Analysis of Early-Retrieved Dual-Mobility Polyethylene Liners for Total Hip Replacement
Abstract
1. Introduction
2. Materials and Methods
2.1. Visual Inspection
2.2. Material Characterisation
2.3. Geometric Assessment
3. Results
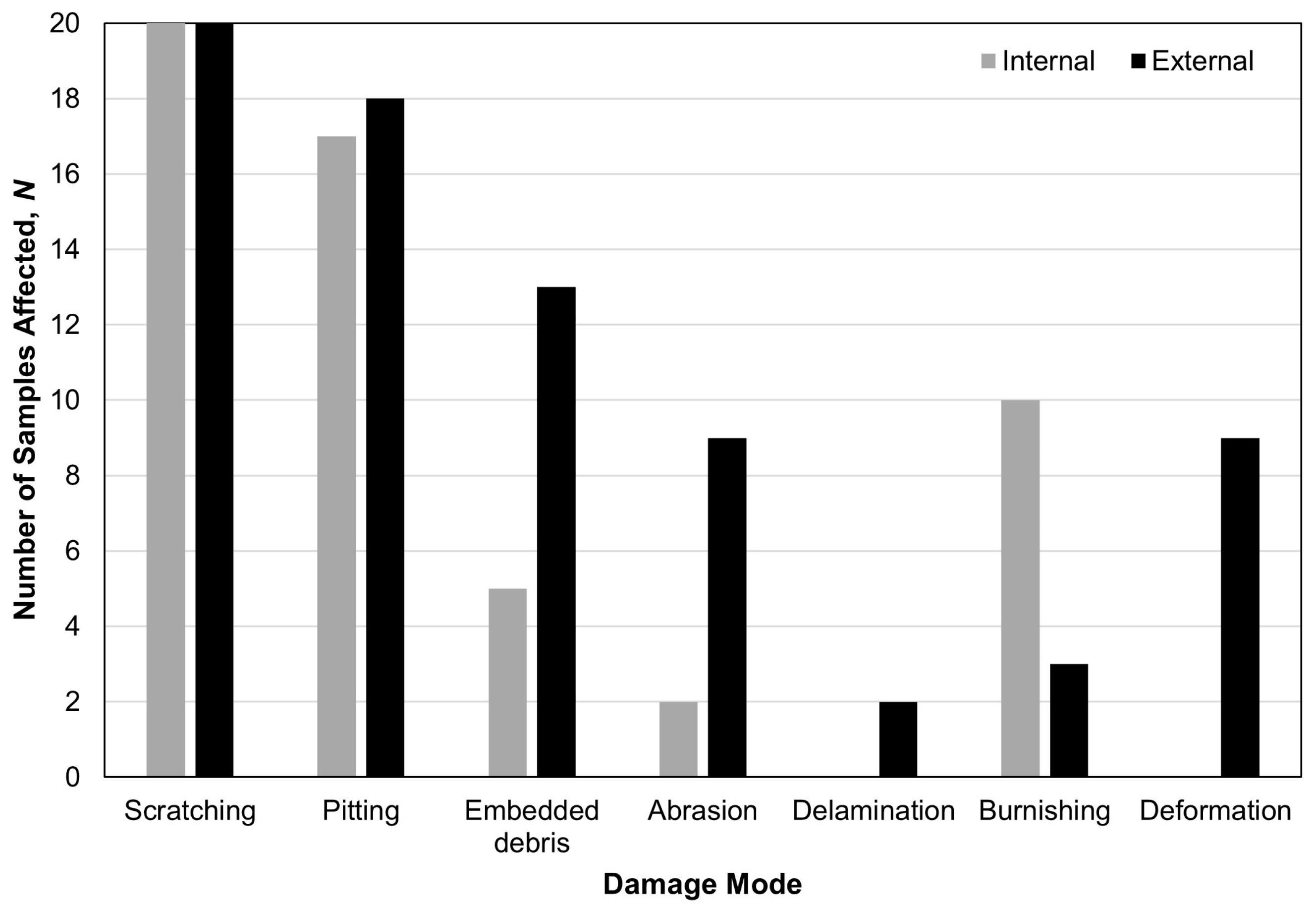
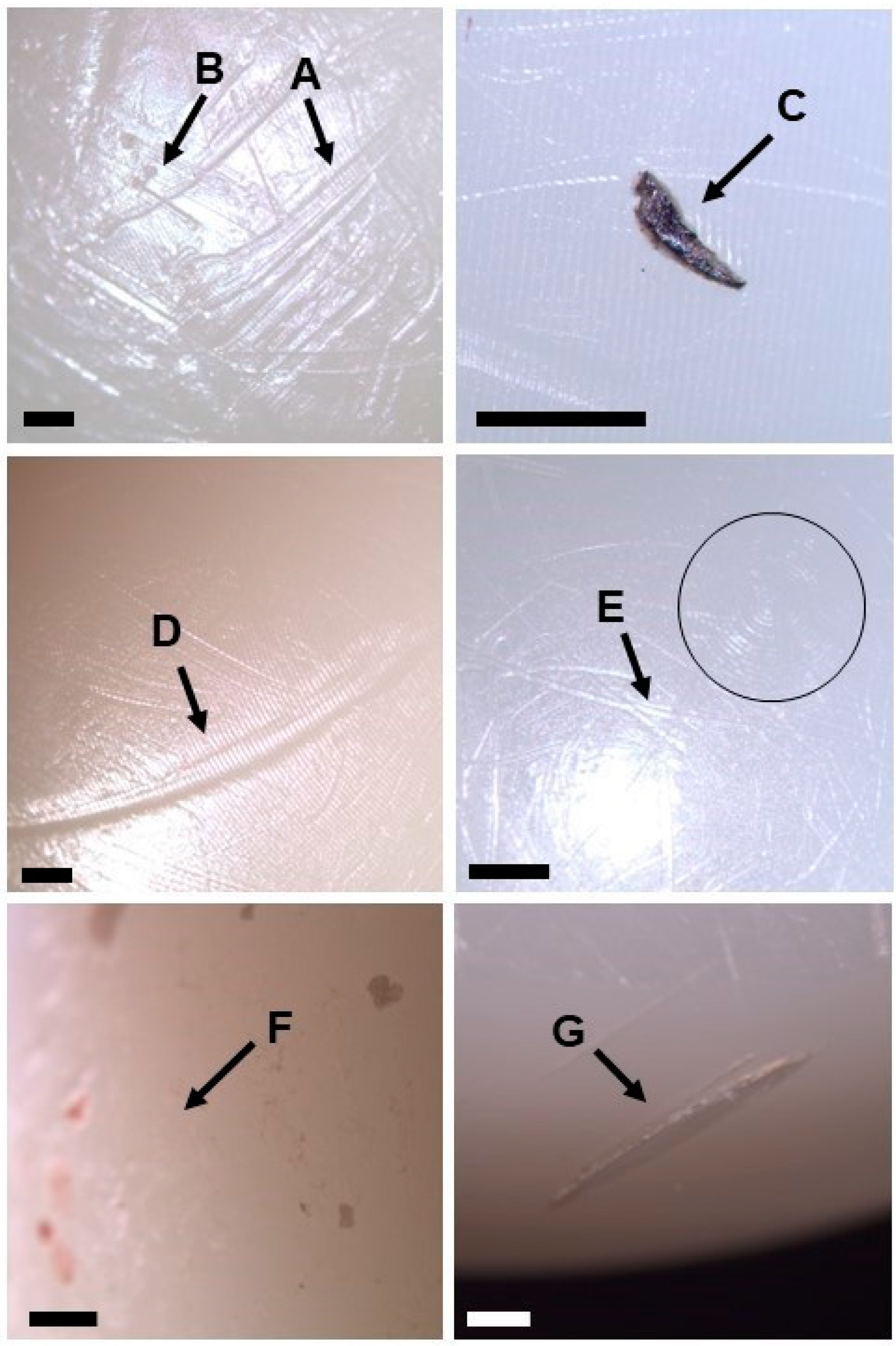
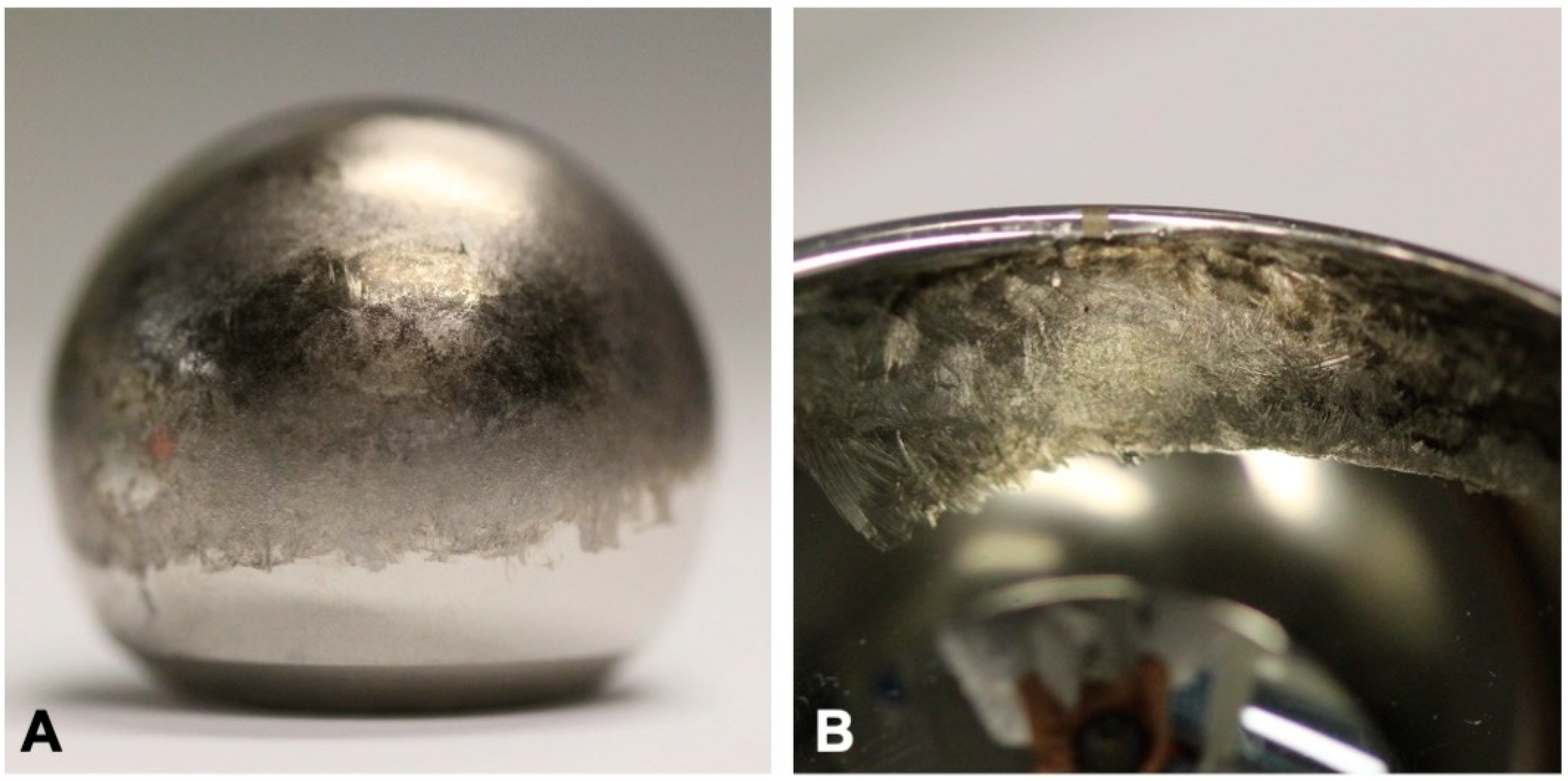
3.1. Visual Inspection
3.2. Material Characterisation of Embedded Particles
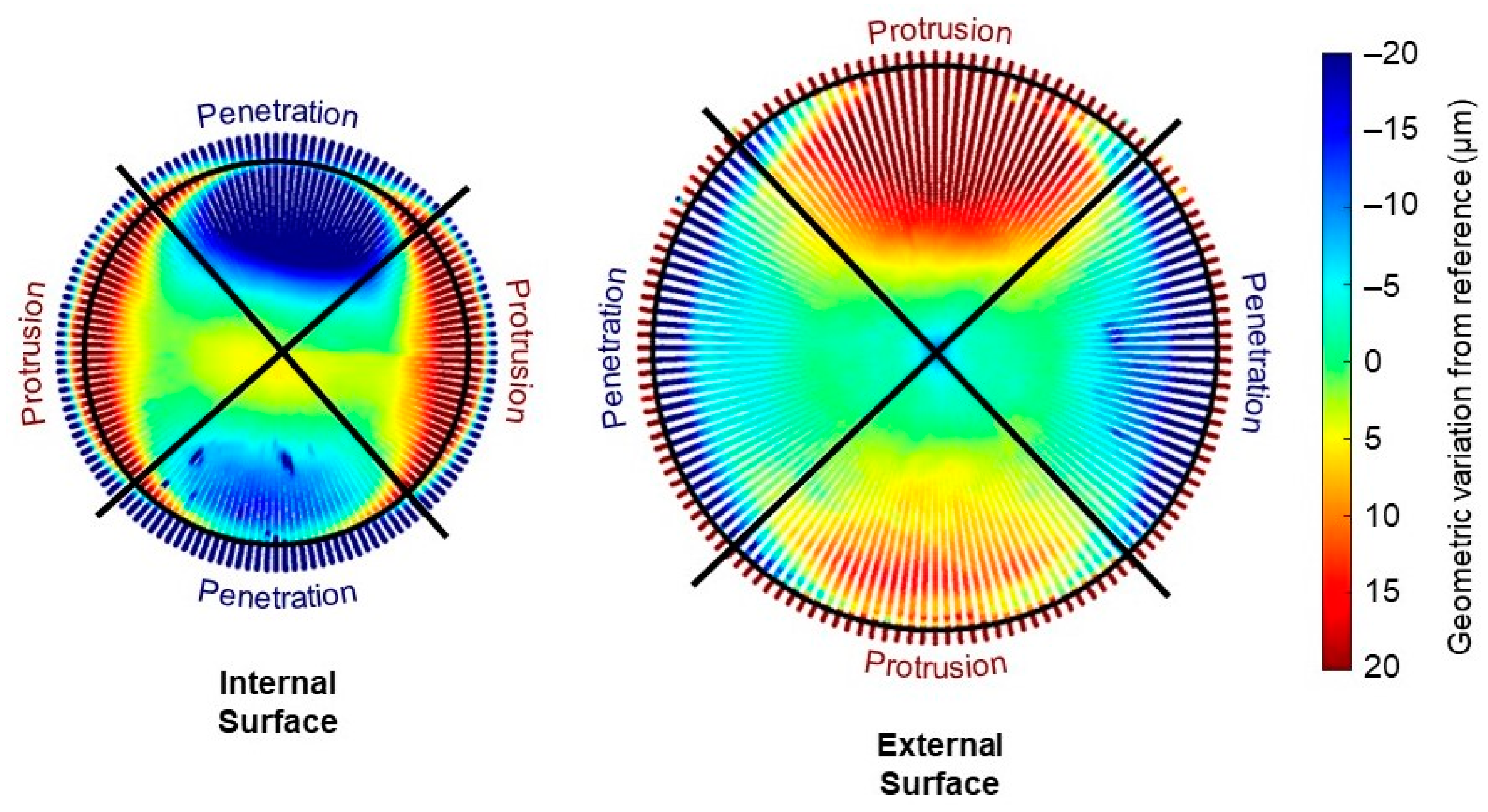
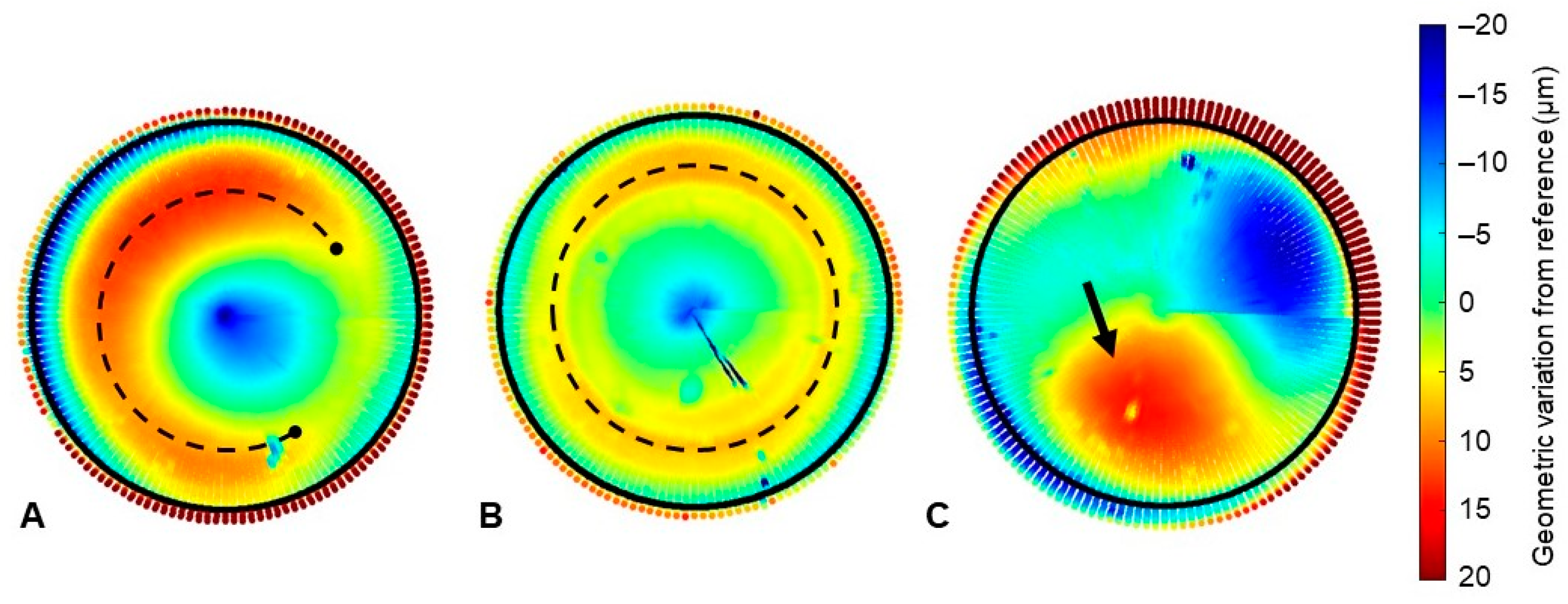
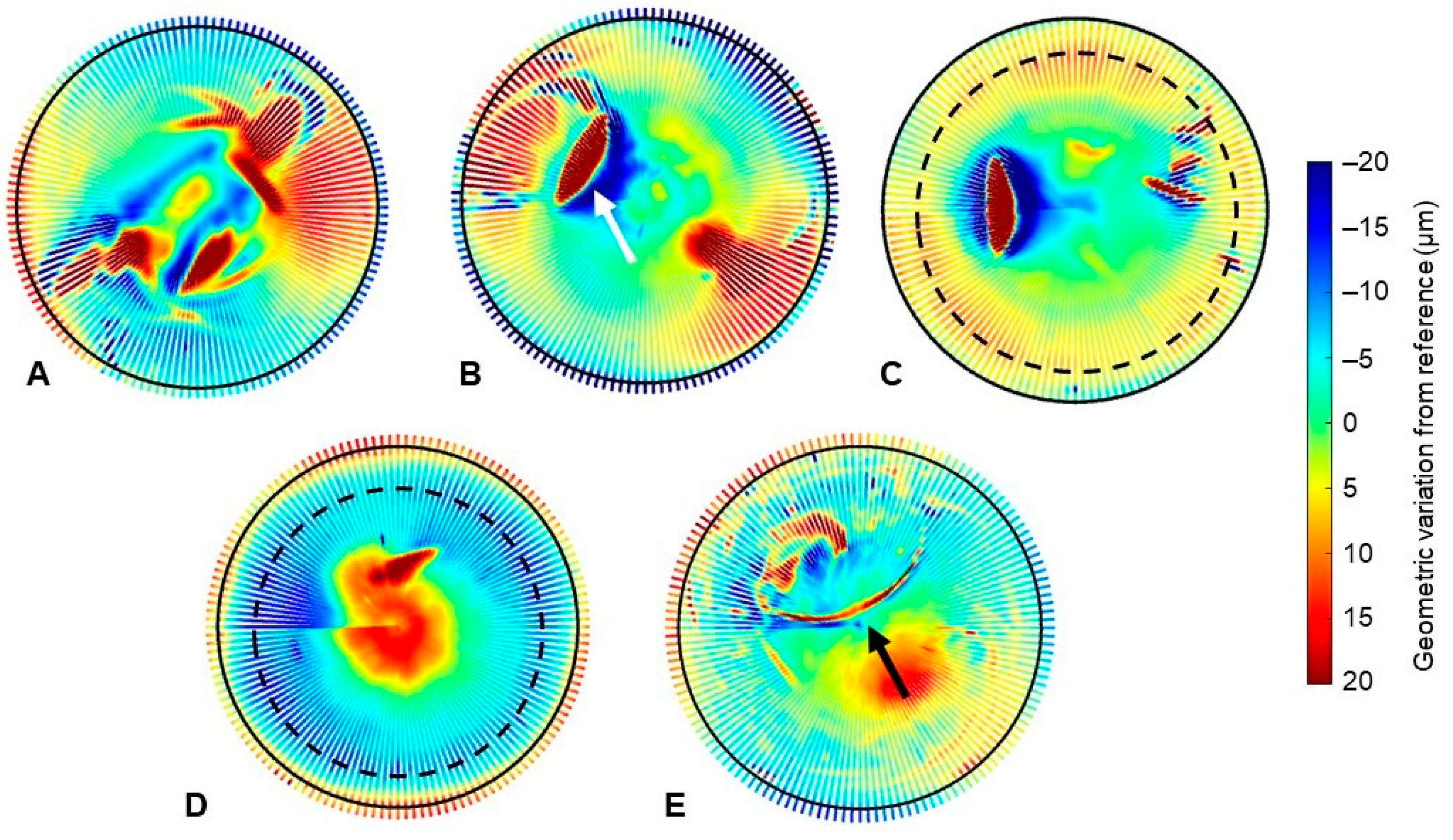
3.3. Geometric Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galvain, T.; Mantel, J.; Kakade, O.; Board, T.N. Treatment patterns and clinical and economic burden of hip dislocation following primary total hip arthroplasty in England. Bone Jt. J. 2022, 104, 811–819. [Google Scholar] [CrossRef]
- National Joint Registry. 20th Annual Report 2023. 2023. Available online: www.njrcentre.org.uk (accessed on 13 February 2024).
- American Joint Replacement Registry. The Tenth Annual Report of the AJRR on Hip and Knee Arthroplasty; American Academy of Orthopaedic Surgeons: Rosemont, IL, USA, 2023. [Google Scholar]
- Laura, A.; Di Hothi, H.; Battisti, C.; Cerquiglini, A.; Henckel, J.; Skinner, J.; Hart, A. Wear of dual-mobility cups: A review article. Int. Orthop. 2017, 41, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Adam, P.; Philippe, R.; Ehlinger, M.; Roche, O.; Bonnomet, F.; Molé, D.; Fessy, M.H. Dual mobility cups hip arthroplasty as a treatment for displaced fracture of the femoral neck in the elderly. A prospective, systematic, multicenter study with specific focus on postoperative dislocation. Orthop. Traumatol. Surg. Res. 2012, 98, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Canton, G.; Moghnie, A.; Cleva, M.; Kostoris, F.M.; Murena, L. Dual mobility total hip arthroplasty in the treatment of femoral neck fractures: A retrospective evaluation at mid-term follow-up. Acta Biomed. 2019, 90, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Klemt, C.; Smith, E.J.; Oganesyan, R.; Limmahakhun, S.; Fitz, D.; Kwon, Y.M. Outcome of Dual Mobility Constructs for Adverse Local Tissue Reaction Associated Abductor Deficiency in Revision Total Hip Arthroplasty. J. Arthroplast. 2020, 35, 3689–3691. [Google Scholar] [CrossRef] [PubMed]
- Mudrick, C.A.; Melvin, J.S.; Springer, B.D. Late posterior hip instability after lumbar spinopelvic fusion. Arthroplast. Today 2015, 1, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Nessler, J.M.; Malkani, A.L.; Sachdeva, S.; Nessler, J.P.; Westrich, G.; Harwin, S.F.; Mayman, D.; Jerabek, S. Use of dual mobility cups in patients undergoing primary total hip arthroplasty with prior lumbar spine fusion. Int. Orthop. 2020, 44, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Ozden, V.E.; Dikmen, G.; Beksac, B.; Tozun, R. Dual-mobility bearings for patients with abductor-trochanteric complex insufficiency. HIP Int. 2018, 28, 491–497. [Google Scholar] [CrossRef]
- Philippeau, J.-M.; Durand, J.-M.; Carret, J.-P.; Leclercq, S.; Waast, D.; Gouin, F. Dual mobility design socket use in preventing total hip replacement dislocation following tumor resection. Orthop. Traumatol. Surg. Res. 2010, 96, 2–8. [Google Scholar] [CrossRef]
- Sanders, R.J.M.; Swierstra, B.A.; Goosen, J.H.M. The use of a dual-mobility concept in total hip arthroplasty patients with spastic disorders: No dislocations in a series of ten cases at midterm follow-up. Arch. Orthop. Trauma Surg. 2013, 133, 1011–1016. [Google Scholar] [CrossRef]
- Zoccali, C.; Attala, D.; Scotto di Uccio, A.; Rossi, B.; Scotto, G.; Biagini, R. The dual mobility cup in muscular skeletal oncology: Rationale and indications. Int. Orthop. 2017, 41, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Loving, L.Q.; Lee, R.K.F.; Herrera, L.; Essner, A.P.; Nevelos, J.E. Wear performance evaluation of a contemporary dual mobility hip bearing using multiple hip simulator testing conditions. J. Arthroplast. 2013, 28, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Saikko, V.; Shen, M. Wear comparison between a dual mobility total hip prosthesis and a typical modular design using a hip joint simulator. Wear 2010, 268, 617–621. [Google Scholar] [CrossRef]
- Adam, P.; Farizon, F.; Fessy, M.H. Dual mobility retentive acetabular liners and wear: Surface analysis of 40 retrieved polyethylene implants. Orthop. Traumatol. Surg. Res. 2014, 100, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Boyer, B.; Neri, T.; Geringer, J.; Di Iorio, A.; Philippot, R.; Farizon, F. Long-term wear of dual mobility total hip replacement cups: Explant study. Int. Orthop. 2018, 42, 41–47. [Google Scholar] [CrossRef] [PubMed]
- D’Apuzzo, M.R.; Koch, C.N.; Esposito, C.I.; Elpers, M.E.; Wright, T.M.; Westrich, G.H. Assessment of Damage on a Dual Mobility Acetabular System. J. Arthroplast. 2016, 31, 1828–1835. [Google Scholar] [CrossRef]
- Darrith, B.; Courtney, P.M.; Della Valle, C.J. Outcomes of dual mobility components in total hip arthroplasty: A systematic review of the literature. Bone Jt. J. 2018, 100, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Neri, T.; Boyer, B.; Geringer, J.; Di Iorio, A.; Caton, J.H.; PhiIippot, R.; Farizon, F. Intraprosthetic dislocation of dual mobility total hip arthroplasty: Still occurring? Int. Orthop. 2019, 43, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Vielpeau, C.; Lebel, B.; Ardouin, L.; Burdin, G.; Lautridou, C. The dual mobility socket concept: Experience with 668 cases. Int. Orthop. 2011, 35, 225–230. [Google Scholar] [CrossRef]
- Hood, R.W.; Wright, T.M.; Burstein, A.H. Retrieval analysis of total knee prostheses: A method and its application to 48 total condylar prostheses. J. Biomed. Mater. Res. 1983, 17, 829–842. [Google Scholar] [CrossRef]
- Smeeton, M.; Isaac, G.; Wilcox, R.; Anderson, J.; Board, T.; Van Citters, D.W.; Williams, S. A geometric assessment method for estimating dimensional change of retrieved dual mobility liners for total hip arthroplasty. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2023, 237, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Smeeton, M. Dataset Associated with “Analysis of Retrieved Dual Mobility Polyethylene Liners for Total Hip Replacement”; [Dataset]. 2024. [Google Scholar] [CrossRef]
- Reina, N.; Pareek, A.; Krych, A.J.; Pagnano, M.W.; Berry, D.J.; Abdel, M.P. Dual-Mobility Constructs in Primary and Revision Total Hip Arthroplasty: A Systematic Review of Comparative Studies. J. Arthroplast. 2019, 34, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Geringer, J.; Boyer, B.; Farizon, F. Understanding the dual mobility concept for total hip arthroplasty. Investigations on a multiscale analysis-highlighting the role of arthrofibrosis. Wear 2011, 271, 2379–2385. [Google Scholar] [CrossRef]
- Al-Hajjar, M.; Fisher, J.; Williams, S.; Tipper, J.L.; Jennings, L.M. Effect of femoral head size on the wear of metal on metal bearings in total hip replacements under adverse edge-loading conditions. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2013, 101, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Leslie, I.J.; Williams, S.; Isaac, G.; Ingham, E.; Fisher, J. High cup angle and microseparation increase the wear of hip surface replacements. Clin. Orthop. Relat. Res. 2009, 467, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer Lancaster-Jones, O.; Williams, S.; Jennings, L.M.; Thompson, J.; Isaac, G.H.; Fisher, J.; Al-Hajjar, M. An in vitro simulation model to assess the severity of edge loading and wear, due to variations in component positioning in hip joint replacements. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2018, 106, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.I.; Walter, W.L.; Roques, A.; Tuke, M.A.; Zicat, B.A.; Walsh, W.R.; Walter, W.K. Wear in alumina-on-alumina ceramic total hip replacements: A retrieval analysis of edge loading. J. Bone Jt. Surg.-Ser. B 2012, 94, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Sariali, E.; Klouche, S.; Mamoudy, P. Ceramic-on-ceramic total hip arthroplasty: Is squeaking related to an inaccurate three-dimensional hip anatomy reconstruction? Orthop. Traumatol. Surg. Res. 2014, 100, 437–440. [Google Scholar] [CrossRef]
- Loving, L.; Herrera, L.; Banerjee, S.; Heffernan, C.; Nevelos, J.; Markel, D.C.; Mont, M.A. Dual mobility bearings withstand loading from steeper cup-inclinations without substantial wear. J. Orthop. Res. 2015, 33, 398–404. [Google Scholar] [CrossRef]
| Factor | Details |
|---|---|
| BMI | 29.5 ± 6.9 Unknown, 3 |
| Length of implantation (months) | 20.0 ± 18.8 Unknown, 3 |
| Implant side (left/right) | Left, 10 (50%) Right, 8 (40%) Unknown, 3 |
| Reason for revision | Loosening, 6 (30%) Infection, 3 (15%) Instability/dislocation, 2 (10%) Periprosthetic fracture, 2 (10%) Intra-prosthetic dislocation, 2 (10%) Metal wear, 1 (5%) Pain, 1 (5%) Unknown, 3 |
| Device type | Stryker ADM/MDM, 13 (65%) Zimmer Biomet Active Articulation, 3 (15%) Serf Novae, 2 (10%) Zimmer Biomet Avantage, 1 (5%) Dedienne Sante ADES, 1 (5%) |
| Bearing combination | MoPoM, 11 (55%) CoPoM, 6 (30%) Unknown, 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smeeton, M.; Isaac, G.; Wilcox, R.; Anderson, J.; Board, T.; Van Citters, D.W.; Williams, S. Analysis of Early-Retrieved Dual-Mobility Polyethylene Liners for Total Hip Replacement. Prosthesis 2024, 6, 841-852. https://doi.org/10.3390/prosthesis6040060
Smeeton M, Isaac G, Wilcox R, Anderson J, Board T, Van Citters DW, Williams S. Analysis of Early-Retrieved Dual-Mobility Polyethylene Liners for Total Hip Replacement. Prosthesis. 2024; 6(4):841-852. https://doi.org/10.3390/prosthesis6040060
Chicago/Turabian StyleSmeeton, Mackenzie, Graham Isaac, Ruth Wilcox, James Anderson, Tim Board, Douglas W. Van Citters, and Sophie Williams. 2024. "Analysis of Early-Retrieved Dual-Mobility Polyethylene Liners for Total Hip Replacement" Prosthesis 6, no. 4: 841-852. https://doi.org/10.3390/prosthesis6040060
APA StyleSmeeton, M., Isaac, G., Wilcox, R., Anderson, J., Board, T., Van Citters, D. W., & Williams, S. (2024). Analysis of Early-Retrieved Dual-Mobility Polyethylene Liners for Total Hip Replacement. Prosthesis, 6(4), 841-852. https://doi.org/10.3390/prosthesis6040060








