Rehabilitation of a Wide Buccal Recession Using a Combination of Adhesive Prosthetic Procedures and Transmucosal Convergent Neck Implant to Replace a Lower Fractured Canine: Case Report with 6 Years Follow-Up
Abstract
1. Introduction
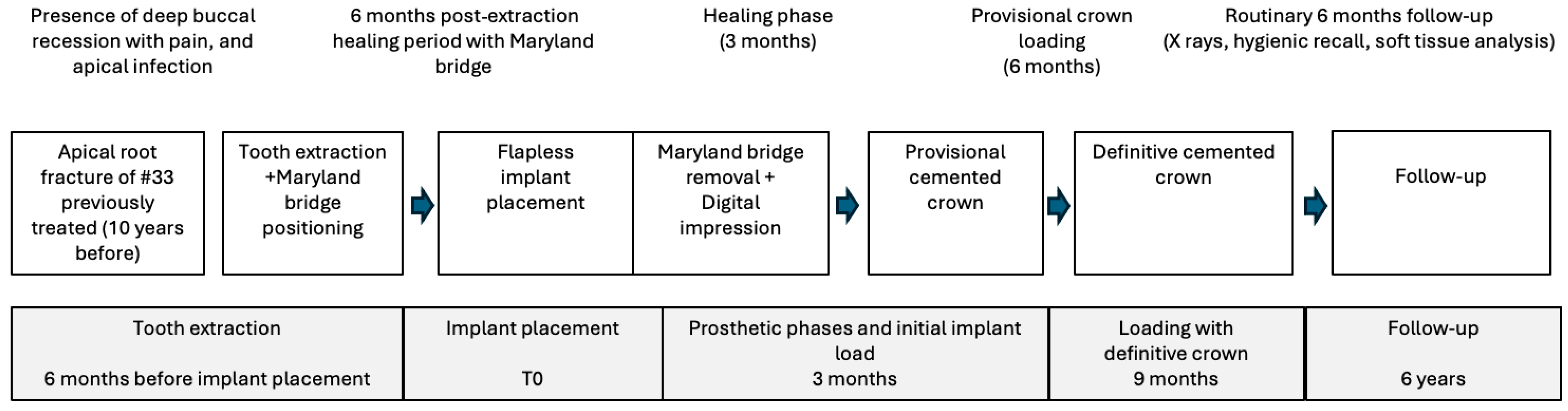
2. Materials and Methods
2.1. Patient Information
2.2. Tooth Extraction and Resin-Bonded Bridge Preparation
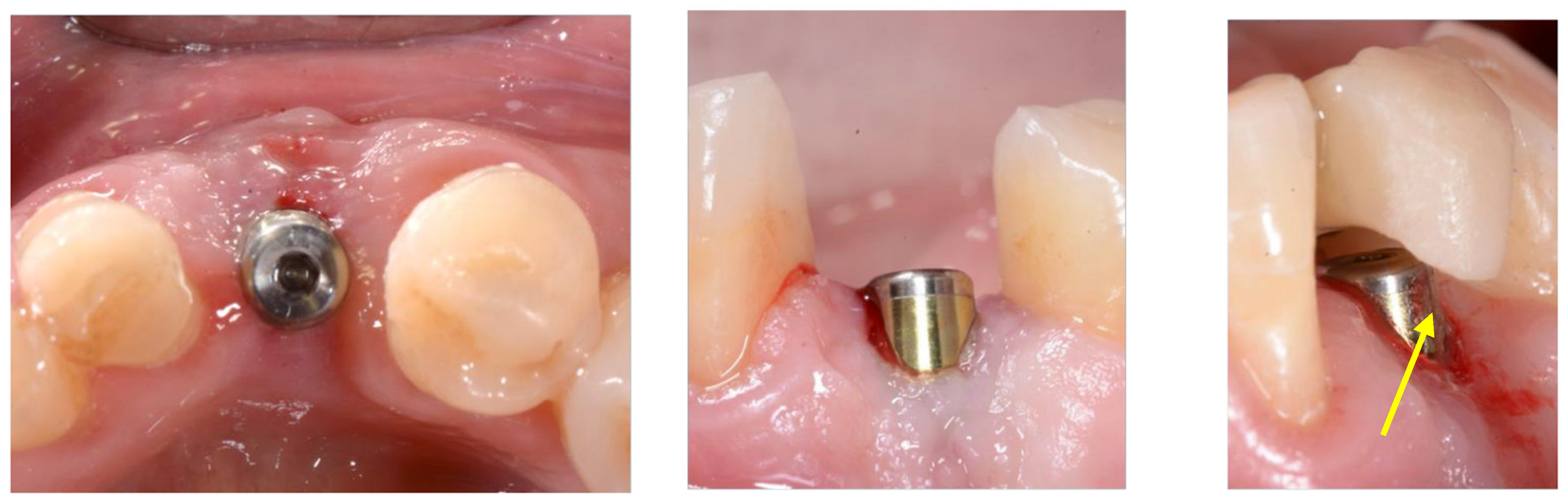
2.3. Implant Insertion
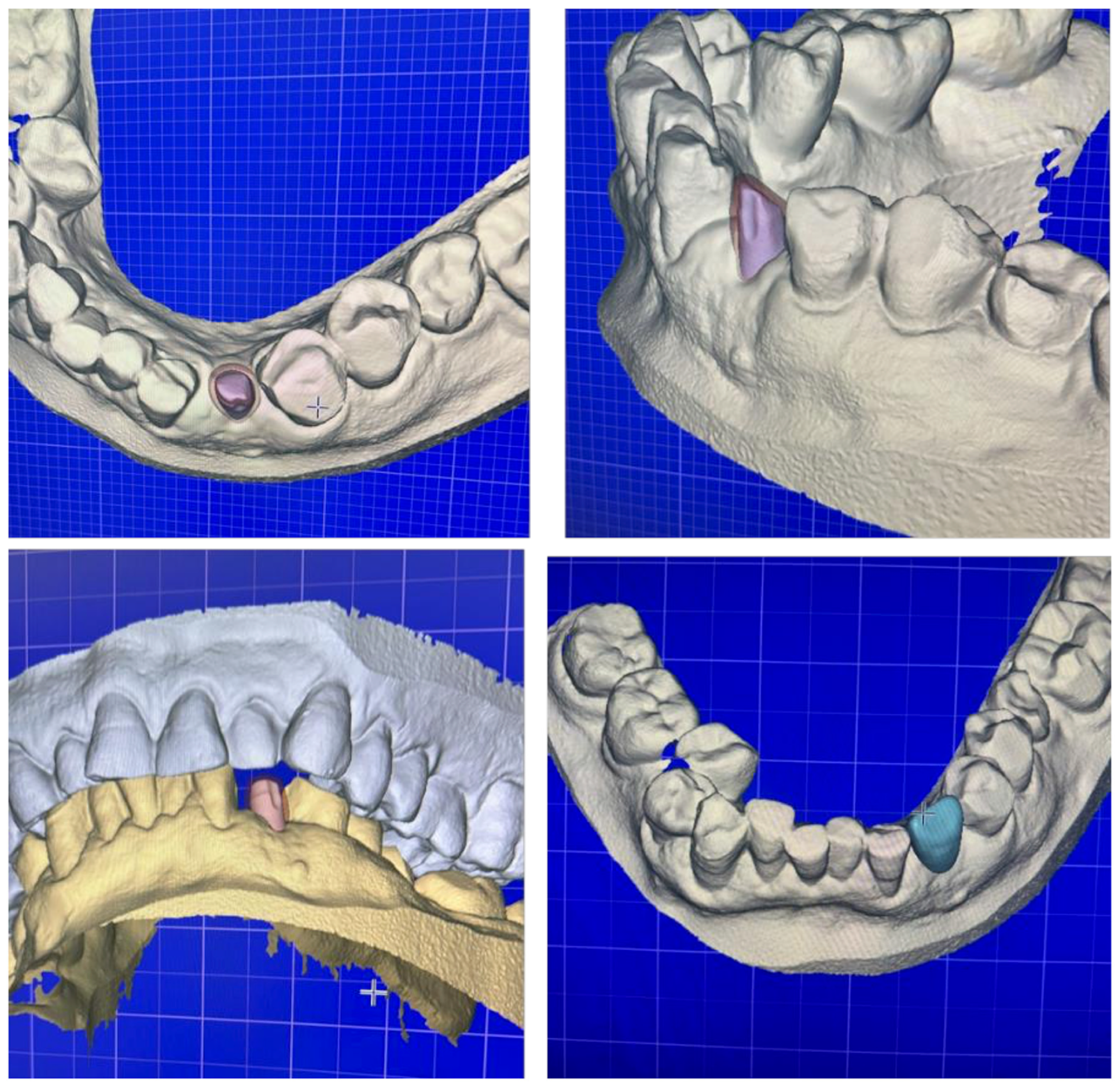
2.4. Digital Workflow for Prosthetic Rehabilitation
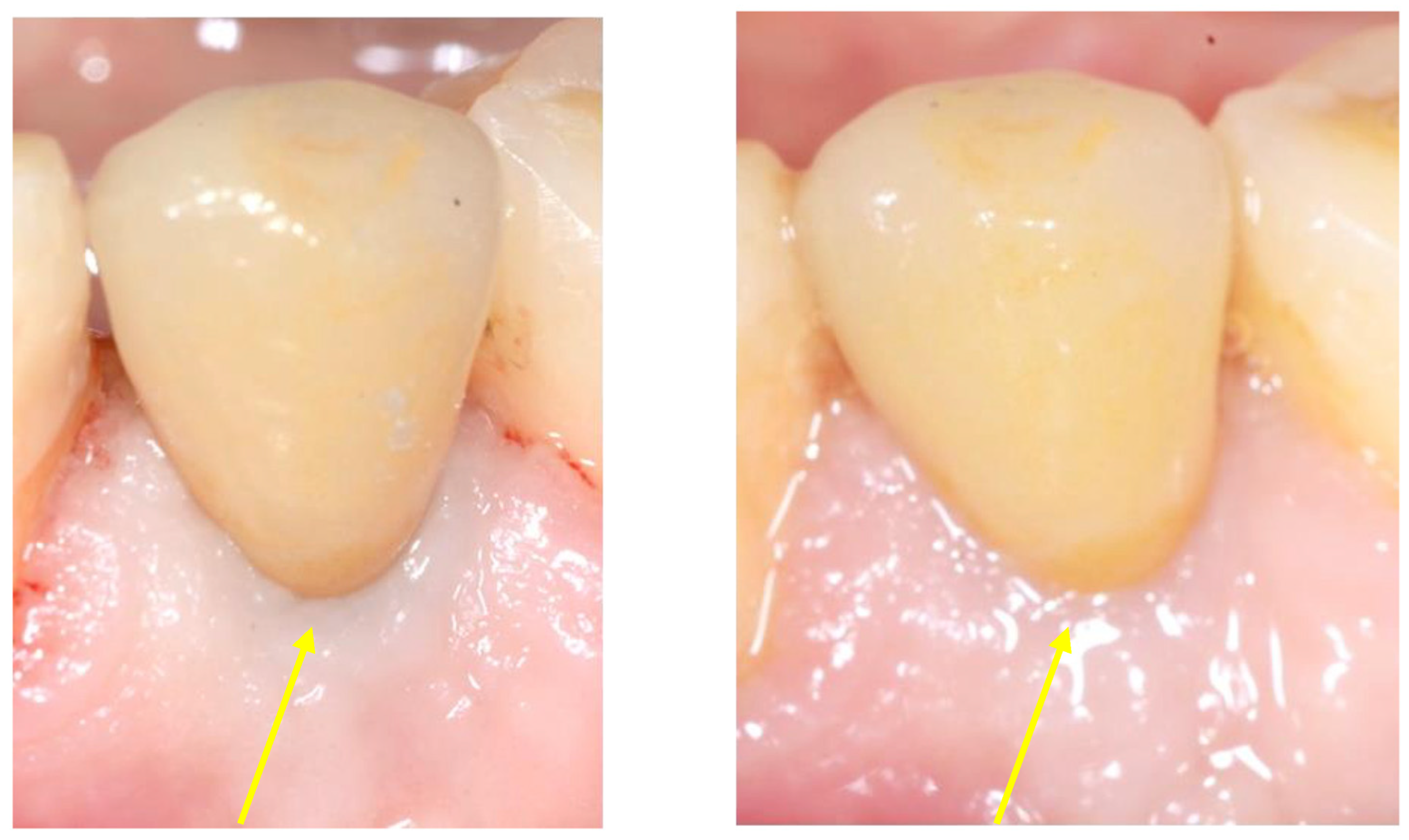
2.5. Follow-Up
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D.S. Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral Implant. Res. 2012, 23, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin. Oral Implant. Res. 2012, 23, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction: An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef]
- Belser, U.C.; Buser, D.; Hess, D.; Schmid, B.; Bernard, J.P.; Lang, N.P. Aesthetic implant restorations in partially edentulous patients—A critical appraisal. Periodontology 2000 1998, 17, 132–150. [Google Scholar] [CrossRef] [PubMed]
- Cardaropoli, G.; Araujo, M.; Lindhe, J. Dynamics of bone tissue formation in tooth extraction sites: An experimental study in dogs. J. Clin. Periodontol. 2003, 30, 809–818. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R.; Marzola, A.; Bozzi, L.; Liljenberg, B.; Lindhe, J. Modeling and remodeling of human extraction sockets. J. Clin. Periodontol. 2008, 35, 630–639. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; León-Cano, A.; Ortega-Oller, I.; Monje, A.; OValle, F.; Catena, A. Marginal bone loss as success criterion in implant dentistry: Beyond 2 mm. Clin. Oral Implant. Res. 2015, 26, 28–34. [Google Scholar] [CrossRef]
- Thoma, D.S.; Mühlemann, S.; Jung, R.E. Critical soft-tissue dimensions with dental implants and treatment concepts. Periodontology 2000 2014, 66, 106–118. [Google Scholar] [CrossRef]
- Botticelli, D.; Berglundh, T.; Lindhe, J. Hard-tissue alterations following immediate implant placement in extraction sites. J. Clin. Periodontol. 2004, 31, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.; Zamparini, F.; Spinelli, A.; Prati, C.; Gandolfi, M.G. ESEM-EDX Microanalysis at Bone-Implant Region on Immediately Loaded Implants Retrieved Postmortem. Int. J. Oral Maxillofac. Implant. 2022, 37, e51–e60. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Zamparini, F.; Iezzi, G.; Degidi, M.; Botticelli, D.; Piattelli, A.; Prati, C. Microchemical and Micromorphologic ESEM-EDX Analysis of Bone Mineralization at the Thread Interface in Human Dental Implants Retrieved for Mechanical Complications After 2 Months to 17 Years. Int. J. Periodontics Restor. Dent. 2018, 38, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kitagawa, N.; Isobe, A. Implant treatment in ultra-aged society. Jpn. Dent. Sci. Rev. 2018, 54, 45–51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogawa, H.; Yoshihara, A.; Hirotomi, T.; Ando, Y.; Miyazaki, H. Risk factors for periodontal disease progression among elderly people. J. Clin. Periodontol. 2002, 29, 592–597. [Google Scholar] [CrossRef]
- Quirynen, M.; Vogels, R.; Alsaadi, G.; Naert, I.; Jacobs, R.; van Steenberghe, D. Predisposing conditions for retrograde peri-implantitis, and treatment suggestions. Clin. Oral Implant. Res. 2005, 16, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wu, Y.; Xu, C.; Zhang, F. Immediate dental implant placement into infected vs. non-infected sockets: A meta-analysis. Clin. Oral Implant. Res. 2016, 27, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Zamparini, F.; Azizi, A.; Spinelli, A.; Gandolfi, M.G. Resin-Bonded Prosthesis in Posterior Area to Prevent Early Marginal Bone Resorption in Implants Placed at Tissue Level. Prosthesis 2022, 4, 575–588. [Google Scholar] [CrossRef]
- Prati, C.; Zamparini, F.; Canullo, L.; Pirani, C.; Botticelli, D.; Gandolfi, M.G. Factors Affecting Soft and Hard Tissues Around Two-Piece Transmucosal Implants: A 3-Year Prospective Cohort Study. Int. J. Oral Maxillofac. Implant. 2020, 35, 1022–1036. [Google Scholar] [CrossRef]
- Prati, C.; Zamparini, F.; Spinelli, A.; Lenzi, J.; Gandolfi, M.G. The Use of Two-Piece Transmucosal Implants Designed with a Convergent Neck: A 6-Year Clinical Prospective Cohort Study Evaluating the Impact on Soft and Hard Tissues. Int. J. Oral Maxillofac. Implant. 2024, 21, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Loi, I.; Di Felice, A. Biologically oriented preparation technique (BOPT): A new approach for prosthetic restoration of periodontically healthy teeth. Eur. J. Esthet. Dent. 2013, 8, 10–23. [Google Scholar]
- Fürhauser, R.; Florescu, D.; Benesch, T.; Haas, R.; Mailath, G.; Watzek, G. Evaluation of soft tissue around single-tooth implant crowns: The pink esthetic score. Clin. Oral Implant. Res. 2005, 16, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E.; Perel, M.L.; Wang, H.L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant success, survival, and failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant. Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Pace, R.; Giuliani, V.; Nieri, M.; Di Nasso, L.; Pagavino, G. Mineral trioxide aggregate as apical plug in teeth with necrotic pulp and immature apices: A 10-year case series. J. Endod. 2014, 40, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41, S6–S22. [Google Scholar] [CrossRef]
- Barootchi, S.; Tavelli, L.; Majzoub, J.; Stefanini, M.; Wang, H.L.; Avila-Ortiz, G. Alveolar ridge preservation: Complications and cost-effectiveness. Periodontology 2000 2023, 92, 235–262. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira-Neto, O.B.; Lemos, C.A.; Barbosa, F.T.; de Sousa-Rodrigues, C.F.; Camello de Lima, F.J. Immediate dental implants placed into infected sites present a higher risk of failure than immediate dental implants placed into non-infected sites: Systematic review and meta-analysis. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e518–e528. [Google Scholar] [CrossRef]
- Hwang, D.; Wang, H.L. Flap thickness as a predictor of root coverage: A systematic review. J. Periodontol. 2006, 77, 1625–1634. [Google Scholar] [CrossRef]
- Evans, C.D.; Chen, S.T. Esthetic outcomes of immediate implant placements. Clin. Oral Implant. Res. 2008, 19, 73–80. [Google Scholar] [CrossRef]
- Buser, D.; Chappuis, V.; Kuchler, U.; Bornstein, M.M.; Wittneben, J.G.; Buser, R.; Cavusoglu, Y.; Belser, U.C. Long-term stability of early implant placement with contour augmentation. J. Dent. Res. 2013, 92, 176S–182S. [Google Scholar] [CrossRef]
- Gurtner, G.; Werner, S.; Barrandon Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef]
- Palombo, D.; Rahmati, M.; Vignoletti, F.; Sanz-Esporrin, J.; Haugen, H.J.; Sanz, M. Hard and soft tissue healing around implants with a modified implant neck configuration: An experimental in vivo preclinical investigation. Clin. Oral Implant. Res. 2021, 32, 1127–1141. [Google Scholar] [CrossRef]
- Canullo, L.; Giuliani, A.; Furlani, M.; Menini, M.; Piattelli, A.; Iezzi, G. Influence of abutment macro- and micro-geometry on morphologic and morphometric features of peri-implant connective tissue. Clin. Oral Implant. Res. 2023, 34, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Valles, C.; Rodríguez-Ciurana, X.; Nart, J.; Santos, A.; Galofre, M.; Tarnow, D. Influence of Implant Neck Surface and Placement Depth on Crestal Bone Changes Around Platform-Switched Implants: A Clinical and Radiographic Study in Dogs. J. Periodontol. 2017, 88, 1200–1210. [Google Scholar] [CrossRef]
- Alomrani, A.N.; Hermann, J.S.; Jones, A.A.; Buser, D.; Schoolfield, J.; Cochran, D.L. The effect of a machined collar on coronal hard tissue around titanium implants: A radiographic study in the canine mandible. Int. J. Oral Maxillofac. Implant. 2005, 20, 677–686. [Google Scholar]
- Schwarz, F.; Mihatovic, I.; Golubovich, V.; Schär, A.; Sager, M.; Becker, J. Impact of abutment microstructure and insertion depth on crestal bone changes at nonsubmerged titanium implants with platform switch. Clin. Oral Implant. Res. 2015, 26, 287–292. [Google Scholar] [CrossRef]
- Chan, D.; Pelekos, G.; Ho, D.; Cortellini, P.; Tonetti, M.S. The depth of the implant mucosal tunnel modifies the development and resolution of experimental peri-implant mucositis: A case-control study. J. Clin. Periodontol. 2019, 46, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Schaller, D.; Hakansson, J.; Wennstrom, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of implant therapy analyzed in a swedish population: Prevalence of peri-implantitis. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Morón-Conejo, B.; Sanz-Sánchez, I.; Salido, M.P.; Martínez-Rus, F.; Pradíes, G. The effect of a convergent transmucosal neck on soft tissues and radiographic outcomes: A 1-year follow-up randomized controlled trial. Clin. Oral Investig. 2023, 27, 2923–2933. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van der Meer, W.J.; Andriessen, F.S.; Wismeijer, D.; Ren, Y. Application of intra-oral dental scanners in the digital workflow of implantology. PLoS ONE 2012, 7, e43312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.J.; Betensky, R.A.; Gianneschi, G.E.; Gallucci, G.O. Accuracy of digital versus conventional implant impressions. Clin. Oral Implant. Res. 2015, 26, 715–719. [Google Scholar] [CrossRef]
- Mangano, F.G.; Hauschild, U.; Veronesi, G.; Imburgia, M.; Mangano, C.; Admakin, O. Trueness and precision of 5 intraoral scanners in the impressions of single and multiple implants: A comparative in vitro study. BMC Oral Health 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Schulze, R.; Heil, U.; Gross, D.; Bruellmann, D.D.; Dranischnikow, E.; Schwanecke, U.; Schoemer, E. Artefacts in CBCT: A review. Dentomaxillofac. Radiol. 2011, 40, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Wanderley, V.A.; de Faria Vasconcelos, K.; Leite, A.F.; Pauwels, R.; Shujaat, S.; Jacobs, R.; Oliveira, M.L. Impact of the blooming artefact on dental implant dimensions in 13 cone-beam computed tomography devices. Int. J. Implant. Dent. 2021, 7, 67. [Google Scholar] [CrossRef] [PubMed]



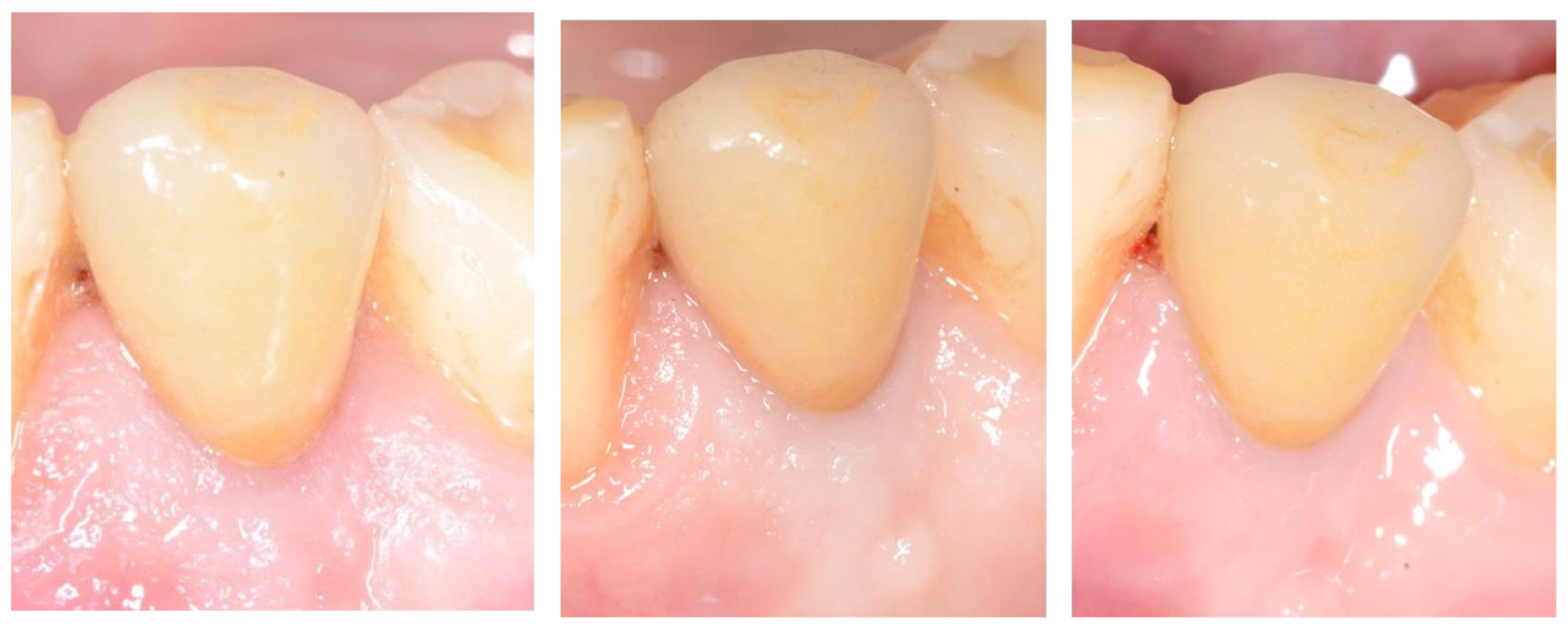
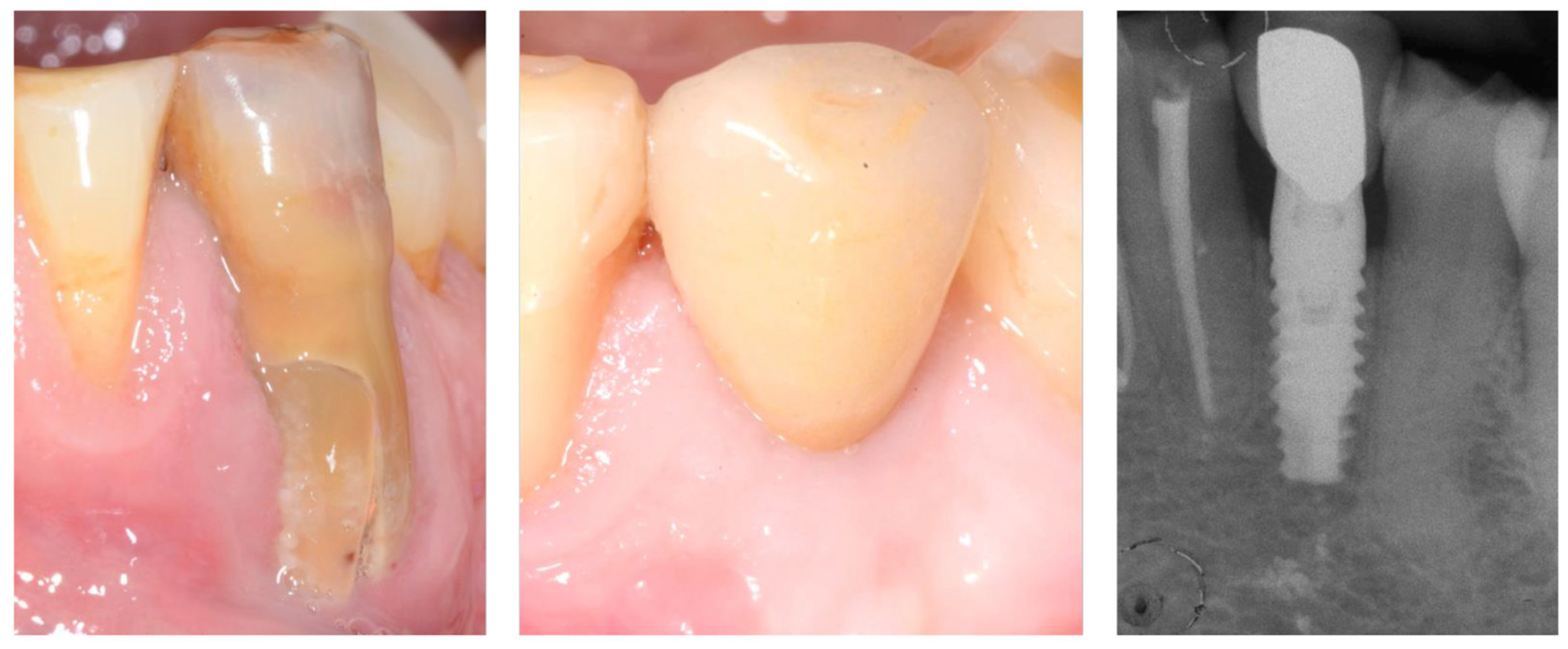
| Q | Parameter | Scoring (0–2) | Preoperative (Before Extraction) | 9 Months | 12 Months | 72 Months |
|---|---|---|---|---|---|---|
| Q1 | Mesial Papilla | 0 = absent, 1 = incomplete, 2 = complete | 2 | 1 | 1 | 2 |
| Q2 | Distal Papilla | 0 = absent, 1 = incomplete, 2 = complete | 2 | 1 | 1 | 1 |
| Q3 | Soft tissue contour | 0 = poor match, 1 = moderate match, 2 = perfect match | 0 | 1 | 2 | 2 |
| Q4 | Soft tissue level | 0 = >2 mm discrepancy, 1 = 1–2 mm discrepancy, 2 = <1 mm discrepancy | 0 | 1 | 1 | 2 |
| Q5 | Alveolar process deficiency | 0 = poor match, 1 = moderate match, 2 = perfect match | 0 | 1 | 1 | 2 |
| Q6 | Soft Tissue Texture | 0 = poor match, 1 = moderate match, 2 = perfect match | 0 | 1 | 2 | 2 |
| Q7 | Soft Tissue Color | 0 = poor match, 1 = moderate match, 2 = perfect match | 0 | 2 | 2 | 2 |
| Total | 12–14 Excellent outcome 9–11 Good outcome 6–8 Moderate outcome 0–5 Poor esthetic outcome | 4 | 8 | 10 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prati, C.; Spinelli, A.; Gandolfi, M.G.; Zamparini, F. Rehabilitation of a Wide Buccal Recession Using a Combination of Adhesive Prosthetic Procedures and Transmucosal Convergent Neck Implant to Replace a Lower Fractured Canine: Case Report with 6 Years Follow-Up. Prosthesis 2025, 7, 117. https://doi.org/10.3390/prosthesis7050117
Prati C, Spinelli A, Gandolfi MG, Zamparini F. Rehabilitation of a Wide Buccal Recession Using a Combination of Adhesive Prosthetic Procedures and Transmucosal Convergent Neck Implant to Replace a Lower Fractured Canine: Case Report with 6 Years Follow-Up. Prosthesis. 2025; 7(5):117. https://doi.org/10.3390/prosthesis7050117
Chicago/Turabian StylePrati, Carlo, Andrea Spinelli, Maria Giovanna Gandolfi, and Fausto Zamparini. 2025. "Rehabilitation of a Wide Buccal Recession Using a Combination of Adhesive Prosthetic Procedures and Transmucosal Convergent Neck Implant to Replace a Lower Fractured Canine: Case Report with 6 Years Follow-Up" Prosthesis 7, no. 5: 117. https://doi.org/10.3390/prosthesis7050117
APA StylePrati, C., Spinelli, A., Gandolfi, M. G., & Zamparini, F. (2025). Rehabilitation of a Wide Buccal Recession Using a Combination of Adhesive Prosthetic Procedures and Transmucosal Convergent Neck Implant to Replace a Lower Fractured Canine: Case Report with 6 Years Follow-Up. Prosthesis, 7(5), 117. https://doi.org/10.3390/prosthesis7050117









