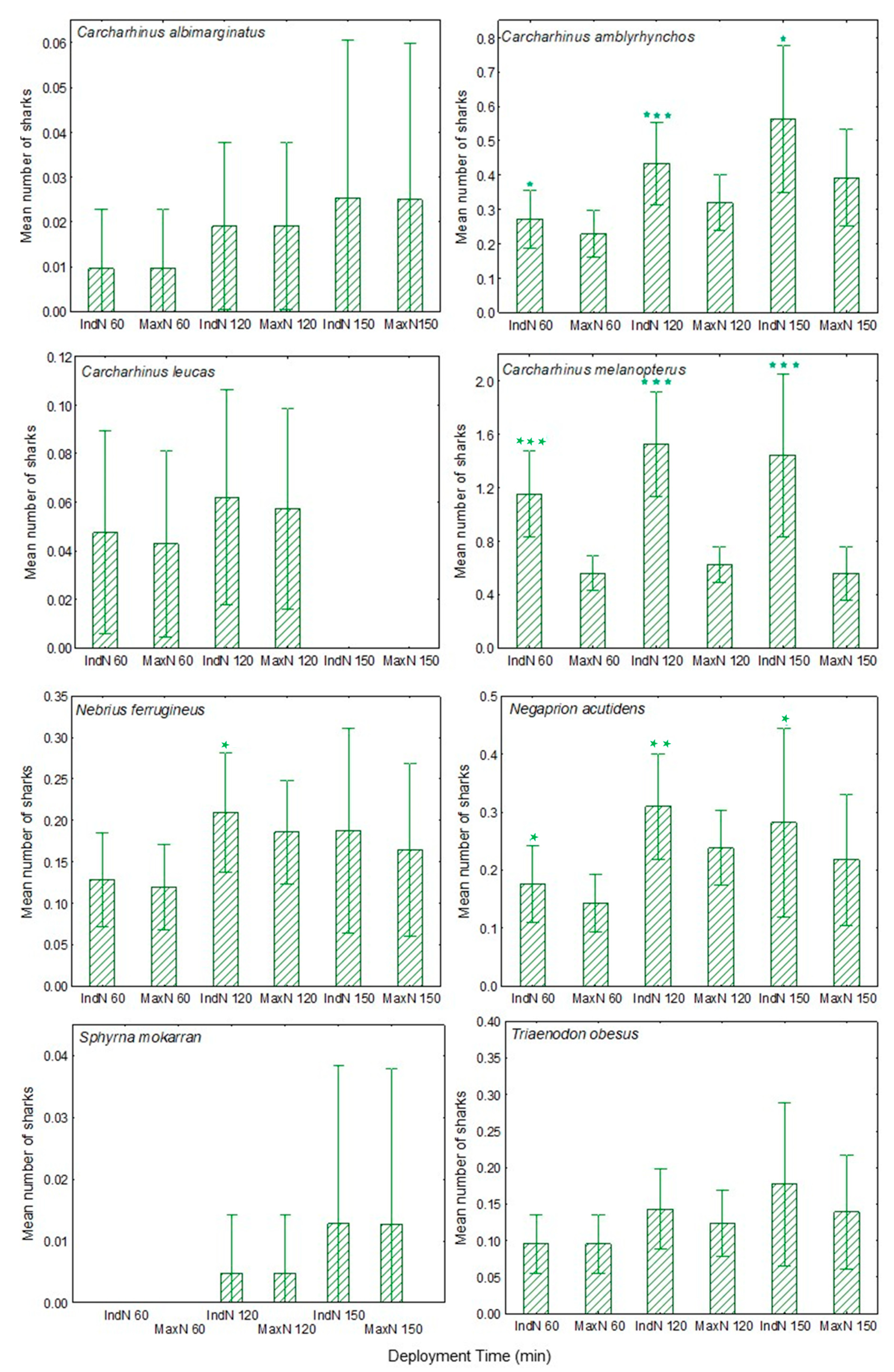
To date, the most commonly used measure of the numbers of fishes coming to the BRUVS has been the maximum number of fishes recorded in a single frame during the viewing period (referred to as MaxN or Nmax). While simple for an observer to record, this measure suffers a number of limitations, most obviously that the observed MaxN may be much lower than the total number of separate individual fish attracted to the bait. Harvey et al. [
15] discussed alternative approaches, including the use of cumulative MaxN (recorded each time MaxN is updated for each species). Campbell et al. [
20] compared MaxN with MeanCount (the mean number of fishes observed in a series of frames or stills). They concluded that these two measures were comparable for abundance estimation, although MeanCount resulted in less precision for all species analysed. Similarly, Schobernd et al. [
21] compared the advantages of using MaxN and MeanCount in both simulations and laboratory experiments, as well as in empirical data. They concluded that an advantage of MeanCount is that it is linearly related to actual abundance, whereas MaxN is not.
Understanding the accuracy and precision of monitoring and surveying methods is important for interpreting any apparent change or lack of change in species number or relative abundance. A weakness of the above counting methods is that they do not record whether different individuals are visiting a BRUVS at different times, as opposed to a single individual making repeat visits. Nor do they detect when the same individual visits two or more different BRUVS. This limitation is most obvious with less abundant but not uncommon species, such as many reef sharks and rays, in which different individuals may visit the same BRUVS, but at different times.
To address this issue, studies on other taxa have experimented with photo-identification as a tool for distinguishing different individuals. Stobart et al. [
22] used BRUVS to record spiny lobster,
Palinurus elephas, and compared three measures of abundance: MaxN, mean MaxN (using 5 min sampling periods) and the true number of individuals per recording based on individual identification (referred to in the present study as IndN). The authors concluded that while all three measures could distinguish areas of contrasting population density, MaxN and MeanN were not appropriate for documenting changes in population abundance because they were liable to sample saturation. That is, an increase in population abundance may not result in any increase in the MaxN. More recently, Irigoyen et al. [
23] used three relative abundance indices in a survey of broadnose sevengill shark,
Notorynchus cepedianus: Nmax, NmaxIND (cumulative number of different sharks and similar to IndN) and Nocc (total number of occurrences in a BRUVS session). The index NmaxIND appeared to show a greater abundance of sharks than Nmax. Sherman et al. [
24] used photo-identification to determine the true numbers of two species of ray visiting BRUVS and found that the actual abundances were for
Neotrygon orientalis 2.4, and for
Taeniura lymma 1.1, times greater than the recorded MaxN values. However, to date this approach has not been generally applied to BRUVS-based monitoring of sharks, in part because the earlier cameras used were of lower resolution, so that it was not feasible to distinguish between most individuals of the same species.
Photo-identification has been found to be practical in some other studies of sharks. Population estimates of basking shark,
Cetorhinus maximus [
25], and great white shark,
Carcharhinus carcharius [
26], have been made based on individual recognition through quality images of dorsal fins. Photo-identification based on images captured by divers or fishers has also been used in ecological studies of blacktip reef shark,
C. melanopterus [
27], Greenland shark,
Somniosus microcephalus [
28], nurse shark,
Ginglymostoma cirratum [
29], sicklefin sharks,
Negaprion acutidens [
30], broadnose sevengill shark,
Notorynchus cepedianus [
23], and whale shark [
31,
32], as well as flapper skate,
Dipturus intermedius [
33]. In addition, BRUVS have been used to monitor the presence and size [
34], as well as site fidelity [
11], of individually identified sharks. Here, the feasibility of applying photo-identification methodology to the video footage recorded by BRUVS is assessed to monitor more effectively the abundance of coral reef shark species and test whether the data can also be used to generate additional information on population size or ranging behaviour.
The deployment period used to record individuals visiting a BRUVS (i.e., the effective length of deployment) has also varied between studies. The majority of previous studies have used deployment periods of 60 min (for example [
24,
35,
36]), often because this was the maximum recording time that could be guaranteed by the batteries in older cameras. Some studies used shorter times, such as 30 min ([
19] fish species), while Brooks et al. ([
10] shark species) used 90 min, Harasti et al. ([
34] white shark) 5 h and Stobart et al. ([
22] lobster) 7 h. After using BRUVS to record coral reef fishes in the Hawaiian Islands, Asher [
37] concluded that while a short deployment of 20 min was sufficient to capture fast-reacting species and residents, longer periods of 60 min were required to capture macropiscivores, including sharks. Our experience suggested that additional individual and species of shark could arrive at a BRUVS even after this time. A study by Torres et al. [
38] exceeded other deployment times in using a duration of 24 h for sharks in the western Mediterranean. As a result of the wide range of deployment times used in different studies, the effect of deployment period on the numbers of individuals and species of shark recorded at a site was also tested in the present study.
In the present study, the BRUVS video recordings obtained during two shark conservation projects, one in the Cayman Islands (western Caribbean) and the other in the Amirante Islands (south-western Seychelles) were reviewed. Photo-identification methodology was applied to distinguish the separate individuals visiting BRUVS over different periods. The aims of the study were to assess whether (a) the use of photo-identification can provide better estimates of the numbers of sharks being recorded, (b) the approach can be used to generate estimates of population size by applying mark–recapture analyses to the data, and (c) the method can also be used to provide information about the movements of individuals. In addition, (d) it was investigated whether for reef sharks the numbers of species and individuals recorded varied significantly with BRUVS deployment period. We found that photo-identification could enhance the value of data obtained in relation to all three questions. However, the present study is not intended to compare abundances between areas or habitats, or across years (hence locations of individual stations are not included); those analyses are being reported on separately.
4. Discussion
Comparison of the present data with those obtained by other researchers in the same ocean regions is possible (
Table 6 [
10,
11,
12,
42,
46,
47,
48]), but confounded by differences in method, such as the time and depth ranges at which the BRUVS were deployed. In particular, while most researchers report MaxN for a deployment period of 60 min, relevant studies have used deployment periods ranging from 54 to 90 min, with one using 6 h [
48]. Some authors (Brooks et al. [
10]) have corrected for differences in deployment period by reporting numbers as catch per unit effort (CPUE) of one hour, on the assumption that MaxN increases linearly with time. However, in the present study, the values of both MaxN and IndN rise exponentially with time, hence longer deployment periods will appear to yield lower values of CPUE if presented this way. Nevertheless, some comment is possible. In the Cayman Islands, the abundance of two species of sharks was greater and of four species less than that reported in Eleuthera, the Bahamas. The abundances of
N. acutidens appear markedly greater in the Amirante Islands than in the eastern Indian Ocean. Abundances of
C. melanopterus and
N. ferrugineus appear broadly comparable. More detailed analysis of the spatial and temporal patterns of abundance for the different species recorded in our study areas will be reported separately.
It is also important to note that when fish visible on BRUVS videos are counted (e.g., to determine MaxN), normally the results provide information only on relative abundance (that is, differences in abundance between locations or between occasions), but not on overall population size or density. Even though such population indices are widely used for wildlife monitoring and are generally assumed to be directly proportional to population density, in many field studies this assumption has been found to be invalid [
49]. It is a weakness of BRUVS methodology as generally employed that the most commonly used measure, MaxN, does not increase linearly with actual abundance, but tends to saturate [
22]. While this would be less of an issue for schooling or non-territorial species, it becomes more of a concern in species which are territorial or keep their distance from each other.
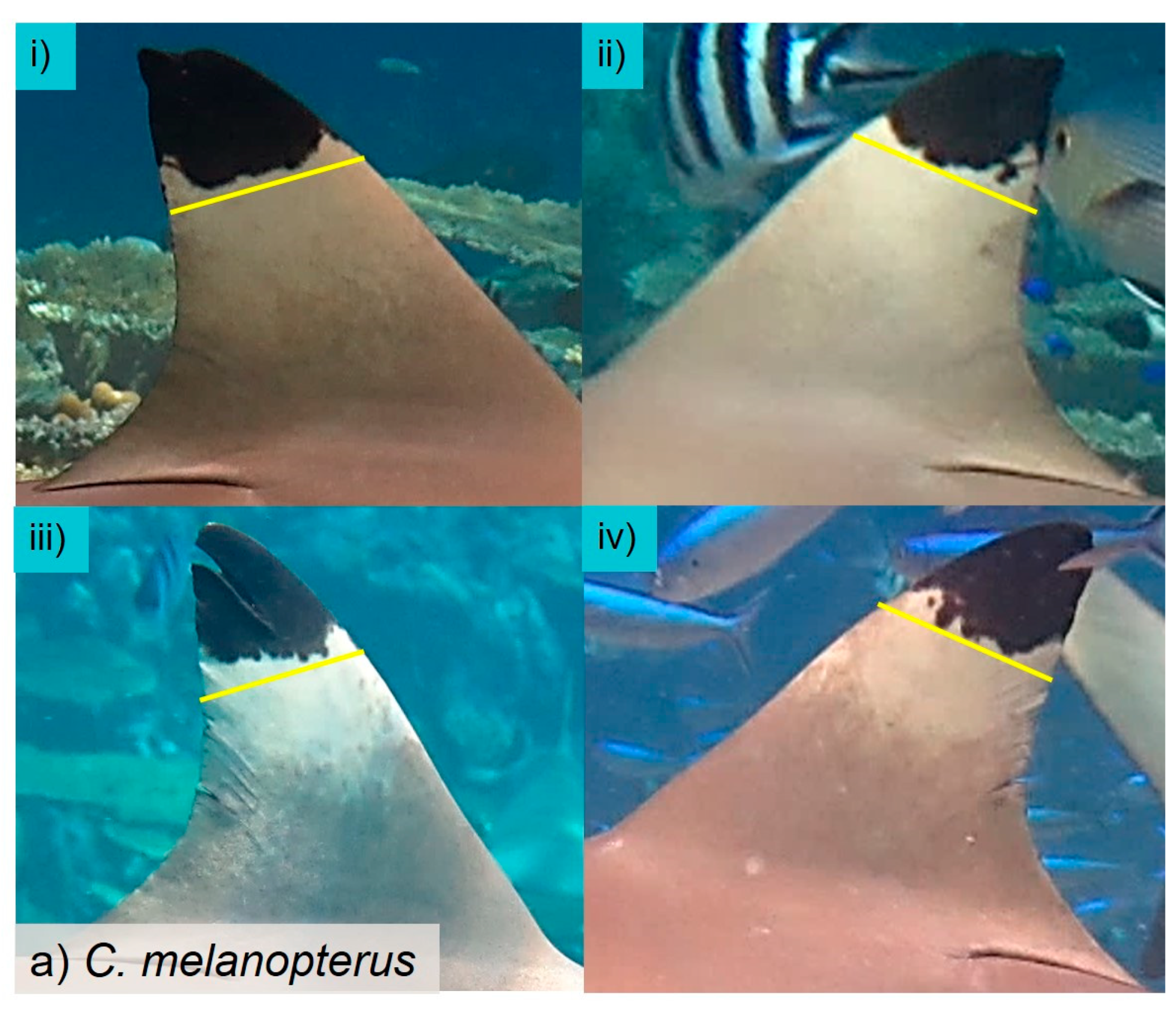
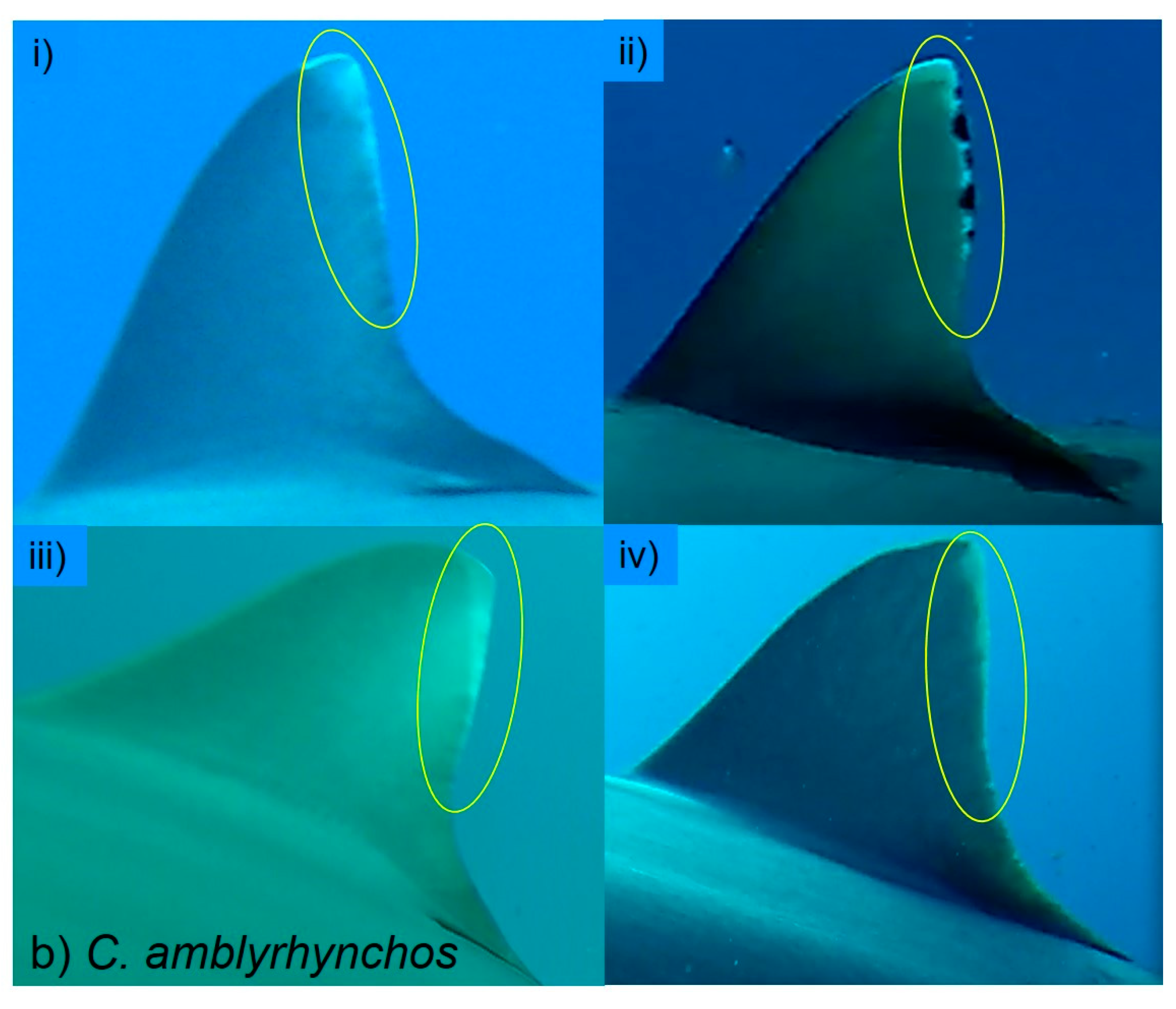
In the present study, we sought to address this issue by identifying sharks as individuals and determining the actual number of sharks visiting each BRUVS. As this study confirmed, most individual sharks carry distinctive marks or features allowing them to be identified as individuals. Some marks are natural while others are acquired, with older individuals tending to have more characteristics through injuries and scars than neonates and juveniles. In 12 of the 14 species recorded, at least two-thirds of the observed sharks could be recognised as individuals through such features. In most cases where it was not possible to discern any distinguishing features, this was because the shark’s appearance on camera was too brief or too distant. Some species, notably
C. albimarginatus and
C. leucas, in which the percentages of distinguishable individuals were the lowest (9.1% and 16.7%, respectively), never closely approached the bait even though they may have been attracted to the vicinity of the BRUVS by olfactory cues. With other species the proportion of individually identified sharks was lower than it might otherwise have been because of high turbidity and poor visibility at some stations. This was especially the case in the sandy lagoon in St. Joseph Atoll, where only a minority of individuals could be identified. In particular, this affected the percentage of individually identifiable
C. melanopterus (85.3%), which otherwise are readily identified as individuals because of the very variable shape of the black patches on their dorsal fins (see also [
50]). In summary, while many sharks can be recognised as individuals with certainty, a proportion of them viewed on BRUVS cannot be distinguished. Thus, in many cases the difference between the true number of visiting sharks and MaxN will be even greater than between MaxN and IndN.
The species for which for most BRUVS IndN was greater than MaxN are those that are largely coastal by habitat. In contrast, more open-water species C. albimarginatus, C. limbatus, G. cuvier and S. mokarran, appeared rarely and only as single individuals. Mean IndN was significantly greater than MaxN for each deployment period overall (60, 120, 150 min). Further, in most species, including C. limbatus, C. perezi and G. cirratum in the Cayman Islands and C. amblyrhynchos, C. melanopterus, N. ferrugineus and T. obesus in the Amirante Islands, the mean ratio of IndN/MaxN increased with recording period. These findings indicate that in at least these two study areas, for most species an increased deployment period resulted in visits by significantly more individuals, not simply in return visits by the same individuals. Thus, IndN may at times be a more useful index than MaxN, especially for assessing ongoing population trends in the least abundant species, such as S. mokarran.
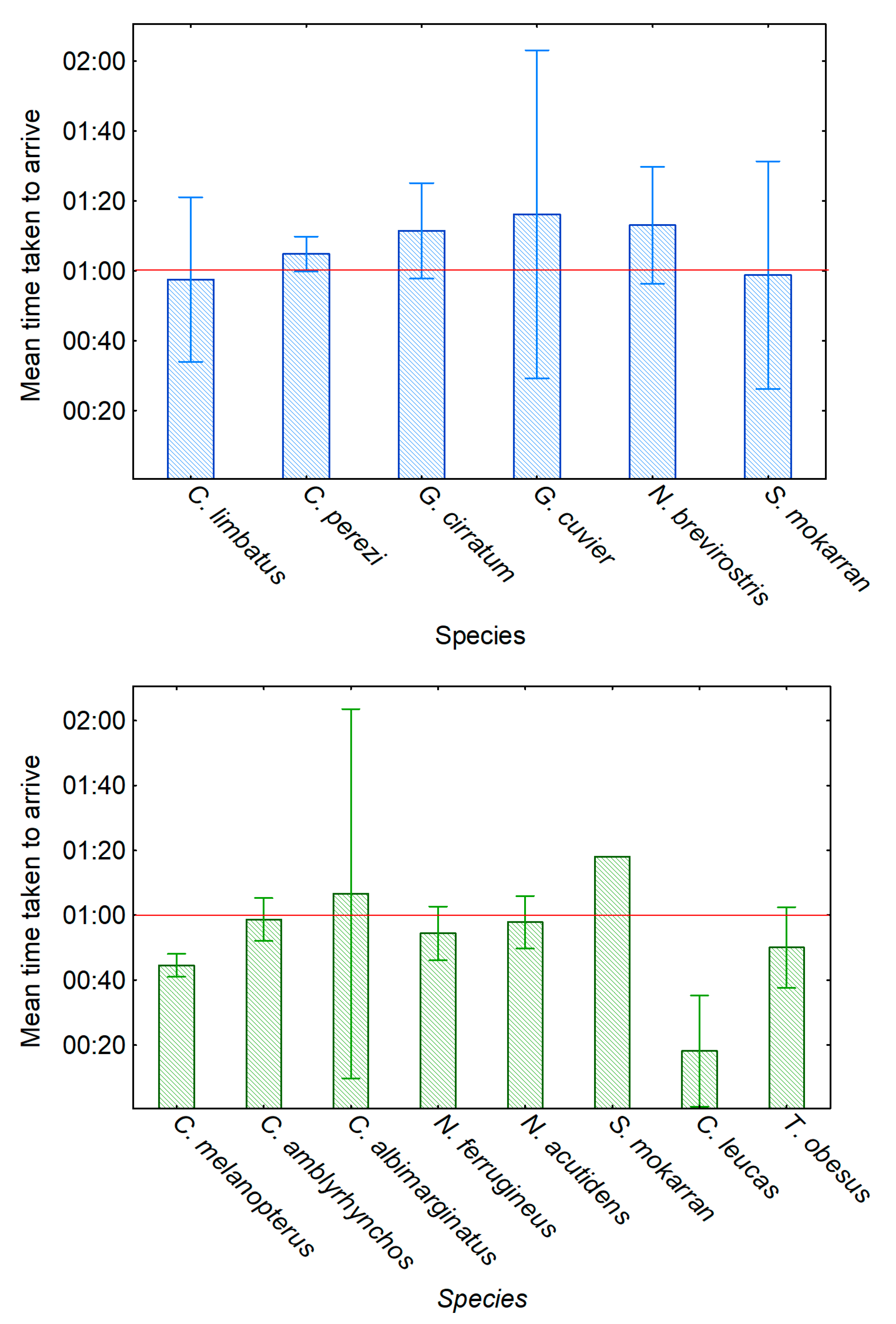
A comparison of the data obtained after different BRUVS deployment periods also showed that while most shark species present might be recorded during the course of survey work using 60 min deployments, nevertheless both the number of species recorded and the numbers of individuals of most species increased with longer BRUVS deployment periods. Interestingly, in a very recent study by Torres et al. [
38], a deployment time of 24 h showed that while no additional species were observed after 60 min deployment, additional individuals continued to arrive for a long time. In the present study, the 120 and 150 min deployment periods showed a greater number of both individuals and species, with some individuals of four species taking over 2 h to arrive. An example of a non-shark elasmobranch arriving late during a recording session was a bowmouth guitarfish,
Rhina ancylostoma, which arrived at a BRUVS only 2 h 17 min 7 s after deployment. Thus we concluded that extended recording periods may be advantageous, provided the fieldwork schedule permits this.
Besides deployment period, the number of sharks attracted to a BRUVS, and hence the precision of monitoring, is also influenced by other factors. Our experience over 10 years suggests that the quality, as well as the quantity, of bait can be important. The oil in bait appears to be a component particularly attractive to sharks, but the oil content can vary greatly not only between bait species, but with the season in which it is caught (relative to fish maturity and reproductive state) for fat content, where they are fished, and with catch storage conditions. For example, the fat content of fished tuna has been reported to vary from 0.4 to 13.5%, and of sardine from 1.2 to 18% [
51,
52,
53,
54]. The quantity of bait may in our experience be less critical. We used less bait than many researchers, but in a pilot study we found that using over five times the amount of bait used here (1550 g as opposed to 300 g) resulted in only a small, non-significant increase in the numbers of sharks arriving at the BRUVS.
The method of containment of bait on the BRUVS is also important. When, during earlier work, bait was enclosed only in a large mesh-size bag, most or all of it could be quickly removed by sharks, but also by scavenging fishes such as species of snapper (
Lutjanus), emperor (
Lethrinus), durgon (
Melichthys) and octopus. In such circumstances, all of the bait could be consumed in well under an hour, in which case extended deployment would not result in further sharks being recorded. In both studies described here, double bags of fine mesh inside larger mesh were employed, so that the scent of the bait could still disperse readily, while the bait itself was difficult for fishes to extract. Other workers have used perforated cans as bait containers [
23,
32]. As in any monitoring program, the data obtained from BRUVS-based shark monitoring programs will be more reliable if such considerations are taken into account and all aspects of the method standardised as far as possible.
As well as providing more precise estimates of abundance, the application of photo-identification to BRUVS can provide information on the behaviour of individuals. Re-sightings showed that some individuals of most species moved distances of up to 33 km across the study area.
C. melanopterus,
G. cirratum and
N. ferrugineus, however, were also often re-sighted close to the stations where they were originally recorded. Data from acoustic tagging work undertaken in both study areas and elsewhere have provided evidence that some of the species studied can move over even greater distances than evident from the BRUVS data. For example, one
C. perezi was recorded moving 125 km from Grand Cayman to Little Cayman and the same distance back again to Grand Cayman [
39]. Similarly, in the present study individual
C. amblyrhynchos in the Amirante Islands were observed on different BRUVS up to 20 km apart. Both long- and short-range movements of this species have also been documented in New Caledonian waters by Bonnin et al. [
55], who suggested that the long-range movement of adults was motivated by mating opportunity. In contrast, only a few of the >50 tagged
C. melanopterus in the Amirante Islands have been recorded crossing the <1 km wide channel between D’Arros and St. Joseph Atoll [
56], even though in Polynesia this species has been recorded crossing open water between islands up to 50 km apart [
57].
If the low rates of re-sighting that were observed in the present study are typical of such photo-identification work, then the movement data generated will be more limited than can be obtained using acoustic tagging, which frequently records the locations of individuals if they are present within a study area, provided that a dense network of receivers is installed over the area of interest. Information derived from photo-identification work may nevertheless be of value where the considerable funding required to mount an effective acoustic tagging study is unavailable. Furthermore, where BRUVS are deployed on a regular basis within a specific area, photo-identification data can reveal whether individual sharks make frequent use of an area or are resident within it.
It was also anticipated that re-sightings data might allow the application of mark–recapture modelling to estimate the population of different species within the study areas. In practice, the numbers of individuals of even the more frequently recorded species re-sighted on a different BRUVS during the same sampling campaign were low (ranging from 2 to 9%), and the numbers re-sighted in the following year even lower (0.4–2.7%). Encouragingly, however, low rates of re-sighting suggest that known individuals must be mixing with a larger population of other individuals. Thus the model used indicated for the Cayman Islands a mean effective population sizes of 76 ± 23 (S.E.)
C. perezi and 199 ± 42 (S.E.)
G. cirratum. These estimates are of interest in light of shark conservation efforts in Cayman, where all shark species have been protected since April 2015 [
39,
40]. It will be important to determine whether estimated population sizes of these species increase with time. Local opinion surveys indicated that the shark protection legislation had overwhelming public support [
39], being opposed by only a small minority of boat-based fishers who may be tempted to ignore it.
There are also two factors which confound the interpretation of the population estimates. Firstly, the calculated population sizes are only of individually identifiable sharks. Following Gore et al. [
25], these numbers need to be corrected upwards to allow for the proportions of sharks (of the species concerned) that lacked distinguishing marks. However, a simple proportional correction based on the proportions of individually identifiable sharks may result in an over-estimate, because some of the sightings of non-identifiable sharks will also have been re-sightings. Furthermore, while some sharks could not be identified as individuals because they lacked distinguishing features, others could not be identified as individuals since they did not approach the BRUVS closely enough or because the water was too turbid, and so could in fact have been known individuals.
A second uncertainty relates to the size of area to which these population estimates apply. The three Cayman Islands have a combined terrestrial area of only 264 km
2 and an estimated length of coast of 160 km, most of which is fringed by coral reef. These reefs drop steeply into water >500 m deep within 2 km of the shore. However,
C. perezi, for example, have been recorded at depths of 150 m or more [
58,
59,
60], and in the Cayman Islands were recorded moving between Grand Cayman and Little Cayman, islands 125 km apart separated by water > 1000 m deep [
39]. Further, it seems from the results of conventional and acoustic tagging studies of sharks on reefs that, while some individuals may be resident or semi-resident in an area, many are only occasionally present in the area or may never be recorded again [
39,
58,
61,
62]. Thus, some of the individuals observed in the present study may be foraging and mixing over a much larger region than the 160–320 km
2 shelf area immediately surrounding the individual Cayman Islands. Comparable analysis is underway of the Amirante Islands data, but is confounded by the facts that the study area covers only a third of the archipelago and that the area did not achieve marine protected area status until 2019.