The Effect of Electrospun PMMA/rGO Fiber Addition on the Improvement of the Physical and Mechanical Properties of PMMA Resin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PMMA Solutions Containing Different Amounts of rGO
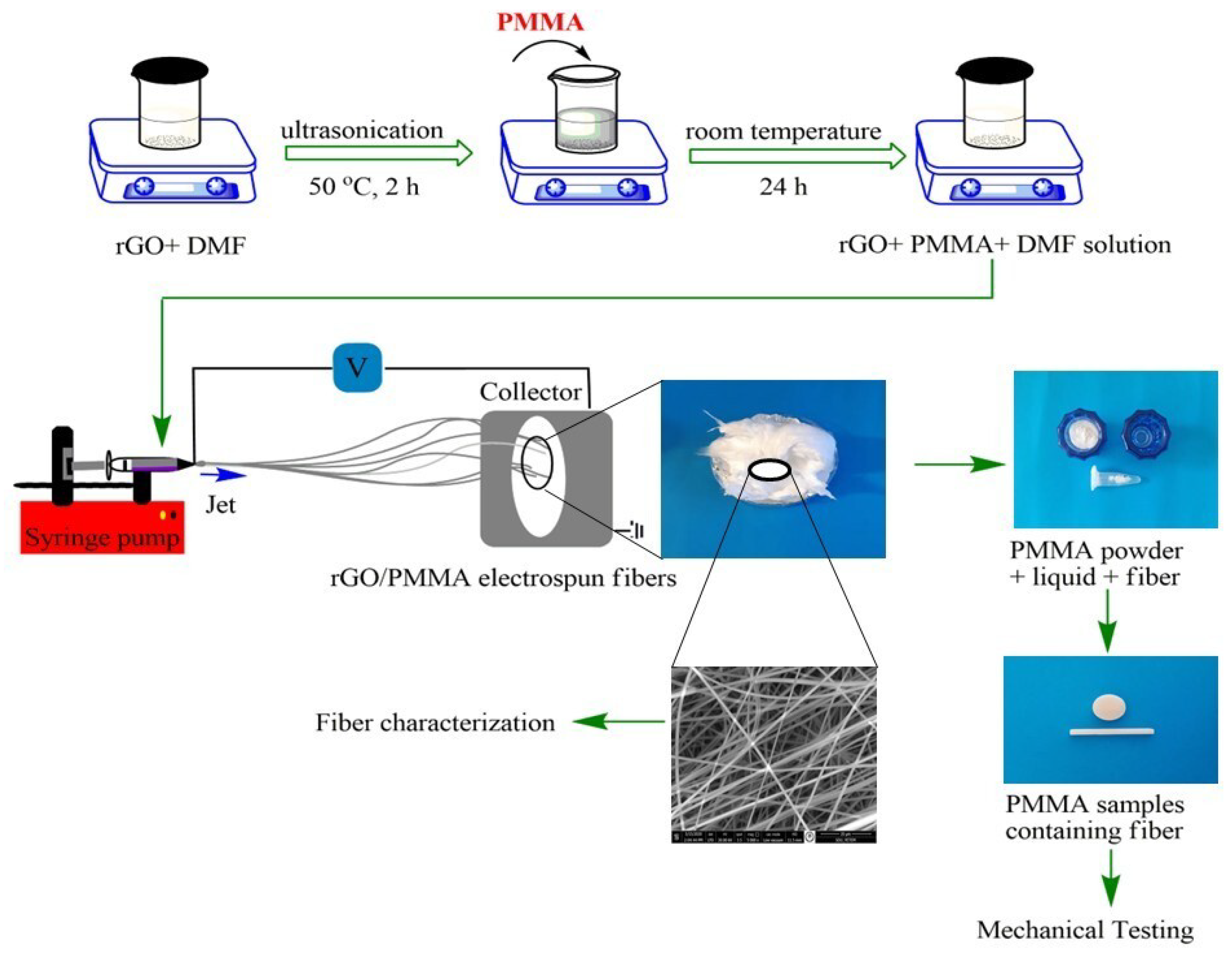
2.3. Synthesis of Electrospun Fibers
2.4. Fiber Characterization
2.5. Silane Functionalization of Fiber Surfaces
2.6. Preparation of Fiber-Modified PMMA Specimens
2.7. Mechanical Testing
2.8. Statistical Analysis
3. Results
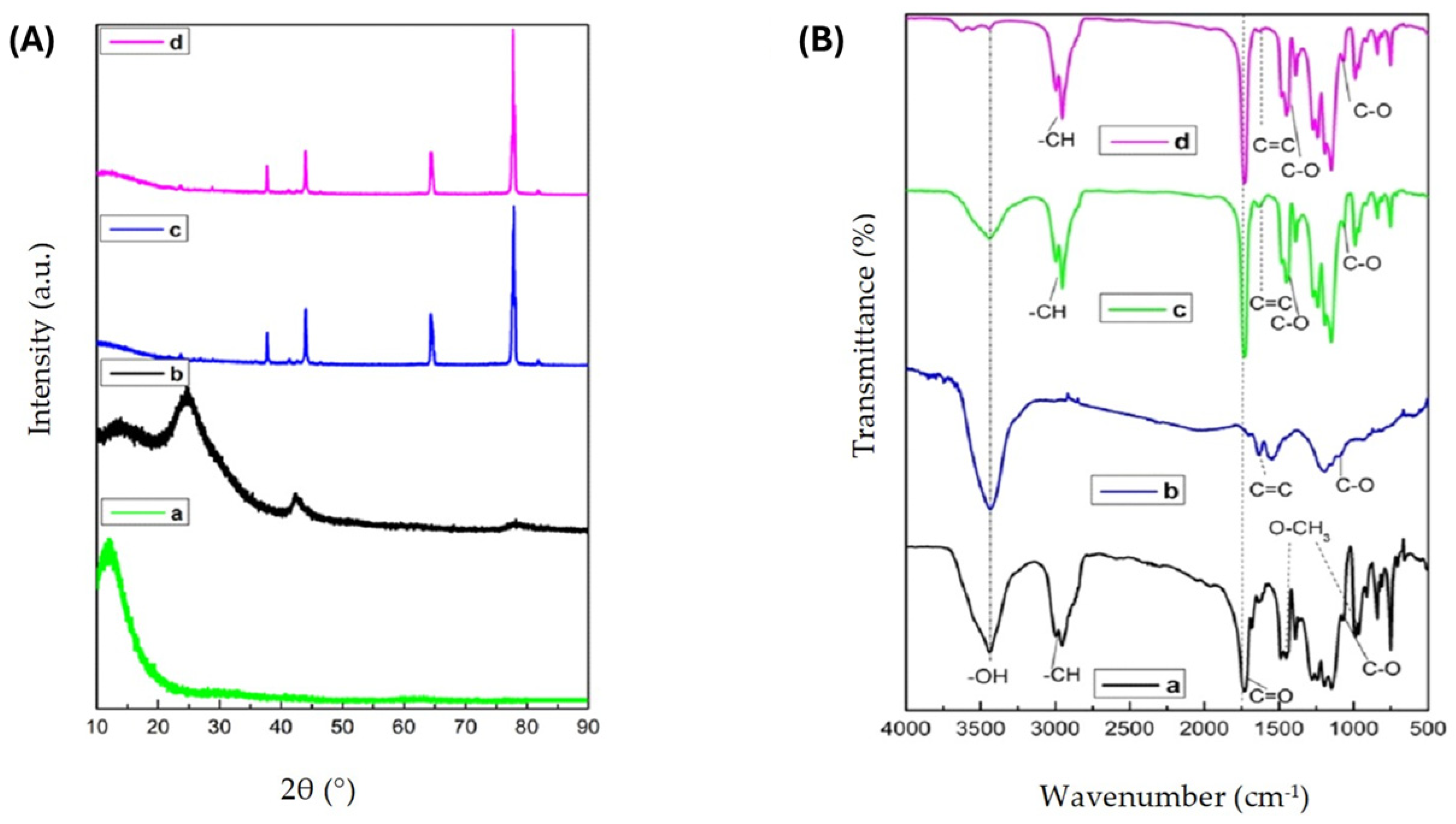
3.1. Fiber Characterization Results
3.2. Mechanical Tests
3.2.1. Flexural Strength and Elastic Modulus
3.2.2. Surface Roughness and Vickers Microhardness
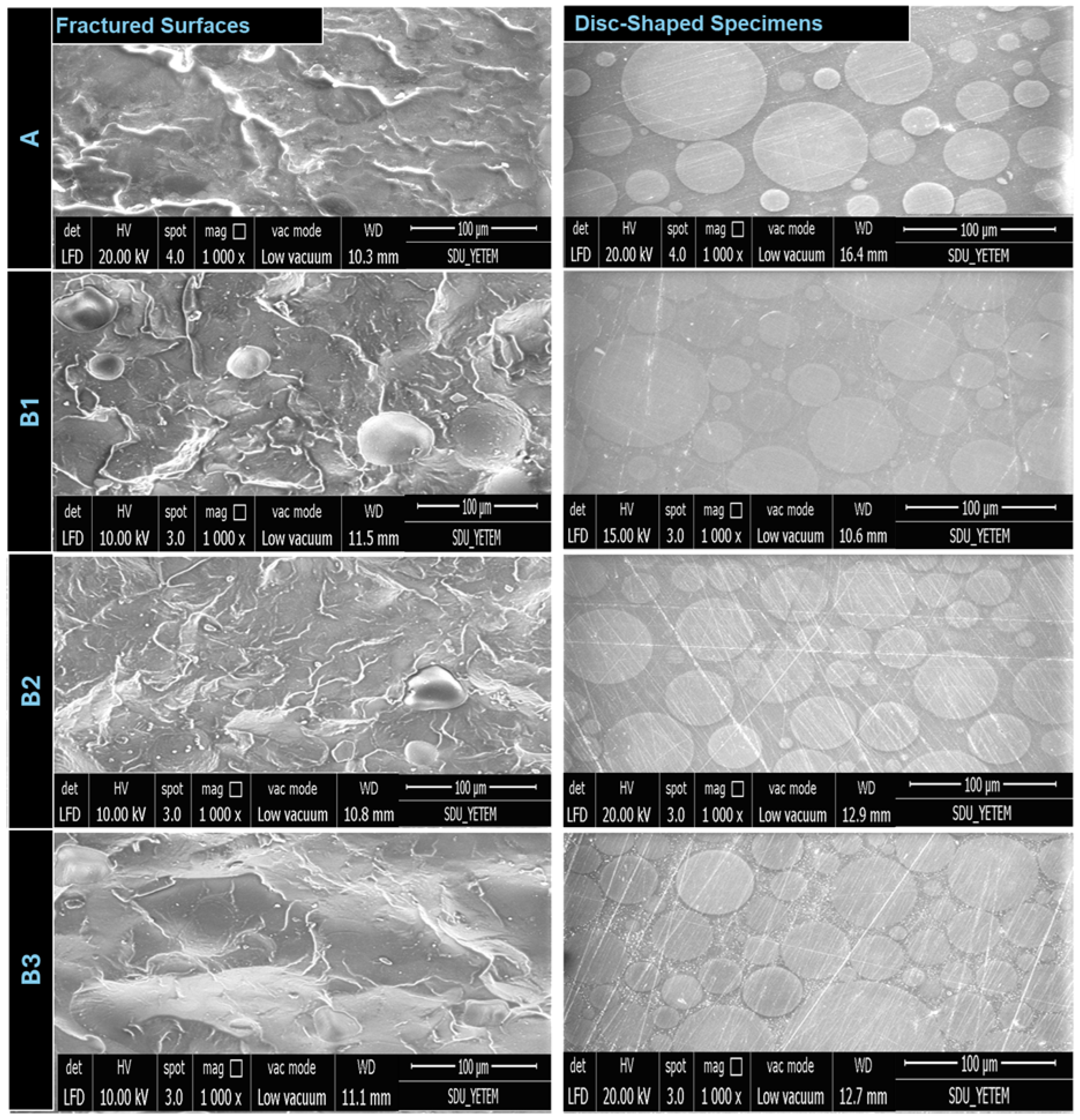
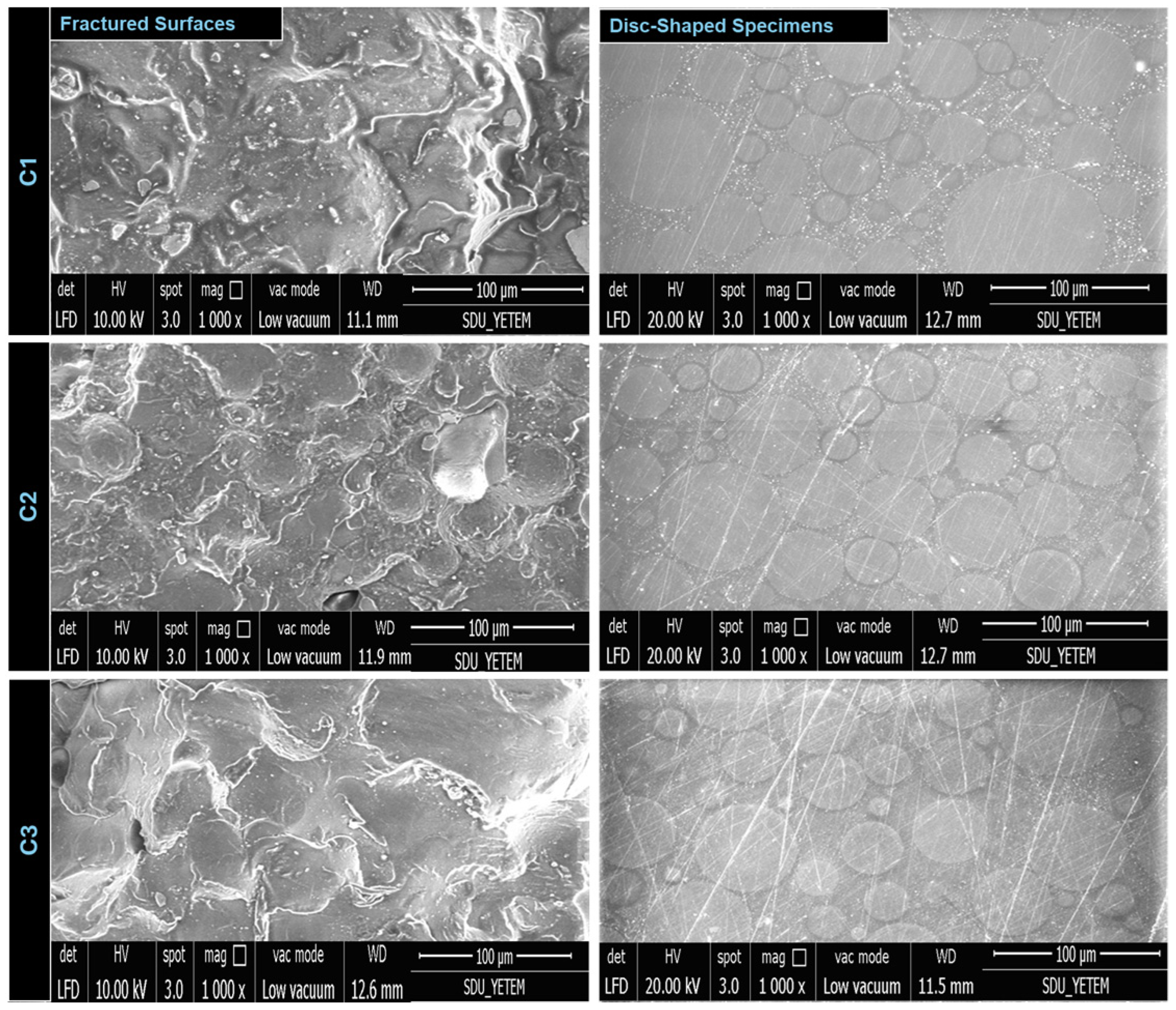
3.3. Microstructural Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Am-rGO | Amino-functionalized reduced graphene oxide |
| DMF | N, N-dimethylformamide |
| FTIR | Fourier transform infrared spectroscopy |
| GO | Graphene oxide |
| IQR | Interquartile Range |
| MMA | Methyl methacrylate |
| MPa | Megapascals |
| N | Newtons |
| PANI | Polyaniline |
| PMMA | Poly(methyl methacrylate) |
| PVC | Polyvinyl chloride |
| Ra | Surface roughness |
| rGO | Reduced graphene oxide |
| SD | Standard Deviation |
| SEM-EDS | Scanning electron microscopy in combination with energy-dispersive X-ray spectroscopy |
| TGA/DTG | Thermogravimetric analysis/Differential thermal analysis |
| XRD | X-ray diffraction |
References
- Bağış, B.; Çizmeci Basmacı, F.; Ustaömer, S.; Özen, B. Sabit geçici restorasyonlar. Ataturk Diş Hekim. Fak. Derg. 2006, 16, 42–49. [Google Scholar]
- Burke, F.J.; Murray, M.C.; Shortall, A.C. Trends in indirect dentistry: 6. provisional restorations, more than just a temporary. Dent. Update 2005, 32, 443–452. [Google Scholar] [CrossRef]
- Garg, P.; Ravi, R.; Ghalaut, P. Outcome of provisional restorations on basis of materials and techniques of choice: A systematic review. Restoration 2021, 3, 6–15. [Google Scholar]
- Yadav, R.; Sonwal, S.; Sharma, R.P.; Saini, S.; Huh, Y.S.; Brambilla, E.; Ionescu, A.C. Ranking analysis of tribological, mechanical, and thermal properties of nano hydroxyapatite filled dental restorative composite materials using the r-method. Polym. Adv. Technol. 2024, 35, e70010. [Google Scholar] [CrossRef]
- Patras, M.; Naka, O.; Doukoudakis, S.; Pissiotis, A. Management of provisional restorations deficiencies: A literature review. J. Esthet. Restor. Dent. 2012, 24, 26–38. [Google Scholar] [CrossRef]
- Astudillo-Rubio, D.; Delgado-Gaete, A.; Bellot-Arcís, C.; Montiel-Company, J.M.; Pascual-Moscardó, A.; Almerich-Silla, J.M. Mechanical properties of provisional dental materials: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0193162. [Google Scholar]
- Dede, D.Ö.; Külünk, Ş.; Şahin, O. Diş hekimliğinde kullanılan termoplastik rezinler. Ondokuz Mayıs Univ. Diş Hekim. Fak. Derg. 2015, 16, 33–43. [Google Scholar]
- Ceylan, G.; Emik, S.; Yalcinyuva, T.; Sunbuloğlu, E.; Bozdag, E.; Unalan, F. The Effects of Cross-Linking Agents on the Mechanical Properties of Poly (Methyl Methacrylate) Resin. Polymers 2023, 15, 2387. [Google Scholar] [CrossRef]
- Gegauff, A.G.; Wilkerson, J.J. Fracture toughness testing of visible light- and chemical-initiated provisional restoration resins. Int. J. Prosthodont. 1995, 8, 62–68. [Google Scholar]
- Alhavaz, A.; Rezaei Dastjerdi, M.; Ghasemi, A.; Ghasemi, A.; Alizadeh Sahraei, A. Effect of untreated zirconium oxide nanofiller on the flexural strength and surface hardness of autopolymerized interim fixed restoration resins. J. Esthet. Restor. Dent. 2017, 29, 264–269. [Google Scholar] [CrossRef]
- Abdulrazzaq Naji, S.; Jafarzadeh Kashi, T.; Behroozibakhsh, M.; Hajizamani, H.; Habibzadeh, S. Recent advances and future perspectives for reinforcement of poly(methyl methacrylate) denture base materials: A literature review. J. Dent. Biomater. 2018, 5, 490–502. [Google Scholar]
- Alamgir, M.; Tiwari, S.K.; Mallick, A.; Nayak, G.C. Graphene oxide and TiO2 based PMMA nanocomposites for dental applications: A comprehensive study of the mechanical properties. IOP Conf. Ser. Mater. Sci. Eng. 2018, 377, 012082. [Google Scholar] [CrossRef]
- Yanıkoğlu, N.; Alawawda, O. Improving PMMA in prosthodontics: A literature review. Dicle Dent. J. 2025, 26, 5–10. [Google Scholar]
- Wang, T.; Tsoi, J.K.; Matinlinna, J.P. A novel zirconia fibre-reinforced resin composite for dental use. J. Mech. Behav. Biomed. Mater. 2016, 53, 151–160. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Li, M.; Liu, Q.; Jia, Z.J.; Xu, X.C.; Cheng, Y.; Zheng, Y.F. Electrophoretic deposition of graphene oxide reinforced chitosan-hydroxyapatite nanocomposite coatings on Ti substrate. J. Mater. Sci. Mater. Med. 2016, 27, 48. [Google Scholar] [CrossRef]
- De Angelis, F.; Vadini, M.; Buonvivere, M.; Valerio, A.; Di Cosola, M.; Piattelli, A.; Biferi, V.; D’Arcangelo, C. In vitro mechanical properties of a novel graphene-reinforced PMMA-based dental restorative material. Polymers 2023, 15, 622. [Google Scholar] [CrossRef]
- Sahm, B.D.; Teixeira, A.B.V.; Dos Reis, A.C. Graphene loaded into dental polymers as reinforcement of mechanical properties: A systematic review. Jpn. Dent. Sci. Rev. 2023, 59, 160–166. [Google Scholar] [CrossRef]
- Mutluay Ünal, S.A. Comparison of the effect of different forms of Graphene and Polyvinylpyrrolidone on physically strengthening PMMA. Int. Dent. Res. 2022, 12, 62–69. [Google Scholar] [CrossRef]
- Tiyek, İ.; Dönmez, U.; Yıldırım, B.; Alma, M.H.; Ersoy, M.S.; Karataş, Ş.; Yazıcı, M. Kimyasal yöntem ile indirgenmiş grafen oksit sentezi ve karakterizasyonu. Sakarya. Univ. J. Sci. 2016, 20, 349–357. [Google Scholar] [CrossRef]
- Bedeloğlu, A.; Taş, M. Grafen ve grafen üretim yöntemleri. AKU J. Sci. Eng. 2016, 16, 544–554. [Google Scholar] [CrossRef]
- ISO 10477; Dentistry-Polymer-Based Crown and Bridge Materials. International Organization for Standardization (ISO): Geneva, Switzerland, 2004.
- Abdel-Karima, U.M.; Kenawy, S.E. Synthesis of zirconia, organic and hybrid nanofibers for reinforcement of polymethyl methacrylate denture base: Evaluation of flexural strength and modulus, fracture toughness and impact strength. Tanta Dent. J. 2019, 16, 12–20. [Google Scholar] [CrossRef]
- Zidan, S.; Silikas, N.; Alhotan, A.; Haider, J.; Yates, J. Investigating the mechanical properties of ZrO2-impregnated PMMA nanocomposite for denture-based applications. Materials 2019, 12, 1344. [Google Scholar] [CrossRef]
- ISO 4287; Geometrical Product Specifications (GPS). Surface texture: Profile Method-Terms, Definitions and Surface Texture Parameters. International Organization for Standardization (ISO): Geneva, Switzerland, 1997.
- Perchyonok, V.T.; Souza, J.; Küll, M.F.; Suzuki, T.Y.U.; Maluly-Proni, A.T.; dos Santos, P.H. Color stability and surface roughness of chitosan- and nanodiamond-modified bisacrylic resin. Braz. Oral. Res. 2019, 33, e024. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Zhu, B.; Lin, K.; Chang, J. Mechanical and thermal properties of denture PMMA reinforced with silanized aluminum borate whiskers. Dent. Mater. J. 2012, 31, 903–908. [Google Scholar] [CrossRef]
- Abdulrazzaq Naji, S.; Behroozibakhsh, M.; Jafarzadeh Kashi, T.S.; Eslami, H.; Masaeli, R.; Mahgoli, H.; Tahriri, M.; Ghavvami Lahiji, M.; Rakhshan, V. Effects of incorporation of 2.5 and 5 wt% TiO2 nanotubes on fracture toughness, flexural strength, and mikrohardness of denture base poly methly methacrylate (PMMA). J. Adv. Prosthodont. 2018, 10, 113–121. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, S.; Agnıhotry, S.A. Synthesis and characterization of in situ prepared poly (methyl methacrylate) nanocomposites. Mater. Sci. 2007, 30, 31–35. [Google Scholar] [CrossRef]
- Hashem, M.; Rez, M.F.A.; Fouad, H.; Elsarnagawy, T.D.; Elsharawy, M.A.; Umar, A.; Assery, M.; Ansari, S.G. Influence of titanium oxide nanoparticles on the physical and thermomechanical behavior of poly methyl methacrylate (PMMA). Sci. Adv. Mater. 2017, 9, 938–939. [Google Scholar] [CrossRef]
- Devikala, S.; Kamaraj, P.; Arthanareeswai, M. Conductivity and dielectric studies of PMMA composites. Chem. Sci. Trans. 2013, 2, 129–134. [Google Scholar]
- Kim, D.H.; Park, M.S.; Cho, H.H.; Park, J.T.; Kim, J.H. Synthesis of organized mesoporous metal oxide films templated by amphiphilic PVA-PMMA comb copolymer. RSC Adv. 2016, 6, 67849–67857. [Google Scholar] [CrossRef]
- Haldorai, Y.; Zong, T.; Shim, J.J. Core-shell ZrO2/PMMA composites via dispersion polymerization in supercritical fluid: Synthesis, characterization and mechanism. J. Appl. 2011, 123, 1176–1183. [Google Scholar] [CrossRef]
- Motaung, T.E.; Luyt, A.S.; Saladino, M.L.; Martino, D.C.; Caponetti, E. Morphology, mechanical properties and thermal degradation kinetics of PMMA-zirconia nanocomposites prepared by melt compounding. Express Polym. Lett. 2012, 6, 871–881. [Google Scholar] [CrossRef]
- Zhu, G.; Ding, Y.; Zhao, D.; Jiang, Y.; Zheng, J. Effect of flake size on thermal properties of graphene oxide/poly(methyl methacrylate) composites prepared via in situ polymerization. J. Appl. 2018, 135, 46290. [Google Scholar] [CrossRef]
- Heo, S.; Cho, S.Y.; Kim, D.H.; Choi, Y.; Park, H.H.; Jin, H.J. Improved thermal properties of graphene oxide-incorporated poly(methyl methacrylate) microspheres. J. Nanosci. Nanotechnol. 2012, 12, 5990–5994. [Google Scholar] [CrossRef]
- Dukali, R.M.; Radovic, I.M.; Stojanovic, D.B.; Sevic, D.M.; Radojevic, V.J.; Jocic, D.M.; Aleksic, R.R. Electrospinning of laser dye Rhodamine B-doped poly(methyl methacrylate) nanofibers. J. Serb. Chem. Soc. 2014, 79, 867–880. [Google Scholar] [CrossRef]
- Shen, J.; Li, T.; Long, Y.; Shi, M.; Li, N.; Ye, M. One-step solid state preparation of reduced graphene oxide. Carbon 2012, 50, 2134–2140. [Google Scholar] [CrossRef]
- Wan, X.; Lu, H.; Kang, J.; Li, S.; Yue, Y. Preparation of graphene-glass fiber-resin composites and its electromagnetic shielding performance. Compos. Interfaces 2018, 25, 883–900. [Google Scholar] [CrossRef]
- Yazıcı, M.; Tiyek, İ.; Ersoy, M.S.; Alma, M.H.; Dönmez, U.; Yıldırım, B.; Salan, T.; Karataş, Ş.; Uruş, S.; Karteri, İ.; et al. Modifiye hummers yöntemiyle grafen oksit (GO) sentezi ve karakterizasyonu. GU J. Sci. Part C 2016, 4, 43–50. [Google Scholar]
- Pramanik, N.; De, J.; Basu, R.K.; Rath, T.; Kundu, P.P. Fabrication of magnetite nanoparticle doped reduced graphene oxide grafted polyhydroxyalkanoate nanocomposites for tissue engineering application. RSC Advance. 2016, 6, 46116–46133. [Google Scholar] [CrossRef]
- Mindivan, F. The Synthesis and characterization of graphene oxide (GO) and reduced graphene oxide (RGO). Mach. Technol. Mater. 2016, 6, 32–35. [Google Scholar]
- Abdali, H.; Ajji, A. Preparation of electrospun nanocomposite nanofibers of polyaniline/poly(methyl methacrylate) with amino-functionalized graphene. Polymers 2017, 9, 453. [Google Scholar] [CrossRef]
- Mhamane, D.; Unni, S.M.; Suryawanshi, A.; Game, O.; Rode, C.; Hannoyer, B.; Kurungot, S.; Ogale, S. Trigol based reduction of graphite oxide to graphene with enhanced charge storage activity. J. Mater. Chem. 2011, 22, 11140–11145. [Google Scholar] [CrossRef]
- Assal, M.E.; Shaik, M.R.; Kuniyil, M.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Khan, S.M.A.; Tremel, W.; Tahir, M.N.; Adil, S.F. A highly reduced graphene oxide/ZrOx–MnCO3 or –Mn2O3 nanocomposite as an efficient catalyst for selective aerial oxidation. RSC Adv. 2017, 7, 55336–55349. [Google Scholar] [CrossRef]
- Köroğlu, A.; Sahin, O.; Dede, D.Ö.; Yilmaz, B. Effect of different surface treatment methods on the surface roughness and color stability of interim prosthodontic materials. J. Prosthet. Dent. 2016, 115, 447–455. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Wang, Y.; Cui, W. Fabrication of gelatin-based electrospun composite fibers for anti-bacterial properties and protein adsorption. Mar. Drugs 2016, 14, 192. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Jiang, Z.; Gu, J.; Zhang, D. Thermal and mechanical performance of electrospun chitosan/poly(vinyl alcohol) nanofibers with graphene oxide. Adv. Compos. Hybrd Mater. 2018, 1, 722–730. [Google Scholar] [CrossRef]
- Elmadani, A.A.; Radović, I.; Tomić, N.Z.; Petrović, M.; Stojanović, D.B.; Heinemann, R.J.; Radojević, V. Hybrid denture acrylic composites with nanozirconia and electrospun polystyrene fibers. PLoS ONE 2019, 14, e0226528. [Google Scholar] [CrossRef]
- Abdel-Karim, U.M.; El-Safty, S.M.; Kenawy, E.R. Surface roughness, hardness, color stability, water sorption and water solubility of PMMA denture base material reinforced with synthesized inorganic, organic, and hybrid nanofibers. Egypt. Dent. J. 2018, 64, 3593–3608. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chong, M.H.; Park, M.; Kim, H.Y.; Park, S.J. Effect of chemically reduced graphene oxide on epoxy nanocomposites for flexural behaviors. Carbon Lett. 2014, 15, 67–70. [Google Scholar] [CrossRef]
- Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The influence of graphene in improvement of physico-mechanical properties in PMMA denture base resins. Materials 2019, 12, 2335. [Google Scholar] [CrossRef]
- Kausar, A. Poly(methyl methacrylate) nanocomposite reinforced with graphene, graphene oxide, and graphite: A review. Polym.-Plast. Technol. Mater. 2019, 58, 821–842. [Google Scholar] [CrossRef]
- Gonçalves, G.; Portolés, M.T.; Ramírez-Santillán, C.; Vallet-Regí, M.; Serro, A.P.; Grácio, J.; Marques, P.A. Evaluation of the in vitro biocompatibility of PMMA/high-load HA/carbon nanostructures bone cement formulations. J. Mater. Sci. Mater. Med. 2013, 24, 2787–2796. [Google Scholar] [CrossRef]
- Joshi, G.M.; Deshmukh, K. Optimized quality factor of graphene oxide-reinforced PVC nanocomposite. J. Electron. Mater. 2014, 43, 1161–1165. [Google Scholar] [CrossRef]
- Mindivan, F. Grafen oksit (GO) ve indirgenmiş grafen oksit (RGO) dolgulu PVC kompozitlerin mekanik özelliklerinin karşılaştırılması. Pamukkale Univ. Muh. Bilim. Derg. 2019, 25, 43–48. [Google Scholar]
- Benevides, A.P.; Campos, A.R.; Vieira, L.C.; Perez, C.R.; Cesar, D.V. Reduced graphene oxide-zinc oxide flower-like composite for glass-ionomer materials reinforcement. Mater. Res. 2020, 23, e20190580. [Google Scholar] [CrossRef]
- Li, B.; Yuan, H.; Zhang, Y. Transparent PMMA-based nanocomposite using electrospun graphene-incorporated PA-6 nanofibers as the reinforcement. Compos. Sci. Technol. 2013, 89, 134–141. [Google Scholar] [CrossRef]
- Lee, J.H.; Jo, J.K.; Kim, D.A.; Patel, K.D.; Kim, H.W.; Lee, H.H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef]
- Aktaş, G.; Göncü Başaran, E.; Güncü, M.B.; Lassilla, L.; Vallittu, P. Fiberle güçlendirme kompozit rezinlerin eğilme direncine etki eder mi? Ataturk Üniv. Diş Hekim. Fak. Derg. 2016, 26, 473–477. [Google Scholar]
- Karlina, E.; Ramadhani, E.; Takarini, V.; Djustiana, N. Diametral tensile strength on restorative dental composite: Contrasting results from the addition of PMMA fiber filler. AIP Conf. Proc. 2019, 2219, 080008. [Google Scholar]
- Sava, S.; Moldovan, M.; Sarosi, C.; Mesaros, A.; Dudea, D.; Alb, C. Effects of graphene addition on the mechanical properties of composites for dental restoration. Mater. Plast. 2015, 52, 90–92. [Google Scholar]
- Vallés, C.; Kinloch, I.A.; Young, R.J.; Wilson, N.R.; Rourke, J.P. Graphene oxide and base-washed graphene oxide as reinforcements in PMMA nanocomposites. Compos. Sci. Technol. 2013, 88, 158–164. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Bacali, C.; Baldea, I.; Moldovan, M.; Carpa, R.; Olteanu, D.E.; Filip, G.A.; Nastase, V.; Lascu, L.; Badea, M.; Constantiniuc, M.; et al. Flexural strength, biocompatibility, and antimicrobial activity of a polymethyl methacrylate denture resin enhanced with graphene and silver nanoparticles. Clin. Oral Investig. 2020, 24, 2713–2725. [Google Scholar] [CrossRef]
- Valenti, C.; Billi, M.; Pancrazi, G.L.; Calabria, E.; Armogida, N.G.; Tortora, G.; Pagano, S.; Barnaba, P.; Marinucci, L. Biological Effects of Cannabidiol on Human Cancer Cells: Systematic Review of the Literature. Pharmacol. Res. 2022, 181, 106267. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of graphene oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef]

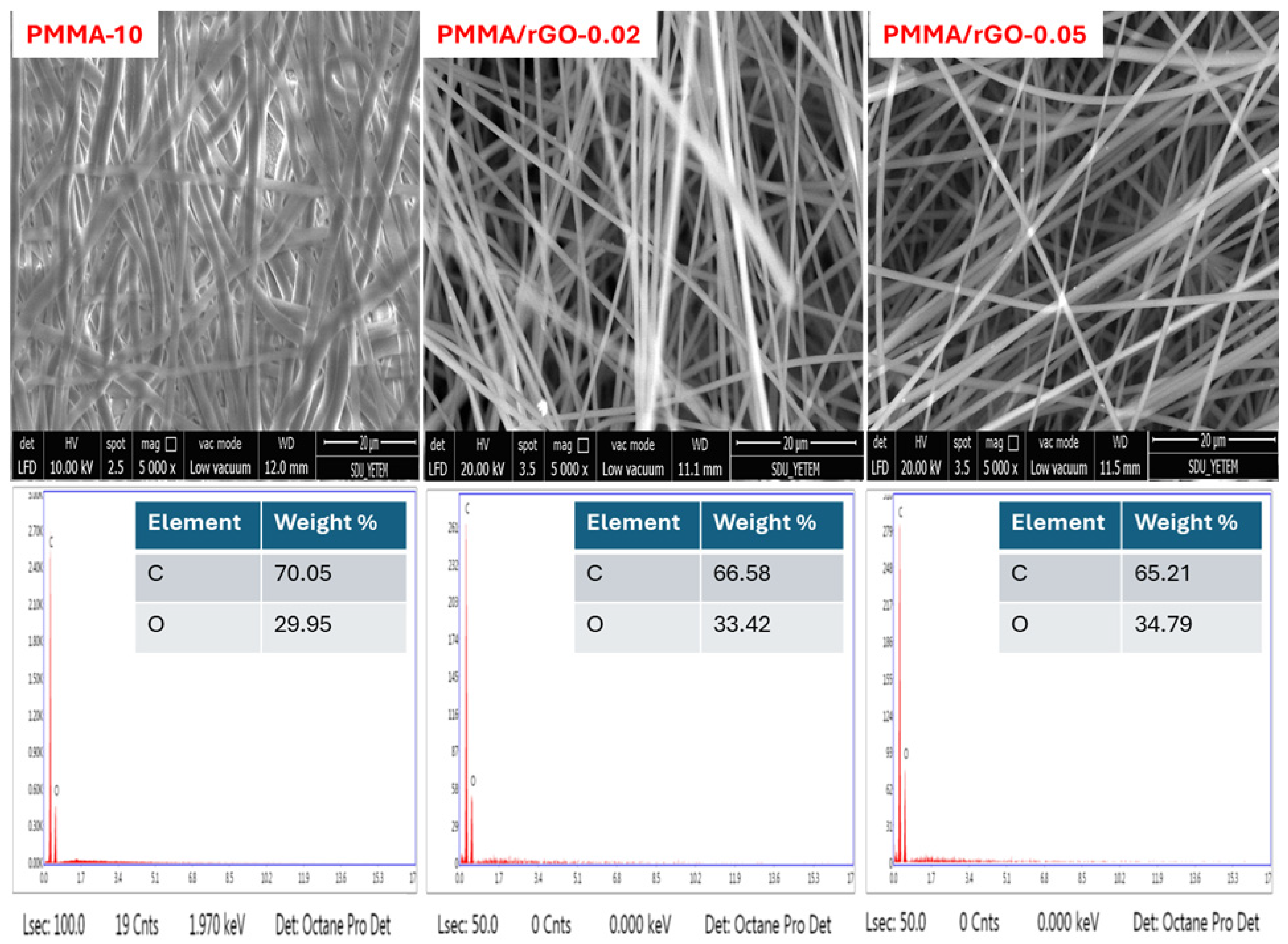

| Sample Code | Needle Tip–Collector Distance (cm) | Solution Flow Rate (µL/h) | Applied Voltage (kV) |
|---|---|---|---|
| PMMA-10 | 15 | 15 | 12 |
| PMMA/rGO-0.02 | 10 | 25 | 12 |
| PMMA/rGO-0.05 | 10 | 25 | 12 |
| Group (n = 15) | Type of Fiber in the Specimens | The Amount of Fiber in the Specimens | PMMA Powder (g) | MMA Monomer (g) | ||
|---|---|---|---|---|---|---|
| (wt%) | (g) | |||||
| Control | A | - | - | - | 1.200 | 0.600 |
| B | B1 | PMMA/rGO-0.02 | 1.0 | 0.012 | 1.188 | 0.600 |
| B2 | 2.5 | 0.030 | 1.170 | 0.600 | ||
| B3 | 5.0 | 0.060 | 1.140 | 0.600 | ||
| C | C1 | PMMA/rGO-0.05 | 1.0 | 0.012 | 1.188 | 0.600 |
| C2 | 2.5 | 0.030 | 1.170 | 0.600 | ||
| C3 | 5.0 | 0.060 | 1.140 | 0.600 | ||
| Materials | Ti (°C) | Tdmax (°C) | Tf (°C) | Mass Remaining at 600 °C (%) |
|---|---|---|---|---|
| rGO | - | - | - | 77 |
| PMMA-10 | 172 | 353 | 537 | 0 |
| PMMA/rGO-0.02 | 185 | 363 | 498 | 0 |
| PMMA/rGO-0.05 | 201 | 359 | 499 | 0.3 |
| Group (n = 15) | Flexural Strength and IQR (MPa) | Elastic Modulus and IQR (MPA) | Surface Roughness and IQR (µm) | Vickers Microhardness and SD (kgf/mm2) |
|---|---|---|---|---|
| A | 65.86 (8.26) A | 2653.31 (407.43) A | 0.10 (0.020) A | 18.19 (0.59) A |
| B1 | 82.14 (23.34) B | 3162.98 (857.52) B | 0.09 (0.023) A | 18.21 (1.22) A |
| B2 | 78.79 (15.35) B | 2899.10 (484.46) A | 0.09 (0.023) A | 18.92 (0.97) A |
| B3 | 78.09 (15.16) A | 2797.17 (623.02) A | 0.12 (0.046) A | 18.77 (1.14) A |
| C1 | 77.43 (19.15) B | 2843.14 (680.14) A | 0.11 (0.023) A | 18.14 (1.27) A |
| C2 | 72.13 (14.71) A | 2776.41 (458.89) A | 0.10 (0.026) A | 17.52 (0.69) A |
| C3 | 74.01 (16.04) A | 2792.98 (368.42) A | 0.11 (0.039) A | 17.84 (0.82) A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmas Alsini, T.G.; Kurkcuoglu, I.; Nohut Maslakci, N.; Uygun Oksuz, A. The Effect of Electrospun PMMA/rGO Fiber Addition on the Improvement of the Physical and Mechanical Properties of PMMA Resin. Prosthesis 2025, 7, 79. https://doi.org/10.3390/prosthesis7040079
Elmas Alsini TG, Kurkcuoglu I, Nohut Maslakci N, Uygun Oksuz A. The Effect of Electrospun PMMA/rGO Fiber Addition on the Improvement of the Physical and Mechanical Properties of PMMA Resin. Prosthesis. 2025; 7(4):79. https://doi.org/10.3390/prosthesis7040079
Chicago/Turabian StyleElmas Alsini, Tugce Gul, Isin Kurkcuoglu, Neslihan Nohut Maslakci, and Aysegul Uygun Oksuz. 2025. "The Effect of Electrospun PMMA/rGO Fiber Addition on the Improvement of the Physical and Mechanical Properties of PMMA Resin" Prosthesis 7, no. 4: 79. https://doi.org/10.3390/prosthesis7040079
APA StyleElmas Alsini, T. G., Kurkcuoglu, I., Nohut Maslakci, N., & Uygun Oksuz, A. (2025). The Effect of Electrospun PMMA/rGO Fiber Addition on the Improvement of the Physical and Mechanical Properties of PMMA Resin. Prosthesis, 7(4), 79. https://doi.org/10.3390/prosthesis7040079






