Accuracy of New-Generation Intraoral Scanners in Digitizing All-on-Four Implant Models with Varying Posterior Implant Angulations: An In Vitro Trueness and Precision Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Sample Size Calculation
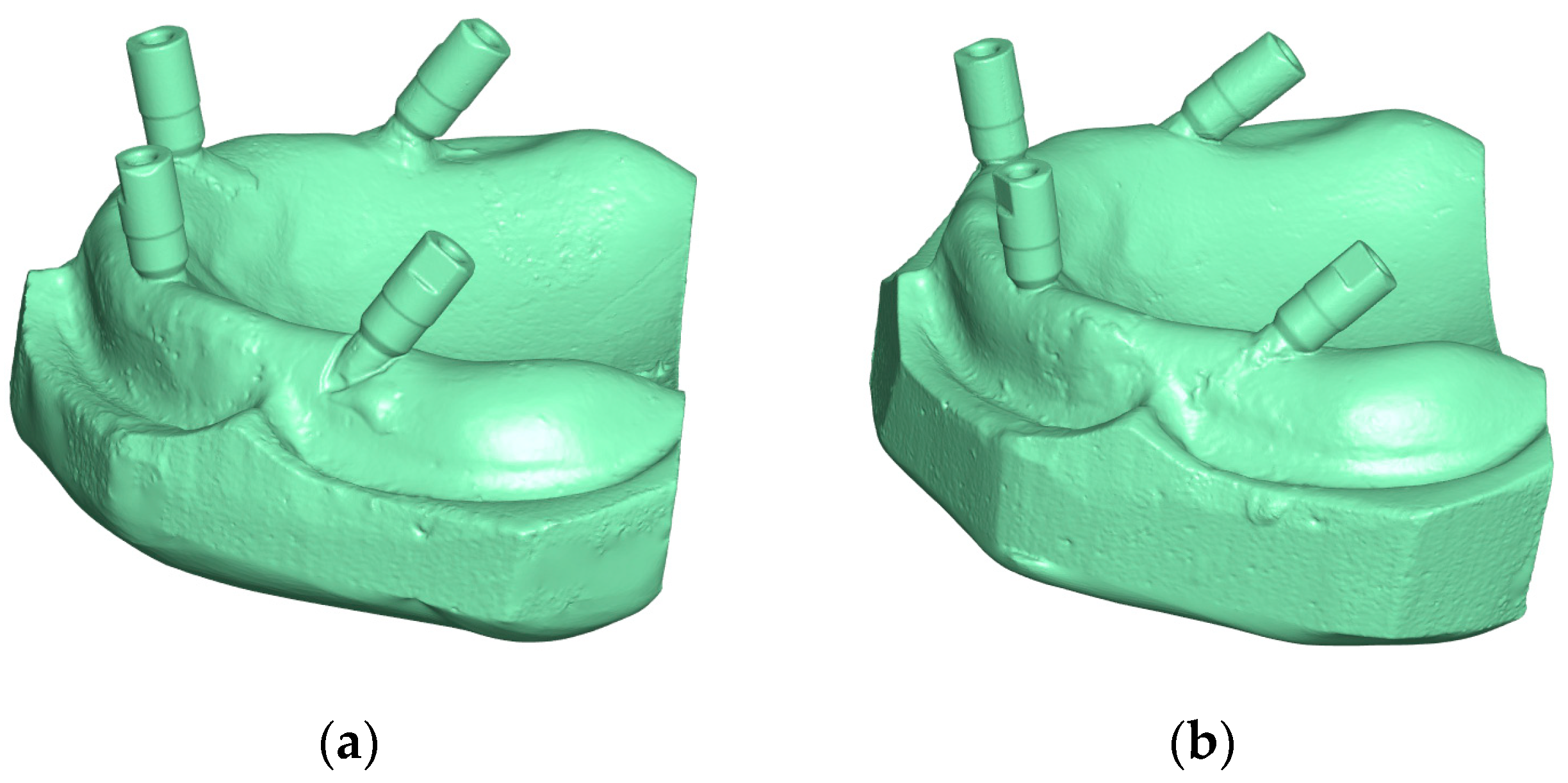
2.3. Fabrication of All-on-Four Master Models
2.4. Generation of Reference Digital Models
2.5. IOS Scanning Procedures
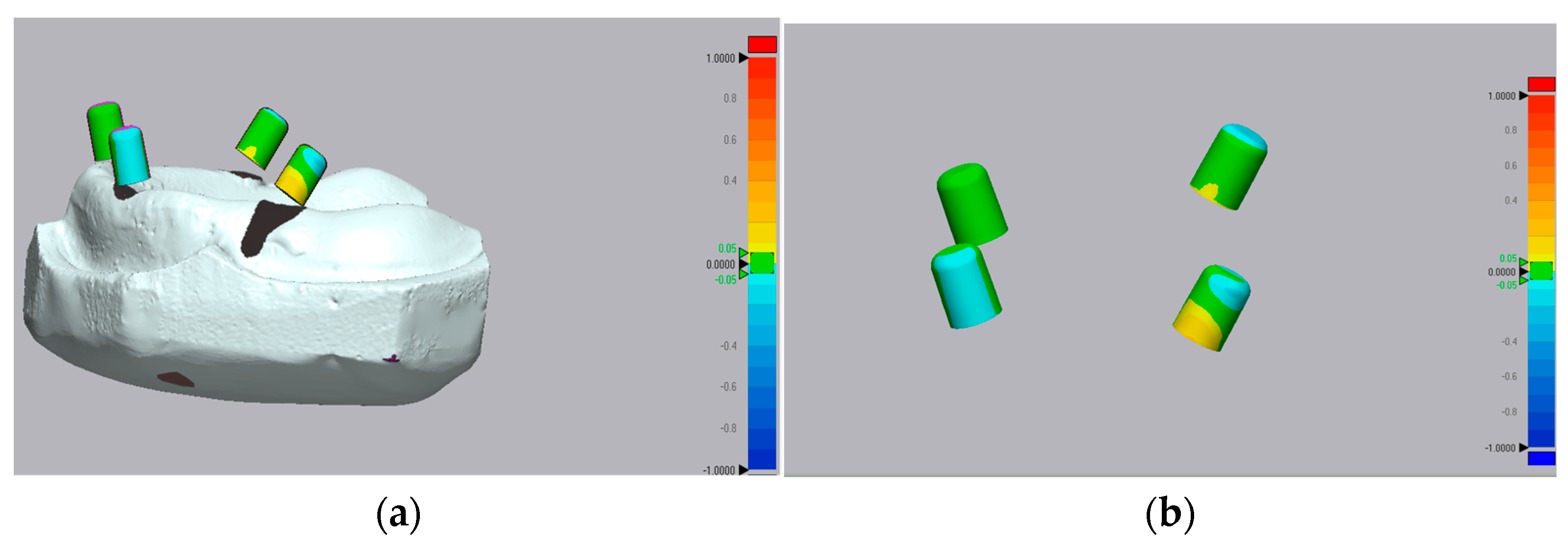
2.6. Accuracy Assessment and 3D Deviation Analysis
2.7. Statistical Analysis
3. Results
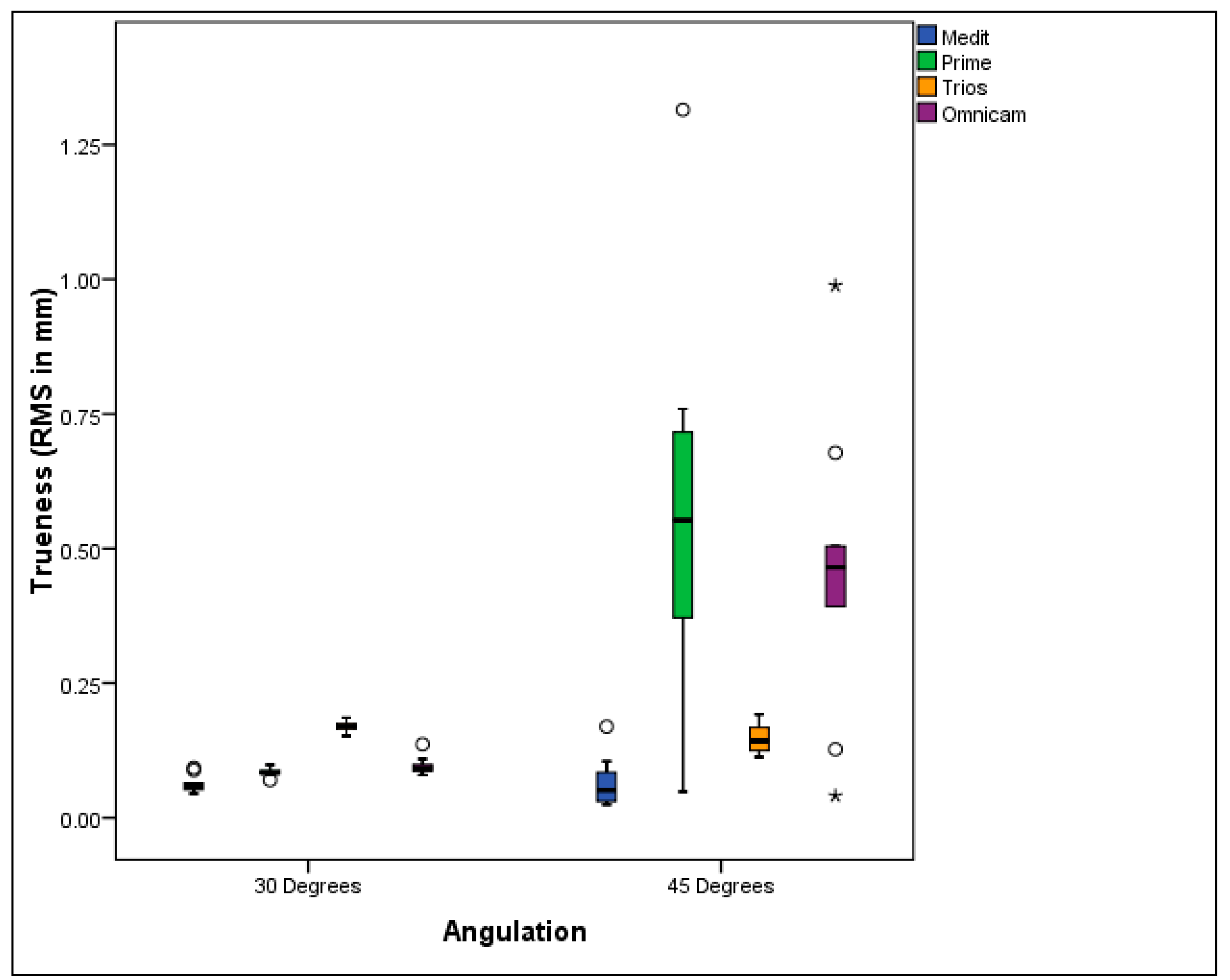
3.1. Trueness Evaluation
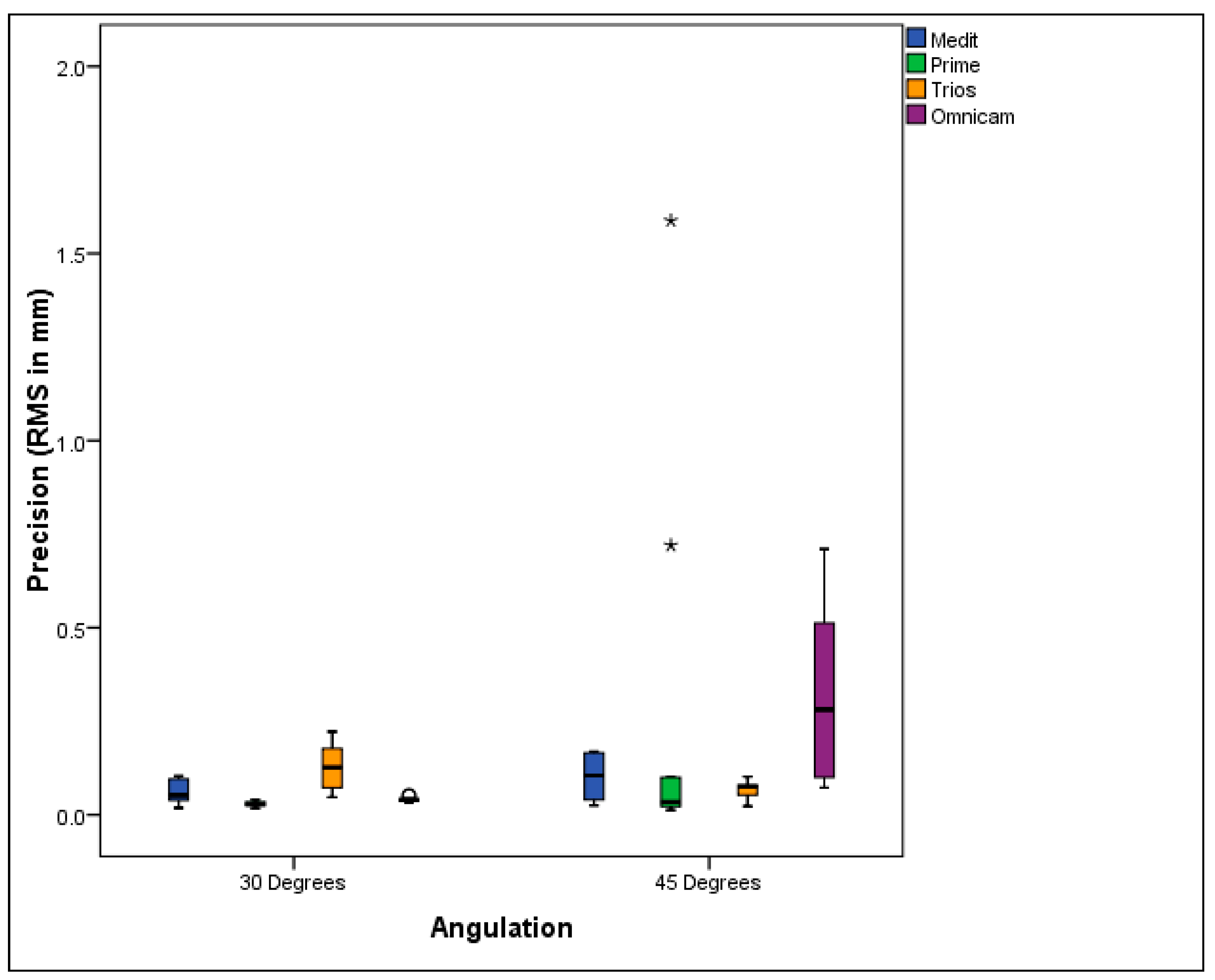
3.2. Precision Evaluation
4. Discussion
5. Conclusions
- The accuracy of some intraoral scanners was affected by the posterior implant angulation in the All-on-Four treatment modality. As the implant angulation increased, the accuracy of the scans decreased. Specifically, Primescan and Omnicam showed lower accuracy as the angulation increased.
- Clinical acceptability was demonstrated for Medit i700, CEREC Primescan, and Omnicam in All-on-Four full-arch digital scans involving posterior implants angled up to 30°, with research indicating these systems maintain accuracy within clinically tolerable thresholds despite angulation-induced challenges. However, for All-on-Four cases with 45-degree posterior implant angulation, the Medit i700 was the only IOS that remained clinically acceptable.
- Medit i700 demonstrated the greatest trueness, while Primescan and Omnicam excelled in precision.
- Advanced accuracy assessment methodologies, including the Hausdorff distance (HD) and dice similarity coefficient (DSC), to provide a more detailed and multidimensional evaluation of intraoral scanner performance.
- In vivo validation to correlate laboratory accuracy with actual clinical outcomes.
- Development of advanced AI-enhanced stitching algorithms to improve scan consistency in long-span edentulous arches.
- Exploration of real-time error detection or compensation features within scanner software to enhance reliability during clinical scanning.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maló, P.; De Araújo Nobre, M.; Lopes, A.; Ferro, A.; Botto, J. The All-on-4 Treatment Concept for the Rehabilitation of the Completely Edentulous Mandible: A Longitudinal Study with 10 to 18 Years of Follow-up. Clin. Implant Dent. Relat. Res. 2019, 21, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Taymour, N.; Fahmy, A.E.; Gepreel, M.A.H.; Kandil, S.; El-Fattah, A.A. Improved Mechanical Properties and Bioactivity of Silicate Based Bioceramics Reinforced Poly(Ether-Ether-Ketone) Nanocomposites for Prosthetic Dental Implantology. Polymers 2022, 14, 1632. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, S.B.M.; Bahat, O.; Reynolds, M.A.; Strub, J.R. The All-on-Four Treatment Concept: A Systematic Review. Clin. Implant Dent. Relat. Res. 2014, 16, 836–855. [Google Scholar] [CrossRef] [PubMed]
- Peñarrocha-Diago, M.A.; Maestre-Ferrín, L.; Demarchi, C.L.; Peñarrocha-Oltra, D.; Peñarrocha-Diago, M. Immediate Versus Nonimmediate Placement of Implants for Full-Arch Fixed Restorations: A Preliminary Study. J. Oral Maxillofac. Surg. 2011, 69, 154–159. [Google Scholar] [CrossRef]
- Korsch, M.; Walther, W.; Hannig, M.; Bartols, A. Evaluation of the Surgical and Prosthetic Success of All-on-4 Restorations: A Retrospective Cohort Study of Provisional vs. Definitive Immediate Restorations. Int. J. Implant Dent. 2021, 7, 48. [Google Scholar] [CrossRef]
- Jemt, T.; Hjalmarsson, L. In Vitro Measurements of Precision of Fit of Implant-Supported Frameworks. A Comparison between “Virtual” and “Physical” Assessments of Fit Using Two Different Techniques of Measurements. Clin. Implant Dent. Relat. Res. 2012, 14, e175–e182. [Google Scholar] [CrossRef]
- Hjalmarsson, L.; Örtorp, A.; Smedberg, J.; Jemt, T. Precision of Fit to Implants: A Comparison of CrescoTM and Procera® Implant Bridge Frameworks. Clin. Implant Dent. Relat. Res. 2010, 12, 271–280. [Google Scholar] [CrossRef]
- Di Fiore, A.; Meneghello, R.; Graiff, L.; Savio, G.; Vigolo, P.; Monaco, C.; Stellini, E. Full Arch Digital Scanning Systems Performances for Implant-Supported Fixed Dental Prostheses: A Comparative Study of 8 Intraoral Scanners. J. Prosthodont. Res. 2019, 63, 396–403. [Google Scholar] [CrossRef]
- Alikhasi, M.; Siadat, H.; Nasirpour, A.; Hasanzade, M. Three-Dimensional Accuracy of Digital Impression versus Conventional Method: Effect of Implant Angulation and Connection Type. Int. J. Dent. 2018, 2018, 3761750. [Google Scholar] [CrossRef]
- Mpikos, P.; Kafantaris, N.; Tortopidis, D.; Galanis, C.; Kaisarlis, G.; Koidis, P. The Effect of Impression Technique and Implant Angulation on the Impression Accuracy of External- and Internal-Connection Implants. Int. J. Oral Maxillofac. Implant. 2012, 27, 1422–1428. [Google Scholar]
- Papaspyridakos, P.; Gallucci, G.O.; Chen, C.; Hanssen, S.; Naert, I.; Vandenberghe, B. Digital versus Conventional Implant Impressions for Edentulous Patients: Accuracy Outcomes. Clin. Oral Implant. Res. 2016, 27, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Yasar, M.N.; Cetinsahin, C.; Bayar, O.; Ozer, H.Y. Implant Impression Techniques Using Different Materials and Methods: A Review. J. Clin. Diagn. Res. 2022, 16, ZE12–ZE17. [Google Scholar] [CrossRef]
- Ender, A.; Attin, T.; Mehl, A. In Vivo Precision of Conventional and Digital Methods of Obtaining Complete-Arch Dental Impressions. J. Prosthet. Dent. 2016, 115, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Awad, E.M.; ElSheikh, M.M.; El-Segai, A.A.E.M. Effect of Soft Tissue Thickness on Accuracy of Conventional and Digital Implant Impression Techniques. BMC Oral Health 2024, 24, 1318. [Google Scholar] [CrossRef]
- Kim, R.J.Y.; Benic, G.I.; Park, J.-M. Trueness of Ten Intraoral Scanners in Determining the Positions of Simulated Implant Scan Bodies. Sci. Rep. 2021, 11, 2606. [Google Scholar] [CrossRef]
- Ahlholm, P.; Sipilä, K.; Vallittu, P.; Jakonen, M.; Kotiranta, U. Digital Versus Conventional Impressions in Fixed Prosthodontics: A Review. J. Prosthodont. 2018, 27, 35–41. [Google Scholar] [CrossRef]
- Mizumoto, R.M.; Alp, G.; Özcan, M.; Yilmaz, B. The Effect of Scanning the Palate and Scan Body Position on the Accuracy of Complete-arch Implant Scans. Clin. Implant Dent. Relat. Res. 2019, 21, 987–994. [Google Scholar] [CrossRef]
- Wulfman, C.; Naveau, A.; Rignon-Bret, C. Digital Scanning for Complete-Arch Implant-Supported Restorations: A Systematic Review. J. Prosthet. Dent. 2020, 124, 161–167. [Google Scholar] [CrossRef]
- Flügge, T.; Att, W.; Metzger, M.; Nelson, K. Precision of Dental Implant Digitization Using Intraoral Scanners. Int. J. Prosthodont. 2016, 29, 277–283. [Google Scholar] [CrossRef]
- Andriessen, F.S.; Rijkens, D.R.; Van Der Meer, W.J.; Wismeijer, D.W. Applicability and Accuracy of an Intraoral Scanner for Scanning Multiple Implants in Edentulous Mandibles: A Pilot Study. J. Prosthet. Dent. 2014, 111, 186–194. [Google Scholar] [CrossRef]
- Iturrate, M.; Eguiraun, H.; Etxaniz, O.; Solaberrieta, E. Accuracy Analysis of Complete-Arch Digital Scans in Edentulous Arches When Using an Auxiliary Geometric Device. J. Prosthet. Dent. 2019, 121, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Suese, K. Progress in Digital Dentistry: The Practical Use of Intraoral Scanners. Dent. Mater. J. 2020, 39, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.; Silva, A.S.; Costa, R.; Barreiros, P.; Mendes, J.; Mendes, J.M. In Vitro Comparison of Three Intraoral Scanners for Implant—Supported Dental Prostheses. Dent. J. 2022, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Arcuri, L.; Pozzi, A.; Lio, F.; Rompen, E.; Zechner, W.; Nardi, A. Influence of Implant Scanbody Material, Position and Operator on the Accuracy of Digital Impression for Complete-Arch: A Randomized in Vitro Trial. J. Prosthodont. Res. 2020, 64, 128–136. [Google Scholar] [CrossRef]
- Amornvit, P.; Rokaya, D.; Sanohkan, S. Comparison of Accuracy of Current Ten Intraoral Scanners. BioMed Res. Int. 2021, 2021, 2673040. [Google Scholar] [CrossRef]
- Mangano, F.G.; Hauschild, U.; Veronesi, G.; Imburgia, M.; Mangano, C.; Admakin, O. Trueness and Precision of 5 Intraoral Scanners in the Impressions of Single and Multiple Implants: A Comparative in Vitro Study. BMC Oral Health 2019, 19, 101. [Google Scholar] [CrossRef]
- Natsubori, R.; Fukazawa, S.; Chiba, T.; Tanabe, N.; Kihara, H.; Kondo, H. In Vitro Comparative Analysis of Scanning Accuracy of Intraoral and Laboratory Scanners in Measuring the Distance between Multiple Implants. Int. J. Implant Dent. 2022, 8, 18. [Google Scholar] [CrossRef]
- Önöral, Ö.; Kurtulmus-Yilmaz, S.; Toksoy, D.; Ozan, O. Effect of Angulation on the 3D Trueness of Conventional and Digital Implant Impressions for Multi-Unit Restorations. J. Adv. Prosthodont. 2023, 15, 290. [Google Scholar] [CrossRef]
- Alikhasi, M.; Siadat, H.; Rahimian, S. The Effect of Implant Angulation on the Transfer Accuracy of External-Connection Implants. Clin. Implant Dent. Relat. Res. 2015, 17, 822–829. [Google Scholar] [CrossRef]
- Martínez-Rus, F.; García, C.; Santamaría, A.; Özcan, M.; Pradíes, G. Accuracy of Definitive Casts Using 4 Implant-Level Impression Techniques in a Scenario of Multi-Implant System with Different Implant Angulations and Subgingival Alignment Levels. Implant Dent. 2013, 22, 268–276. [Google Scholar] [CrossRef]
- Lin, W.-S.; Harris, B.; Elathamna, E.; Abdel-Azim, T.; Morton, D. Effect of Implant Divergence on the Accuracy of Definitive Casts Created from Traditional and Digital Implant-Level Impressions: An In Vitro Comparative Study. Int. J. Oral Maxillofac. Implant. 2015, 30, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Weber, H.P.; Finkelman, M.; El Rafie, K.; Kudara, Y.; Papaspyridakos, P. Digital vs. Conventional Full-arch Implant Impressions: A Comparative Study. Clin. Oral Implant. Res. 2017, 28, 1360–1367. [Google Scholar] [CrossRef]
- Ashraf, Y.; Abo El Fadl, A.; Hamdy, A.; Ebeid, K. Effect of Different Intraoral Scanners and Scanbody Splinting on Accuracy of Scanning Implant-supported Full Arch Fixed Prosthesis. J. Esthet. Restor. Dent. 2023, 35, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Rotar, R.N.; Jivanescu, A.; Ille, C.; Podariu, A.C.; Jumanca, D.E.; Matichescu, A.-M.; Balean, O.; Rusu, L.C. Trueness and Precision of Two Intraoral Scanners: A Comparative In Vitro Study. Scanning 2019, 2019, 1289570. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.M.; Alawaji, S.A.; Yehya, M.R.; Alenezi, A.A. Evaluation of the Accuracy of Different Digital Scanning Systems and Intraoral Scanning Strategies: An in Vitro Study. Saudi J. Oral Sci. 2024, 11, 125–132. [Google Scholar] [CrossRef]
- El-Refay, H.M.; Abdelaziz, M.S.; Cheta, N.M.; Abdallah, M.F. The Accuracy of Digital Impression with Different Intraoral Scanners on Maxillary All on Four Implants: An in Vitro Study. BMC Res. Notes 2025, 18, 186. [Google Scholar] [CrossRef]
- Diker, B.; Tak, Ö. Accuracy of Six Intraoral Scanners for Scanning Complete-Arch and 4-Unit Fixed Partial Dentures: An in Vitro Study. J. Prosthet. Dent. 2022, 128, 187–194. [Google Scholar] [CrossRef]
- Joda, T.; Brägger, U. Patient-centered Outcomes Comparing Digital and Conventional Implant Impression Procedures: A Randomized Crossover Trial. Clin. Oral Implant. Res. 2016, 27, e185–e189. [Google Scholar] [CrossRef]
- Gherlone, E.F.; Ferrini, F.; Crespi, R.; Gastaldi, G.; Capparé, P. Digital Impressions for Fabrication of Definitive “All-on-Four” Restorations. Implant Dent. 2015, 24, 125–129. [Google Scholar] [CrossRef]
- Van Der Meer, W.J.; Andriessen, F.S.; Wismeijer, D.; Ren, Y. Application of Intra-Oral Dental Scanners in the Digital Workflow of Implantology. PLoS ONE 2012, 7, e43312. [Google Scholar] [CrossRef]
- Lyu, M.; Di, P.; Lin, Y.; Jiang, X. Accuracy of Impressions for Multiple Implants: A Comparative Study of Digital and Conventional Techniques. J. Prosthet. Dent. 2022, 128, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Yadav, H.; Pathak, A.; Bhatnagar, A.; Kumar, V. Comparative Evaluation of Biting Force and Chewing Efficiency of All-on-Four Treatment Concept with Other Treatment Modalities in Completely Edentulous Individuals. J. Indian Prosthodont. Soc. 2020, 20, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Pesce, P.; Nicolini, P.; Caponio, V.C.A.; Zecca, P.A.; Canullo, L.; Isola, G.; Baldi, D.; De Angelis, N.; Menini, M. Accuracy of Full-Arch Intraoral Scans Versus Conventional Impression: A Systematic Review with a Meta-Analysis and a Proposal to Standardise the Analysis of the Accuracy. J. Clin. Med. 2024, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Róth, I.; Czigola, A.; Fehér, D.; Vitai, V.; Joós-Kovács, G.L.; Hermann, P.; Borbély, J.; Vecsei, B. Digital Intraoral Scanner Devices: A Validation Study Based on Common Evaluation Criteria. BMC Oral Health 2022, 22, 140. [Google Scholar] [CrossRef]
- Turkyilmaz, I.; Abdullah, J.Y.; Wilkins, G.N.; Bernardi, S.; Varvara, G. Analysis of the Effects of Mesh Reduction of Digital Files on the Surface Area and Volume Accuracy of Complete Dentures Using an Intraoral Scanner. Minerva Dent. Oral Sci. 2025, 74, 12–19. [Google Scholar] [CrossRef]
- Akkal, O.; Korkmaz, I.H.; Bayindir, F. Comparison of 3D Accuracy of Three Different Digital Intraoral Scanners in Full-Arch Implant Impressions. J. Adv. Prosthodont. 2023, 15, 179–188. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, H.; Liu, H.; Li, J.; Wang, H.; Tao, X. Accuracy of Edentulous Full-arch Implant Impression: An in Vitro Comparison between Conventional Impression, Intraoral Scan with and without Splinting, and Photogrammetry. Clin. Oral Implant. Res. 2024, 35, 560–572. [Google Scholar] [CrossRef]
- Jivănescu, A.; Bara, A.; Faur, A.-B.; Rotar, R.N. Is There a Significant Difference in Accuracy of Four Intraoral Scanners for Short-Span Fixed Dental Prosthesis? A Comparative In Vitro Study. Appl. Sci. 2021, 11, 8280. [Google Scholar] [CrossRef]
- Kaya, G.; Bilmenoglu, C. Accuracy of 14 Intraoral Scanners for the All-on-4 Treatment Concept: A Comparative in Vitro Study. J. Adv. Prosthodont. 2022, 14, 388. [Google Scholar] [CrossRef]
- Revell, G.; Simon, B.; Mennito, A.; Evans, Z.P.; Renne, W.; Ludlow, M.; Vág, J. Evaluation of Complete-Arch Implant Scanning with 5 Different Intraoral Scanners in Terms of Trueness and Operator Experience. J. Prosthet. Dent. 2022, 128, 632–638. [Google Scholar] [CrossRef]
- Gimenez-Gonzalez, B.; Hassan, B.; Özcan, M.; Pradíes, G. An In Vitro Study of Factors Influencing the Performance of Digital Intraoral Impressions Operating on Active Wavefront Sampling Technology with Multiple Implants in the Edentulous Maxilla. J. Prosthodont. 2017, 26, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Arcuri, L.; Lio, F.; Papa, A.; Nardi, A.; Barlattani, A. Influence of Implant Scanbody Material and Operator on Scanning Fluency and Polygonal Mesh Numbers of Digital Impression: An in vitro study. J. Biol. Regul. Homeost. Agents 2019, 33, 179–188. [Google Scholar] [PubMed]
- Giménez, B.; Özcan, M.; Martínez-Rus, F.; Pradíes, G. Accuracy of a Digital Impression System Based on Parallel Confocal Laser Technology for Implants with Consideration of Operator Experience and Implant Angulation and Depth. Int. J. Oral Maxillofac. Implant. 2014, 29, 853–862. [Google Scholar] [CrossRef] [PubMed]

| IOS Type | 30° | 45° | p-Value | Effect Size (d) | ||
|---|---|---|---|---|---|---|
| Median (Range) | Mean (SD) | Median (Range) | Mean (SD) | |||
| MED | 0.0575 (0.0448, 0.0927) C | 0.0625 (0.0169) | 0.0514 (0.024, 0.1692) C | 0.0644 (0.0481) | 0.402 | 0.403 |
| PRI | 0.0835 (0.07, 0.0986) B | 0.0843 (0.0079) | 0.5526 (0.0487, 1.3149) A | 0.5327 (0.3901) | 0.047 * | 1.06 |
| TRI | 0.1695 (0.1521, 0.1861) A | 0.1702 (0.0109) | 0.1428 (0.1128, 0.1835) B | 0.1482 (0.0282) | 0.070 | 0.944 |
| OMN | 0.0924 (0.0792, 0.1364) B | 0.0969 (0.0172) | 0.465 (0.0407, 0.9884) A | 0.4582 (0.2784) | 0.007 * | 1.643 |
| p-value | <0.001 * | 0.006 * | ||||
| Effect size (w) | 0.778 | 0.467 | ||||
| IOS Type | 30° | 45° | p-Value | Effect Size (d) | ||
|---|---|---|---|---|---|---|
| Median (Range) | Mean (SD) | Median (Range) | Mean (SD) | |||
| MED | 0.0524 (0.0184, 0.1035) B | 0.0613 (0.0306) | 0.1047 (0.025, 0.1692) | 0.0995 (0.0618) | 0.200 | 0.633 |
| PRI | 0.0279 (0.0178, 0.0393) C | 0.0287 (0.0076) | 0.0346 (0.0129, 1.5877) | 0.2846 (0.5391) | 0.402 | 0.403 |
| TRI | 0.1264 (0.0473, 0.2224) A | 0.1258 (0.0651) | 0.0748 (0.0228, 0.1015) | 0.0664 (0.0238) | 0.112 | 0.808 |
| OMN | 0.04 (0.0315, 0.0525) C | 0.041 (0.0071) | 0.2812 (0.0726, 0.7104) | 0.3122 (0.2472) | <0.001 * | 3.133 |
| p-value | <0.001 * | 0.057 | ||||
| Effect size (w) | 0.793 | 0.279 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taymour, N.; Abdul Hameed, S.M.; AlGhamdi, M.A.; El Sharkawy, Z.R.; Farid, Z.S.; Ahmed, Y. Accuracy of New-Generation Intraoral Scanners in Digitizing All-on-Four Implant Models with Varying Posterior Implant Angulations: An In Vitro Trueness and Precision Evaluation. Prosthesis 2025, 7, 74. https://doi.org/10.3390/prosthesis7040074
Taymour N, Abdul Hameed SM, AlGhamdi MA, El Sharkawy ZR, Farid ZS, Ahmed Y. Accuracy of New-Generation Intraoral Scanners in Digitizing All-on-Four Implant Models with Varying Posterior Implant Angulations: An In Vitro Trueness and Precision Evaluation. Prosthesis. 2025; 7(4):74. https://doi.org/10.3390/prosthesis7040074
Chicago/Turabian StyleTaymour, Noha, Shereen Moselhy Abdul Hameed, Maram A. AlGhamdi, Zainab Refaey El Sharkawy, Zienab S. Farid, and Yousra Ahmed. 2025. "Accuracy of New-Generation Intraoral Scanners in Digitizing All-on-Four Implant Models with Varying Posterior Implant Angulations: An In Vitro Trueness and Precision Evaluation" Prosthesis 7, no. 4: 74. https://doi.org/10.3390/prosthesis7040074
APA StyleTaymour, N., Abdul Hameed, S. M., AlGhamdi, M. A., El Sharkawy, Z. R., Farid, Z. S., & Ahmed, Y. (2025). Accuracy of New-Generation Intraoral Scanners in Digitizing All-on-Four Implant Models with Varying Posterior Implant Angulations: An In Vitro Trueness and Precision Evaluation. Prosthesis, 7(4), 74. https://doi.org/10.3390/prosthesis7040074








