Adaptation of 3D-Printed and Milled Titanium Custom Post and Core
Abstract
1. Introduction
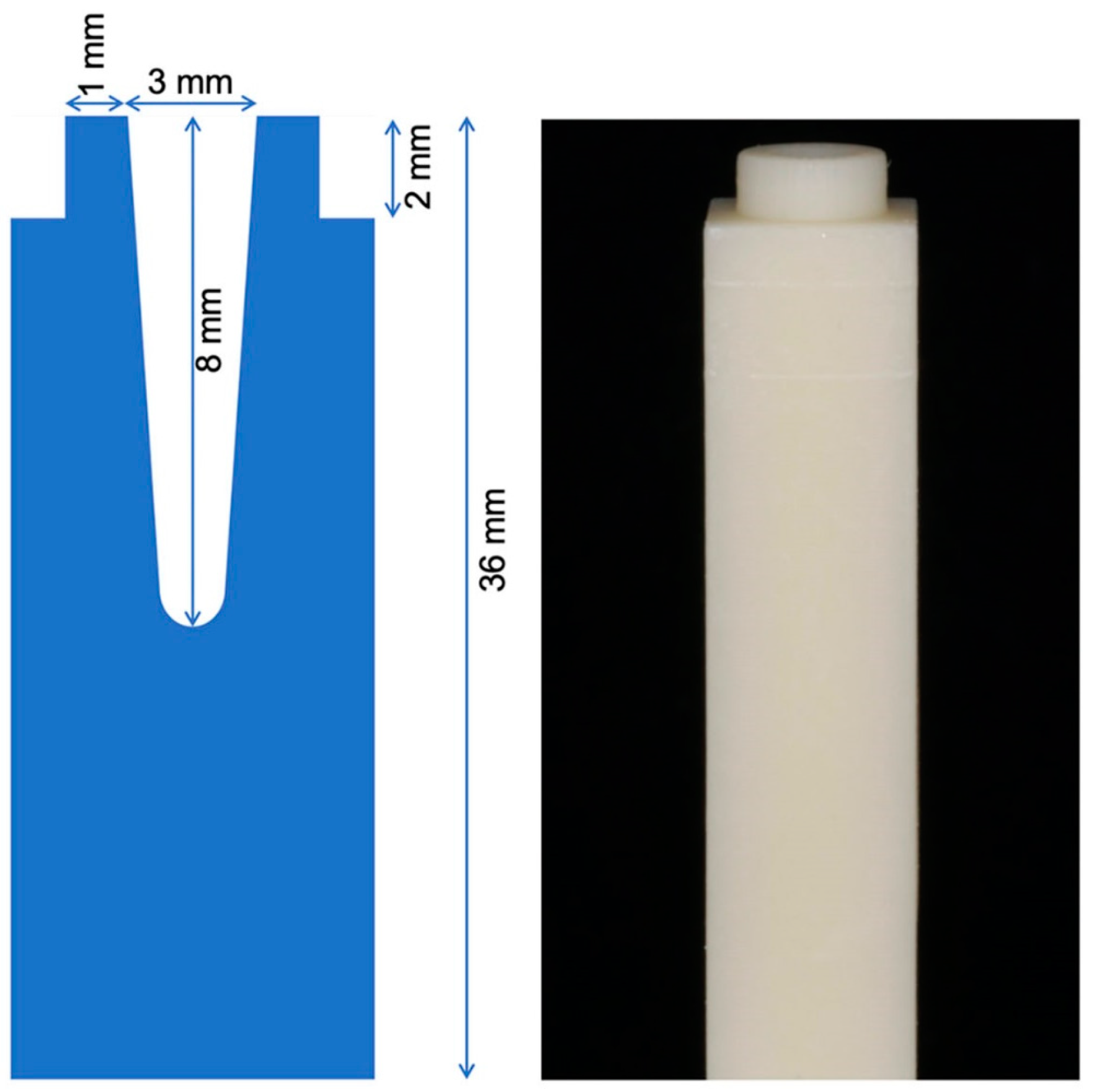
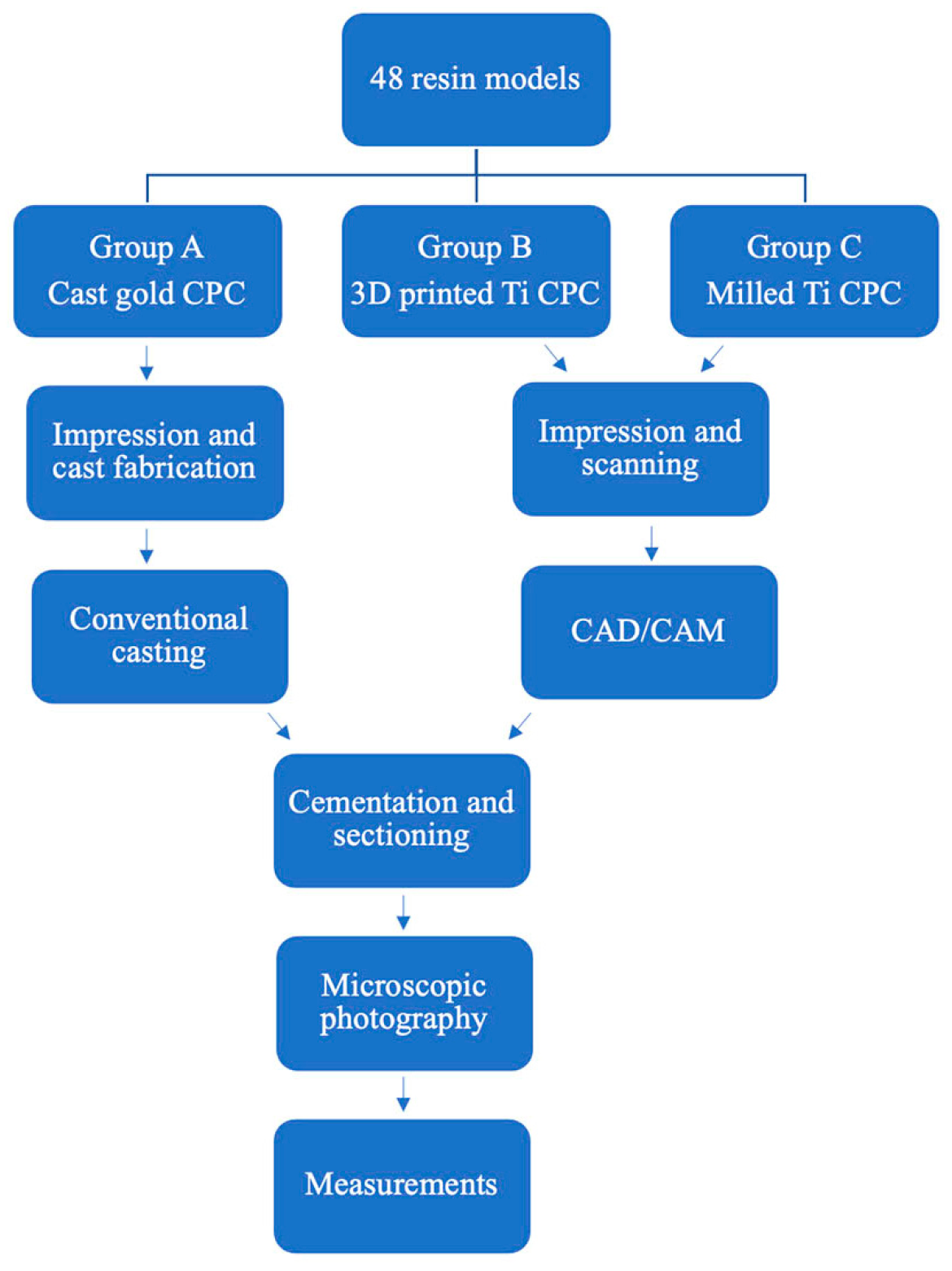
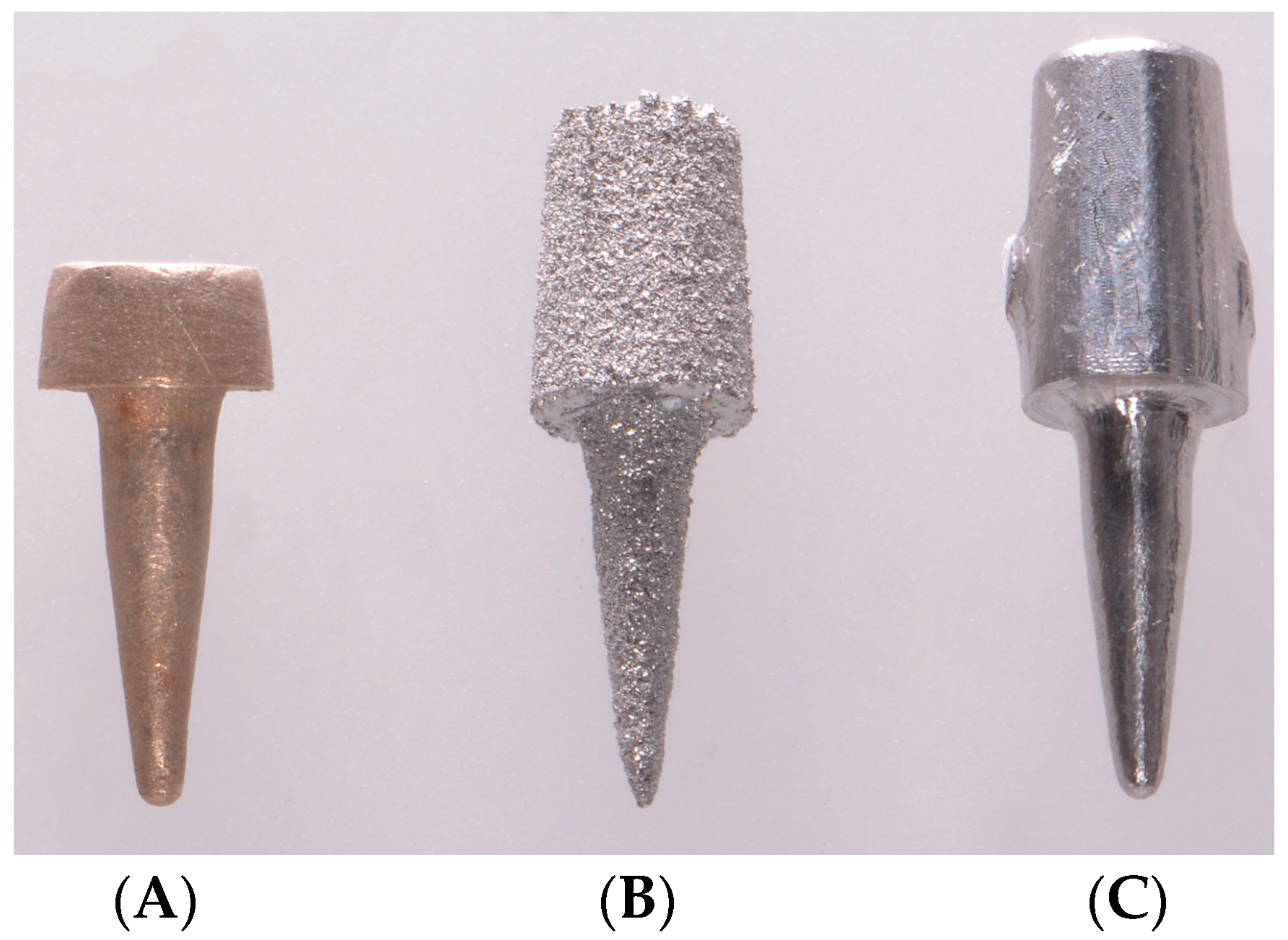
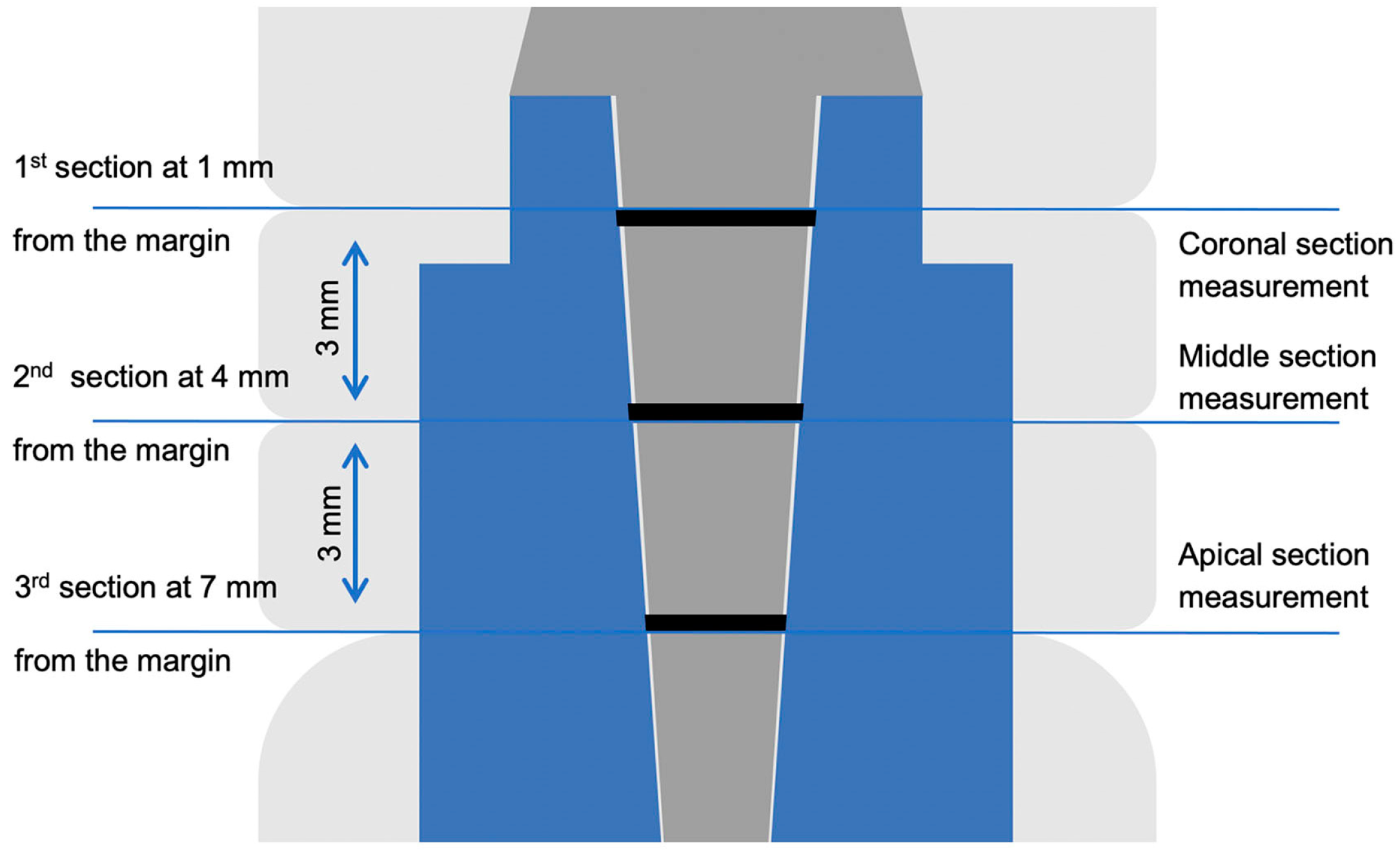
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenstiel, S.F.; Land, M.F.; Fujimoto, J. Contemporary Fixed Prosthodontics, 5th ed.; Mosby: St. Louis, MO, USA, 2015. [Google Scholar]
- Ng, C.C.; Dumbrigue, H.B.; Al-Bayat, M.I.; Griggs, J.A.; Wakefield, C.H. Influence of remaining coronal tooth structure location on the fracture resistance of restored endodontically treated anterior teeth. J. Prosthet. Dent. 2006, 95, 290–296. [Google Scholar] [CrossRef]
- Fokkinga, W.A.; Kreulen, C.M.; Le Bell-Rönnlöf, A.M.; Lassila, L.V.; Vallittu, P.K.; Creugers, N.H. In vitro fracture behavior of maxillary premolars with metal crowns and several post-and-core systems. Eur. J. Oral Sci. 2006, 11, 250–256. [Google Scholar] [CrossRef]
- Hegde, J.; Bashetty, K.; Ramakrishna, S.; Lekha, C. An in vitro evaluation of fracture strength of endodontically treated teeth with simulated flared root canals restored with different post and core systems. J. Conserv. Dent. 2012, 15, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.; Lundquist, P.; Sjögren, U.; Sundquist, G. Restorative and endodontic results after treatment with cast posts and cores. J. Prosthet. Dent. 1989, 61, 10–15. [Google Scholar] [CrossRef]
- Creugers, N.H.; Mentink, A.G.; Käyser, A.F. An analysis of durability data on post and core restorations. J. Dent. 1993, 21, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Raedel, M.; Fiedler, C.; Jacoby, S.; Boening, K.W. Survival of teeth treated with cast post and cores: A retrospective analysis over an observation period of up to 19. 5 years. J. Prosthet. Dent. 2015, 114, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Linde, L.A. The use of composites as core material in root-filled teeth. II. Clinical investigation. Swed. Dent. J. 1984, 8, 209–216. [Google Scholar]
- Worni, A.; Kolgeci, L.; Rentsch-Kollar, A.; Katsoulis, J.; Mericske-Stern, R. Zirconia-based screw-retained prostheses supported by implants: A Retrospective study on technical complications and failures. Clin. Implant. Dent. Relat. Res. 2015, 17, 1073–1081. [Google Scholar] [CrossRef]
- Moscovitch, M. Consecutive case series of monolithic and minimally veneered zirconia restorations on teeth and implants: Up to 68 months. Int. J. Periodontics Restor. Dent. 2015, 35, 314–323. [Google Scholar] [CrossRef]
- Maló, P.; de Araújo Nobre, M.; Borges, J.; Almeda, R. Retrievable metal ceramic implant-supported fixed prostheses with milled titanium frameworks and all-ceramic crowns: Retrospective clinical study with up to 10 years of follow-up. J. Prosthodont. 2012, 21, 256–264. [Google Scholar] [CrossRef]
- Paniz, G.; Stellini, E.; Meneghello, R.; Cerardi, A.; Gobbato, E.A.; Bressan, E. The precision of fit of cast and milled full-arch implant-supported restorations. Int. J. Oral Maxillofac. Implant. 2013, 28, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiao, Z.; Huang, C.; Wen, L.; Zhang, W. Influence of ultrasonic surface rolling on microstructure and wear behavior of selective laser melted Ti-6Al-4V alloy. Materials 2017, 10, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, F.Q. The application of rapid prototyping in prosthodontics. J. Prosthodont. 2012, 21, 641–644. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.W.; von Fraunhofer, J.A. The estimation of cement film thickness by an in vivo technique. Br. Dent. J. 1971, 131, 107–111. [Google Scholar] [CrossRef]
- Ferrari, M.; Vichi, A.; Mannocci, F.; Mason, P.N. Retrospective study of the clinical performance of fiber posts. Am. J. Dent. 2000, 13, 9B–13B. [Google Scholar]
- Ferrari, M.; Cagidiaco, M.C.; Goracci, C.; Vichi, A.; Mason, P.N.; Radovic, I.; Tay, F. Long-term retrospective study of the clinical performance of fiber posts. Am. J. Dent. 2007, 20, 287–291. [Google Scholar]
- Grandini, S.; Goracci, C.; Monticelli, F.; Borracchini, A.; Ferrari, M. SEM evaluation of the cement layer thickness after luting two different posts. J. Adhes. Dent. 2005, 7, 235–240. [Google Scholar]
- Sorensen, J.A.; Engelman, M.J. Effect of post adaptation on fracture resistance of endodontically treated teeth. J. Prosthet. Dent. 1990, 64, 419–424. [Google Scholar] [CrossRef]
- Geramipanah, F.; Rezaei, S.M.; Sichani, S.F.; Sichani, B.F.; Sadighpour, L. Microleakage of different post systems and a custom adapted fiber post. J. Dent. 2013, 10, 94–102. [Google Scholar]
- Musikant, B.L.; Deutsch, A.S. Endodontic posts and cores. Part II. Design of the Flexi-post. J. Ala. Dent. Assoc. 1985, 69, 42–46. [Google Scholar]
- Duc, O.; Krejci, I. Effects of adhesive composite core systems on adaptation of adhesive post and cores under load. J. Dent. 2009, 37, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.; Walton, R.; Drake, D. Coronal leakage: Endotoxin penetration from mixed bacterial communities through obturated, post-prepared root canals. J. Endod. 1998, 24, 587–591. [Google Scholar] [CrossRef]
- Mannocci, F.; Ferrari, M.; Watson, T.F. Microleakage of endodontically treated teeth restored with fiber posts and composite cores after cyclic loading: A confocal microscopic study. J. Prosthet. Dent. 2001, 85, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Muttlib, N.A.; Azman, A.N.; Seng, Y.T.; Alawi, R.; Ariffin, Z. Intracanal Adaptation of a Fiber Reinforced Post System as Compared to a Cast Post-and-Core. Acta Stomatol. Croat. 2016, 50, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Jamovi. The Jamovi Project. (Version 1.2). 2020. Available online: https://www.jamovi.org (accessed on 9 January 2020).
- R Core Team. R: A Language and Environment for Statistical Computing. (Version 3.6). 2019. Available online: https://cran.r-project.org/ (accessed on 9 January 2020).
- Patzelt, S.B.; Bishti, S.; Stampf, S.; Att, W. Accuracy of computer-aided design/computer-aided manufacturing-generated dental casts based on intraoral scanner data. J. Am. Dent. Assoc. 2014, 145, 1133–1140. [Google Scholar] [CrossRef]
- Revilla-León, M.; Gonzalez-Martín, Ó.; López, J.P.; Sánchez-Rubio, J.L.; Özcan, M. Position Accuracy of Implant Analogs on 3D Printed Polymer versus Conventional Dental Stone Casts Measured Using a Coordinate Measuring Machine. J. Prosthodont. 2018, 27, 560–567. [Google Scholar] [CrossRef]
- Linkevicius, T.; Vindasiute, E.; Puisys, A.; Maslova, N.; Puriene, A. The influence of the cementation margin position on the amount of undetected cement. A prospective clinical study. Clin. Oral Implant. Res. 2013, 24, 71–76. [Google Scholar] [CrossRef]
- Bukhari, S.A.; AlHelal, A.; Kattadiyil, M.T.; Wadhwani, C.P.K.; Taleb, A.; Dehom, S. An in vitro investigation comparing methods of minimizing excess luting agent for cement-retained implant-supported fixed partial dentures. J. Prosthet. Dent. 2020, 124, 706–715. [Google Scholar] [CrossRef]
- Goto, Y.; Nicholls, J.I.; Phillips, K.M.; Junge, T. Fatigue resistance of endodontically treated teeth restored with three dowel-and-core systems. J. Prosthet. Dent. 2005, 93, 45–50. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Shetty, S.; Coutinho, I. Factors determining post selection: A literature review. J. Prosthet. Dent. 2003, 90, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Pontius, O.; Nathanson, D.; Giordano, R.; Hutter, J.W. Survival rate and fracture strength of incisors restored with different post and core systems and endodontically treated incisors without coronoradicular reinforcement. J. Endod. 2002, 28, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Anusavice, K.J.; Shen, C.; Rawls, H.R. Phillips’ Science of Dental Materials, 12th ed.; Saunders: Philadelphia, PA, USA, 2013. [Google Scholar]
- Knosp, H.; Holliday, R.J.; Corti, C.W. Gold in Dentistry: Alloys, Uses and Performance. Gold Bull. 2003, 36, 93–102. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2007, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Steinemann, S. Titanium—The material of choice. Periodontology 2000 1998, 17, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, C.; Brindis, M.; Kattadiyil, M.T.; O’Brien, R.; Chung, K. Colorizing titanium-6aluminum-4vanadium alloy using electrochemical anodization: Developing a color chart. J. Prosthet. Dent. 2018, 119, 26–28. [Google Scholar] [CrossRef]
- ASTM Standard F67; Standard Specification for Unalloyed Titanium, for Surgical Implant Applications. ASTM International: West Conshohocken, PA, USA, 2017. Available online: http://www.astm.org/ (accessed on 9 January 2020).
- Koizumi, H.; Takeuchi, Y.; Imai, H.; Kawai, T.; Yoneyama, T. Application of titanium and titanium alloys to fixed dental prostheses. J. Prosthodont. Res. 2019, 63, 266–270. [Google Scholar] [CrossRef]
- Liu, W.; Qing, H.; Pei, X.; Wang, J. Internal adaptation of cobalt-chromium posts fabricated by selective laser melting technology. J. Prosthet. Dent. 2019, 121, 455–460. [Google Scholar] [CrossRef]
- Pulskamp, F.E. A comparison of the casting accuracy of base metal and gold alloys. J. Prosthet. Dent. 1979, 41, 272–276. [Google Scholar] [CrossRef]
- Tamac, E.; Toksavul, S.; Toman, M. Clinical marginal and internal adaptation of CAD/CAM milling, laser sintering, and cast metal ceramic crowns. J. Prosthet. Dent. 2014, 112, 909–913. [Google Scholar] [CrossRef]
- Kocaağaoğlu, H.; Kılınç, H.İ.; Albayrak, H.; Kara, M. In vitro evaluation of marginal, axial, and occlusal discrepancies in metal ceramic restorations produced with new technologies. J. Prosthet. Dent. 2016, 116, 368–374. [Google Scholar] [CrossRef]
- Akçin, E.T.; Güncü, M.B.; Aktaş, G.; Aslan, Y. Effect of manufacturing techniques on the marginal and internal fit of cobalt-chromium implant-supported multiunit frameworks. J. Prosthet. Dent. 2018, 120, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Nesse, H.; Ulstein, D.M.; Vaage, M.M.; Øilo, M. Internal and marginal fit of cobalt-chromium fixed dental prostheses fabricated with 3 different techniques. J. Prosthet. Dent. 2015, 114, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Katsoulis, J.; Mericske-Stern, R.; Yates, D.M.; Izutani, N.; Enkling, N.; Blatz, M.B. In vitro precision of fit of computer-aided design and computer-aided manufacturing titanium and zirconium dioxide bars. Dent. Mater. 2013, 29, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubaye, T.; Elsubeihi, E. The Accuracy of Custom-Made Milled Metal Posts as Compared to Conventional Cast Metal Posts. Dent. J. 2024, 12, 309. [Google Scholar] [CrossRef] [PubMed]
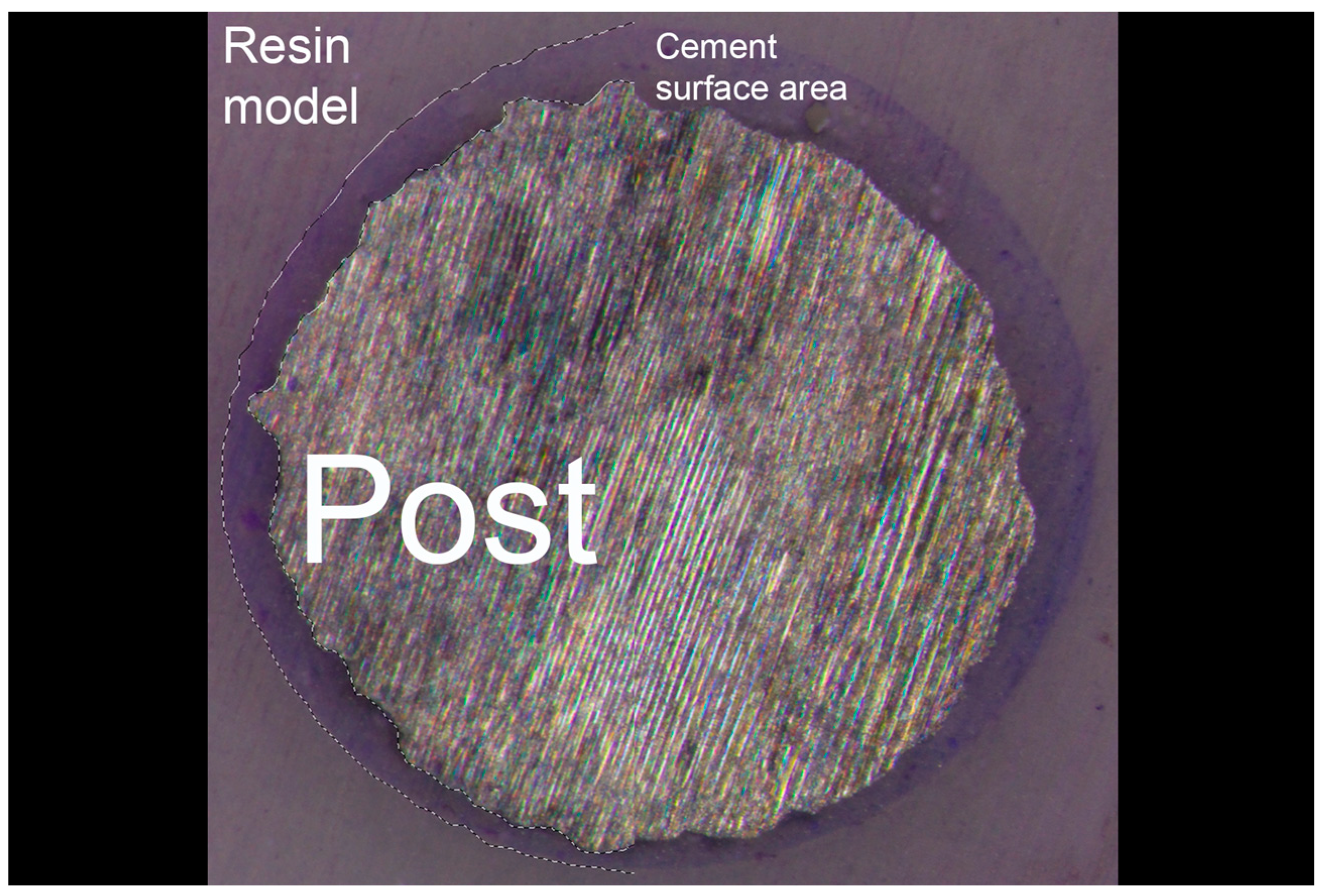
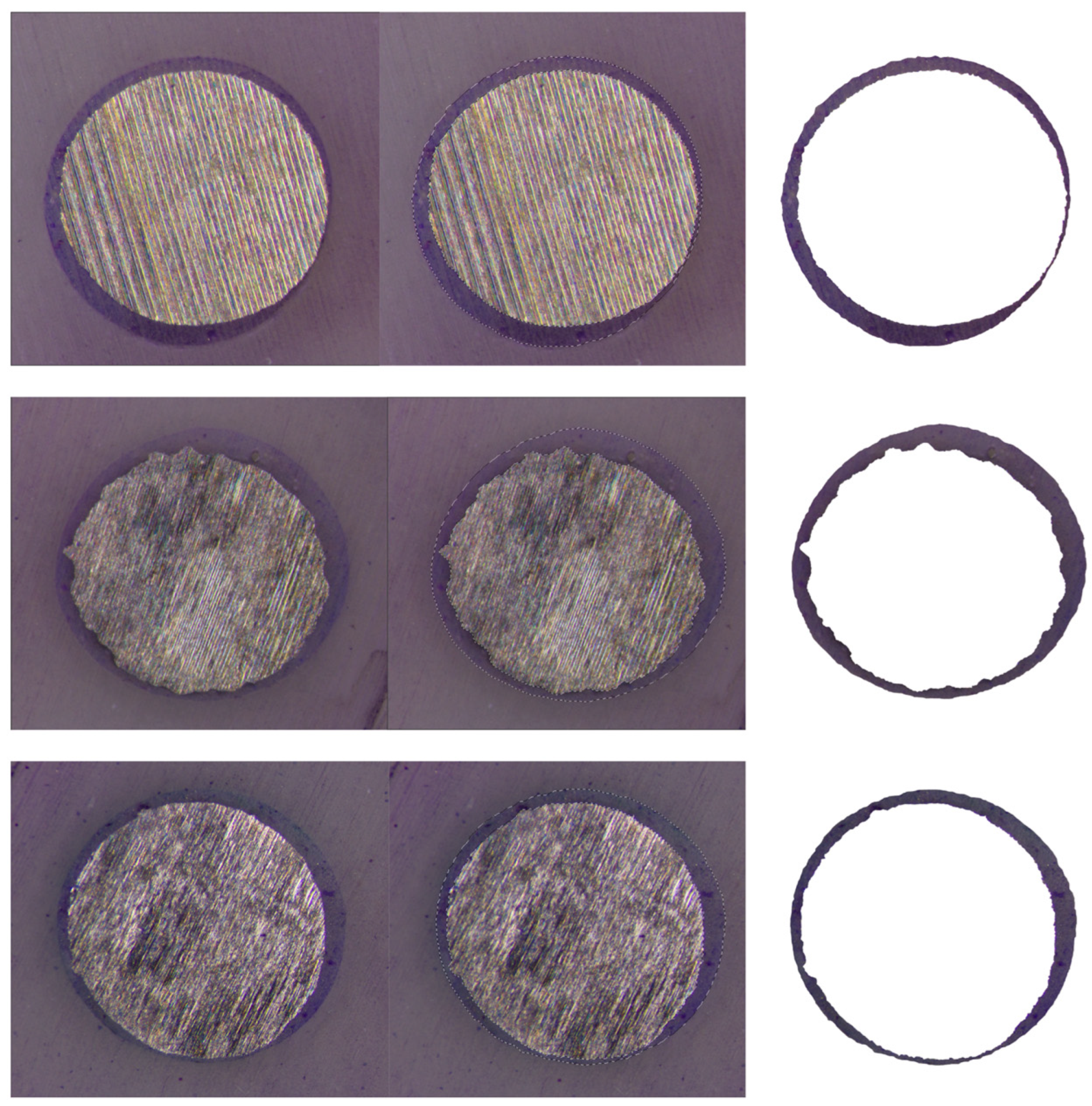
| Groups | Group A * | Group B # | Group C ^ |
|---|---|---|---|
| Coronal | 10,451 ± 4701 a | 26,044 ± 4464 | 13,992 ± 4344 a |
| Middle | 11,412 ± 8164 | 30,458 ± 21,955 b | 23,337 ± 8860 b |
| Apical | 17,737 ± 6391 | 51,106 ± 5949 | 31,193 ± 9609 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzaid, A.A.; Bukhari, S.; Kattadiyil, M.T.; Alqarni, H.; AlHelal, A.A.; Alanazi, K.K.; Suprono, M.S.; Jekki, R.; Sahl, E.F. Adaptation of 3D-Printed and Milled Titanium Custom Post and Core. Prosthesis 2024, 6, 1448-1458. https://doi.org/10.3390/prosthesis6060105
Alzaid AA, Bukhari S, Kattadiyil MT, Alqarni H, AlHelal AA, Alanazi KK, Suprono MS, Jekki R, Sahl EF. Adaptation of 3D-Printed and Milled Titanium Custom Post and Core. Prosthesis. 2024; 6(6):1448-1458. https://doi.org/10.3390/prosthesis6060105
Chicago/Turabian StyleAlzaid, Abdulaziz A., Sarah Bukhari, Mathew T. Kattadiyil, Hatem Alqarni, Abdulaziz A. AlHelal, Khalid K. Alanazi, Montry S. Suprono, Rami Jekki, and Erik F. Sahl. 2024. "Adaptation of 3D-Printed and Milled Titanium Custom Post and Core" Prosthesis 6, no. 6: 1448-1458. https://doi.org/10.3390/prosthesis6060105
APA StyleAlzaid, A. A., Bukhari, S., Kattadiyil, M. T., Alqarni, H., AlHelal, A. A., Alanazi, K. K., Suprono, M. S., Jekki, R., & Sahl, E. F. (2024). Adaptation of 3D-Printed and Milled Titanium Custom Post and Core. Prosthesis, 6(6), 1448-1458. https://doi.org/10.3390/prosthesis6060105






