Using Bioimpedance Analysis as a Clinical Predictive Tool for the Assessment of Limb Fluid Volume Fluctuation: An Initial Investigation of Transtibial Prosthesis Users
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Limb Fluid Volume Monitor
2.3. Protocol
2.3.1. Preliminary Session
2.3.2. Pre-Modification Session
2.3.3. Post-Modification Session
2.3.4. Data Analysis
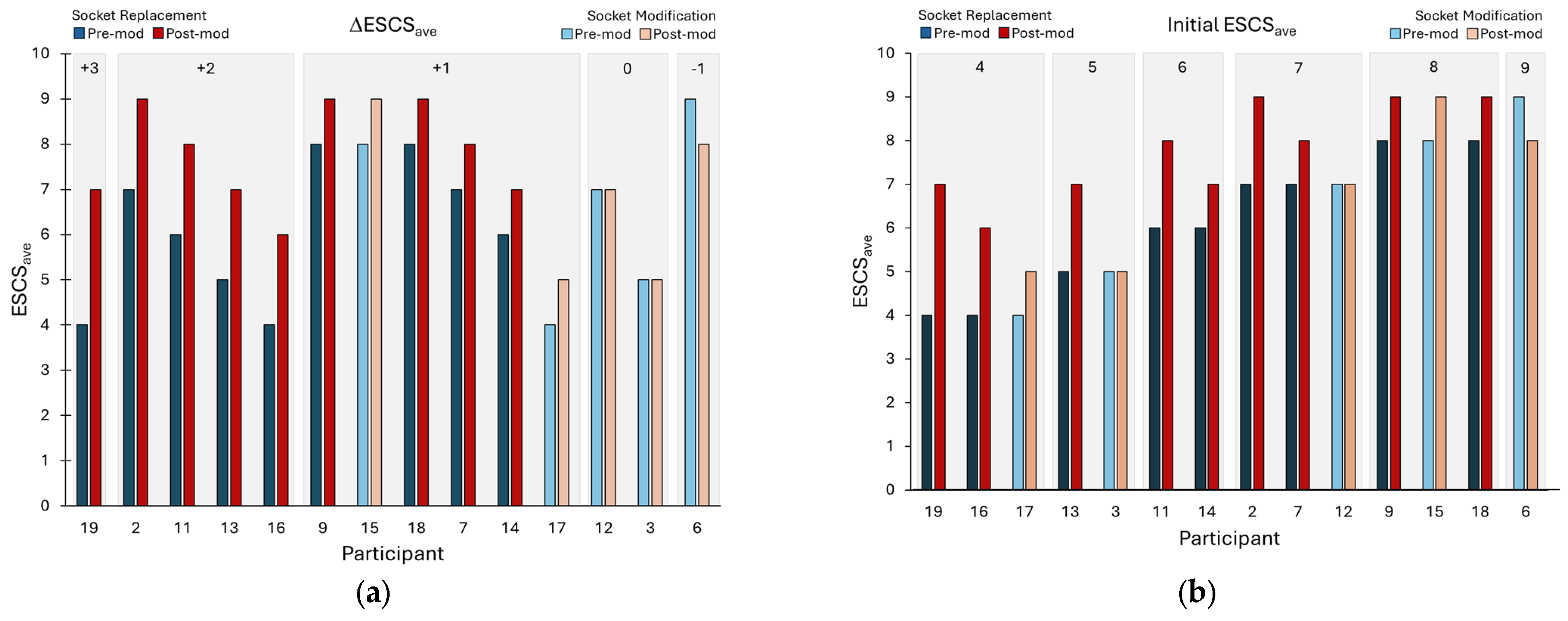
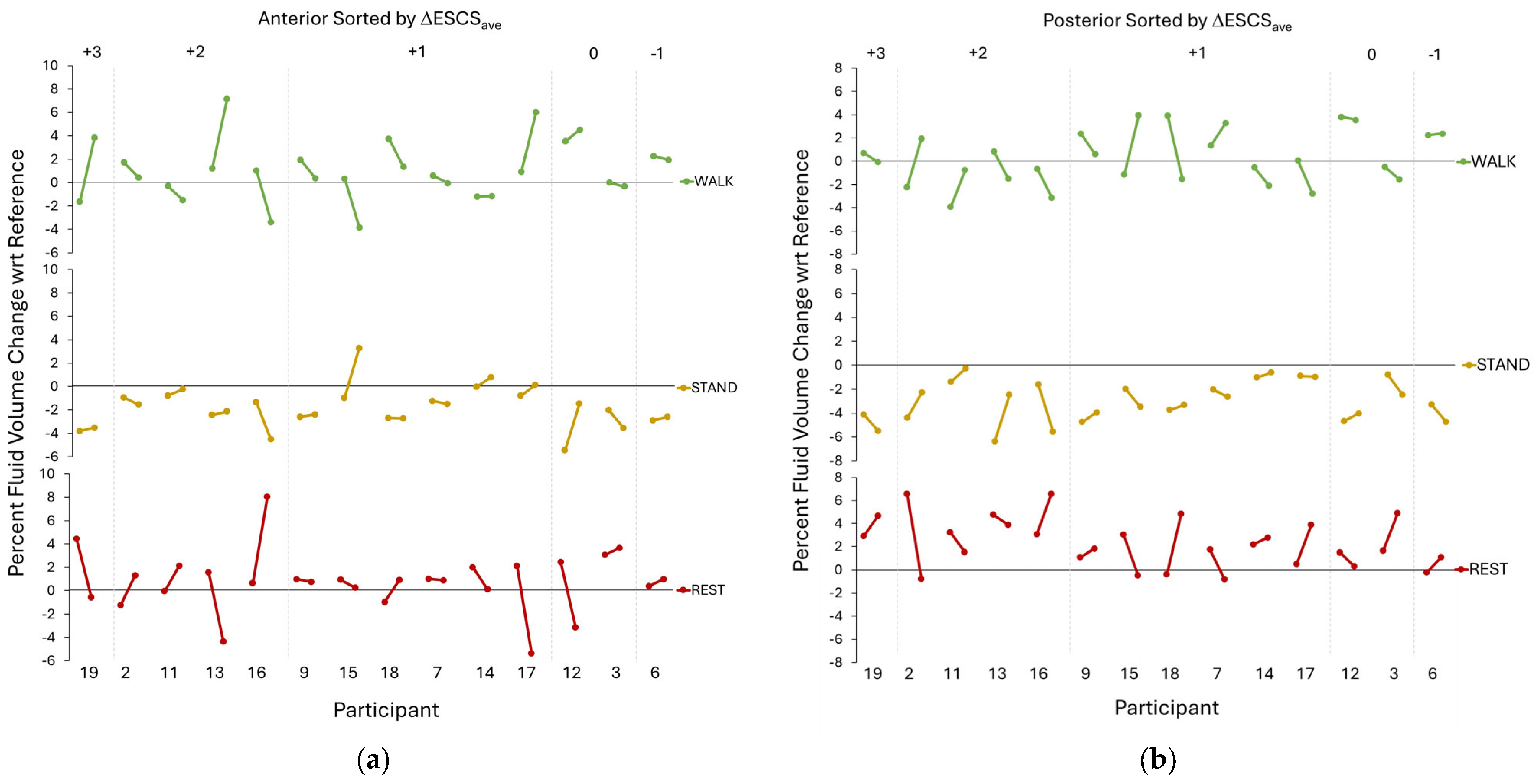
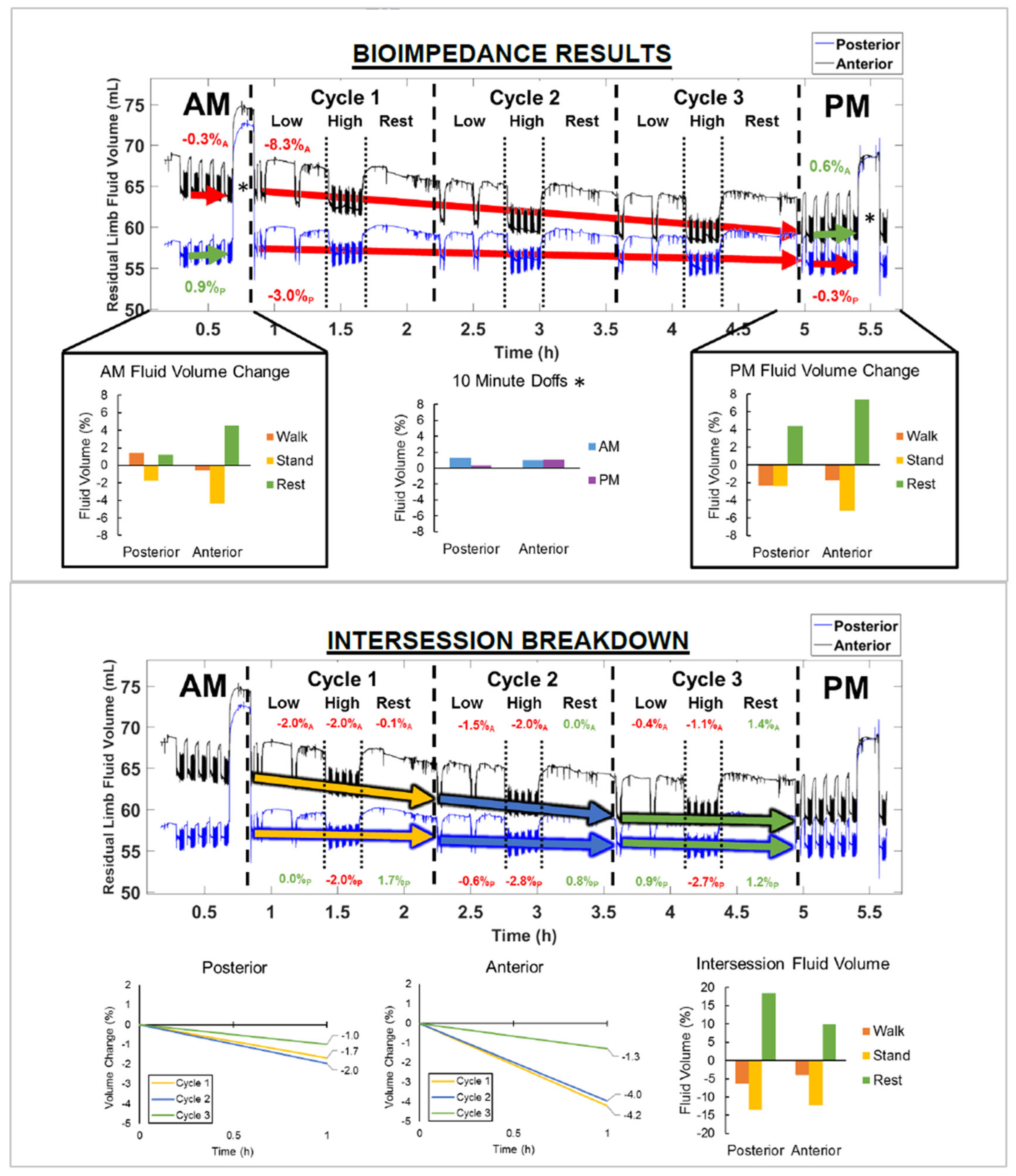
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| ESCS | Expanded Socket Comfort Score |
| MDCs | Minimal Detectable Changes |
| MFCL | Medicare Functional Classification Level |
| PEQ | Prosthesis Evaluation Questionnaire |
| PTB | Patellar Tendon Bearing |
| TSB | Total Surface Bearing |
| USB | Universal Serial Bus |
Appendix A. Electrode Fabrication
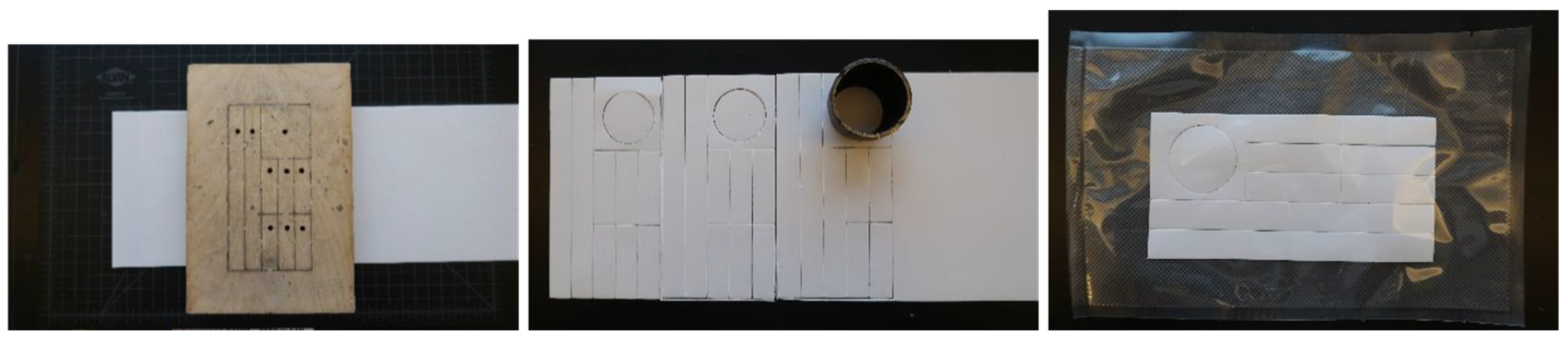
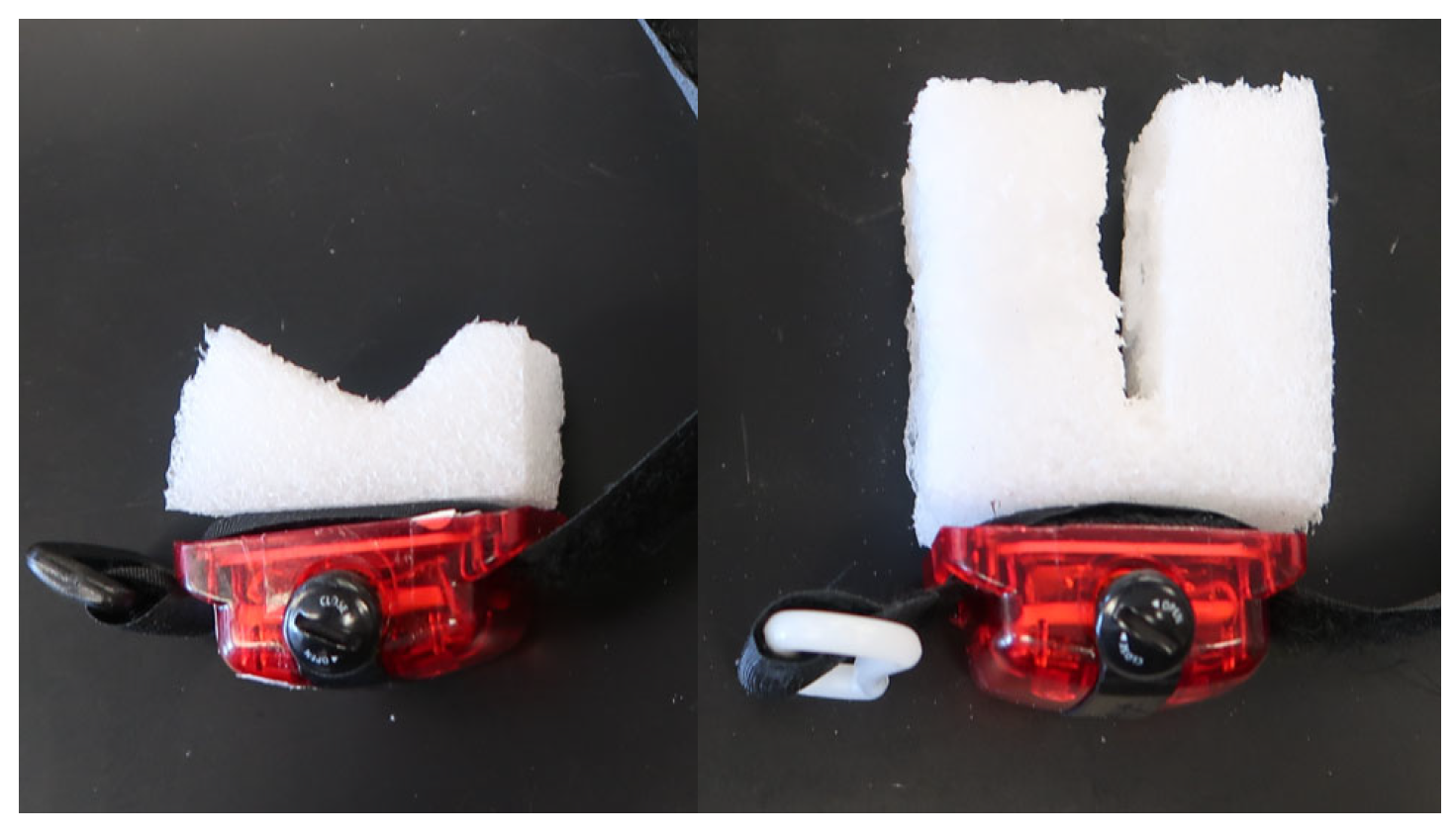
Appendix B. Attaching the Activity Monitor to the Liner
Appendix B.1. Liner/Sleeve ActiGraph Attachment


Appendix B.2. Ankle ActiGraph Attachment

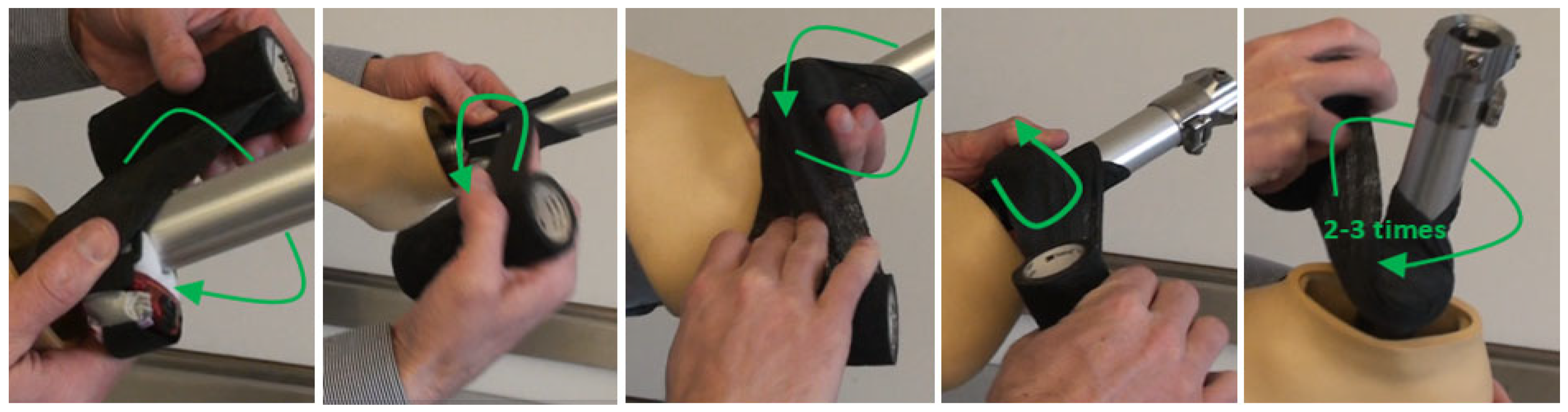
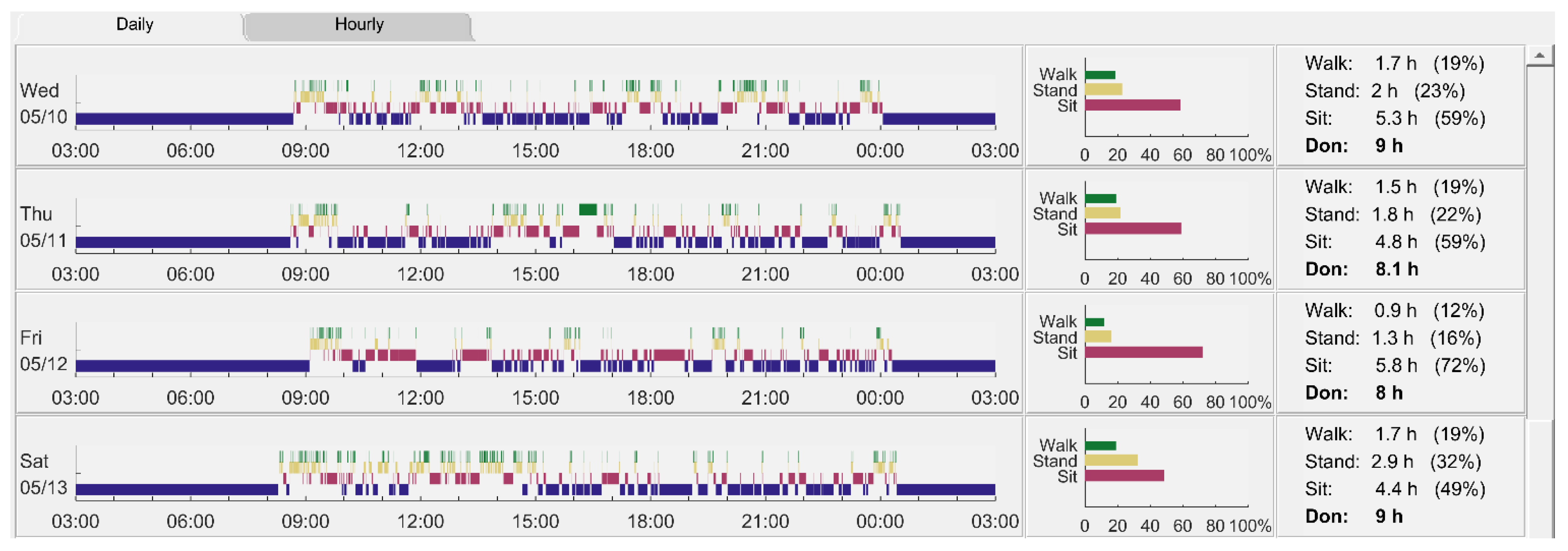
Appendix C. Example Visual Display of Activity Data
References
- Legro, M.W.; Reiber, G.; del Aguila, M.; Ajax, M.J.; Boone, D.A.; Larsen, J.A.; Smith, D.G.; Sangeorzan, B. Issues of importance reported by persons with lower limb amputations and prostheses. J. Rehabil. Res. Dev. 1999, 36, 155–163. [Google Scholar]
- Turner, S.; Gregor, A.H. Perceived effect of socket fit on major lower limb prosthetic rehabilitation: A clinician and amputee perspective. Arch. Rehabil. Res. Clin. Transl. 2020, 2, 100059. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.E.; Allyn, K.J.; Harrison, D.S.; Myers, T.R.; Ciol, M.A.; Tsai, E.C. Preliminary investigation of residual-limb fluid volume changes within one day. J. Rehabil. Res. Dev. 2012, 49, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.E.; Youngblood, R.T.; Hafner, B.J.; Ciol, M.A.; Allyn, K.J.; Gardner, D.; Cagle, J.C.; Redd, C.B.; Dietrich, C.R. Residual limb fluid volume change and volume accommodation: Relationships to activity and self-report outcomes in people with trans-tibial amputation. Prosthet. Orthot. Int. 2018, 42, 415–427. [Google Scholar] [CrossRef]
- Youngblood, R.T.; Hafner, B.J.; Allyn, K.J.; Cagle, J.C.; Hinrichs, P.; Redd, C.; Vamos, A.C.; Ciol, M.A.; Bean, N.; Sanders, J.E. Effects of activity intensity, time, and intermittent doffing on daily limb fluid volume change in people with transtibial amputation. Prosthet. Orthot. Int. 2019, 43, 28–38. [Google Scholar] [CrossRef]
- Díaz, N.; Galván Duque, C.; Zavaleta, J.; Moreno, J. Impact of residual limb volume loss on socket internal pressures: A preliminary study. J. Prosthet. Orthot. 2023, 35, 106–113. [Google Scholar] [CrossRef]
- Sanders, J.E.; Moehring, M.A.; Rothlisberger, T.M.; Phillips, R.H.; Hartley, T.; Dietrich, C.R.; Redd, C.B.; Gardner, D.W.; Cagle, J.C. A bioimpedance analysis platform for amputee residual limb assessment. IEEE Trans. Biomed. Eng. 2016, 63, 1760–1770. [Google Scholar] [CrossRef]
- Hinrichs, P.; Cagle, J.C.; Sanders, J.E. A portable bioimpedance instrument for monitoring residual limb fluid volume in people with transtibial limb loss: A technical note. Med. Eng. Phys. 2019, 68, 101–107. [Google Scholar] [CrossRef]
- Sanders, J.E.; Rogers, E.L.; Abrahamson, D.C. Assessment of residual-limb volume change using bioimpedence. J. Rehabil. Res. Dev. 2007, 44, 525–535. [Google Scholar] [CrossRef]
- Joan, E.S.; Timothy, R.M.; Katheryn, J.A.; Daniel, S.H. Clinical utility of in-socket residual limb volume change measurement: Case study results. Prosthet. Orthot. Int. 2009, 33, 378–390. [Google Scholar]
- Sanders, J.E.; Cagle, J.C.; Allyn, K.J.; Harrison, D.S.; Ciol, M.A. How do walking, standing, and resting influence transtibial amputee residual limb fluid volume? J. Rehabil. Res. Dev. 2014, 51, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.E.; Severance, M.R.; Swartzendruber, D.L.; Allyn, K.J.; Ciol, M.A. Influence of prior activity on residual limb volume and shape measured using plaster casting: Results from individuals with transtibial limb loss. J. Rehabil. Res. Dev. 2013, 50, 1007–1016. [Google Scholar] [CrossRef]
- McLean, J.B.; Redd, C.B.; Larsen, B.G.; Garbini, J.L.; Brzostowski, J.T.; Hafner, B.J.; Sanders, J.E. Effects of socket size on metrics of socket fit in trans-tibial prosthesis users. Med. Eng. Phys. 2017, 44, 32–43. [Google Scholar]
- Larsen, B.G.; McLean, J.B.; Allyn, K.J.; Brzostowski, J.T.; Garbini, J.L.; Sanders, J.E. How does adding and removing liquid from socket bladders affect residual-limb fluid volume? J. Rehabil. Res. Dev. 2013, 50, 845–860. [Google Scholar]
- Sanders, J.E.; Redd, C.B.; Cagle, J.C.; Hafner, B.J.; Gardner, D.; Allyn, K.J.; Harrison, D.S.; Ciol, M.A. Preliminary evaluation of a novel bladder-liner for facilitating residual limb fluid volume recovery without doffing. J. Rehabil. Res. Dev. 2016, 53, 1107–1120. [Google Scholar] [CrossRef]
- McLean, J.B.; Redd, C.B.; Larsen, B.G.; Garbini, J.L.; Brzostowski, J.T.; Hafner, B.J.; Sanders, J.E. Socket size adjustments in people with transtibial amputation: Effects on residual limb fluid volume and limb-socket distance. Clin. Biomech. 2019, 63, 161–171. [Google Scholar] [CrossRef]
- Larsen, B.G.; McLean, J.B.; Allyn, K.J.; Brzostowski, J.T.; Garbini, J.L.; Sanders, J.E. How do transtibial residual limbs adjust to intermittent incremental socket volume changes? Prosthet. Orthot. Int. 2019, 43, 528–539. [Google Scholar] [CrossRef]
- Ballesteros, D.; Youngblood, R.T.; Vamos, A.C.; Garbini, J.L.; Allyn, K.J.; Hafner, B.J.; Larsen, B.G.; Ciol, M.A.; Friedly, J.L.; Sanders, J.E. Cyclic socket enlargement and reduction during walking to minimize limb fluid volume loss in transtibial prosthesis users. Med. Eng. Phys. 2022, 103, 103787. [Google Scholar] [CrossRef]
- Weathersby, E.J.; Vamos, A.C.; Larsen, B.G.; McLean, J.B.; Carter, R.V.; Allyn, K.J.; Ballesteros, D.; Wang, H.; deGrasse, N.S.; Friedly, J.L.; et al. Performance of an auto-adjusting prosthetic socket during walking with intermittent socket release. J. Rehabil. Assist. Technol. 2022, 9, 20556683221093271. [Google Scholar] [CrossRef]
- Brian, L.; McLean, J.; Redd, C.; Brzostowski, J.; Allyn, K.; Sanders, J. How do socket size adjustments during ambulation affect residual limb fluid volume? Case study results. J. Prosthet. Orthot. 2019, 31, 58–66. [Google Scholar]
- Sanders, J.E.; Harrison, D.S.; Allyn, K.J.; Myers, T.R.; Ciol, M.A.; Tsai, E.C. How do sock ply changes affect residual-limb fluid volume in people with transtibial amputation? J. Rehabil. Res. Dev. 2012, 49, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Medicare Region C Durable Medical Equipment Prosthetics Orthotic Supplier (DMEPOS) Manual; Palmetto GBA: Columbia, SC, USA, 2005; pp. 53.5–53.6.
- Gardner, D.W.; Redd, C.B.; Cagle, J.C.; Hafner, B.J.; Sanders, J.E. Monitoring prosthesis user activity and doffing using an activity monitor and proximity sensors. J. Prosthet. Orthot. 2016, 28, 68–77. [Google Scholar] [CrossRef]
- Redfield, M.T.; Cagle, J.C.; Hafner, B.J.; Sanders, J.E. Classifying prosthetic use via accelerometry in persons with transtibial amputations. J. Rehabil. Res. Dev. 2013, 50, 1201–1212. [Google Scholar] [CrossRef]
- Legro, M.W.; Reiber, G.D.; Smith, D.G.; Aguila, M.; Larsen, J.; Boone, D. Prosthesis evaluation questionnaire for persons with lower limb amputations: Assessing prosthesis-related quality of life. Arch. Phys. Med. Rehabil. 1998, 79, 931–938. [Google Scholar] [CrossRef]
- Morgan, S.J.; Askew, R.L.; Hafner, B.J. Measurements of best, worst, and average socket comfort are more reliable than current socket comfort in established lower limb prosthesis users. Arch. Phys. Med. Rehabil. 2022, 103, 1201–1204. [Google Scholar] [CrossRef]
- Hanspal, R.S.; Fisher, K.; Nieveen, R. Prosthetic socket fit comfort score. Disabil. Rehabil. 2003, 25, 1278–1280. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Andreoli, A.; Matthie, J.; Withers, P. Predicting body cell mass with bioimpedance by using theoretical methods: A technological review. J. Appl. Physiol. 1997, 82, 1542–1558. [Google Scholar] [CrossRef]
- Fenech, M.; Jaffrin, M.Y. Extracellular and intracellular volume variations during postural change measured by segmental and wrist-ankle bioimpedance spectroscopy. IEEE Trans. Biomed. Eng. 2004, 51, 166–175. [Google Scholar] [CrossRef]
- Hanai, T. Electrical Properties of Emulsions; Sherman, P., Ed.; Academic Press: London, UK, 1968. [Google Scholar]
- Grimnes, S.; Martinsen, Ø.G. Bioimpedance and Bioelectricity Basics; Academic Press: San Diego, CA, USA, 2000; Available online: http://site.ebrary.com/id/10206346 (accessed on 10 January 2024).
- Amputee Coalition. About Body Mass Index (BMI). 2016. Available online: https://amputee-coalition.org/ (accessed on 21 April 2021).
- Mertens, J.C.; Brzostowski, J.T.; Vamos, A.; Allyn, K.J.; Hafner, B.J.; Friedly, J.L.; DeGrasse, N.S.; Ballesteros, D.; Krout, A.; Larsen, B.G.; et al. A novel portable sensor to monitor bodily positions and activities in transtibial prosthesis users. Clin. Biomech. 2022, 99, 105741. [Google Scholar] [CrossRef]
- DeGrasse, N.S.; Mertens, J.C.; Brzostowski, J.T.; Allyn, K.J.; Vamos, A.C.; Krout, A.J.; Hafner, B.J.; Garbini, J.L.; Sanders, J.E. Beyond step counts: Including wear time in prosthesis use assessment for lower-limb amputation. J. Rehabil. Assist. Technol. Eng. 2023, 10, 20556683231163337. [Google Scholar] [CrossRef]
- Levine, J.A.; Schleusner, S.J.; Jensen, M.D. Energy expenditure of nonexercise activity. Am. J. Clin. Nutr. 2000, 72, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Haskell, W. The exercise stress test: Needs for standardization. In Cardiology: Current Topics and Progress. 6; Academic Press: New York, NY, USA, 1970; pp. 149–154. [Google Scholar]
- Lanahan, C.R.; Coburn, K.A.; Hafner, B.J.; Ballesteros, D.; Allyn, K.J.; Friedly, J.L.; Carter, R.V.; Mertens, J.C.; Krout, A.J.; Sanders, J.E. Normalizing variance of self-selected effort. J. Prosthet. Orthot. 2025. submitted. [Google Scholar]
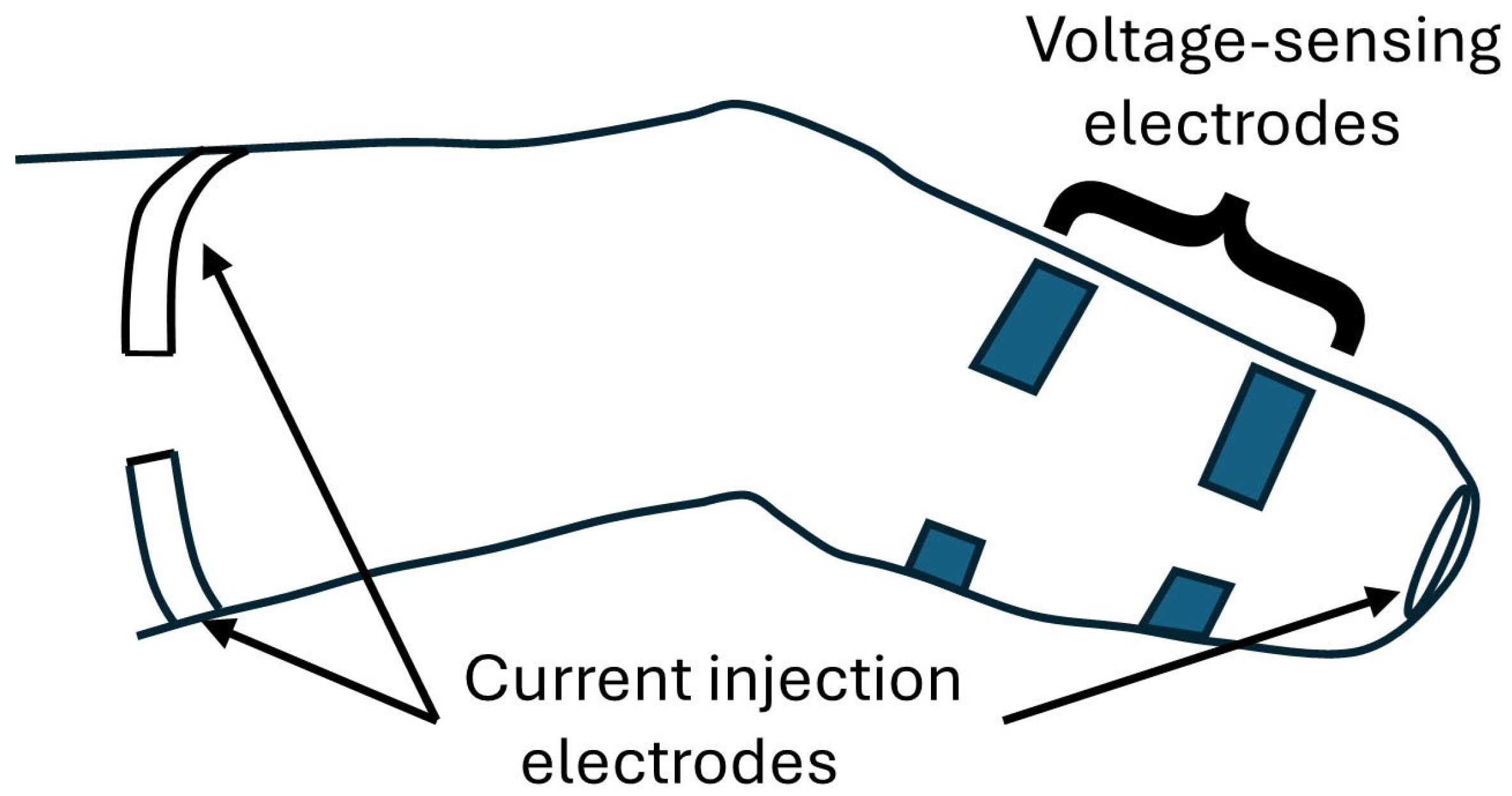


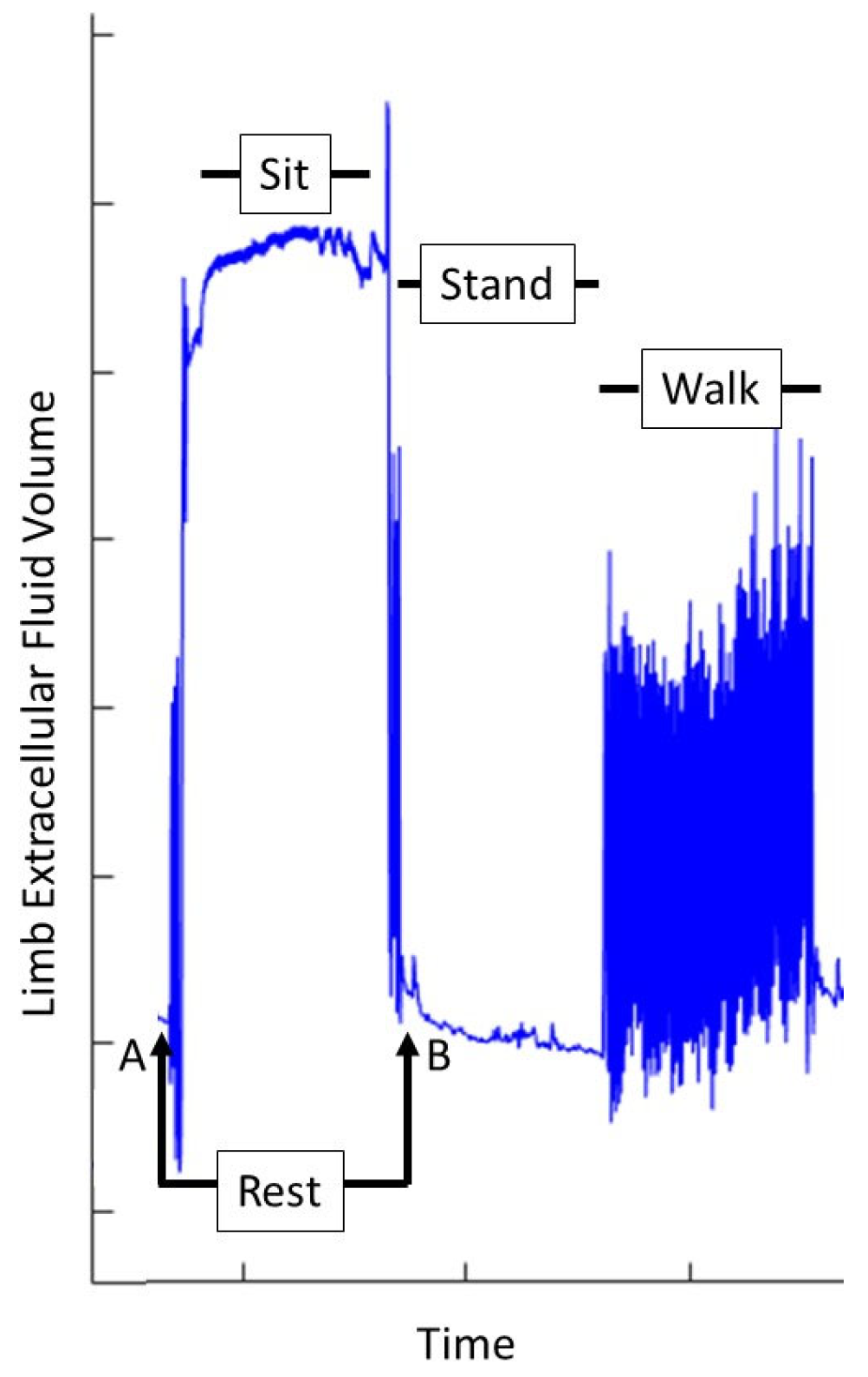

| Mean Measured Active Use Time per Prosthesis Day (Recorded for ~2 wk) | Active Use Time Implemented During Intersession Testing Protocol |
|---|---|
| <11.0% | 5% |
| 11.0–15.9% | 11% |
| 16.0–20.9% | 16% |
| >21.0% | 21% |
| Characteristic | Description of Sample |
|---|---|
| Gender | 12 Male, 2 Female |
| Age (y) | Median 46, Range 26 to 70 |
| Time since amputation (y) | Median 7, Range 2 to 43 |
| BMI [32] | Median 29, Range 22 to 41 |
| MFCL | 9 K3 (unlimited community ambulator), 5 K4 (active adult) |
| Residual Limb Length (cm) | Median 15.6, Range 9.0 to 17.0 |
| Etiology | 9 Trauma, 2 Infection following Trauma, 3 Non-Trauma Infection |
| Residual limb Shape | 11 Cylindrical, 1 Conical, 2 Bulbous |
| Co-Morbidities | 4 Diabetic, 7 High Blood Pressure, 3 Vascular Disease |
| Tobacco Products | 6 Users |
| Participant # | Socket | Description of Socket Modification | Socket ΔV |
|---|---|---|---|
| 2 | New | Changed from PTB to Hybrid PTB/TSB, smaller socket, new liner (from Alpha hybrid to Alpha Classic M+) | 1.6% |
| 3 | Mod | Modified socket to reduce brim height and added more padding; ground out crest of tibia, fibular head; new pads in distal end | NA |
| 6 | Mod | Changed sock ply because of fit issues—no new socket | NA |
| 7 | New | Socket replaced. Old socket had a poor fit and a large posterior pad; new socket did not | 0.1% |
| 9 | New | Changed from a PTB to a TSB socket—new socket has no PTB contours but no discernable TSB characteristics that indicated suction; both pylons (bilateral amputations) lengthened 6 cm | 13.2% |
| 11 | New | Changed from a PTB socket with a flexible inner liner and pin-lock suspension to a TSB with a 1-way valve and sleeve. Changed from an Alpha Classic to an Alpha Hybrid Cushion M liner | 5.0% |
| 12 | Mod | Socket shape changed by lowering posterior trimlines; new foot. Added a 1-way valve to a Seal-In V liner. Changed from a Cheetah foot to a Multiaxis Aeris Performance 2 foot | NA |
| 13 | New | Changed from a TSB+PTB hybrid to a TSB with flexible inner liner. Added an Ossur Iceross Seal In to an Ossur Proflex Foot and mechanical vacuum | −2.5% |
| 14 | New | Changed to a smaller socket (about 8-ply reduction). Added a flexible inner liner | −1.0% |
| 15 | Mod | Changed from a SmartTemp Liner to an Alpha liner (ΔV= −2.3%) | NA |
| 16 | New | Changed from a TSB with flexible inner liner suction socket to a TSB with foam inner (supracondylar suspension). Changed from an Ossur Seal In X to no liner | NA |
| 17 | Mod | Changed socket. Medial brim trimmed because of irritation there. Changed from a Renegade Freedom Innovations foot to an Endolite Javelin foot | 0.4% |
| 18 | New | Changed from a 1-way valve suspension, liner and sleeve, and energy storage and return foot to a locking pin socket with a modified Cheetah foot directly attached to a carbon-fiber socket | 1.5% |
| 19 | New | Changed from a flexible inner socket suspension sleeve, cushion liner with locking pin and an Eschelon foot (Endolite) to a flexible inner socket, liner with locking pin suspension and a RUSH foot | −5.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vamos, A.C.; Youngblood, R.T.; Lanahan, C.R.; Allyn, K.J.; Friedly, J.L.; Sanders, J.E. Using Bioimpedance Analysis as a Clinical Predictive Tool for the Assessment of Limb Fluid Volume Fluctuation: An Initial Investigation of Transtibial Prosthesis Users. Prosthesis 2025, 7, 53. https://doi.org/10.3390/prosthesis7030053
Vamos AC, Youngblood RT, Lanahan CR, Allyn KJ, Friedly JL, Sanders JE. Using Bioimpedance Analysis as a Clinical Predictive Tool for the Assessment of Limb Fluid Volume Fluctuation: An Initial Investigation of Transtibial Prosthesis Users. Prosthesis. 2025; 7(3):53. https://doi.org/10.3390/prosthesis7030053
Chicago/Turabian StyleVamos, Andrew C., Robert T. Youngblood, Conor R. Lanahan, Katheryn J. Allyn, Janna L. Friedly, and Joan E. Sanders. 2025. "Using Bioimpedance Analysis as a Clinical Predictive Tool for the Assessment of Limb Fluid Volume Fluctuation: An Initial Investigation of Transtibial Prosthesis Users" Prosthesis 7, no. 3: 53. https://doi.org/10.3390/prosthesis7030053
APA StyleVamos, A. C., Youngblood, R. T., Lanahan, C. R., Allyn, K. J., Friedly, J. L., & Sanders, J. E. (2025). Using Bioimpedance Analysis as a Clinical Predictive Tool for the Assessment of Limb Fluid Volume Fluctuation: An Initial Investigation of Transtibial Prosthesis Users. Prosthesis, 7(3), 53. https://doi.org/10.3390/prosthesis7030053






