Prosthetic Management of Peri-Implant Mucositis via CRD Optimization: A Split-Mouth Case Report
Abstract
1. Introduction
- Subcrestally Placed Implants (SPIs) to promote soft tissue adaptation [22].
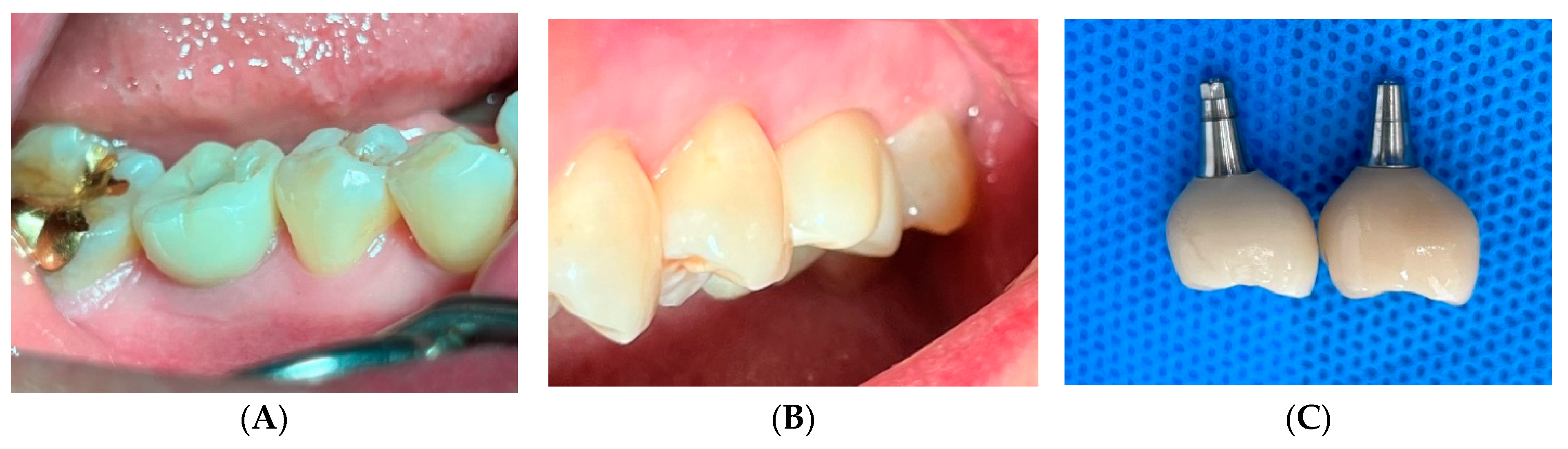
2. Case Presentation
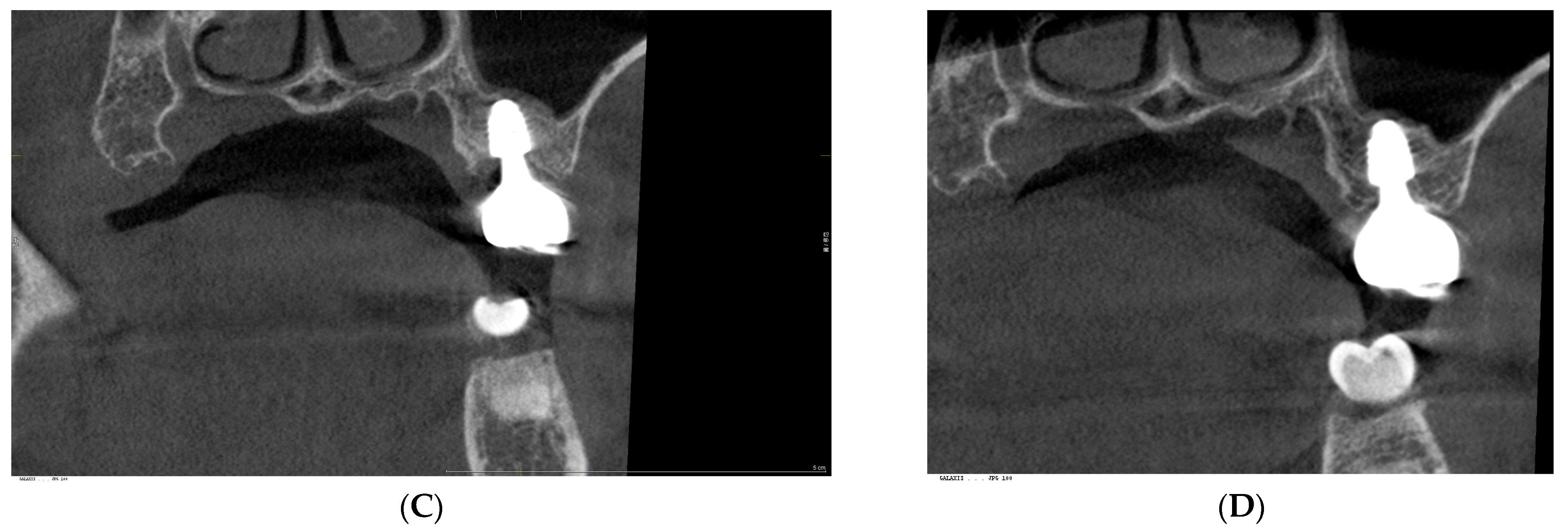
3. Radiographic and Clinical Findings
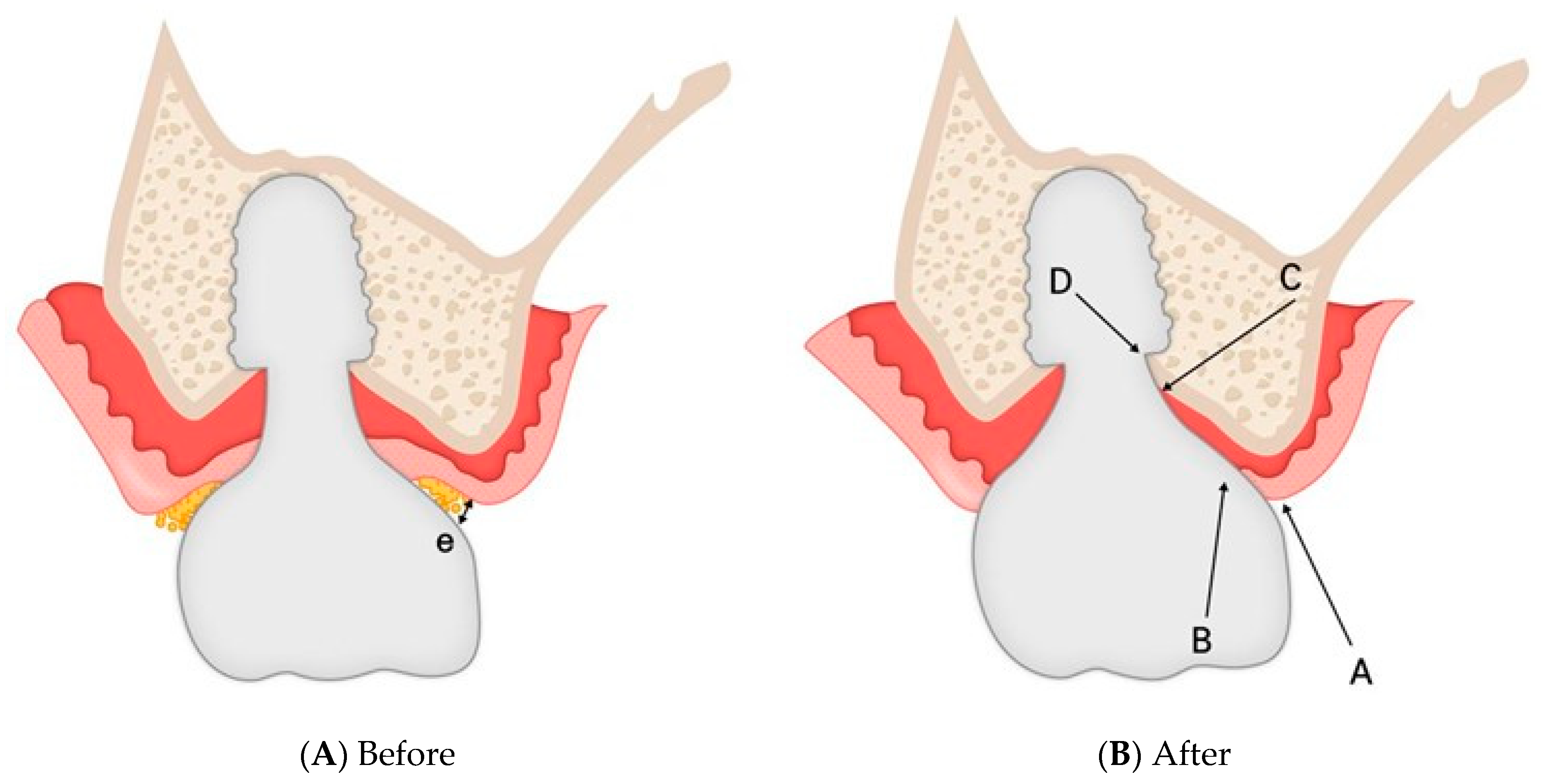
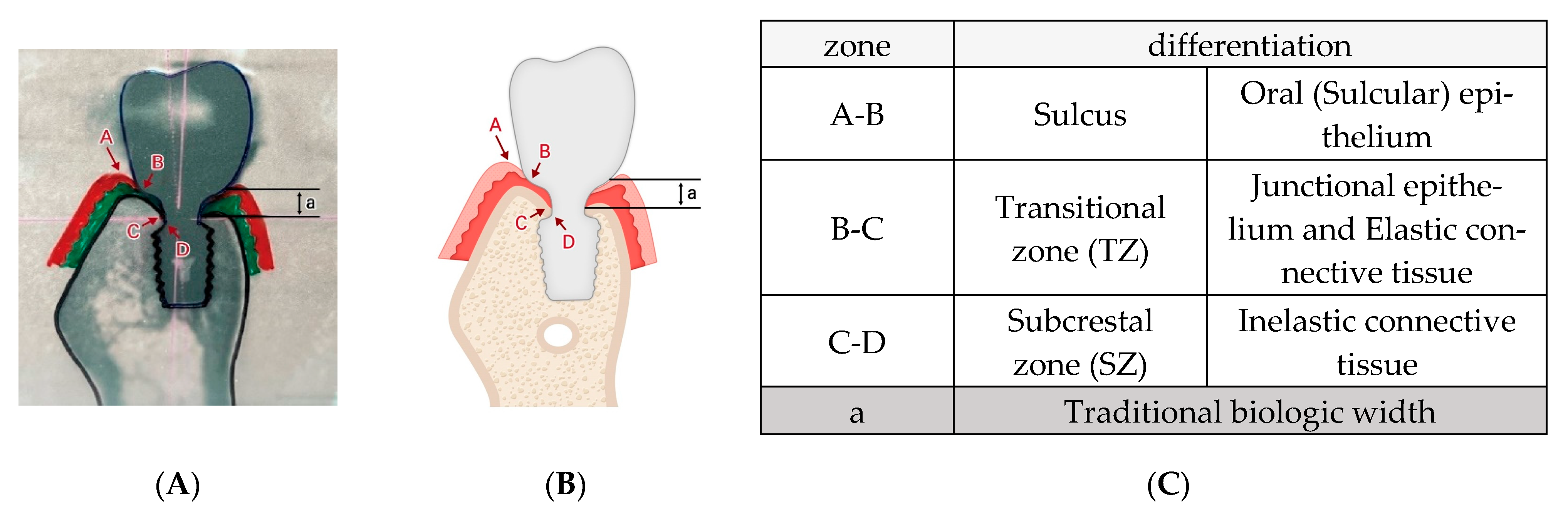
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SPI | Subcrestally Placed Implant |
| STT | Soft Tissue Thickness |
| CRD | Crest to Restoration Distance |
| cCRD | Central Crest to Restoration Distance |
| pCRD | Peripheral Crest to Restoration Distance |
| E/CT ratio | Epithelium-to-Connective tissue ratio |
| SZ | Subcrestal Zone |
| TZ | Transitional Zone |
| CBCT | Cone Beam Computed Tomography |
| PPD | Probing Pocket Depth |
| BOP | Bleeding on Probing |
| SOP | Suppuration on Probing |
| IPPP | Implant Paper Point Probing |
| CAC | Crown-Abutment Complex |
| STH | Supracrestal Tissue Height |
| STA | Supracrestal Soft Tissue Attachment |
References
- Gomez-Meda, R.; Esquivel, J.; Blatz, M.B. The esthetic biological contour concept for implant restoration emergence profile design. J. Esthet. Restor. Dent. 2021, 33, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends, and open questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Kunrath, M.F.; Gerhardt, M.N. Trans-mucosal platforms for dental implants: Strategies to induce muco-integration and shield peri-implant diseases. Dent. Mater. 2023, 39, 846–859. [Google Scholar] [CrossRef]
- Abdallah, M.N.; Badran, Z.; Ciobanu, O.; Hamdan, N.; Tamimi, F. Strategies for optimizing the soft tissue seal around osseointegrated implants. Adv. Healthc. Mater. 2017, 6, 1700549. [Google Scholar] [CrossRef]
- Zheng, Z.; Ao, X.; Xie, P.; Jiang, F.; Chen, W. The biological width around implants. J. Prosthodont. Res. 2021, 65, 11–18. [Google Scholar] [CrossRef]
- Monje, A.; González-Martín, O.; Ávila-Ortiz, G. Impact of peri-implant soft tissue characteristics on health and esthetics. J. Esthet. Restor. Dent. 2023, 35, 183–196. [Google Scholar] [CrossRef]
- Ávila-Ortiz, G.; González-Martín, O.; Couso-Queiruga, E.; Wang, H.L. The peri-implant phenotype. J. Periodontol. 2020, 91, 283–288. [Google Scholar] [CrossRef]
- Gibbs, S.; Roffel, S.; Meyer, M.; Gasser, A. Biology of soft tissue repair: Gingival epithelium in wound healing and attachment to the tooth and abutment surface. Eur. Cell. Mater. 2019, 38, 63–78. [Google Scholar] [CrossRef]
- Aellos, F.; Grauer, J.A.; Harder, K.G.; Dworan, J.S.; Fabbri, G.; Cuevas, P.L.; Yuan, X.; Liu, B.; Brunski, J.B.; Helms, J.A. Dynamic analyses of a soft tissue-implant interface: Biological responses to immediate versus delayed dental implants. J. Clin. Periodontol. 2024, 51, 806–817. [Google Scholar] [CrossRef]
- Lang, N.P.; Kinane, D.F.; Lindhe, J.; Sanz, M.; Tonetti, M.S. Sixth European Workshop on Periodontology of the European Academy of Periodontology at the Charterhouse at Ittingen, Thurgau, Switzerland. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 1–2. [Google Scholar] [CrossRef]
- Sanz, M.; Chapple, I.L. Clinical research on peri-implant diseases: Consensus report of Working Group 4. J. Clin. Periodontol. 2012, 39 (Suppl. S12), 202–206. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of Workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef]
- Linkevicius, T.; Apse, P.; Grybauskas, S.; Puisys, A. The influence of soft tissue thickness on crestal bone changes around implants: A 1-year prospective controlled clinical trial. Int. J. Oral Maxillofac. Implants 2009, 24, 712–719. [Google Scholar]
- Al Rezk, F.; Trimpou, G.; Lauer, H.C.; Weigl, P.; Krockow, N. Response of soft tissue to different abutment materials with different surface topographies: A review of the literature. Gen. Dent. 2018, 66, 18–25. [Google Scholar]
- Jin, S.; Yu, Y.; Zhang, T.; Xie, D.; Zheng, Y.; Wang, C.; Liu, Y.; Xia, D. Surface modification strategies to reinforce the soft tissue seal at the transmucosal region of dental implants. Bioact. Mater. 2024, 42, 404–432. [Google Scholar] [CrossRef]
- Rompen, E.; Domken, O.; Degidi, M.; Farias Pontes, A.E.; Piattelli, A. The effect of material characteristics, surface topography, and implant components and connections on soft tissue integration: A literature review. Clin. Oral Implants Res. 2006, 17 (Suppl. S2), 55–67. [Google Scholar] [CrossRef]
- Baus-Domínguez, M.; Oliva-Ferrusola, E.; Maza-Solano, S.; Ruiz-De-León, G.; Serrera-Figallo, M.; Gutiérrez-Perez, J.-L.; Torres-Lagares, D.; Macías-García, L. Biological response of the peri-implant mucosa to different definitive implant rehabilitation materials. Polymers 2024, 16, 1534. [Google Scholar] [CrossRef]
- González-Martín, O.; Lee, E.; Weisgold, A.; Veltri, M.; Su, H. Contour management of implant restorations for optimal emergence profiles: Guidelines for immediate and delayed provisional restorations. Int. J. Periodontics Restor. Dent. 2020, 40, 61–70. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Lin, G.H.; Wang, H.L. Effects of platform-switching on peri-implant soft and hard tissue outcomes: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implants 2017, 32, e9–e24. [Google Scholar] [CrossRef]
- Esquivel, J.; Gomez Meda, R.; Blatz, M.B. The impact of 3D implant position on emergence profile design. Int. J. Periodontics Restor. Dent. 2021, 41, 79–86. [Google Scholar] [CrossRef]
- Palacios-Garzón, N.; Velasco-Ortega, E.; López-López, J. Bone loss in implants placed at subcrestal and crestal level: A systematic review and meta-analysis. Materials 2019, 12, 154. [Google Scholar] [CrossRef]
- Vervaeke, S.; Dierens, M.; Besseler, J.; De Bruyn, H. The influence of initial soft tissue thickness on peri-implant bone remodeling. Clin. Implant Dent. Relat. Res. 2014, 16, 238–247. [Google Scholar] [CrossRef]
- Paolantoni, G.; Tatullo, M.; Miniello, A.; Sammartino, G.; Marenzi, G. Influence of crestal and subcrestal implant position on development of peri-implant diseases: A 5-year retrospective analysis. Clin. Oral Investig. 2023, 28, 16. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, J.; Chen, K.; Li, Z.; Chen, Z.; Huang, B. Clinical and radiographic results of crestal vs. subcrestal placement of implants in posterior areas: A split-mouth randomized controlled clinical trial. Clin. Implant Dent. Relat. Res. 2023, 25, 948–959. [Google Scholar] [CrossRef]
- Valles, C.; Rodríguez-Ciurana, X.; Clementini, M.; Baglivo, M.; Paniagua, B.; Nart, J. Influence of subcrestal implant placement compared with equicrestal position on the peri-implant hard and soft tissues around platform-switched implants: A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 555–570. [Google Scholar] [CrossRef]
- Wang, I.C.; Barootchi, S.; Tavelli, L.; Wang, H.L. The peri-implant phenotype and implant esthetic complications: Contemporary overview. J. Esthet. Restor. Dent. 2021, 33, 212–223. [Google Scholar] [CrossRef]
- Bosshardt, D.D. The periodontal pocket: Pathogenesis, histopathology, and consequences. Periodontology 2000 2018, 76, 43–50. [Google Scholar] [CrossRef]
- Won, C. A novel framework for optimizing peri-implant soft tissue in subcrestally placed implants in single molar cases: Integrating transitional and subcrestal zones for biological stability. J. Clin. Med. 2025, 14, 2435. [Google Scholar] [CrossRef]
- Monje, A.; Amerio, E.; Farina, R.; Nart, J.; Ramanauskaite, A.; Renvert, S.; Roccuzzo, A.; Salvi, G.E.; Schwarz, F.; Trombelli, L.; et al. Significance of probing for monitoring peri-implant diseases. Int. J. Oral Implantol. 2021, 14, 385–399. [Google Scholar]
- Fischer, N.G.; Aparicio, C. Junctional epithelium and hemidesmosomes: Tape and rivets for solving the “percutaneous device dilemma” in dental and other permanent implants. Bioact. Mater. 2022, 18, 178–198. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J. Dimension of the peri-implant mucosa: Biological width revisited. J. Clin. Periodontol. 1996, 23, 971–973. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Soldini, C. Probe penetration in periodontal and peri-implant tissues: An experimental study in the beagle dog. Clin. Oral Implants Res. 2006, 17, 601–605. [Google Scholar] [CrossRef]
- Barendregt, D.S.; Van der Velden, U.; Timmerman, M.F.; Van der Weijden, G.A. Comparison of two automated periodontal probes and two probes with a conventional readout in periodontal maintenance patients. J. Clin. Periodontol. 2006, 33, 276–282. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Kondo, R.; Oshiro, W.; Matsuura, Y.; Furuhashi, A.; Tsukiyama, Y.; Koyano, K. Soft tissue sealing around dental implants based on histological interpretation. J. Prosthodont. Res. 2016, 60, 3–11. [Google Scholar] [CrossRef]
- Gargiulo, A.W.; Arrocha, R. Histo-clinical evaluation of free gingival grafts. Periodontics 1967, 5, 285–291. [Google Scholar]
- Vacek, J.S.; Gher, M.E.; Assad, D.A.; Richardson, A.C.; Giambarresi, L.I. The dimensions of the human dentogingival junction. Int. J. Periodontics Restor. Dent. 1994, 14, 154–165. [Google Scholar]
- Ivanovski, S.; Lee, R. Comparison of peri-implant and periodontal marginal soft tissues in health and disease. Periodontology 2000 2018, 76, 116–130. [Google Scholar] [CrossRef]
- Lin, G.H.; Madi, I.M. Soft-tissue conditions around dental implants: A literature review. Implant Dent. 2019, 28, 138–143. [Google Scholar] [CrossRef]
- Fuchigami, K.; Munakata, M.; Kitazume, T.; Tachikawa, N.; Kasugai, S.; Kuroda, S. A diversity of peri-implant mucosal thickness by site. Clin. Oral Implants Res. 2017, 28, 171–176. [Google Scholar] [CrossRef]
- Won, C. 3-Dimensional Soft Tissue Analysis (3DSTA) of Subcrestally Placed Implants: Relating Biologic Stability with Morphologic Achievements. Preprints 2024, 2024041116, (Version 1). [Google Scholar] [CrossRef]
- Domic, M.; Bertl, K.; Ahmad, K.; Schopper, C.; Schropp, L.; Stavropoulos, A.; Hellén-Halme, K. Accuracy of cone-beam computed tomography is limited at implant sites with a thin buccal bone: A laboratory study. Clin. Oral Implants Res. 2021, 32, 1092–1100. [Google Scholar] [CrossRef] [PubMed]




| Upper Left 1st Molar | Lower Right 1st Molar | |||
|---|---|---|---|---|
| cCRD | pCRD | cCRD | pCRD | |
| M | 1.52 | 1.91 | 0.36 | 0.91 |
| D | 1.54 | 1.87 | 0.78 | 1.66 |
| B | 1.88 | 2.04 | 0.3 | 0.3 |
| L | 0.98 | 2.05 | 0.6 | 1.41 |
| Average | 1.48 | 1.97 | 0.51 | 1.07 |
| Site | Change in cCRD (mm) | Change in pCRD (mm) | Probing Depth | BOP | Redness | Swelling |
|---|---|---|---|---|---|---|
| Lower right first molar (control) | –0.08 | –0.10 | <1 mm | No | No | No |
| Upper left first molar (treated) | –0.93 | –1.09 | <1 mm (post-treatment) | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, C. Prosthetic Management of Peri-Implant Mucositis via CRD Optimization: A Split-Mouth Case Report. Prosthesis 2025, 7, 146. https://doi.org/10.3390/prosthesis7060146
Won C. Prosthetic Management of Peri-Implant Mucositis via CRD Optimization: A Split-Mouth Case Report. Prosthesis. 2025; 7(6):146. https://doi.org/10.3390/prosthesis7060146
Chicago/Turabian StyleWon, Chiyun. 2025. "Prosthetic Management of Peri-Implant Mucositis via CRD Optimization: A Split-Mouth Case Report" Prosthesis 7, no. 6: 146. https://doi.org/10.3390/prosthesis7060146
APA StyleWon, C. (2025). Prosthetic Management of Peri-Implant Mucositis via CRD Optimization: A Split-Mouth Case Report. Prosthesis, 7(6), 146. https://doi.org/10.3390/prosthesis7060146





