Effectiveness of a New Microprocessor-Controlled Knee–Ankle–Foot System for Transfemoral Amputees: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Trial Registration
2.2. Trial Design
2.3. Participants
2.3.1. Eligibility Criteria
2.3.2. Consent to Participate
2.4. Setting and Location
2.5. Prosthetic System
2.6. Interventions
2.7. Outcomes
2.8. Sample Size
2.9. Randomization
2.10. Blinding
2.11. Statistical Methods
3. Results
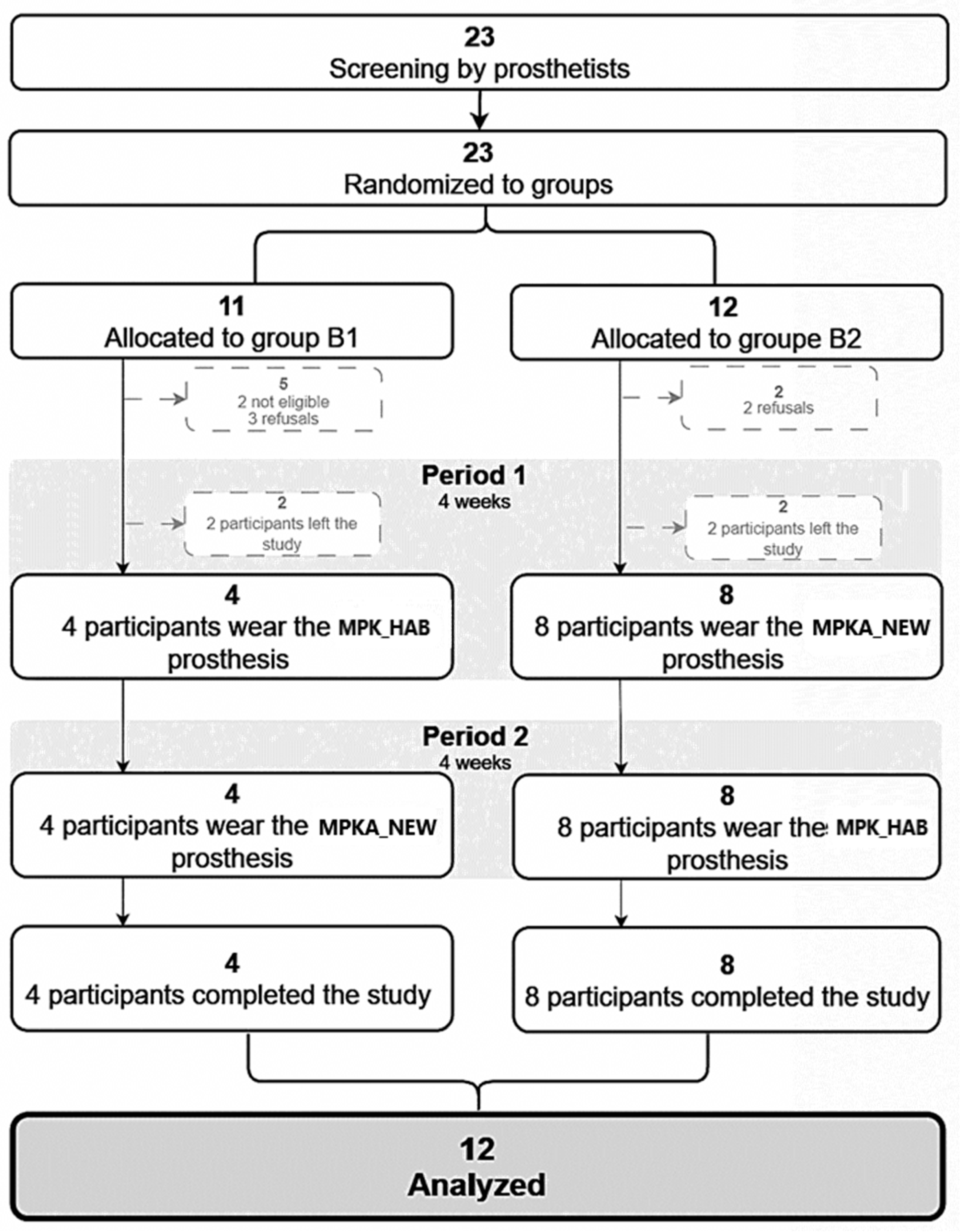
3.1. Recruitment
3.2. Population Characteristics
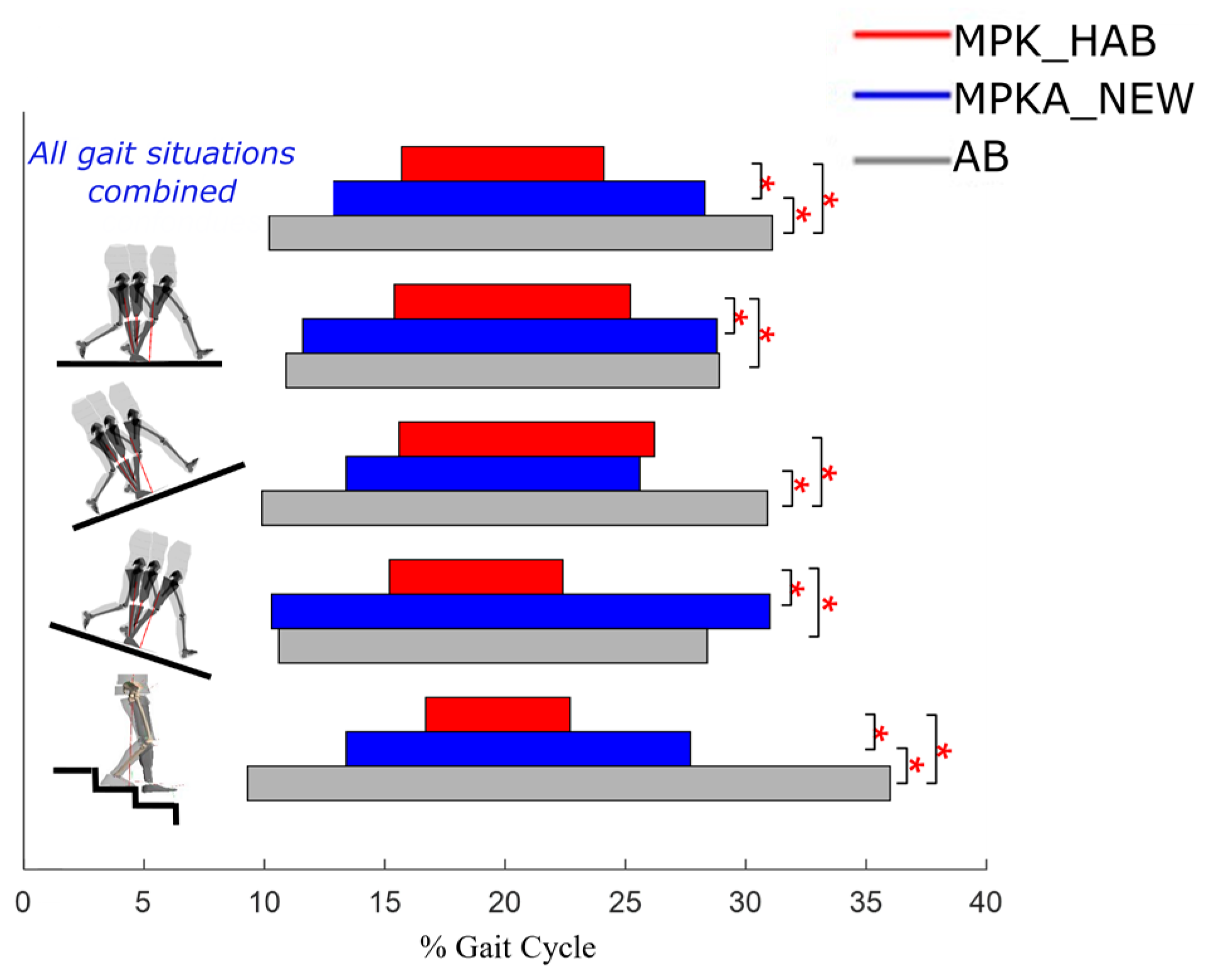
3.3. Stance Phase
3.4. Swing Phase
3.5. Locomotor Skills and Performances
3.6. Quality of Life
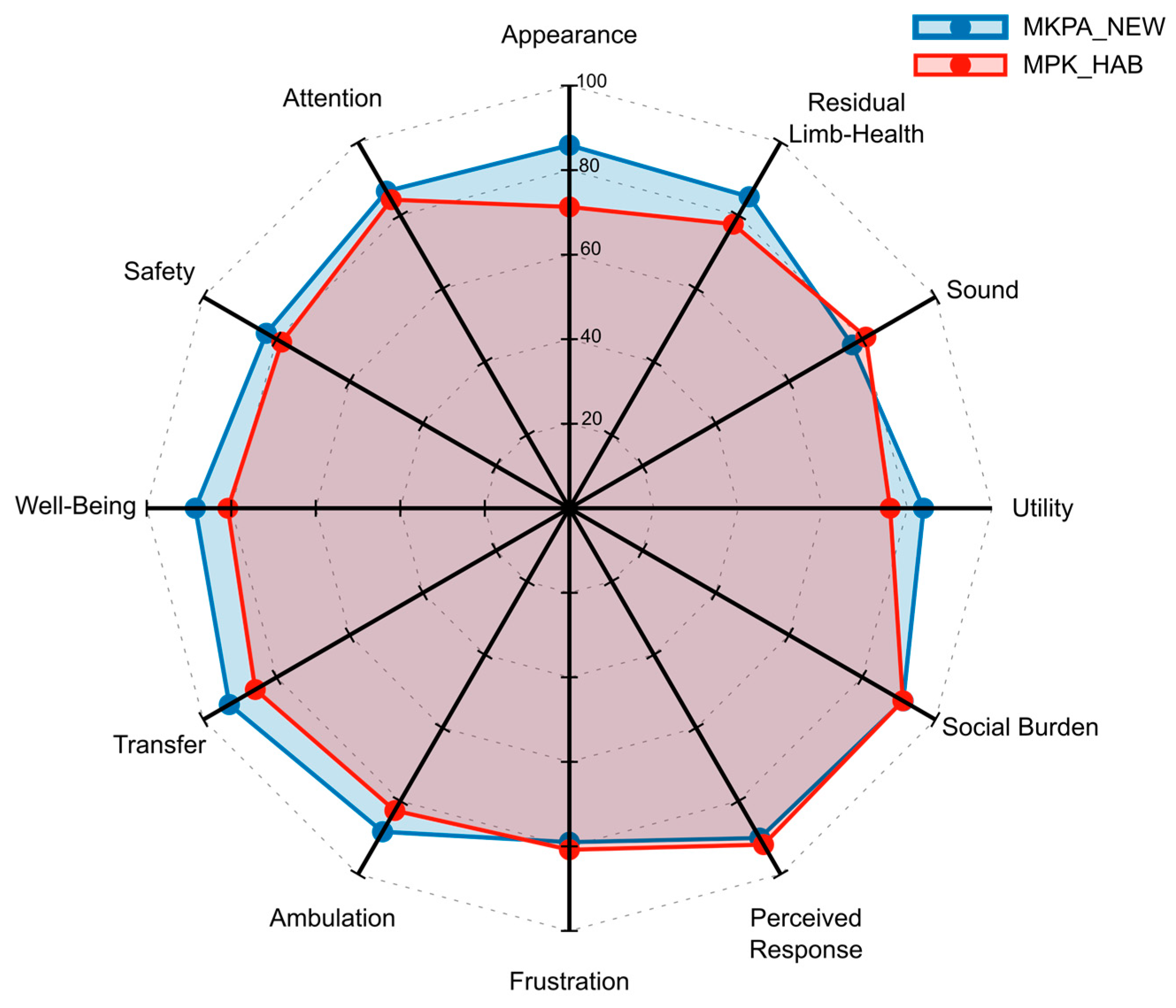
3.7. Prosthesis Evaluation Questionnaire
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sawers, A.B.; Hafner, B.J. Outcomes associated with the use of microprocessor-controlled prosthetic knees among individuals with unilateral transfemoral limb loss: A systematic review. J. Rehabil. Res. Dev. 2013, 50, 273–314. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, K.R.; Levine, J.A.; Brey, R.H.; Iverson, B.K.; McCrady, S.K.; Padgett, D.J.; Joyner, M.J. Gait and balance of transfemoral amputees using passive mechanical and microprocessor-controlled prosthetic knees. Gait Posture 2007, 26, 489–493. [Google Scholar] [CrossRef]
- Schmalz, T.; Blumentritt, S.; Jarasch, R. Energy expenditure and biomechanical characteristics of lower limb amputee gait:: The influence of prosthetic alignment and different prosthetic components. Gait Posture 2002, 16, 255–263. [Google Scholar] [CrossRef]
- Steinberg, N.; Gottlieb, A.; Siev-Ner, I.; Plotnik, M. Fall incidence and associated risk factors among people with a lower limb amputation during various stages of recovery—A systematic review. Disabil. Rehabil. 2019, 41, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.C.; Speechley, M.; Deathe, B. The prevalence and risk factors of falling and fear of falling among lower extremity amputees. Arch. Phys. Med. Rehabil. 2001, 82, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Wurdeman, S.R.; Stevens, P.M.; Campbell, J.H. Mobility Analysis of AmpuTees (MAAT I): Quality of life and satisfaction are strongly related to mobility for patients with a lower limb prosthesis. Prosthet. Orthot. Int. 2018, 42, 498–503. [Google Scholar] [CrossRef]
- Samuelsson, K.A.; Töytäri, O.; Salminen, A.-L.; Brandt, Å. Effects of lower limb prosthesis on activity, participation, and quality of life: A systematic review. Prosthet. Orthot. Int. 2012, 36, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Vickers, D.R.; Palk, C.; McIntosh, A.S.; Beatty, K.T. Elderly unilateral transtibial amputee gait on an inclined walkway: A biomechanical analysis. Gait Posture 2008, 27, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Gailey, R.; Gaunaurd, I.A.; O’Toole, C.; Finnieston, A.A. Comparison between microprocessor-controlled ankle/foot and conventional prosthetic feet during stair negotiation in people with unilateral transtibial amputation. J. Rehabil. Res. Dev. 2013, 50, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Dauriac, B.; Bonnet, X.; Villa, C.; Pillet, H.; Lavaste, F. Foot-flat period estimation during daily living situations of asymptomatic and lower limb amputee subjects. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 1920–1921. [Google Scholar] [CrossRef]
- Ernst, M.; Altenburg, B.; Schmalz, T.; Kannenberg, A.; Bellmann, M. Benefits of a microprocessor-controlled prosthetic foot for ascending and descending slopes. J. Neuroeng. Rehabil. 2022, 19, 9. [Google Scholar] [CrossRef]
- Palmer, M.L. Sagittal Plane Characterization of Normal Human Ankle Function Across a Range of Walking Gait Speeds. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2002. Available online: https://dspace.mit.edu/handle/1721.1/16802 (accessed on 17 October 2023).
- Gates, D.H.; Lelas, J.; Croce, U.D.; Herr, H.; Bonato, P. Characterization of ankle function during stair ambulation. In Proceedings of the the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; pp. 4248–4251. [Google Scholar] [CrossRef]
- Major, M.; Twiste, M.; Kenney, L.; Howard, D. The effects of prosthetic ankle stiffness on stability of gait in people with trans-tibial amputation. J. Rehabil. Res. Dev. 2016, 53, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Pillet, H.; Drevelle, X.; Bonnet, X.; Villa, C.; Martinet, N.; Sauret, C.; Bascou, J.; Loiret, I.; Djian, F.; Rapin, N.; et al. APSIC: Training and fitting amputees during situations of daily living. IRBM 2014, 35, 60–65. [Google Scholar] [CrossRef]
- Burnfield, J.M.; Eberly, V.J.; Gronely, J.K.; Perry, J.; Yule, W.J.; Mulroy, S.J. Impact of stance phase microprocessor-controlled knee prosthesis on ramp negotiation and community walking function in K2 level transfemoral amputees. Prosthet. Orthot. Int. 2012, 36, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, T.; Blumentritt, S.; Marx, B. Biomechanical analysis of stair ambulation in lower limb amputees. Gait Posture 2007, 25, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, N.J.; Bauer, A.; Rotter, D.; Grabiner, M.D. Active dorsiflexing prostheses may reduce trip-related fall risk in people with transtibial amputation. J. Rehabil. Res. Dev. 2014, 51, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; De Asha, A.R.; Munjal, R.; Kulkarni, J.; Buckley, J.G. Toe clearance when walking in people with unilateral transtibial amputation: Effects of passive hydraulic ankle. J. Rehabil. Res. Dev. 2014, 51, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Rábago, C.A.; Whitehead, J.A.; Wilken, J.M. Evaluation of a Powered Ankle-Foot Prosthesis during Slope Ascent Gait. PLoS ONE 2016, 11, e0166815. [Google Scholar] [CrossRef] [PubMed]
- Russell Esposito, E.; Aldridge Whitehead, J.M.; Wilken, J.M. Step-to-step transition work during level and inclined walking using passive and powered ankle–foot prostheses. Prosthet. Orthot. Int. 2016, 40, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Struchkov, V.; Buckley, J.G. Biomechanics of ramp descent in unilateral trans-tibial amputees: Comparison of a microprocessor controlled foot with conventional ankle–foot mechanisms. Clin. Biomech. 2016, 32, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Kaluf, B.; Duncan, A.; Bridges, W. Comparative Effectiveness of Microprocessor-Controlled and Carbon-Fiber Energy-Storing-and-Returning Prosthetic Feet in Persons with Unilateral Transtibial Amputation: Patient-Reported Outcome Measures. JPO J. Prosthet. Orthot. 2020, 32, 214–221. [Google Scholar] [CrossRef]
- Bai, X.; Ewins, D.; Crocombe, A.D.; Xu, W. A biomechanical assessment of hydraulic ankle-foot devices with and without micro-processor control during slope ambulation in trans-femoral amputees. PLoS ONE 2018, 13, e0205093. [Google Scholar] [CrossRef]
- “SYNSYS”, Proteor France. Available online: https://fr.proteor.com/composants/synsys/ (accessed on 29 November 2024).
- Bonnet, X.; Djian, F.; Drevelle, X.; Villa, C.; Pillet, H. Design and preliminary evaluation of a microprocessor controlled ankle-knee prosthetic system for above knee amputees. In Proceedings of the 13th International Symposium on 3D Analysis of Human Movement, Lausanne, Switzerland, 14–17 July 2014; p. 29. [Google Scholar]
- Bonnet, X.; Djian, F. Hydraulic System for a Knee-Ankle Assembly Controlled by a Microprocessor. EP2877130B1, 22 February 2017. Available online: https://patents.google.com/patent/EP2877130B1/en (accessed on 3 December 2024).
- Bellmann, M.; Schmalz, T.; Ludwigs, E.; Blumentritt, S. Immediate Effects of a New Microprocessor-Controlled Prosthetic Knee Joint: A Comparative Biomechanical Evaluation. Arch. Phys. Med. Rehabil. 2012, 93, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Kahle, J.T.; Highsmith, M.J.; Hubbard, S.L. Comparison of nonmicroprocessor knee mechanism versus C-Leg on Prosthesis Evaluation Questionnaire, stumbles, falls, walking tests, stair descent, and knee preference. J. Rehabil. Res. Dev. 2008, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sagawa, Y.; Turcot, K.; Armand, S.; Thevenon, A.; Vuillerme, N.; Watelain, E. Biomechanics and physiological parameters during gait in lower-limb amputees: A systematic review. Gait Posture 2011, 33, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, A.H.; van Keeken, H.G.; Schoppen, T.; Otten, E.; Halbertsma, J.P.K.; Hof, A.L.; Postema, K. Gait termination in lower limb amputees. Gait Posture 2008, 27, 82–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lechler, K.; Kristjansson, K. The importance of additional mid swing toe clearance for amputees. Can. Prosthet. Orthot. J. 2018, 1, 1. [Google Scholar] [CrossRef]
- Riveras, M.; Ravera, E.; Ewins, D.; Shaheen, A.F.; Catalfamo-Formento, P. Minimum toe clearance and tripping probability in people with unilateral transtibial amputation walking on ramps with different prosthetic designs. Gait Posture 2020, 81, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, N.J.; Bauer, A.; Grabiner, M.D. Relating minimum toe clearance to prospective, self-reported, trip-related stumbles in the community. Prosthet. Orthot. Int. 2017, 41, 387–392. [Google Scholar] [CrossRef]
- Dwan, K.; Li, T.; Altman, D.G.; Elbourne, D. CONSORT 2010 statement: Extension to randomised crossover trials. BMJ 2019, 366, l4378. [Google Scholar] [CrossRef] [PubMed]
- Legifrance. Arrêté du 19 Mars 2013 Portant Modification des Modalités d’Inscription des Pieds à Restitution d’Énergie Inscrits au Chapitre 7 du Titre II de la Liste Prévue à l’Article L. 165-1 (LPPR) du Code de la Sécurité Sociale. Bulletin Officiel. Available online: https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000027243099 (accessed on 2 August 2024).
- Legro, M.W.; Reiber, G.D.; Smith, D.G.; Del Aguila, M.; Larsen, J.; Boone, D. Prosthesis evaluation questionnaire for persons with lower limb amputations: Assessing prosthesis-related quality of life. Arch. Phys. Med. Rehabil. 1998, 79, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Hafner, B.J.; Smith, D.G. Differences in function and safety between Medicare Functional Classification Level-2 and -3 transfemoral amputees and influence of prosthetic knee joint control. J. Rehabil. Res. Dev. 2009, 46, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.D.; States, U. Rand-36 Health Status Inventory; Psychological Corporation: San Antonio, TX, USA, 1998. [Google Scholar]
- Leplège, A.; Ecosse, E.; Verdier, A.; Perneger, T.V. The French SF-36 Health Survey: Translation, cultural adaptation and preliminary psychometric evaluation. J. Clin. Epidemiol. 1998, 51, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Dauriac, B. Contribution à la Mise en Œuvre et l’Évaluation de Technologies Embarquées Pour l’Appareillage de Personnes Amputées du Membre Inférieur. Ph.D. Thesis, ENSAM, Paris, France, 2018. Available online: https://www.theses.fr/2018ENAM0017 (accessed on 12 January 2024).
- Hafner, B.J.; Gaunaurd, I.A.; Morgan, S.J.; Amtmann, D.; Salem, R.; Gailey, R.S. Construct validity of the Prosthetic Limb Users Survey of Mobility (PLUS-M) in adults with lower limb amputation. Arch. Phys. Med. Rehabil. 2017, 98, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Resnik, L.; Borgia, M. Reliability of outcome measures for people with lower-limb amputations: Distinguishing true change from statistical error. Phys. Ther. 2011, 91, 555–565. [Google Scholar] [CrossRef]
- Hansen, A.; Nickel, E.; Medvec, J.; Brielmaier, S.; Pike, A.; Weber, M. Effects of a flat prosthetic foot rocker section on balance and mobility. J. Rehabil. Res. Dev. 2014, 51, 137–148. [Google Scholar] [CrossRef]
- Perry, J.; Boyd, L.A.; Rao, S.S.; Mulroy, S.J. Prosthetic weight acceptance mechanics in transtibial amputees wearing the Single Axis, Seattle Lite, and Flex Foot. IEEE Trans. Rehabil. Eng. 1997, 5, 283–289. [Google Scholar] [CrossRef] [PubMed]
- De Asha, A.R.; Munjal, R.; Kulkarni, J.; Buckley, J.G. Impact on the biomechanics of overground gait of using an ‘Echelon’ hydraulic ankle–foot device in unilateral trans-tibial and trans-femoral amputees. Clin. Biomech. 2014, 29, 728–734. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alexander, N.; Strutzenberger, G.; Kroell, J.; Barnett, C.T.; Schwameder, H. Joint Moments During Downhill and Uphill Walking of a Person with Transfemoral Amputation with a Hydraulic Articulating and a Rigid Prosthetic Ankle—A Case Study. JPO J. Prosthet. Orthot. 2018, 30, 46–54. [Google Scholar] [CrossRef]
- McGrath, M.; Laszczak, P.; Zahedi, S.; Moser, D. Microprocessor knees with ‘standing support’ and articulating, hydraulic ankles improve balance control and inter-limb loading during quiet standing. J. Rehabil. Assist. Technol. Eng. 2018, 5, 2055668318795396. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A. Foot Trajectory in Human Gait: A Precise and Multifactorial Motor Control Task. Phys. Ther. 1992, 72, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Drevelle, X.; Villa, C.; Bonnet, X.; Loiret, I.; Fodé, P.; Pillet, H. Vaulting quantification during level walking of transfemoral amputees. Clin. Biomech. 2014, 29, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Goujon-Pillet, H.; Sapin, E.; Fodé, P.; Lavaste, F. Three-Dimensional Motions of Trunk and Pelvis During Transfemoral Amputee Gait. Arch. Phys. Med. Rehabil. 2008, 89, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Michaud, S.B.; Gard, S.A.; Childress, D.S. A preliminary investigation of pelvic obliquity patterns during gait in persons with transtibial and transfemoral amputation. J. Rehabil. Res. Dev. 2000, 37, 1–10. [Google Scholar] [PubMed]
- Villa, C.; Loiret, I.; Langlois, K.; Bonnet, X.; Lavaste, F.; Fodé, P.; Pillet, H. Cross-Slope and Level Walking Strategies During Swing in Individuals With Lower Limb Amputation. Arch. Phys. Med. Rehabil. 2017, 98, 1149–1157. [Google Scholar] [CrossRef]
- Blumentritt, S.; Schmalz, T.; Jarasch, R. The Safety of C-Leg: Biomechanical Tests. JPO J. Prosthet. Orthot. 2009, 21, 2–15. [Google Scholar] [CrossRef]
- Asano, M.; Rushton, P.; Miller, W.C.; Deathe, B.A. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet. Orthot. Int. 2008, 32, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; van den Heuvel, W.J.; Arokiasamy, P. Factors affecting quality of life in lower limb amputees. Prosthet. Orthot. Int. 2011, 35, 90–96. [Google Scholar] [CrossRef]
- Sinha, R.; Van Den Heuvel, W.J.A. A systematic literature review of quality of life in lower limb amputees. Disabil. Rehabil. 2011, 33, 883–899. [Google Scholar] [CrossRef]



| Age (yrs) | Height (cm) | Weight (kg) | Time Since 1st Prosthesis (yrs) | Cause of Amputation | Current Prosthetic Knee | Current Prosthetic Foot | |
|---|---|---|---|---|---|---|---|
| 1 | 65 | 175 | 92 | 40 | Traumatic | CLeg 3 | Variflex |
| 2 | 53 | 184 | 72 | 22 | Traumatic | CLeg 3 | Proflex XC |
| 3 | 44 | 178 | 70 | 7 | Traumatic | CLeg 4 | Proflex XC |
| 4 | 60 | 163 | 77 | 4 | Traumatic | Rheoknee | Proflex Rotate |
| 5 | 39 | 180 | 74 | 9 | Traumatic | Rheoknee | Proflex XC |
| 6 | 48 | 196 | 74 | 1.5 | Traumatic | CLeg 4 | Proflex XC |
| 7 | 56 | 172 | 68 | 39 | Traumatic | Rheoknee | Variflex |
| 8 | 67 | 182 | 62 | 3 | Traumatic | CLeg 4 | Triton |
| 9 | 30 | 183 | 80 | 2 | Traumatic | CLeg 4 | HiPro |
| 10 | 21 | 167 | 65 | 20 | Congenital | Rheoknee | Proflex XC |
| 11 | 25 | 169 | 78 | 20 | Congenital | Rheoknee | Proflex XC |
| 12 | 41 | 179 | 87 | 6 | Traumatic | Rheoknee | Proflex XC |
| MPK_HAB | MPKA_NEW | p-Value | Effect Size |r| | ||
|---|---|---|---|---|---|
| WALKING SPEED (M/S) | Level Ground | 1.16 (0.1) | 1.2 (0.1) | 0.28 | 1.12 |
| Uphill 12% | 1.04 (0.1) | 1.01 (0.1) | 0.55 | 0.62 | |
| Downhill 12% | 1.05 (0.2) | 0.98 (0.2) | 0.15 | 1.58 | |
| Stairs | 0.46 (0.1) | 0.42 (0.1) | 0.07 | 2.01 | |
| STEP LENGTH P (CM) | Level Ground | 67.6 (7.8) | 68.8 (8.3) | 0.49 | 0.71 |
| Uphill 12% | 67.7 (8.1) | 64.8 (9.4) | 0.17 | 1.5 | |
| Downhill 12% | 62.1 (5.9) | 64 (4.8) | 0.44 | 0.80 | |
| Stairs | 39.9 (5.2) | 40 (6.7) | 0.95 | 0.06 | |
| STEP LENGTH C (CM) | Level Ground | 65.4 (4.9) | 64.3 (5.4) | 0.50 | 0.70 |
| Uphill 12% | 66.7 (6) | 62.9 (6.2) | 0.01 * | 3.02 | |
| Downhill 12% | 58.6 (7.6) | 51.4 (10.4) | 0.02 * | 2.74 | |
| Stairs | 24.2 (6.7) | 29 (7.2) | 0.03 * | 2.54 | |
| STEP WIDTH P (CM) | Level Ground | 20.1 (4.4) | 20.9 (4.9) | 0.35 | 0.97 |
| Uphill 12% | 21.4 (4.9) | 23.9 (5.8) | 0.03 * | 2.59 | |
| Downhill 12% | 20.3 (4.5) | 20.7 (4.6) | 0.41 | 0.85 | |
| Stairs | 21.9 (3.3) | 23.7 (3.4) | <0.01 * | 3.48 | |
| STEP WIDTH C (CM) | Level Ground | 22.5 (3.9) | 23.4 (4.6) | 0.30 | 1.1 |
| Uphill 12% | 22.0 (6.8) | 23.3 (5.3) | 0.07 | 1.97 | |
| Downhill 12% | 21.8 (4.5) | 23.4 (5.7) | 0.21 | 1.32 | |
| Stairs | 23.7 (3.1) | 22.9 (2.2) | 0.52 | 0.67 | |
| STANCE PHASE (% GC) | Level Ground | 58.7 (1.1) | 57.2 (1.3) | 0.03 * | 2.69 |
| Uphill 12% | 60.0 (2.1) | 59.6 (2.2) | 0.43 | 0.82 | |
| Downhill 12% | 56.2 (3.8) | 56.3 (3) | 0.33 | 1.02 | |
| Stairs | 60.8 (5.4) | 63.3 (5.9) | 0.17 | 1.47 | |
| FLAT-FOOT TIME (% GC) | Level Ground | 9.8 (5.7) | 17.2 (6.3) | 0.02 * | 2.65 |
| Uphill 12% | 10.6 (4.3) | 12.2 (4.2) | 0.36 | 0.96 | |
| Downhill 12% | 7.2 (3.3) | 20.7 (4.7) | <0.01 * | 7.66 | |
| Stairs | 6 (3.6) | 14.3 (6.4) | <0.01 * | 5.28 |
| MTC_C | MPK_HAB | MPKA_NEW | p-Value | Effect Size |r| | |
|---|---|---|---|---|---|
| Level ground | 2.2 (1.5) | 1.8 (8) | 4.6 (2.1) | 0.01 * | 3.08 |
| 12% uphill | 3 (1.2) | 1.8 (6) | 4.1 (1.5) | <0.01 * | 3.45 |
| MPK_HAB | MPKA_NEW | p-Value | Effect Size |r| | |
|---|---|---|---|---|
| Physical functionning | 86.7 (13.2) | 91.7 (9.8) | 0.04 * | 2.43 |
| Role physical | 68.8 (42.8) | 91.7 (28.9) | 0.06 | 2.09 |
| Body pain | 74.8 (27) | 78.5 (24) | 1.00 | 3.50 |
| General health | 77.9 (21.5) | 80.4 (18.6) | 0.31 | 1.08 |
| Vitality | 70.4 (23.8) | 72.1 (23.8) | 0.66 | 0.45 |
| Social functional | 90.6 (10.8) | 96.9 (7.8) | 0.06 | 2.10 |
| Role emotional | 83.3 (33.3) | 91.7 (28.7) | 0.04 * | 2.33 |
| Mental health | 64.6 (24.9) | 68.8 (24.1) | 0.70 | 0.39 |
| MHC | 70.2 (15.3) | 77.7 (9.7) | 0.07 | 2.01 |
| PHC | 75.3 (12.4) | 78.5 (9.4) | 0.04 * | 2.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Requena, C.; Bascou, J.; Loiret, I.; Bonnet, X.; Thomas-Pohl, M.; Duraffourg, C.; Calistri, L.; Pillet, H. Effectiveness of a New Microprocessor-Controlled Knee–Ankle–Foot System for Transfemoral Amputees: A Randomized Controlled Trial. Prosthesis 2024, 6, 1591-1606. https://doi.org/10.3390/prosthesis6060115
Requena C, Bascou J, Loiret I, Bonnet X, Thomas-Pohl M, Duraffourg C, Calistri L, Pillet H. Effectiveness of a New Microprocessor-Controlled Knee–Ankle–Foot System for Transfemoral Amputees: A Randomized Controlled Trial. Prosthesis. 2024; 6(6):1591-1606. https://doi.org/10.3390/prosthesis6060115
Chicago/Turabian StyleRequena, Christelle, Joseph Bascou, Isabelle Loiret, Xavier Bonnet, Marie Thomas-Pohl, Clément Duraffourg, Laurine Calistri, and Hélène Pillet. 2024. "Effectiveness of a New Microprocessor-Controlled Knee–Ankle–Foot System for Transfemoral Amputees: A Randomized Controlled Trial" Prosthesis 6, no. 6: 1591-1606. https://doi.org/10.3390/prosthesis6060115
APA StyleRequena, C., Bascou, J., Loiret, I., Bonnet, X., Thomas-Pohl, M., Duraffourg, C., Calistri, L., & Pillet, H. (2024). Effectiveness of a New Microprocessor-Controlled Knee–Ankle–Foot System for Transfemoral Amputees: A Randomized Controlled Trial. Prosthesis, 6(6), 1591-1606. https://doi.org/10.3390/prosthesis6060115







