Mechanical Behavior of Oil-Saturated Silicone Membranes for Adipose Tissue Synthesis in Clinical and Theatrical Prosthesis
Abstract
1. Introduction
Background
2. Materials and Methods
2.1. Test Stamdards Used
2.2. Shore Hardness
2.3. Spring Force
2.4. Tensile Stress–Strain
2.5. Multi-Axial Compression
2.6. Materials
2.7. Porcine Fat
2.8. PDMS Membranes
2.9. General Material Preparation
3. Results
3.1. The Effect of Oil Dispersal in PDMS Gel Membrane Cure Time
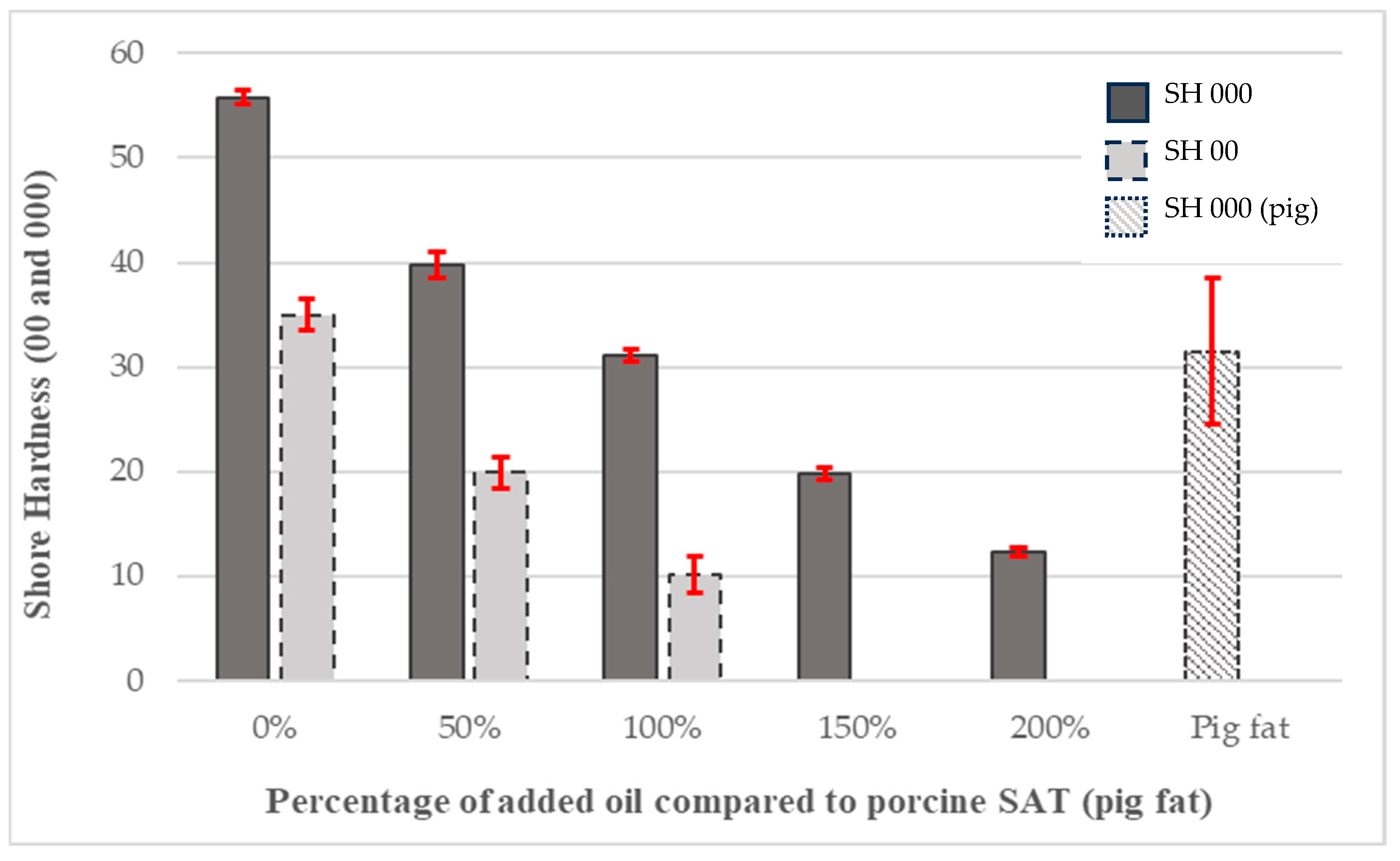
3.2. Membrane Hardnesses
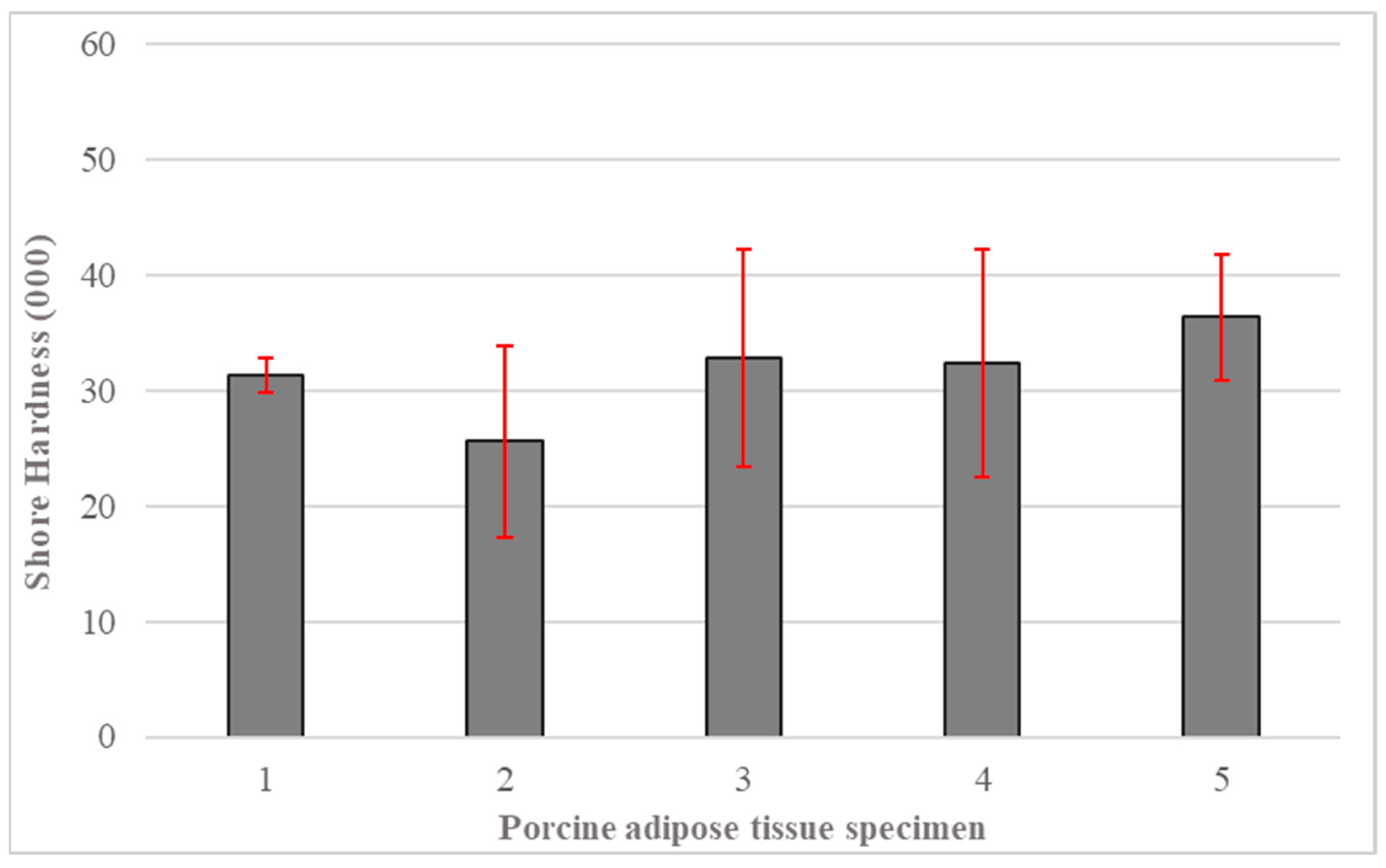
3.3. Porcine SAT Membrane Hardness
3.4. PDMS Membrane Hardness
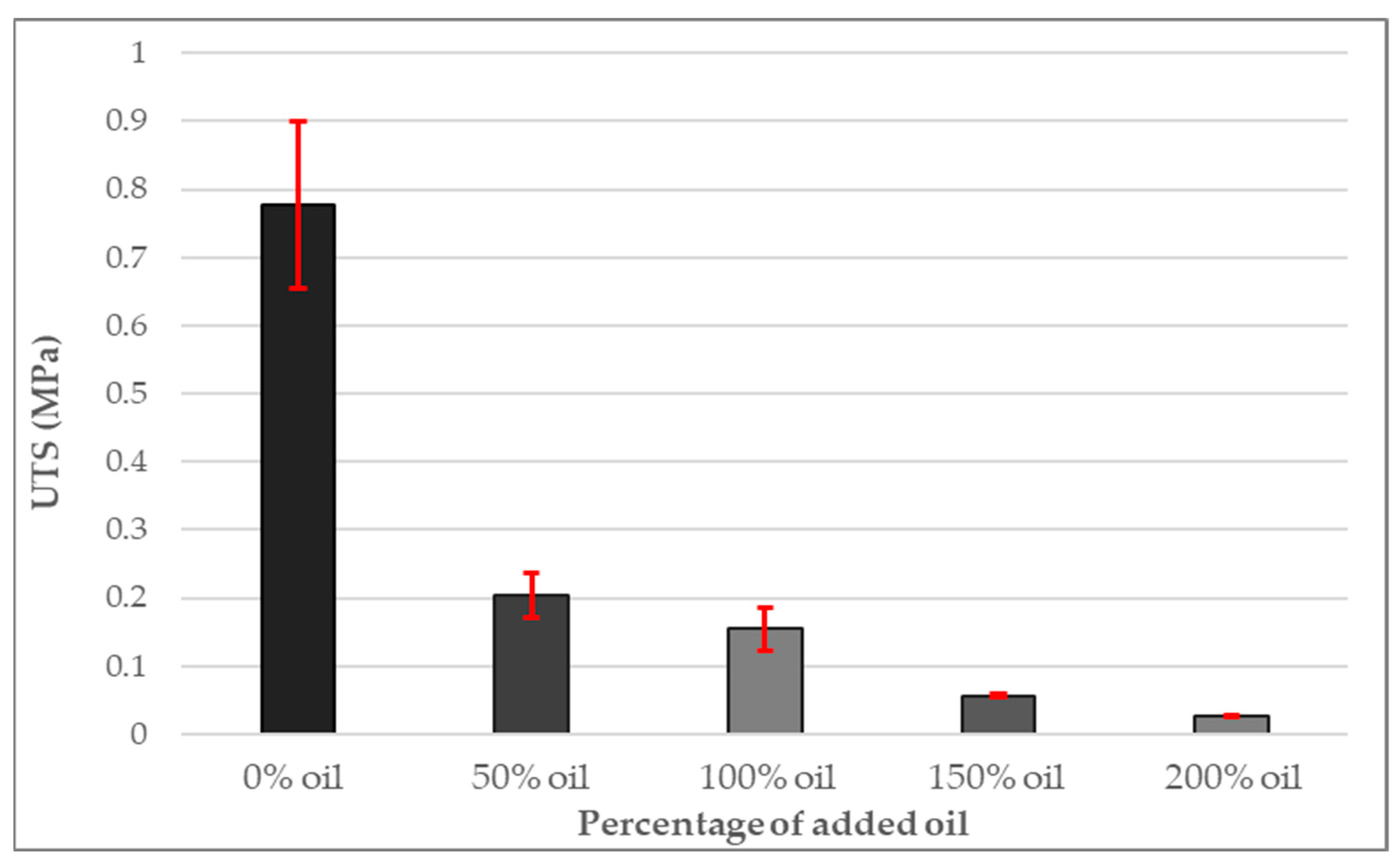
3.5. Ultimate Tensile Strength of PDMS Gel Membranes with Dispersed Oil
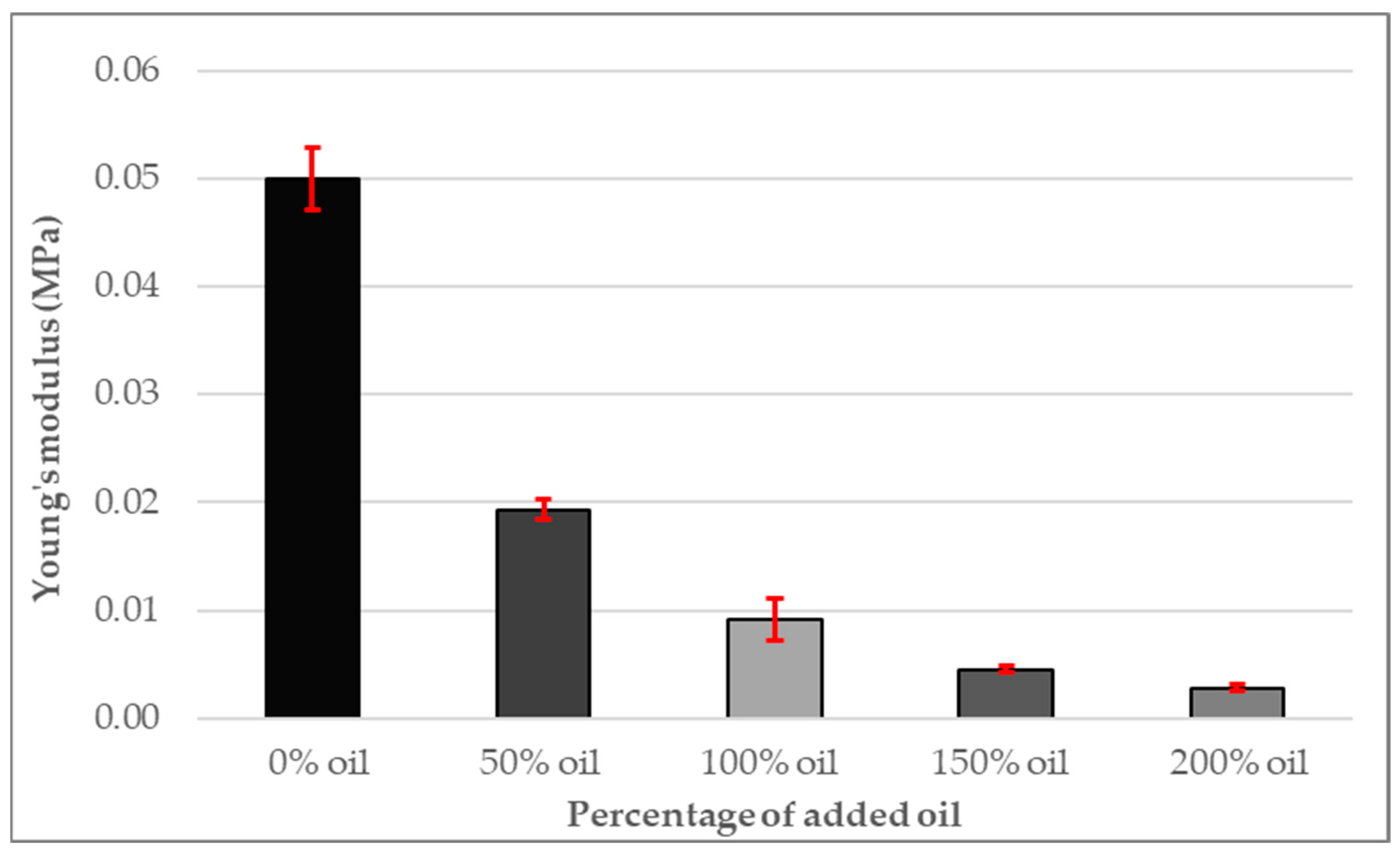
3.6. Young’s Modulus of PDMS Gel Membranes with Dispersed Oil
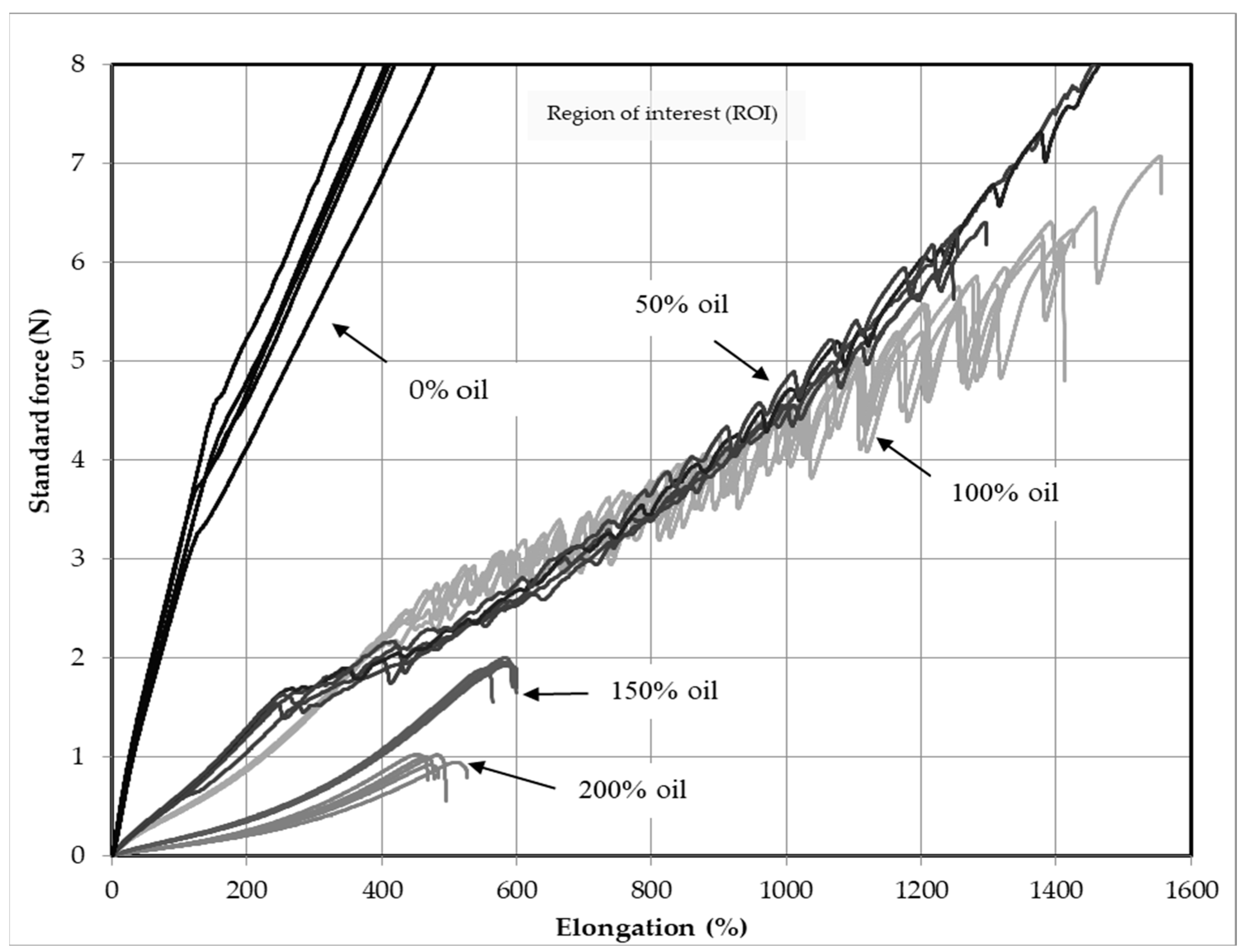
3.7. Uniaxial Characteristics of PDMS Gel Membranes with Dispersed Oil
3.8. Multi-Axial Test Results of PDMS Gel Membranes with Dispersed Oil
3.9. Hysteresis of PDMS Gel Membranes with Dispersed Oil
3.10. Force Degradation of PDMS Gel Membranes with Dispersed Oil
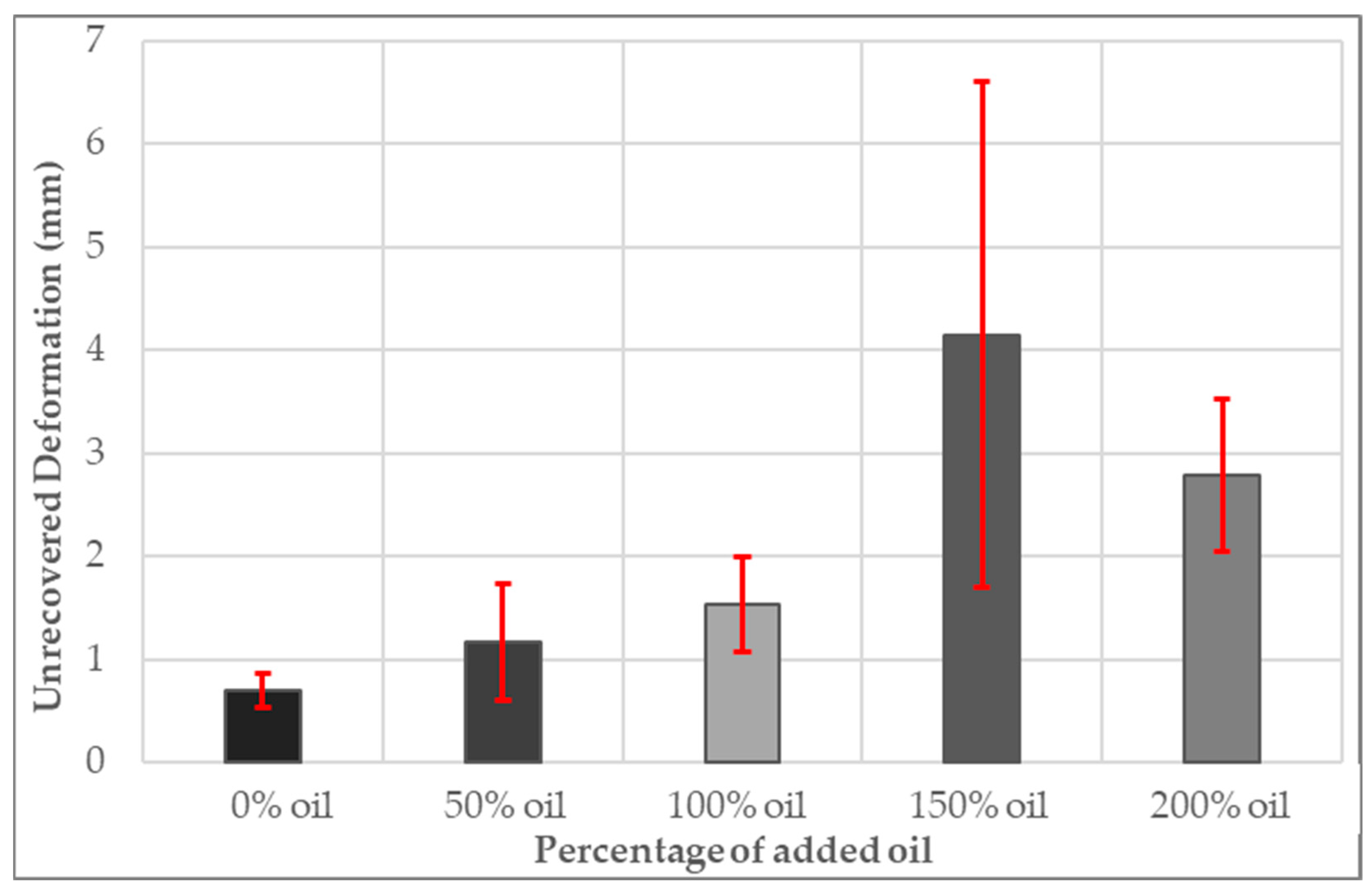
3.11. Unrecovered Deformation of PDMS Gel Membranes with Dispersed Oil
3.12. Summary of Mechanical Changes in Oily PDMS Membranes
4. Discussion
4.1. Hardness Characteristics
4.2. Uniaxial Characteristics
4.3. Multi-Axial Characteristics
5. Conclusions
Summary
- Significant differences were observed in all tests when comparing specimens with and without oil, except in force decay and force degradation experiments.
- A strong correlation was found between increasing oil content and decreasing hardness, UTS and elastic modulus.
- A strong correlation was found between increasing oil content and increasing extensibility and cure time.
- A weak relationship was found between increasing oil content relaxation and elastic recoverability.
- Evidence of the Lüders and PLC effects were observed, for the very first time, in soft gel membranes with 50% and 100% added oil.
- PDMS gel (Shore A-10) membranes with 100% added oil were best at mimicking subcutaneous adipose tissue. They exhibited the same hardness observed in identical tests on warmed porcine fat and the Young’s modulus of human fat given in the literature.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanzara, R.; Viswambaran, M.; Kumar, D. Maxillofacial prosthetic materials: Current status and recent advances: A comprehensive review. Int. J. Appl. Dent. Sci. 2021, 7, 255–259. [Google Scholar] [CrossRef]
- Arm, R.; Shahidi, A.; Clarke, C.; Alabraba, E. Synthesis and characterisation of a cancerous liver for presurgical planning and training applications. BMJ Open Gastroenterol. 2022, 9, e000909. [Google Scholar] [CrossRef] [PubMed]
- Debreceni, T. Special Makeup Effects for Stage and Screen: Making and Applying Prosthetics.; Routledge: London, UK; Focal Press: Oxen, UK, 2013; pp. 14–17. [Google Scholar]
- Agache, P.G.; Monneur, C.; Leveque, J.L.; De Rigal, J. Mechanical properties and Young’s modulus of human skin in vivo. Arch. Dermatol. Res. 1980, 269, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J. A System of Human Anatomy; Blackwood and Anderson: London, UK, 1815; Volume 1, pp. 223–225. [Google Scholar]
- Comley, K.; Fleck, N. The compressive response of porcine adipose tissue from low to high strain rate. Int. J. Impact Eng. 2012, 46, 1–10. [Google Scholar] [CrossRef]
- Comley, K.; Fleck, N.A. A micromechanical model for the Young’s modulus of adipose tissue. Int. J. Solids Struct. 2010, 47, 2982–2990. [Google Scholar] [CrossRef]
- Comley, K.; Fleck, N.A. The toughness of adipose tissue: Measurements and physical basis. J. Biomech. 2010, 43, 1823–1826. [Google Scholar] [CrossRef]
- Fung, Y.-C. Biomechanics: Mechanical Properties of Living Tissues, 2nd ed.; Behaviour of Soft Tissues Under Uniaxial Loading; Springer Science & Business Media: New York, NY, USA, 2013; Volume 7, pp. 269–271. [Google Scholar]
- Kerdok, A.E.; Ottensmeyer, M.P.; Howe, R.D. Effects of perfusion on the viscoelastic characteristics of liver. J. Biomech. 2006, 39, 2221–2231. [Google Scholar] [CrossRef]
- Falanga, V.; Bucalo, B. Use of a durometer to assess skin hardness. J. Am. Acad. Dermatol. 1993, 29, 47–51. [Google Scholar] [CrossRef]
- Yoon, Y.C.; Lee, J.S.; Park, S.U.; Kwon, J.H.; Hong, T.H.; Kim, D.G. Quantitative assessment of liver fibrosis using shore durometer. Ann. Surg. Treat. Res. 2017, 93, 300–304. [Google Scholar] [CrossRef][Green Version]
- Chatzistergos, P.E.; Allan, D.; Chockalingam, N.; Naemi, R. Shore hardness is a more representative measurement of bulk tissue biomechanics than of skin biomechanics. Med. Eng. Phys. 2022, 105, 103816. [Google Scholar] [CrossRef]
- Kissin, E.Y.; Schiller, A.M.; Gelbard, R.B.; Anderson, J.J.; Falanga, V.; Simms, R.W.; Korn, J.H.; Merkel, P.A. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Care Res. Off. J. Am. Coll. Rheumatol. 2006, 55, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, M.; Falanga, V. Use of a durometer to measure the degree of skin induration in lipodermatosclerosis. J. Am. Acad. Dermatol. 1995, 32, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Piaggesi, A.; Romanelli, M.; Schipani, E.; Campi, F.; Magliaro, A.; Baccetti, F.; Navalesi, R. Hardness of plantar skin in diabetic neuropathic feet. J. Diabetes Its Complicat. 1999, 13, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Strzalkowski, N.D.J.; Triano, J.J.; Lam, C.K.; Templeton, C.A.; Bent, L.R. Thresholds of skin sensitivity are partially influenced by mechanical properties of the skin on the foot sole. Physiol. Rep. 2015, 3, e12425. [Google Scholar] [CrossRef] [PubMed]
- Aghassi, D.; Monoson, T.; Braverman, I. Reproducible measurements to quantify cutaneous involvement in scleroderma. Arch. Dermatol. 1995, 131, 1160–1166. [Google Scholar] [CrossRef]
- Periyasamy, R.; Sneh, A.; Ammini, A. The effect of aging on the hardness of foot sole skin: A preliminary study. Foot 2012, 22, 95–99. [Google Scholar] [CrossRef]
- Reisfeld, P.L. A hard subject: Use of a durometer to assess skin hardness. J. Am. Acad. Dermatol. 1994, 31 Pt 1, 515. [Google Scholar] [CrossRef]
- Tong, J.; Lim, C.S.; Goh, O.L. Technique to study the biomechanical properties of the human calcaneal heel pad. Foot 2003, 13, 83–91. [Google Scholar] [CrossRef]
- Chanda, A.; McClain, S. Mechanical modelling of healthy and diseased calcaneal fat pad surrogates. Biomimetics 2019, 4, 1. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.M.H.; Hukins, D.W. Feasibility of using mixtures of silicone elastomers and silicone oils to model the mechanical behaviour of biological tissues. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 730–734. [Google Scholar] [CrossRef]
- Cai, Z. Drop Wetting and Sliding on Soft, Swollen Elastomers. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 2022. Available online: https://uknowledge.uky.edu/cme_etds/148/ (accessed on 13 July 2023).
- Anon. Tissue-Mimicking Phantoms. OBEL (Optical And Biomedical Engineering Laboratory), School of Electrical, Electronic and Computer Engineering, The University of Western Australia, Australia. 2023. Available online: https://obel.ee.uwa.edu.au/research/techniques/tissue-mimicking-phantoms/ (accessed on 14 December 2023).
- Oldenburg, A.L.; Toublan, F.J.J.; Suslick, K.S.; Wei, A.; Boppart, S.A. Magnetomotive contrast for in vivo optical coherence tomography. Opt. Express 2005, 13, 6597–6614. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Oldenburg, A.L.; Crecea, V.; Chaney, E.J.; Boppart, S.A. Optical micro-scale mapping of dynamic biomechanical tissue properties. Opt. Express 2008, 16, 11052–11065. [Google Scholar] [CrossRef]
- Moučka, R.; Sedlačík, M.; Osička, J.; Pata, V. Mechanical properties of bulk Sylgard 184 and its extension with silicone oil. Sci. Rep. 2021, 11, 19090. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, E.; Montazerian, H.; Khademhosseini, A.; Toyserkani, E. Sacrificial 3D printing of shrinkable silicone elastomers for enhanced feature resolution in flexible tissue scaffolds. Acta Biomater. 2020, 117, 261–272. [Google Scholar] [CrossRef]
- Ustbas, B.; Kilic, D.; Bozkurt, A.; Aribal, M.E.; Akbulut, O.; Ustbas, B.; Kilic, D.; Bozkurt, A.; Aribal, M.E.; Akbulut, O. Silicone-based composite materials simulate breast tissue to be used as ultrasonography training phantoms. Ultrasonics 2018, 88, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, L.; Zhang, Z.; Pal, K.; Kim, J.K. Effect of silica and silicone oil on the mechanical and thermal properties of silicone rubber. J. Macromol. Sci. Part B 2011, 50, 1144–1153. [Google Scholar] [CrossRef]
- Liu, Z.; Yeung, K. The preconditioning and stress relaxation of skin tissue. J. Biomed. Pharm. Eng. 2008, 2, 22–28. [Google Scholar]
- Wolf, K.J.; Weiss, J.D.; Uzel, S.G.; Skylar-Scott, M.A.; Lewis, J.A. Biomanufacturing human tissues via organ building blocks. Cell Stem Cell 2022, 29, 667–677. [Google Scholar] [CrossRef]
- Maclean, C.; Brodie, R.; Nash, D.H. Shore OO Hardness Measurement of Bovine Aorta and Mock Vessel Materials for Endovascular Device Design. 2019. Available online: https://www.bssm.org/media/z11jr0sq/88_craig_maclean_formatted.pdf (accessed on 2 March 2023).
- ASTM D2240-15(2021); Standard Test Method for Rubber Property—Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2021. Available online: https://www.astm.org/d2240-15r21.html (accessed on 5 May 2023).
- ISO 37:2024; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2024. Available online: https://www.iso.org/standard/86892.html (accessed on 14 April 2023).
- ASTM D412-16(2021); Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers—Tension. ASTM International: West Conshohocken, PA, USA, 2021. Available online: https://www.astm.org/standards/d412 (accessed on 14 April 2023).
- ISO 5893:2019; Rubber and Plastics Test Equipment—Tensile, Flexural and Compression Types (Constant Rate of Traverse)—Specification. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/77424.html (accessed on 15 April 2023).
- ISO 20932-2:2018; Textiles—Determination of the Elasticity of Fabrics Part 2: Multiaxial Tests. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/69490.html (accessed on 9 October 2023).
- ISO 23529:2016; Rubber—General Procedures for Preparing and Conditioning Test Pieces for Physical Test Methods. ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/70323.html (accessed on 9 October 2023).
- Shergold, O.A.; Fleck, N.A.; Radford, D. The uniaxial stress versus strain response of pig skin and silicone rubber at low and high strain rates. Int. J. Impact Eng. 2006, 32, 1384–1402. [Google Scholar] [CrossRef]
- Bergström, J.S.; Boyce, M.C. Constitutive modelling of the large strain time-dependent behaviour of elastomers. J. Mech. Phys. Solids 1998, 46, 931–954. [Google Scholar] [CrossRef]
- Annaidh, A.N.; Bruyère, K.; Destrade, M.; Gilchrist, M.D.; Otténio, M. Characterization of the anisotropic mechanical properties of excised human skin. J. Mech. Behav. Biomed. Mater. 2012, 5, 139–148. [Google Scholar] [CrossRef]
- Jacquemoud, C.; Bruyere-Garnier, K.; Coret, M. Methodology to determine failure characteristics of planar soft tissues using a dynamic tensile test. J. Biomech. 2007, 40, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Ziraki, S.; Zebarjad, S.M.; Hadianfard, M.J. A study on the tensile properties of silicone rubber/polypropylene fibres/silica hybrid nanocomposites. J. Mech. Behav. Biomed. Mater. 2016, 57, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Haider, Y.M.; Abdullah, Z.S.; Jani, G.H.; Mokhtar, N. Evaluation of some mechanical properties of a maxillofacial silicon elastomer reinforced with polyester powder. Int. J. Dent. 2019, 2019, 2948457. [Google Scholar] [CrossRef]
- Arm, R.; Shahidi, A.; Dias, T. Mechanical behaviour of silicone membranes saturated with short strand, loose polyester fibres for prosthetic and rehabilitative surrogate skin applications. Materials 2019, 12, 3647. [Google Scholar] [CrossRef] [PubMed]
- Madhani, A.J.; Niemeyer, G.; Salisbury, J.K. The black falcon: A teleoperated surgical instrument for minimally invasive surgery. In Proceedings of the 1998 IEEE/RSJ International Conference on Intelligent Robots and Systems. Innovations in Theory, Practice and Applications (Cat. No. 98ch36190), Victoria, BC, Canada, 17 October 1998; IEEE: Piscataway, NJ, USA, 1998; Volume 2, pp. 936–944. [Google Scholar]
- Brown, J.D.; Rosen, J.; Kim, Y.S.; Chang, L.; Sinanan, M.N.; Hannaford, B. In-vivo and in-situ compressive properties of porcine abdominal soft tissues. In Medicine Meets Virtual Reality 11; IOS Press: Amsterdam, The Netherlands, 2003; pp. 26–32. [Google Scholar]
- Bello, M.; Welch, C.F.; Goodwin, L.A.; Keller, J. Sylgard® Mixing Study; No. LA-UR-14-26638; Los Alamos National Laboratory (LANL): Los Alamos, NM, USA, 2014. [Google Scholar]
- Humphrey, J.D. Mechanics of the arterial wall: Review and directions. Crit. Rev. Biomed. Eng. 1995, 23, 1–162. [Google Scholar] [CrossRef]
- Ramphal, P.S.; Coore, D.N.; Craven, M.P.; Forbes, N.F.; Newman, S.M.; Coye, A.A.; Little, S.G.; Silvera, B.C. A high-fidelity tissue-based cardiac surgical simulator. Eur. J. Cardio-Thorac. Surg. 2005, 27, 910–916. [Google Scholar] [CrossRef]
- Wei, J.C.; Cartmill, I.D.; Kendall, M.A.; Crichton, M.L. In vivo, in situ and ex vivo comparison of porcine skin for micro projection array penetration depth, delivery efficiency and elastic modulus assessment. J. Mech. Behav. Biomed. Mater. 2022, 130, 105187. [Google Scholar] [CrossRef]
- Trotta, A.; Annaidh, A.N. Mechanical characterisation of human and porcine scalp tissue at dynamic strain rates. J. Mech. Behav. Biomed. Mater. 2019, 100, 103381. [Google Scholar] [CrossRef]
- Portevin, A.; Le Chatelier, F. Le traitement thermique des alliages légers d’aluminium à base de cuivre. (“Heat treatment of copper-based light aluminium alloys”). Rev. Métallurgie 1924, 21, 233–246. [Google Scholar] [CrossRef]
- Lüders, W. Über die äusserung der elasticität an stahlartigen eisenstäben und stahlstäben, und über eine beim biegen solcher stäbe beobachtete molecularbewegung. (“About the expression of elasticity in steel-like iron rods and steel rods, and about a molecular movement observed when bending such rods”). Dinglers. Polytech. J. 1860, 5, 18–22. [Google Scholar]
- Abbadi, M.; Hähner, P.; Zeghloul, A. On the characteristics of Portevin–Le Chatelier bands in aluminium alloy 5182 under stress-controlled and strain-controlled tensile testing. Mater. Sci. Eng. A 2002, 337, 194–201. [Google Scholar] [CrossRef]
- Conrad, H. Effect of stress on the Lüders band velocity in low carbon steels. J. Mech. Phys. Solids 1963, 11, 437–440. [Google Scholar] [CrossRef]
- Ren, S.; Morgeneyer, T.; Mazière, M.; Forest, S.; Rousselier, G. Effect of Lüders and Portevin–Le Chatelier localization bands on plasticity and fracture of notched steel specimens studied by DIC and FE simulations. Int. J. Plast. 2021, 136, 102880. [Google Scholar] [CrossRef]
- Coër, J.; Manach, P.Y.; Laurent, H.; Oliveira, M.C.; Menezes, L.F. Piobert–Lüders plateau and Portevin–Le Chatelier effect in an Al–Mg alloy in simple shear. Mech. Res. Commun. 2013, 48, 1–7. [Google Scholar] [CrossRef]
| Name and Purpose of Test | Test Assignment Code |
|---|---|
| Shore Hardness. Standard test method for rubber property. Durometer hardness [35]. | ASTM D2240-15 (2021) |
| Tensile stress-strain. Rubber, vulcanized or thermoplastic. Determination of tensile stress–strain properties [36]. | ISO 37:2024 |
| Tensile stress-strain. Standard test methods for vulcanized rubber and thermoplastic elastomers. Tension [37]. | ASTM D412-16 (2021) |
| Apperatus configuration. Test equipment. Tensile, flexural and compression types (constant rate of traverse). Specification [38]. | ISO 5893:2019 |
| Multi-axial compression. Determination of the elasticity of fabrics Part 2: Multiaxial tests [39]. | ISO 20932-2:2018 |
| Material storage and preparation. General procedures for preparing and conditioning test pieces for physical test methods [40]. | ISO 23529:2016 |
| Amount of Oil | Observed Mechanical Characteristics |
|---|---|
| No added oil | Soft, high elasticity, high extensibility, good recoverability, poor viscoelasticity. |
| 50–100% added oil | Moderately softer, lower elasticity, higher extensibility, moderate recoverability, PVC effect evident, moderate viscoelasticity. |
| 150–200% added oil | Very soft, lower elasticity, lower extensibility, low recoverability, higher viscoelasticity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arm, R.; Shahidi, A.; Pislaru, A.; Marasinghe, K.; Bibb, R.; Hughes-Riley, T. Mechanical Behavior of Oil-Saturated Silicone Membranes for Adipose Tissue Synthesis in Clinical and Theatrical Prosthesis. Prosthesis 2024, 6, 1340-1358. https://doi.org/10.3390/prosthesis6060097
Arm R, Shahidi A, Pislaru A, Marasinghe K, Bibb R, Hughes-Riley T. Mechanical Behavior of Oil-Saturated Silicone Membranes for Adipose Tissue Synthesis in Clinical and Theatrical Prosthesis. Prosthesis. 2024; 6(6):1340-1358. https://doi.org/10.3390/prosthesis6060097
Chicago/Turabian StyleArm, Richard, Arash Shahidi, Andreea Pislaru, Kalana Marasinghe, Richard Bibb, and Theodore Hughes-Riley. 2024. "Mechanical Behavior of Oil-Saturated Silicone Membranes for Adipose Tissue Synthesis in Clinical and Theatrical Prosthesis" Prosthesis 6, no. 6: 1340-1358. https://doi.org/10.3390/prosthesis6060097
APA StyleArm, R., Shahidi, A., Pislaru, A., Marasinghe, K., Bibb, R., & Hughes-Riley, T. (2024). Mechanical Behavior of Oil-Saturated Silicone Membranes for Adipose Tissue Synthesis in Clinical and Theatrical Prosthesis. Prosthesis, 6(6), 1340-1358. https://doi.org/10.3390/prosthesis6060097








