Abstract
Background: Tooth development or odontogenesis is a complicated, multi-staged process, regulated by a plethora of genes. Disruptions during the early stages of odontogenesis may cause the complete absence of one or more teeth, known as tooth agenesis (TA). Except for PAX9, alterations in MSX1, AXIN2, WNT10A, and EDA/EDAR/EDARADD have gathered an increasing amount of interest. Objectives: This systematic review aims to investigate whether non-syndromic tooth agenesis (NSTA) is associated with MSX1, AXIN2, WNT10A, and EDA/EDAR/EDARADD mutations and to list the related phenotypic patterns of these alterations with regard to missing teeth. Methods: MEDLINE, Scopus, and Web of Science were the three selected databases. Duplicates were removed using Mendeley, and the records were assessed via the Rayyan platform. The Newcastle–Ottawa Scale was used to evaluate the quality of the evidence. Results: Fifteen case–control studies were eligible for this systematic review. The MSX1 gene was examined in most studies, whereas second premolars and lateral incisors were the most commonly missing teeth among TA cases. In total, 61.29% to 84.9% of the cases included one or two absent teeth. Conclusions: Due to the considerable heterogeneity in reporting results across the included studies, along with the high risk of bias present in most of them, it was not feasible to conduct a meta-analysis of the data. Nonetheless, the findings suggest that the NSTA phenotypes linked to the studied genes are similar to those associated with other forms of TA and share a common pattern of missing teeth. Future research should adopt a more standardized approach in presenting findings by adhering to established terminology and definitions and by utilizing common cut-off points to categorize results.
Keywords:
AXIN2; EDA; hypodontia; MSX1; oligodontia; single-nucleotide polymorphisms; systematic review; tooth agenesis; WNT10A 1. Introduction
The process of teeth development during embryonic growth, referred to as odontogenesis, is regarded as a fundamental pillar of organogenesis [1]. It is based on reciprocal interactions between two embryonic tissue types, ectoderm and mesenchyme [2,3]. Tooth development begins with the thickening of the epithelium in the regions that will become the future maxilla and mandible [2,4]. Further immersion of dental epithelial thickening into the underlying mesenchyme leads to the bud, cap, and bell stages of odontogenesis [1,4,5]. Cell differentiation of the epithelium and mesenchyme forms ameloblasts and odontoblasts, respectively [1,4]; these create the hard tissues of the crown, enamel, and dentine. The complex multi-step process of odontogenesis is regulated by various genes [1,4]. Homeobox genes seem to play a pivotal role during odontogenesis [6], and four main signaling pathways (BMP, FGF, WNT, and SHH) and their receptors are of crucial importance as well [1,3,4].
Complications during the initial stages of tooth development may cause the congenital absence of one or more teeth [7,8]. This disorder is called tooth agenesis (TA) and is one of the most common dental anomalies [9,10], with its prevalence varying according to sex and ethnicity [11]. Based on the number of missing teeth, TA is classified as hypodontia (less than six missing teeth, excluding third molars), oligodontia (six or more missing teeth, excluding third molars), or anodontia (all teeth missing) [9,12]. TA is presented either as part of a syndrome (syndromic) or as an individual trait (non-syndromic tooth agenesis—NSTA) [10]. It may appear bilaterally or unilaterally [13] and may affect not only permanent but also, more rarely, deciduous teeth [14].
Third molars excluded, the most frequently missing teeth are the lateral incisors and second premolars [15,16,17,18], and several theories have been proposed to interpret this pattern [19]. Severe TA can significantly impact oral function, aesthetics, nutrition, and psychological well-being, in addition to disrupting normal craniofacial development. Therefore, it requires continuous treatment with targeted interventions from a multidisciplinary team of dental and medical professionals [13,20].
The prosthodontic management of children and adolescents with missing teeth constitutes a challenging task, especially in cases of severe TA. Intraoral changes, as well as ongoing craniofacial growth, should be taken into account when planning and monitoring prosthodontic interventions [11,21]. Furthermore, TA cases often involve other dental abnormalities, such as teeth with atypical shapes or reduced sizes, which can complicate treatment planning [22,23]. Removable prostheses, typically mucosa-borne acrylic partial dentures, are generally regarded as the preferred treatment option for children and adolescents, as they serve as interim appliances until the growth is complete and permanent prosthodontic options can be realized [24,25].
The etiology of TA is mainly attributed to genetic and environmental factors [7], and mutations of specific genes have been identified in many cases [26]. In NSTA, mutations in PAX9, MSX1, AXIN2, WNT10A, and EDA/EDAR/EDARADD appear to be responsible for the majority of the cases [9,27]. These genes play various roles during the early stages of odontogenesis, such as transcription factors (PAX9; MSX1) [28,29], regulators of the Wnt signaling pathway (AXIN2; WNT10A) [6,14,30], or members of the EDA pathway (EDA-ligand, EDAR-receptor, and EDARADD-adaptor protein) [31,32]. The combined (synergistic or antagonistic) action of these molecules and their corresponding pathways could possibly explain the considerable heterogeneity regarding phenotype patterns of NSTA [10,18,33].
According to the existing literature, PAX9 appears to be the most prevalent gene linked to NSTA [28,34,35]. More than 50 PAX9 mutations have been associated with TA and impairments in specific gene loci, resulting in varying severity and phenotype [28,34]. However, regarding the rest of the genes that have been studied in connection with NSTA, the scientific evidence has not yet been systematically reviewed.
The purpose of this systematic review was to explore the relationships between mutations in the MSX1, AXIN2, WNT10A, EDA, EDAR, and EDARADD genes and NSTA, with a particular focus on the phenotypic aspects. Specifically, we aimed to determine whether alterations in these genes are linked to distinct patterns of agenesis, differing from those associated with PAX9 mutations or syndrome-related TA.
2. Materials and Methods
2.1. Protocol Registration
The protocol of this systematic review was developed in accordance with the latest version of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [36] and has been uploaded to the PROSPERO database for the prospective registration of systematic reviews and meta-analyses (CRD42023430408).
2.2. Eligibility Criteria
Studies were eligible for this systematic review under the following prerequisites:
- Case–control study design;
- English language;
- Two study arms: participants with diagnosed NSTA and healthy controls;
- Outcome of interest: prevalence of gene mutations (MSX1, AXIN2, WNT10A, EDA/EDAR/EDARADD) in both study groups.
The diagnosis of TA had to be verified by radiographs and/or clinical examination, while gene mutations had to be confirmed by a proper genotyping or sequencing method. Studies assessing participants with syndromic TA, oral cleft defects, or missing third molars only were excluded.
2.3. Information Sources
Regarding electronic search, MEDLINE (via PubMed), Scopus, and Web of Science were assessed. Handsearching was conducted on Google Scholar, whereas opengrey.eu was deployed to assess gray literature. The detailed search strategy on MEDLINE can be seen in Supplementary Table S1.
2.4. Study Records
Search records were inserted into Mendeley, where duplicates were removed. Data were exported from Mendeley and imported into the Rayyan platform [37]. Records were assessed according to their title and abstract, and the next step involved full-text assessment. Study selection was performed by two independent reviewers (F.B.K., I.T.), with disagreements resolved by a third one (E.K.). Figure 1 provides a detailed overview of the process using a PRISMA 2020 flow diagram.
2.5. Data Items
Data extraction was performed by two independent reviewers (F.B.K., I.T.) and verified by a third independent reviewer (E.K.). The data were inserted into a Microsoft Excel spreadsheet under the following categories:
- PMID, first author, year of publication, age, sex, gene of interest, single-nucleotide polymorphisms (SNPs) of interest, sample size, TA cases and controls, number of TA cases and controls with mutations, number of missing teeth, phenotype, diagnostic method of TA, and verification method of gene mutations.
2.6. Risk of Bias
The quality of included studies was assessed via the Newcastle–Ottawa Scale. Biases were judged as “low”, “high”, or “unclear” in each of the three domains (criteria), namely, selection, comparability, and exposure bias [38]. This step was performed by two independent reviewers (F.B.K., I.T.), with a third reviewer (E.K.) solving any differences.
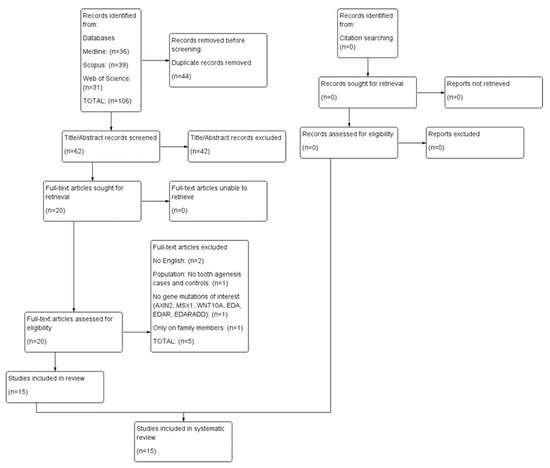
Figure 1.
PRISMA 2020 flow diagram.
3. Results
The electronic search of the three databases yielded 106 records. After duplicate removal, 62 records were screened according to title and abstract, and 20 of them progressed toward full-text evaluation. The final number of studies eligible for the systematic review was 15 [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]; however, a meta-analysis could not be performed due to the complexity and heterogeneity of the numerical results of gene mutations in cases of TA and their controls. Table 1 and Table 2 present the characteristics of the included studies, and Table 3 lists the full-text studies excluded from the review and the reasons for exclusion.

Table 1.
List of included studies—demographic data.
3.1. Demographic Characteristics
Table 1 contains the general demographic characteristics of the included studies. In total, 11 out of 15 studies were published in the decade 2010–2020. Four studies were conducted on Chinese populations, while Brazil followed closely behind with three studies.
3.2. Gene Mutations, Samples, and Methods of Detection
Table 2 provides detailed information about the genes of interest, the number of TA cases and controls, and methods of detecting TA and gene mutations. Among the genes examined, MSX1 has attracted considerable attention, as nine studies refer to it, followed by WNT10A and six studies. A total of 77 SNPs have been reported across the studies, with MSX1 rs12532 appearing to be the most common, as it was found in seven publications. The total sample size was 5186, comprising 1576 TA cases and 3610 controls. TA was confirmed by clinical and radiographic examination (13 studies each), and gene aberrations were detected by DNA extraction and genotyping (14 studies).
3.3. Tooth Agenesis Patterns and Phenotype
Table 2 provides information about missing teeth in TA cases. Based on 13 studies that provided relevant data, 5164 teeth were missing in total. With regard to TA types, Figure 2 depicts the percentage of TA cases with one, two, or at least three missing teeth in each study [39,41,44,45,49,50,52]. The publication of Chen et al. [46] was not included in Figure 2 due to the classification of cases into different groups regarding the number of missing teeth (one to five: 5.36%; six or more: 94.64%).
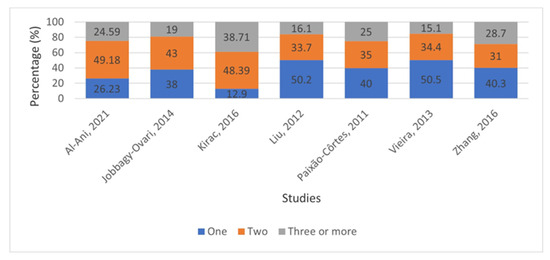
Figure 2.
Percentage (%) of tooth agenesis cases with one, two, or at least three permanent missing teeth (excluding third molars) [39,41,44,45,49,50,52].

Table 2.
List of included studies—study samples, missing teeth, and genes of interest.
Table 2.
List of included studies—study samples, missing teeth, and genes of interest.
| Genes of Interest | SNPs of Interest | Sample Size | Tooth Agenesis Cases/Control Group | Total Number of Missing Teeth | Means for Tooth Agenesis Diagnosis | How Were the Mutations Investigated? | |
|---|---|---|---|---|---|---|---|
| 1 | AXIN2, MSX1, EDA | AXIN2: rs4128941, rs4791171. MSX1: rs8670, rs12532, rs1042484, rs36059701, rs3775261, rs3821949, rs186861426. EDA: rs1160315, rs12853659, rs2274469, rs2428151, rs2520378, rs62604271 | 360 | 61/299 | 141 | Clinical examination, X-ray, dental history | DNA extraction and genotyping |
| 2 | EDA, WNT10A | EDA: rs1160315, rs2428151, rs2520378, rs12853659, rs5936523. WNT10A: rs11680244, rs2385199, rs7349332 | 306 | 102/204 | 188 | Clinical examination, X-ray | DNA extraction and genotyping |
| 3 | EDAR, EDARADD | EDAR: rs151195196, rs759735008, rs61761321, rs3749108, rs3749098, rs3749099, rs200267845, rs10432616, rs3827760, rs146567337. EDARADD: rs966365, rs60808129, rs200569815, rs604070, rs777172467, rs753890063, rs74942492, rs753408117 | 224 | 112/112 | 1766 | Clinical examination, X-ray, dental history | DNA extraction and genotyping |
| 4 | WNT10A | rs147680216 | 1386 | 191/1195 | 502 | Clinical examination, X-ray, dental history | DNA extraction and genotyping |
| 5 | AXIN2, WNT10A | AΧΙΝ2: rs190687283, rs2240308, rs9915936, rs1133683, rs63533624, rs139316692, rs35415678, rs143243661. WNT10A: rs77583146, rs147680216, rs121908120 | 108 | 60/48 | 136 | Clinical examination, X-ray, medical history, family history | DNA extraction and genotyping |
| 6 | MSX1, AXIN2 | MSX1: rs12532. AXIN2: rs2240308, rs2240307, rs35415678 | 469 | 209/260 | 363 (in hypodontia group) | Clinical examination, X-ray, family history | DNA extraction and genotyping |
| 7 | MSX1 | rs8670, rs12532 | 61 | 31/30 | 80 | X-ray, dental history, family history | DNA extraction and genotyping |
| 8 | MSX1 | rs12532 | 210 | 53/157 | 96 | Clinical examination, X-ray | DNA extraction and genotyping |
| 9 | AXIN2, EDAR, EDARADD, MSX1, WNT10A | AXIN2: rs2240308. EDAR: rs3749096, rs3749110, rs3827760, rs6749207. EDARADD: rs966365, rs3916983, rs6428955, rs7513402. MSX1: rs12532, rs3821947, rs3821949. WNT10A: rs1057306, rs6744926, rs34972707 | 473 | 273/200 | 585 | Clinical examination, X-ray | DNA extraction and genotyping |
| 10 | WNT10A | rs121908119, rs121908120 | 587 | 157/430 | 801 | Clinical examination, X-ray, medical history, family history | DNA extraction and genotyping |
| 11 | MSX1 | rs8670, rs1095, rs12532 | 50 | 35/15 | 66 | Clinical examination, X-ray | DNA extraction and genotyping |
| 12 | MSX1 | N/A | 100 | 50/50 | N/A | X-ray, dental history | DNA extraction and genotyping |
| 13 | MSX1 | N/A | 40 | 20/20 | N/A | Clinical examination, X-ray, medical history, family history | DNA extraction and sequencing |
| 14 | MSX1, EDA, EDAR | MSX1: rs62636562, rs1042484, rs10213286, rs13127820, rs12532. EDA: rs760041, rs6625561, Hcv992421, rs2804361, rs6625546, hCV27026158. EDAR: hCV790922, rs3827760, rs13029834, rs7585138, rs12992554, rs899259, rs17269487 | 465 | 93/372 | 175 | Clinical examination | DNA extraction and genotyping |
| 15 | WNT10A | rs116998555, rs147680216 | 347 | 129/218 | 265 | Clinical examination, dental history | DNA extraction and genotyping |

Table 3.
List of excluded studies.
Table 3.
List of excluded studies.
| First Author et al., Year | Citation | Reason for Exclusion | |
|---|---|---|---|
| 1 | Wang, 2010 | [54] | No English language |
| 2 | Wong, 2014 | [55] | No English language |
| 3 | Ross, 2023 | [56] | Population: No tooth agenesis cases and controls |
| 4 | Wang, 2013 | [57] | No gene mutations of interest |
| 5 | Wang, 2016 | [58] | Only on family members |
In Figure 3, information is given about the distribution of missing teeth between the maxilla and mandible, based on nine studies that provided relevant data [40,41,46,47,48,49,50,51,52]. The total number of missing maxillary and mandibular teeth was 1865 and 1917, respectively.
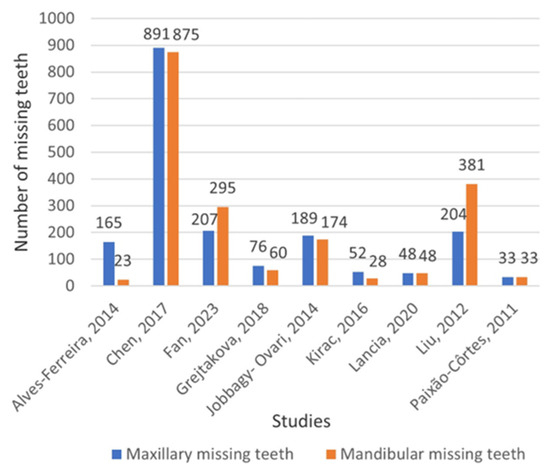
Figure 3.
Distribution of permanent missing teeth between maxilla and mandible (excluding third molars) [40,41,46,47,48,49,50,51,52].
Figure 4 depicts the distribution of missing teeth among tooth groups, based on seven studies that provided data [40,41,45,49,50,52,53]. Furthermore, Figure 5 illustrates the relevant findings from the study by Chen et al. [46], as the composition of their sample appears to vary, with 94.64% of TA cases identified as oligodontia.
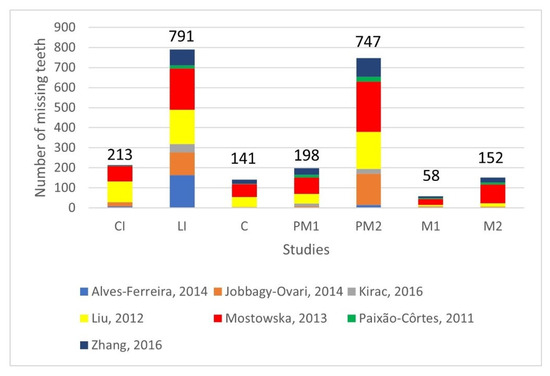
Figure 4.
Distribution of permanent missing teeth among tooth groups (excluding third molars). CI: central incisor, LI: lateral incisor, C: canine, PM1: first premolar, PM2: second premolar, M1: first molar, M2: second molar [40,41,45,49,50,52,53].
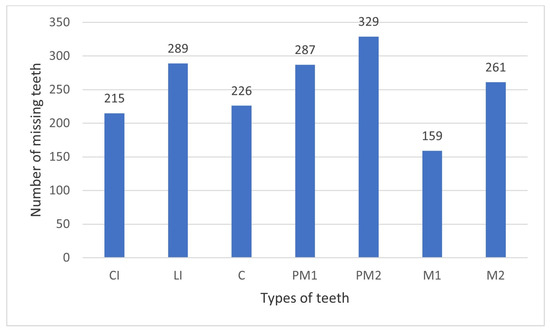
Figure 5.
Distribution of permanent missing teeth among tooth groups in the study of Chen et al., 2017 [46] (excluding third molars). CI: central incisor, LI: lateral incisor, C: canine, PM1: first premolar, PM2: second premolar, M1: first molar, M2: second molar.
3.4. Risk of Bias Assessment
The Newcastle–Ottawa Scale was assessed to evaluate the quality of the evidence. In most of the studies, the risk of bias was characterized as “high” because of the “comparability bias” domain, whereas two studies had a low risk of bias (Figure 6 and Figure 7).
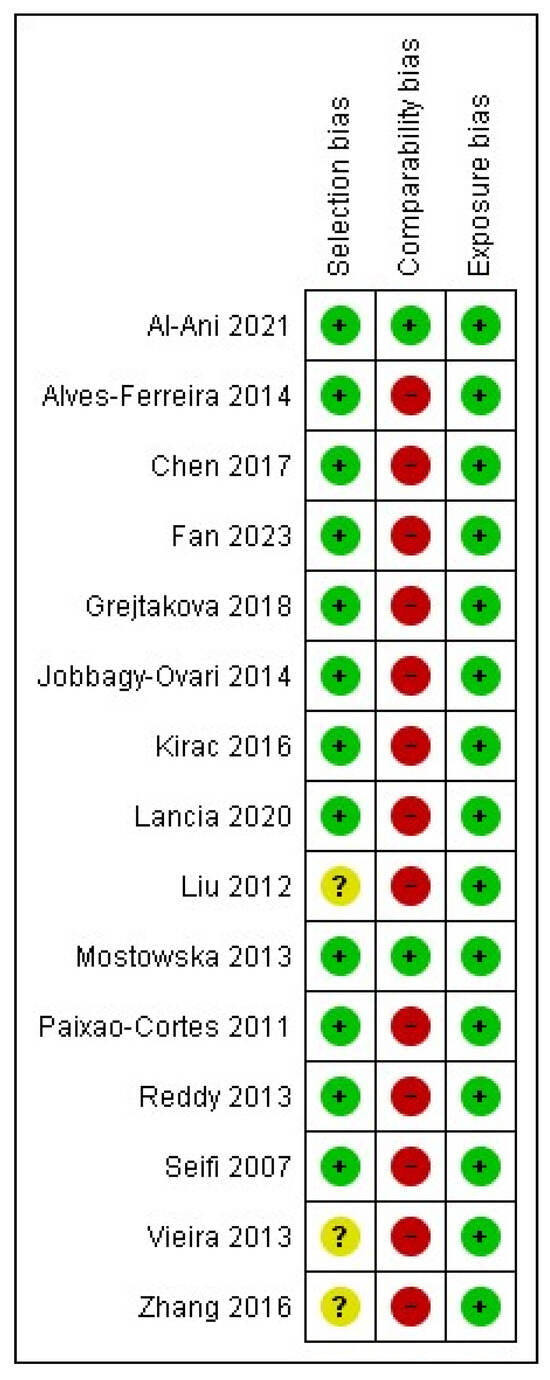
Figure 6.
Risk of bias summary: Review of authors’ judgments about each risk of bias for each included study, based on Newcastle–Ottawa Scale [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53].
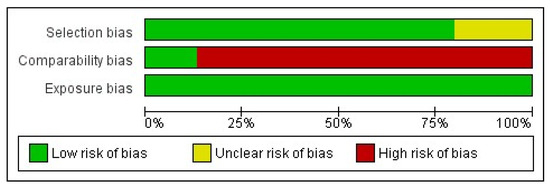
Figure 7.
Risk of bias graph: Review of authors’ judgments about each risk of bias item presented as percentages across all included studies, based on Newcastle–Ottawa Scale.
4. Discussion
The present study aimed to investigate the association between NSTA and the MSX1, AXIN2, WNT10A, EDA, EDAR, and EDARADD mutations, focusing on phenotypic patterns. Our findings highlight the clinical relevance of identifying gene mutations associated with NSTA, as they can inform diagnosis and genetic counseling. The identification of specific patterns may contribute to the development of standardized guidelines for optimal design and manufacturing of prosthetic appliances [26]. By gaining a better understanding of the connection between NSTA phenotypes and the underlying gene mutations, dental prosthodontists will be better equipped to assess the pattern and severity of missing teeth and select restorative approaches tailored to each patient’s genetic and developmental profile. [11].
Although the current systematic review included 15 studies, a meta-analysis could not be performed due to the variability and heterogeneity of the data. Nearly all studies define TA cases as “participants with at least one permanent tooth, excluding third molars,” aligning with the accepted standard. Many studies specify the type of tooth examined, such as “participants with at least one missing (permanent) lateral incisor or premolar,” among others. One study [41] deviates from this standard by including third molars in the count of missing teeth. By analyzing the published data, we were able to include the cases from this study that involved missing teeth other than third molars. Additionally, variability is evident in the cut-off points used across studies, with common thresholds being one, two, or three or more missing teeth. No studies employed a cut-off at the boundary between hypodontia and oligodontia—specifically, six missing teeth—which marks the point where clinical consequences typically become more severe.
This outcome aligns with the report of Rakhshan [59], who also attempted a meta-analysis and characterized the results of the studies as controversial, stating that differing definitions of TA and the absence of standardized cut-offs are fundamental factors contributing to heterogeneity. Therefore, to make a proper quantitative synthesis of the results, it is preferable that the papers apply the definition of TA in a uniform manner and present the number of TA cases and controls with or without gene mutation. Such a presentation would facilitate the synthesis of the results among studies dealing with the same genes and SNPs, e.g., rs12532 located in the MSX1 gene and appearing in 7 of the 15 included studies.
To assess all the available information on a rare condition, such as TA, it was considered necessary to include case–control studies. However, this fact could introduce bias into the research. As most of the included studies were judged as “high risk”, meta-analyses would not offer reliable results, even if it was possible to be conducted [60]. Given that systematic reviews represent the qualitative synthesis of multiple primary studies, a high risk of bias in most of the selected studies could negatively impact the overall reliability of the systematic review [61]. However, it is important to emphasize that presenting the heterogeneous findings of these studies—despite their high risk of bias—highlights the lack of robust evidence and underscores the necessity for well-designed research, as well as the adoption of a standardized definition of TA and universally accepted cut-off values.
This systematic review highlighted MSX1 as the most studied gene and SNP rs12532 as the most examined SNP. It is widely recognized that MSX1 is essential in the early stages of odontogenesis [62], and it has attracted considerable interest from the scientific community. Recent research indicates that MSX1, particularly the rs12532 variant, is linked to the agenesis of the second premolar and third molar [62,63]. However, no statistically significant relationship has been identified between MSX1 rs12532 and TA to date [51,64]. Additionally, MSX1 has been studied in relation to cleft defects, cardiovascular diseases, and various syndromes [63,65,66].
In the studies examined, the proportion of cases with fewer than three missing teeth ranged from 61.29% to 84.9%, suggesting that the samples predominantly consisted of individuals with mild TA. This range is in total agreement with the data provided by Khalaf et al. [16], in which the percentage of participants with less than three missing teeth was 81.6%.
The source of the cases was not specified as a sampling criterion, and the studies we included encompassed both general population samples and exclusively orthodontic patients. Although it may be hypothesized that orthodontic patients might be associated with more severe cases of TA, research has shown that their epidemiology does not differ from that of the general population [16,59,67].
The present study noted a slight difference in favor of mandibular missing teeth. This finding is consistent with the outcomes of an earlier systematic review conducted by Polder et al. [15]; however, the systematic review by Khalaf et al. [16] observed a greater prevalence of missing maxillary teeth. It appears that there is no significant difference in the prevalence of TA between the upper and lower jaws, as both are susceptible to TA, and neither is more affected than the other.
In one of the included studies [40], all participants had maxillary lateral incisor agenesis (MLIA); thus, the calculated results of our study were biased toward maxillary TA. Nevertheless, the prevalence of mandibular tooth agenesis remained unchanged, even after excluding the data from this specific study.
Our findings indicate that lateral incisors and second premolars are the most commonly absent teeth. This observation aligns with the typical distribution of missing teeth in TA cases, irrespective of their genetic background [15,16]. The total counts of missing lateral incisors and second premolars are comparable, with the latter being slightly lower (791 lateral incisors compared to 747 second premolars). In a study by Alves-Ferreira et al. [40], where the sample consisted entirely of cases of MLIA, the number of missing lateral incisors was considerably higher compared to the missing second premolars, with 165 lateral incisors missing compared to 15 premolars. By excluding this study, the count of second premolars exceeds that of lateral incisors (626 lateral incisors compared to 732 premolars), consistent with the findings from previous publications [15,16].
The evaluation of the six genes that have been thoroughly investigated as underlying factors of TA and the assessment of current research findings are the strengths of this systematic review. Although the results were diverse, the review effectively summarized the main research findings, with an emphasis on the resultant phenotypes. Conversely, some limitations are recognized, specifically the limited number of included studies and the inability to conduct meta-analyses because of the heterogeneity and high risk of bias. In addition, the inability to clarify which mutation types are associated with the severity of phenotypic patterns of TA is an additional limitation of this study.
5. Conclusions
- Few case–control studies have investigated the association between TA and gene mutations, and many of these exhibit a high risk of bias, indicating concerns about their quality.
- The distribution of missing teeth by jaw or type of tooth is in accordance with that reported for all TA cases, whether syndromic or non-syndromic. Therefore, the phenotype associated with mutations of the genes related to NSTA is similar to that observed in all TA cases.
- The varying sampling methods and the lack of clear cut-off points do not permit the extraction of valid and clinically relevant results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/prosthesis7060142/s1, Table S1: Search strategy in MEDLINE (PubMed).
Author Contributions
Conceptualization, F.B.-K., I.T., and E.K.; methodology, F.B.-K., I.T., and E.K.; software, F.B.-K., I.T., and E.K.; validation, F.B.-K., I.T., and E.K.; formal analysis, F.B.-K., I.T., and E.K.; investigation, F.B.-K., I.T., and E.K.; resources, F.B.-K., I.T., and E.K.; data curation, F.B.-K., I.T., and E.K.; writing—original draft preparation, F.B.-K., I.T., and E.K.; writing—review and editing, F.B.-K., I.T., and E.K.; visualization, F.B.-K., I.T., and E.K.; supervision, E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data were derived from previously published studies included in the systematic review and are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bei, M. Molecular genetics of tooth development. Curr. Opin. Genet. Dev. 2009, 19, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Bonczek, O.; Krejci, P.; Izakovicova-Holla, L.; Cernochova, P.; Kiss, I.; Vojtesek, B. Tooth agenesis: What do we know and is there a connection to cancer? Clin. Genet. 2021, 99, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Miletich, I.; Sharpe, P.T. Normal and abnormal dental development. Hum. Mol. Genet. 2003, 12, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Thesleff, I. The genetic basis of tooth development and dental defects. Am. J. Med Genet. Part A 2006, 140A, 2530–2535. [Google Scholar] [CrossRef]
- Harris, E.F. Odontogenesis. In A Companion to Dental Anthropology; Irish, J.D., Scott, G.R., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 142–158. [Google Scholar] [CrossRef]
- Kulkarni, M.; Agrawal, T.; Kheur, S. Tooth agenesis: Newer concept. J. Clin. Pediatr. Dent. 2011, 36, 65–70. [Google Scholar] [CrossRef]
- Kiziltan Eliacik, B.; Atas, C.; Guven Polat, G. Prevalence and patterns of tooth agenesis among patients aged 12–22 years: A retrospective study. Korean J. Orthod. 2021, 51, 355–362. [Google Scholar] [CrossRef]
- Bilgin, N.; Kaya, B. Etiology and treatment alternatives in tooth agenesis: A comprehensive review. Stomatol. Dis. Sci. 2018, 2, 9. [Google Scholar] [CrossRef][Green Version]
- Klupś, D.; Kaźmierczak, J.; Torlińska-Walkowiak, N. Tooth agenesis: Genes and syndromic diseases –literature review. J. Pre-Clin. Clin. Res. 2022, 16, 149–152. [Google Scholar] [CrossRef]
- Shimizu, T.; Maeda, T. Prevalence and genetic basis of tooth agenesis. Jpn. Dent. Sci. Rev. 2009, 45, 52–58. [Google Scholar] [CrossRef]
- Meade, M.J.; Dreyer, C.W. Tooth agenesis: An overview of diagnosis, aetiology and management. Jpn. Dent. Sci. Rev. 2023, 59, 209–218. [Google Scholar] [CrossRef]
- Williams, M.A.; Letra, A. The changing landscape in the genetic etiology of human tooth agenesis. Genes 2018, 9, 255. [Google Scholar] [CrossRef]
- Mărgărit, R.; Andrei, O.-C.; Tanasescu, L.A.; Farcasiu, C.; Bisoc, A.; Dina, M.-N.; Burlibasa, M.; Bodnar, D.-C. Non-syndromic familial hypodontia: Rare case reports and literature review. Rom. J. Morphol. Embryol. 2019, 60, 1355–1360. [Google Scholar]
- Matalova, E.; Fleischmannova, J.; Sharpe, P.T.; Tucker, A.S. Tooth agenesis: From molecular genetics to molecular dentistry. J. Dent. Res. 2008, 87, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Polder, B.J.; Van’t Hof, M.A.; Van Der Linden, F.P.G.M.; Kuijpers-Jagtman, A.M. A meta-analysis of the prevalence of dental agenesis of permanent teeth. Community Dent. Oral. Epidemiol. 2004, 32, 217–226. [Google Scholar] [CrossRef]
- Khalaf, K.; Miskelly, J.; Voge, E.; Macfarlane, T.V. Prevalence of hypodontia and associated factors: A systematic review and meta-analysis. J. Orthod. 2014, 41, 299–316. [Google Scholar] [CrossRef]
- Al-Ani, A.H.; Antoun, J.S.; Stacknik, S.; Farella, M. Management of missing mandibular second premolars: A review. Aust. Orthod. J. 2017, 87–98. [Google Scholar]
- Fournier, B.P.; Bruneau, M.H.; Toupenay, S.; Kerner, S.; Berdal, A.; Cormier-Daire, V.; Hadj-Rabia, S.; Coudert, A.; de La Dure-Molla, M. Patterns of Dental Agenesis Highlight the Nature of the Causative Mutated Genes. J. Dent. Res. 2018, 97, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Brook, A.H.; Jernvall, J.; Smith, R.N.; Hughes, T.E.; Townsend, G.C. The dentition: The outcomes of morphogenesis leading to variations of tooth number, size and shape. Aust. Dent. J. 2014, 59 (Suppl. 1), 131–142. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, D.; Grunert, I.; Schmuth, M.; Kapferer-Seebacher, I. Prosthetic rehabilitation of patients with hypohidrotic ectodermal dysplasia: A systematic review. J. Oral. Rehabil. 2018, 45, 555–570. [Google Scholar] [CrossRef]
- Heuberer, S.; Watzak, G.; Zechner, W.; Vasak, C.; Laky, B.; Ulm, C. Oral rehabilitation of tooth agenesis frequently starts in paediatric dentistry. A retrospective analysis of 625 patients. Eur. J. Paediatr. Dent. 2022, 23, 303–314. [Google Scholar]
- Kotsiomiti, E.; Kassa, D.; Kapari, D. Oligodontia and associated characteristics: Assessment in view of prosthodontic rehabilitation. Eur. J. Prosthodont. Restor. Dent. 2007, 15, 55–60. [Google Scholar] [PubMed]
- Yordanova, G. Tooth Agenesis—The Problem and Its Solving in Our Practice, Prevalence and Relation With Other Deformities. J. IMAB—Annu. Proceeding 2015, 21, 859–863. [Google Scholar] [CrossRef]
- Kotsiomiti, E.; Kolokitha, O.E.; Lazaridis, N. Interim prosthodontic management of surgery-induced dental agenesis: A clinical report of 8 years of treatment. J. Prosthodont. 2013, 22, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Varghese, A.A.; Xavier, A.M.; Ramanarayanan, V. Removable prosthetic management for tooth agenesis in the pediatric population: A systematic review of case reports and case series. J. Prosthet. Dent. 2023, 132, e1–e1250. [Google Scholar] [CrossRef]
- Heuberer, S.; Ulm, C.; Zechner, W.; Laky, B.; Watzak, G. Patterns of congenitally missing teeth of non-syndromic and syndromic patients treated at a single-center over the past thirty years. Arch. Oral. Biol. 2019, 98, 140–147. [Google Scholar] [CrossRef]
- Arte, S.; Parmanen, S.; Pirinen, S.; Alaluusua, S.; Nieminen, P. Candidate Gene Analysis of Tooth Agenesis Identifies Novel Mutations in Six Genes and Suggests Significant Role for WNT and EDA Signaling and Allele Combinations. PLoS ONE 2013, 8, 1–12. [Google Scholar] [CrossRef]
- Intarak, N.; Tongchairati, K.; Termteerapornpimol, K.; Chantarangsu, S.; Porntaveetus, T. Tooth agenesis patterns and variants in PAX9: A systematic review. Jpn. Dent. Sci. Rev. 2023, 59, 129–137. [Google Scholar] [CrossRef]
- Lee, J.M.; Qin, C.; Chai, O.H.; Lan, Y.; Jiang, R.; Kwon, H.J.E. MSX1 Drives Tooth Morphogenesis Through Controlling Wnt Signaling Activity. J. Dent. Res. 2022, 101, 832–839. [Google Scholar] [CrossRef]
- Callahan, N.; Modesto, A.; Meira, R.; Seymen, F.; Patir, A.; Vieira, A.R. Axis inhibition protein 2 (AXIN2) polymorphisms and tooth agenesis. Arch. Oral. Biol. 2009, 54, 45–49. [Google Scholar] [CrossRef]
- Fons Romero, J.M.; Star, H.; Lav, R.; Watkins, S.; Harrison, M.; Hovorakova, M.; Headon, D.; Tucker, A.S. The Impact of the Eda Pathway on Tooth Root Development. J. Dent. Res. 2017, 96, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, X.; Wei, Z.; Long, H.; Lai, W. The EDA/EDAR/NF-κB pathway in non-syndromic tooth agenesis: A genetic perspective. Front. Genet. 2023, 14, 1168538. [Google Scholar] [CrossRef]
- Yu, M.; Wong, S.W.; Han, D.; Cai, T. Genetic analysis: Wnt and other pathways in nonsyndromic tooth agenesis. Oral. Dis. 2019, 25, 646–651. [Google Scholar] [CrossRef]
- Bonczek, O.; Balcar, V.J.; Šerý, O. PAX9 gene mutations and tooth agenesis: A review. Clin. Genet. 2017, 92, 467–476. [Google Scholar] [CrossRef]
- Ruf, S.; Klimas, D.; Hönemann, M.; Jabir, S. Genetic background of nonsyndromic oligodontia: A systematic review and meta-analysis. J. Orofac. Orthop. 2013, 74, 295–308. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Stubbs, B.; Solmi, M.; Veronese, N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 2017, 5, 80. [Google Scholar] [CrossRef]
- Al-Ani, A.H.; Antoun, J.S.; Thomson, W.M.; Topless, R.; Merriman, T.R.; Farella, M. Common variants of EDA are associated with non-syndromic hypodontia. Orthod. Craniofacial Res. 2021, 24, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Alves-Ferreira, M.; Pinho, T.; Sousa, A.; Sequeiros, J.; Lemos, C.; Alonso, I. Identification of genetic risk factors for maxillary lateral incisor agenesis. J. Dent. Res. 2014, 93, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Paixão-Côrtes, V.R.; Braga, T.; Salzano, F.M.; Mundstock, K.; Mundstock, C.A.; Bortolini, M.C. PAX9 and MSX1 transcription factor genes in non-syndromic dental agenesis. Arch. Oral. Biol. 2011, 56, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.A.; Adusumilli, G.; Devanna, R.; Pichai, S.; Rohra, M.G.; Arjunan, S. MSX1 gene variant—Its presence in tooth absence—A case control genetic study. J. Int. Oral Heal. JIOH 2013, 5, 20–26. [Google Scholar]
- Seifi, M.; Kazemi, B.; Golkar, P. The role of MSX1 in tooth agenesis in Iranians. Int. J. Paediatr. Dent. 2007, 17, 254–258. [Google Scholar] [CrossRef]
- Vieira, A.R.; D’Souza, R.N.; Mues, G.; Deeley, K.; Hsin, H.Y.; Küchler, E.C.; Meira, R.; Patir, A.; Tannure, P.N.; Lips, A.; et al. Candidate gene studies in hypodontia suggest role for FGF3. Eur. Arch. Paediatr. Dent. 2013, 14, 405–410. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.J.; Wu, Z.Z. WNT10A polymorphism may be a risk factor for non-syndromic hypodontia. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Chen, Y.T.; Liu, H.C.; Han, D.; Liu, Y.; Feng, H.L. Association between EDAR Polymorphisms and Non-Syndromic Tooth Agenesis in the Chinese Han Population. Chin. J. Dent. Res. 2017, 20, 153–159. [Google Scholar] [PubMed]
- Fan, L.; Ma, L.; Zhu, G.; Yao, S.; Li, X.; Yu, X.; Pan, Y.; Wang, L. A Genome-wide association study of premolar agenesis in a chinese population. Oral. Dis. 2023, 29, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Grejtakova, D.; Gabrikova-Dojcakova, D.; Boronova, I.; Kyjovska, L.; Hubcejova, J.; Fecenkova, M.; Zigova, M.; Priganc, M.; Bernasovska, J. WNT10A variants in relation to nonsyndromic hypodontia in eastern Slovak population. J. Genet. 2018, 97, 1169–1177. [Google Scholar] [CrossRef]
- Jobbágy-Óvári, G.; Páska, C.; Stiedl, P.; Trimmel, B.; Hontvári, D.; Soós, B.; Hermann, P.; Tóth, Z.; Kerekes-Máthé, B.; Nagy, D.; et al. Complex analysis of multiple single nucleotide polymorphisms as putative risk factors of tooth agenesis in the Hungarian population. Acta Odontol. Scand. 2014, 72, 216–227. [Google Scholar] [CrossRef]
- Kirac, D.; Eraydin, F.; Avcilar, T.; Ulucan, K.; Özdemir, F.; Guney, A.I.; Kaspar, E.Ç.; Keshi, E.; Isbir, T. Effects of PAX9 and MSX1 gene variants to hypodontia, tooth size and the type of congenitally missing teeth. Cell Mol. Biol. 2016, 62, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Lancia, M.; Machado, R.A.; Dionísio, T.J.; Garib, D.G.; Santos, C.F.d.; Coletta, R.D.; das Neves, L.T. Association between MSX1 rs12532 polymorphism with nonsyndromic unilateral complete cleft lip and palate and tooth agenesis. Arch Oral. Biol. 2020, 109, 104556. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Song, S.; Zhao, H.; Han, D.; Feng, H. A case-control study of the association between tooth-development gene polymorphisms and non-syndromic hypodontia in the Chinese Han population. Eur. J. Oral. Sci. 2012, 120, 378–385. [Google Scholar] [CrossRef]
- Mostowska, A.; Biedziak, B.; Zadurska, M.; Dunin-Wilczynska, I.; Lianeri, M.; Jagodzinski, P. Nucleotide variants of genes encoding components of the Wnt signalling pathway and the risk of non-syndromic tooth agenesis. Clin. Genet. 2013, 84, 429–440. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Pan, Y.C.; Ma, J.Q.; Zhang, W.B. Msh Homebox-1 Polymorphisms and Susceptibility to 198 Sporadic Tooth Agenesis: A Case-Control Study. Zhonghua Kou Qiang Yi Xue Za Zhi 2010, 45, 135–140. [Google Scholar]
- Wong, S.; Liu, H.; Li, Y.; Han, D.; Feng, H. Association between AXIN2 Polymorphism and Oligodontia. Beijing Da Xue Xue Bao 2014, 46, 269–273. [Google Scholar]
- Ross, J.N.; Ruigrok, L.C.; Fennis, W.M.M.; Cune, M.S.; Rosenberg, A.J.W.P.; van Nunen, A.B.; Créton, M.A.; Ploos van Amstel, H.-K.; van den Boogaard, M.-J.J.H. Gastrointestinal Symptoms in Patients with Isolated Oligodontia and a Wnt Gene Mutation. Oral Dis. 2023, 29, 300–307. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Chen, J.; Wang, F.; Huang, R.; Wu, S.; Shu, L.; Qiu, J.; Yang, Z.; Xue, J.; et al. PAX9 Polymorphism and Susceptibility to Sporadic Non-Syndromic Severe Anodontia: A Case-Control Study in Southwest China. J. Appl. Oral Sci. 2013, 21, 256–264. [Google Scholar] [CrossRef]
- Wang, J.; Sun, K.; Shen, Y.; Xu, Y.; Xie, J.; Huang, R.; Zhang, Y.; Xu, C.; Zhang, X.; Wang, R.; et al. DNA Methylation Is Critical for Tooth Agenesis: Implications for Sporadic Non-Syndromic Anodontia and Hypodontia. Sci. Rep. 2016, 6, 19162. [Google Scholar] [CrossRef]
- Rakhshan, V. Meta-analysis and systematic review of factors biasing the observed prevalence of congenitally missing teeth in permanent dentition excluding third molars. Prog. Orthod. 2013, 14, 1–12. [Google Scholar] [CrossRef]
- Lensen, S. When to pool data in a meta-analysis (and when not to)? Fertil. Steril. 2023, 119, 902–903. [Google Scholar] [CrossRef]
- Drucker, A.M.; Fleming, P.; Chan, A.W. Research Techniques Made Simple: Assessing Risk of Bias in Systematic Reviews. J. Investig. Dermatol. 2016, 136, e109–e114. [Google Scholar] [CrossRef]
- Boeira Junior, B.R.; Echeverrigaray, S. Dentistry and molecular biology: A promising field for tooth agenesis management. Tohoku J. Exp. Med. 2012, 226, 243–249. [Google Scholar] [CrossRef]
- Cudney, S.M.; Vieira, A.R. Molecular factors resulting in tooth agenesis and contemporary approaches for regeneration: A review. Eur. Arch. Paediatr. Dent. 2012, 13, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Mártha, K.; Kerekes Máthé, B.; Moldovan, V.G.; Bǎnescu, C. Study of rs12532, rs8670 Polymorphism of Msh Homeobox 1 (MSX1), rs61754301, rs4904155 Polymorphism of Paired Box Gene 9 (PAX9), and rs2240308 Polymorphism of Axis Inhibitor Protein 2 (AXIN2) Genes in Nonsyndromic Hypodontia. Biomed. Res. Int. 2019, 2019, 2183720. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Von Den Hoff, J.; Lange, J.; Ren, Y.; Bian, Z.; Carels, C.E.L. MSX1 mutations and associated disease phenotypes: Genotype-phenotype relations. Eur. J. Hum. Genet. 2016, 24, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-F.; Han, Y.; Shi, S.; Li, X.; Zhu, X.-D.; Zhou, J.; Shao, Q.-L.; Li, X.-Q.; Liu, S.-L. Characterization of transcriptional repressor gene MSX1 variations for possible associations with congenital heart diseases. PLoS ONE 2015, 10, e0142666. [Google Scholar] [CrossRef][Green Version]
- Sisman, Y.; Uysal, T.; Gelgor, I.E. Hypodontia. Does the Prevalence and Distribution Pattern Differ in Orthodontic Patients? Eur. J. Dent. 2007, 1, 167–173. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).