Abstract
Background: This pilot study aimed to evaluate the effectiveness of digitally crafted customized healing abutments in stabilizing peri-implant soft tissues following tooth extraction and assess the preservation of peri-implant soft tissue architecture over 5 years. Material and Methods: Forty patients (age ≥ 25 years) were divided into test (n = 20) and control (n = 20) groups. The test group received dental implants with immediate loading after tooth extraction, along with customized healing abutments fabricated using CAD/CAM technology. The control group received dental implants with immediate loading without customized healing abutments. The primary outcome was the change in distance between the peri-implant soft tissue margin and implant fixture surface from baseline to 5 years post implantation. Results: In the test group, there was a significant decrease in the distance between the peri-implant soft tissue margin and fixture surface from baseline to 5 years (p < 0.001), with pairwise comparisons showing significant differences between multiple time points (p < 0.05). The control group showed less pronounced changes over time. Conclusions: Within the limitations of this pilot study, digitally fabricated customized healing abutments appear effective in stabilizing peri-implant soft tissues and preserving soft tissue architecture around dental implants over 5 years following immediate implant placement. Randomized controlled trials are needed to confirm these findings.
1. Introduction
Dimensional changes in alveolar bone following tooth extraction are well documented [1,2]. These changes exhibit significant variations based on anatomical zones, especially during the initial six months [3,4]. Flapless implant placement reduces this phenomenon by preventing the physiological resorption associated with flap elevation [5,6,7]. Immediate loading of single implants demonstrates survival rates ranging from 95% to 98% [8,9], attributed to the precision of prosthodontic margins and correct occlusal design of the crowns [10,11,12]. Immediate loading of provisional prostheses can be obtained analogically or through pre-surgical impressions [13,14]. However, these procedures involve repetitive stresses and elongated time [15]. Tarnow et al. investigated horizontal volume changes following post-extraction implant placement [16]. They proposed four scenarios: bone grafting with or without a provisional prosthesis, and no grafting with or without a provisional prosthesis. The best results were achieved by inserting a bone graft stabilized with a healing screw or a customized provisional [17]. When immediate loading is not feasible, a customized healing screw is a valid alternative [18], minimally impacting masticatory forces during early osseointegration [19]. However, the analogue creation of customized healing screws can be complex [20]. Digital techniques aim to achieve results comparable to or better than analogue methods [5]. These techniques also reduce chairside times and improve patient comfort [21,22]. Moreover, computer-aided design and manufacturing (CAD/CAM) technologies have shown improved time efficiency in implant-supported reconstructions compared to manual techniques [23]. Recent advancements in CAD/CAM technologies have led to improved time efficiency in implant-supported reconstructions compared to manual techniques. Subtractive CAM (s-CAM), such as CNC milling, is the current gold standard but has limitations, including material waste and accuracy issues influenced by the milling process and bur characteristics [5]. In contrast, additive CAM (a-CAM), which involves 3D printing, has emerged as a promising alternative that overcomes geometric restrictions and reduces material waste [24]. This study influences these digital CAD/CAM techniques to fabricate customized healing abutments, taking advantage of the improved efficiency and precision offered by these cutting-edge technologies.
2. Materials and Methods
2.1. Ethics
This study protocol was approved by the institutional scientific review board of Galeazzi Hospital (Milan, Italy; Prot. no. 75/2019-L2058). All participants provided informed consent and were fully aware of the study’s purpose, procedures, risks, and benefits. Appropriate data were handled and security measures were implemented to protect participant privacy and confidentiality.
2.2. Patients
This prospective study comprised a test group (n = 20) receiving implants with immediate loading and CAD/CAM customized healing abutments and a control group (n = 20) receiving implants with immediate loading without customized abutments. The control group underwent the same surgical procedure as the test group, including tooth extraction and immediate implant placement. However, instead of receiving customized healing abutments fabricated using CAD/CAM technology, the control group received standard, non-customized healing abutments provided by the implant manufacturer. All other aspects of the treatment, including the implant system, surgical protocol, and postoperative care, were identical for both groups. The use of a control group receiving standard healing abutments allows for the evaluation of the specific effects of the customized healing abutments on the stability and preservation of the peri-implant soft tissue architecture over time.
2.3. Inclusion and Exclusion Criteria
Exclusion criteria included a history of head/neck radiation therapy, the use of medications that might carry a risk of osteonecrosis of the jaw (ONJ), hematologic coagulation disorders, bruxism, poor at-home oral hygiene, unstable occlusion, untreated dental caries, uncontrolled periodontal disease, teeth adjacent to those to be extracted with mobility grade I or higher, unrealistic outcome expectations, inability, or unwillingness to attend routine follow-up visits. The inclusion criteria of good general health, absence of chronic systemic illnesses, and non-smoking status ensured that the study participants were in a stable medical condition and did not have any underlying factors that could potentially confound the results or increase the risk of complications. These criteria contributed to the study’s objectives by minimizing the influence of systemic factors on the outcomes of interest, allowing for a more focused evaluation of the peri-implant soft tissue architecture. The exclusion criteria were designed to control for potential confounding factors that could negatively impact the success and stability of dental implants. These conditions could alter the healing process, increase the risk of implant failure, or compromise the maintenance of peri-implant soft tissue architecture over time.
2.4. Outcome Assessment
The primary outcome measure was the maintenance of peri-implant soft tissue architecture over a period of five years, assessed by measuring the distance between the peri-implant soft tissue margin (defined as the gingival and peri-implant connective tissue) and the implant fixture surface (defined as the surface of the implant that is surgically placed into the jawbone) at baseline (T0), three months (T1), one year (T2), and five years (T3). T0 represents the situation after the implant insertion. Upper premolars (teeth 1.4 and 1.5) and molars (teeth 1.6 and 1.7) designated for extraction had ≥5 mm apical bone, ≥12 mm height, ≥5.5 mm alveolar bone thickness, and D2 or D3 bone quality (Misch classification). Teeth lacking vestibular/palatal cortical bone, with periapical/periodontal lesions, or Class B/C (Tarnow classification) were excluded (Figure 1, Figure 2 and Figure 3A).
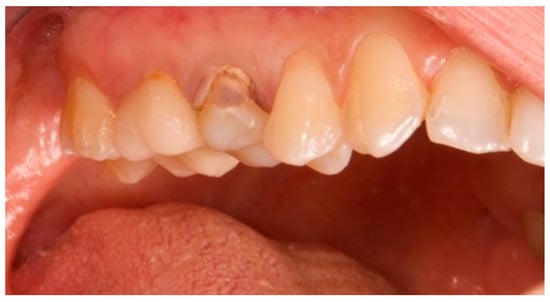
Figure 1.
Vestibular view prior to implant insertion of tooth 1.5.
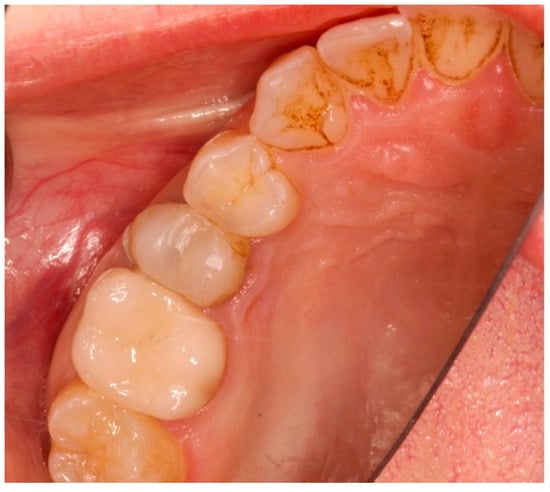
Figure 2.
Occlusal view prior to implant insertion of tooth 1.5.
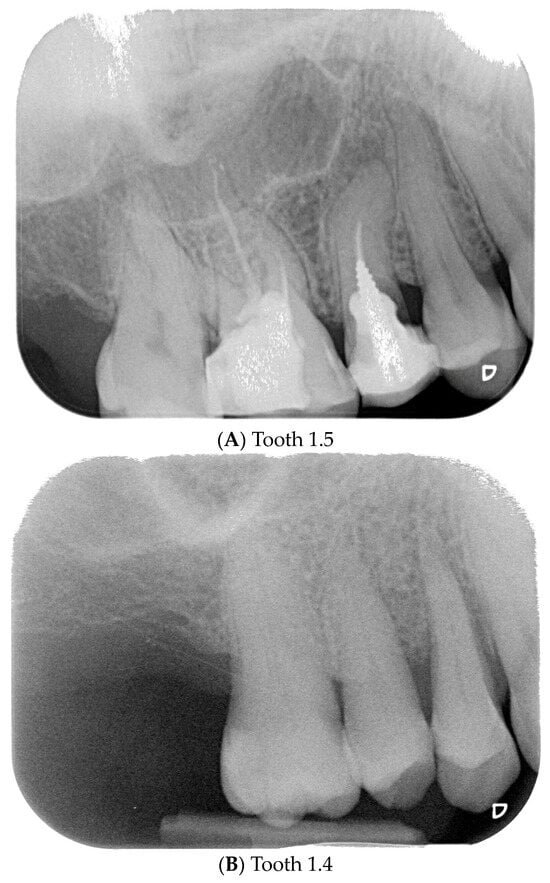
Figure 3.
Endoral RX prior to implant insertion: test group (A) vs. control group (B).
2.5. Oral Examination and Imaging Diagnostics
Preoperative examination addressed smile line, inter-arch relationship, and bucco-lingual bone thickness. Periapical radiographs and cone beam computed tomography (CBCT) were obtained to assess root dimensions, bone availability, adjacent structures, and implant planning. Preoperative periapical radiographs were taken using the VistaScan Mini Plus device (Figure 3) (Dürr Dental SE, Bietigheim-Bissingen, Germany).
Cone beam computed tomography (CBCT) was performed to assess various anatomical aspects, including mesio-distal root width, remaining bone in the apical region and root angulation, proximity to adjacent roots, and for calculating the diameter of the implants to be placed. Implant planning was based on the coronal anatomy and guided the implant position, diameter, and length using the On-Demand3D software (Version: (34104) T 02-6959-1553 | F 02-6959; Cybermed, Daejeon, Republic of Korea).
2.6. Surgical Procedure
In the three months leading up to the surgical intervention, patients were given comprehensive instructions on proper at-home oral hygiene practices, including detailed post-surgical care procedures. Additionally, they underwent a thorough professional oral hygiene session to ensure optimal oral health prior to the implant placement. As a prophylactic measure, patients were administered one gramme of amoxicillin one hour before the surgical procedure, and continued the antibiotic treatment for six days post surgery, taking one gramme every eight hours. To further reduce the risk of infection, patients were instructed to rinse with a 0.20% chlorhexidine mouthwash for one minute just before the administration of anaesthesia and to continue this regimen twice daily for five days following the surgery. Prior to the extraction of the tooth, an intraoperative impression was taken using the TRIOS device (3SHAPE, Copenhagen, Denmark), which captured the tooth to be extracted, adjacent teeth, the antagonist hemi-arch, and their interocclusal relationship. The TRIOS device (3SHAPE) allows for accurate digital impressions, facilitating precise planning and fabrication of the provisional restoration. Following the administration of local anesthesia, the tooth was carefully removed, and the alveolus was meticulously cleaned to remove any remnants of the periodontal ligament, ensuring a clean and healthy implant site. In all patients, a Zimmer TSV implant (Zimmer, Parsippany, NJ, USA) with a size of 4.1 × 11.5 mm was placed following the manufacturer’s recommended protocol. The Zimmer TSV implant (4.1 × 11.5 mm) is designed for optimal stability and osseointegration, with specific dimensions chosen based on the anatomical characteristics of the implant site. The implants were strategically positioned at the crest of the alveolar ridge to ensure optimal stability and osseointegration. To facilitate the fabrication of the provisional restoration, a scan abutment (Zfx Intrascan Matchholder, Zfx, Dachau, Germany) was screwed onto the implant, and an intraoperative optical impression of the hemi-arch containing the implant with the scan abutment was taken. The Intrascan abutment enables the acquisition of a digital impression of the implant site with the scan abutment in place, crucial for the precise design and milling of the provisional restoration. In cases where gaps were present between the vestibular bone and the implant, heterologous bone grafting material (CopiOs, Zimmer, Parsippany, NJ, USA) was used to fill these spaces, promoting better osseointegration and long-term stability of the implant. Following the completion of the surgical procedure, the provisional restoration was designed using advanced CAD/CAM technology, milled from a high-quality material, and applied to the implant as soon as it was ready. This immediate provisionalization helps to maintain the soft tissue contours and promotes better healing and esthetic outcomes. The CAD/CAM technology for provisional restoration allows for the precise design and milling of the provisional restoration, ensuring a high-quality, well-fitting restoration that maintains soft tissue contours and promotes better healing and esthetic outcomes. Finally, to ensure proper healing and to minimize the risk of complications, the mesial and distal papillae were carefully sutured using Vicryl 4-0 thread (Vicryl Ethicon, Johnson and Johnson, Sommerville, NJ, USA), which is a resorbable suture material that provides adequate strength and promotes tissue approximation during the healing process.
2.7. Customized Healing Abutment and Final Crown
The custom-made healing abutment was fabricated using advanced Computer-Aided Manufacturing (CAM) techniques. The abutment was precisely milled from a solid block of high-quality Polymethylmethacrylate (PMMA) (IESS, Pozzuolo del Friuli, UD, Italy), which had a minimal monomer residue of less than 0.02%, ensuring excellent biocompatibility and durability. The intricate CAD design process was carried out using state-of-the-art software (Performa, Pozzuolo del Friuli, UD, Italy), which allowed for the seamless integration of pre-operative (Figure 4) and intra-operative impressions to create the optimal design for the healing abutment [25,26].
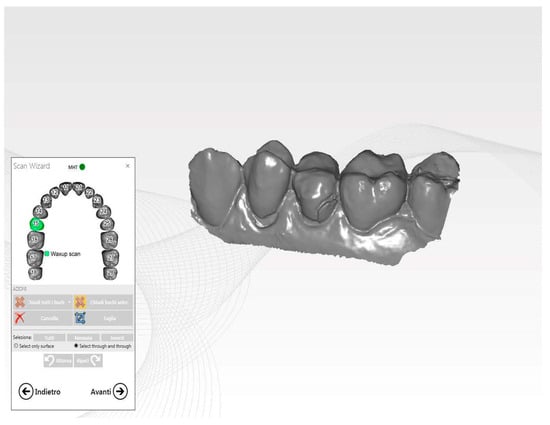
Figure 4.
Pre-operative STL image.
The pre-operative impression of the tooth scheduled for extraction served as the foundation for the provisional shape. The only modification made was a slight reduction in the occlusal plane to prevent any interference with the patient’s occlusion (Figure 5).
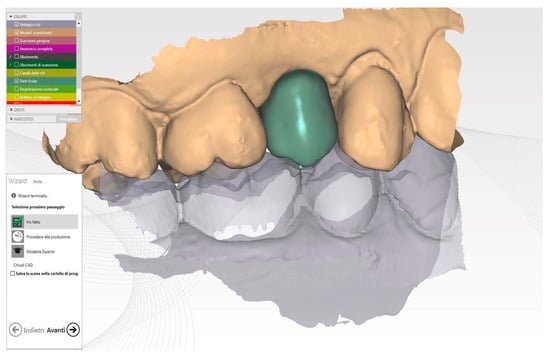
Figure 5.
Healing screw CAD modelling starting to future final crown shape.
To ensure the precise positioning of the implant, the intra-operative impression was utilized. The CAD software, using this crucial information, accurately positioned the virtual ti-base within the selected provisional, determining its ideal shape and orientation based on the correct coordinates. The finalized CAD file was then sent to a cutting-edge milling machine (Zfx Mill Inhouse X4, Zfx, Dachau, Germany) for fabrication. The healing abutment underwent a meticulous finishing and polishing process, taking great care not to interfere with the closure margins (Figure 6).
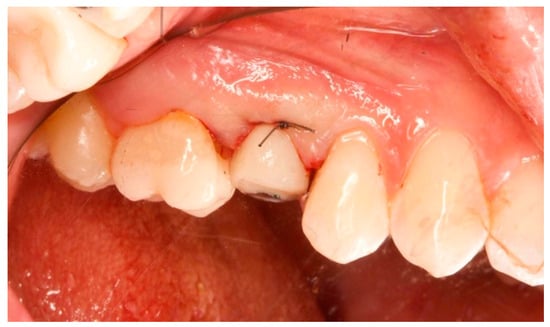
Figure 6.
Healing abutment in situ after implant insertion.
This attention to detail ensured precise fit and optimal soft tissue healing around the abutment.
2.8. Final Crown
Three months after the provisional crown was placed (Figure 7), the final crown was inserted. The same scan of the tooth before extraction was used for its creation, and a scan of the healed mucosal tunnel was performed. The same scan of the tooth before extraction was used for its creation, and a scan of the healed mucosal tunnel was performed.
Figure 7.
Occlusal view after implant insertion at 3 months (T1).
Using the same software, the final crown was designed, matching the unaltered pre-operative impression, the mucosal tunnel impression, and the provisional impression. The monolithic zirconia crown (Figure 8) (Zirconium BionX2, Zfx, Dachau, Germany) was milled, stained, and sintered (AUSTROMAT 674i, Dekema Dental Keramiköfen GmbH, Freilassing, Germany).
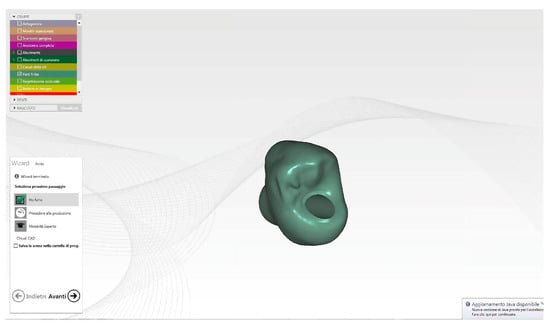
Figure 8.
STL image of final crown Cad modelling.
A titanium ti-base (Zimmer) was used to which the crown was attached and then screwed into the implant (Figure 9).
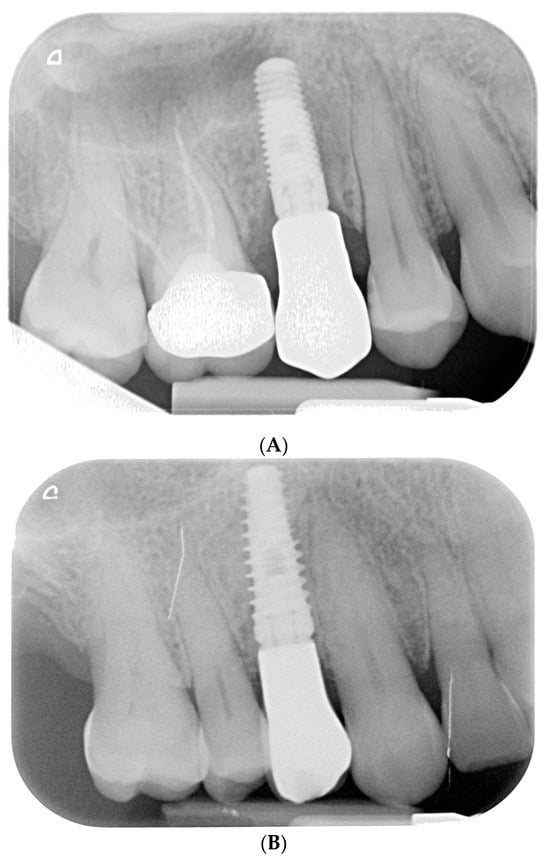
Figure 9.
Endoral RX after final crown delivery. (A) Tooth 1.5; (B) Tooth 1.4.
2.9. Statistical Analysis
The statistical analysis employed a Linear Mixed Model to evaluate the effects of group (test vs. control) and time (T0, T1, T2, T3) on the dependent variable, which was the distance between the peri-implant soft tissue margin (gingival and peri-implant connective tissue) and the implant fixture surface. The assumptions of normality and homogeneity of variance were assessed to ensure the validity of the model. Normality was evaluated using Q-Q plots, comparing the quantiles of model residuals against those of a normal distribution. Homogeneity of variance was assessed using Levene’s test to check whether the variance of the dependent variable was consistent across groups. Pairwise comparisons were conducted within each group over time to examine the simple main effects. The Bonferroni adjustment was applied to control for the increased risk of Type I errors due to multiple comparisons. Additionally, a repeated measures ANOVA was conducted to determine whether there were significant differences among the time points (T0, T1, T2, T3) (Figure 10).
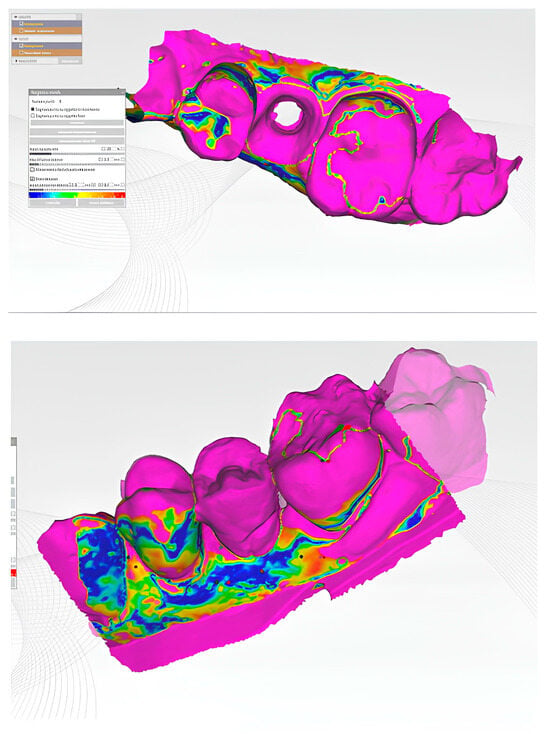
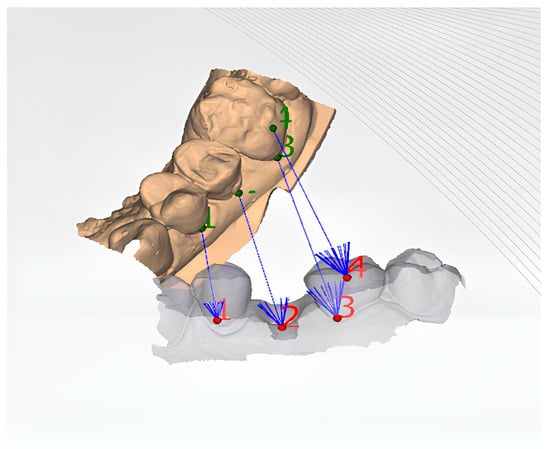
Figure 10.
Illustration of the matching of different STL files to evaluate the 3D changes in the peri-implant soft tissue margin at different time points. By superimposing and comparing the STL files from T0, T1, T2, and T3, the study can assess the spatial changes in the soft tissue margin over time, providing a comprehensive understanding of the intervention’s effects on the peri-implant tissues.
This analysis further validated the assumption of normality for the model residuals using a Q-Q scatterplot, ensuring that the residuals closely followed the diagonal line, indicating a normal distribution. The alignment suggests that the assumption of normality is satisfied for the model residuals (Figure 11).
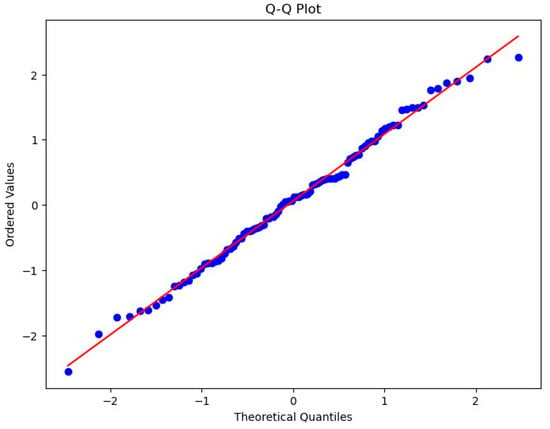
Figure 11.
Q-Q scatterplot for normality of the residuals for the regression model.
The statistical analysis was performed using IBM SPSS Statistics for Windows, version 19.0 (IBM Corp., Armonk, NY, USA).
The rationale for the selection of measurement time points (baseline [T0], three months [T1], one year [T2], and five years [T3]) justifies their suitability for evaluating the long-term maintenance of peri-implant soft tissue architecture in the context of immediate implant placement and provisionalization. The baseline measurement was crucial for understanding the starting condition of the soft tissues and provided a basis for comparison with subsequent measurements. The three-month time point (T1) was crucial for evaluating the short-term response of the peri-implant soft tissues to the immediate implant placement and provisionalization procedure. This time point allowed for the assessment of early healing and the stability of the soft tissue architecture during the critical initial months following the surgical intervention. The one-year time point (T2) provided insights into the medium-term stability of the peri-implant soft tissue architecture. By measuring the distance between the soft tissue margin and the implant fixture surface at this time point, the study aimed to evaluate the effectiveness of the immediate implant placement and provisionalization protocol in maintaining soft tissue health and esthetics over a longer period. Finally, the five-year time point (T3) was essential for assessing the long-term success of the immediate implant placement and provisionalization protocol in maintaining peri-implant soft tissue architecture.
2.10. Assessment of Inter- and Intra-Examiner Reliability
Assessment of inter- and intro-examiner reliability to ensure consistency among the examiners and regular update training sessions throughout the study period to maintain the skills and knowledge of the examiners were carried out. The intraclass correlation coefficients (ICCs) provided a high level of inter-examiner agreement and intra-examiner agreement. The overall inter-examiner agreement was 0.86 (p < 0.001), indicating a strong level of consistency between different examiners when measuring the same subjects or samples. Similarly, the intra-examiner agreement was 0.83 (p < 0.05), suggesting that individual examiners were highly consistent in their measurements when assessing the same subjects or samples on different occasions.
2.11. Sample Size Calculation
The study aimed to investigate the feasibility of stabilizing post-extraction peri-implant soft tissues with a CAD/CAM customized healing screw and assess the maintenance of peri-implant soft tissue architecture over five years. A moderate effect size was assumed, reflecting typical expectations in clinical research where large effects are uncommon. This assumption was based on previous literature. The statistical power was set at 80%, ensuring a high probability of detecting a true effect if it exists. The significance level was established at 0.05, allowing a 5% risk of Type I error. Recognizing the practical challenges of clinical research, a 10% dropout rate was factored into the calculations. This adjustment ensures that the study maintains its statistical integrity, even with potential participant attrition. By integrating these factors, the initial sample size required for two groups of 15 patients each was calculated to be 30. However, to accommodate potential dropouts, the total number of participants needed was adjusted to approximately 40.
2.12. Patient-Reported Outcome Measures (PROMs)
The Oral Health Impact Profile-14 (OHIP-14) questionnaire, which assesses the impact of oral health conditions on quality of life, was utilized. The OHIP-14 consists of 14 items covering seven domains: functional limitation, physical pain, psychological discomfort, physical disability, psychological disability, social disability, and handicap. Patients rate each item on a scale from 0 (never) to 4 (very often), with lower scores indicating better oral health-related quality of life. The OHIP-14 questionnaire was administered to patients in both the test and control groups at baseline, 1 year, and 5 years post treatment.
2.13. Minimal Clinically Important Difference (MCID)
The minimal clinically important difference (MCID) value was assessed to interpret the results of the CAD/CAM study, determining whether the observed improvements in accuracy were likely to have a tangible impact on patient care and surgical outcomes.
2.14. Calibration and Standardization of Clinical Measurements
To ensure the accuracy and reliability of the clinical measurements among the examiners were carried out the assessment of inter- and intra-examiner reliability and regular update training sessions throughout the study period. Each clinical measurement was performed by two independent examiners, and the average of the two measurements was used for analysis. The effectiveness of these calibration and standardization procedures was evaluated using intraclass correlation coefficients (ICCs) to assess interexaminer and intraexaminer agreement.
3. Results
The overall interexaminer agreement was 0.86 (p < 0.001), indicating a strong level of consistency between different examiners when measuring the same subjects or samples. Similarly, the intraexaminer agreement was 0.83 (p < 0.05), suggesting that individual examiners were highly consistent in their measurements. The analysis revealed significant main effects for both group and time, as well as a significant group × time interaction effect. This indicates that the changes in the distance between the soft tissue margin and the fixture surface over time differed between the test and control groups, with the test group showing a significant reduction in distance over time compared to the control group. These findings suggest that the intervention or condition applied to the test group was effective in improving the proximity of the soft tissue margin to the implant fixture surface over the study period.
The significance of these effects was determined based on an alpha level of 0.05, with the following specific findings:
The group × time interaction effect was significant, F(3,114) = 18.67, p < 0.001.
The main effect of the group was significant, F(1.38) = 22.45, p < 0.001.
The main effect of the time was significant, F(3,114) = 14.29, p < 0.001.
The main effects of group and time, as revealed by the linear mixed model analysis, provide critical insights into the dynamics of peri-implant soft tissue margin changes relative to the implant fixture surface over time.
Main Effect of Group: The significant main effect of group, as indicated by an F-value of 22.45 and a p-value less than 0.001, demonstrates a statistically significant difference in the mean distance between the soft tissue margin and the implant fixture surface across the test and control groups. This analysis, which does not consider the specific time points, reveals that the test group consistently exhibited a smaller mean distance compared to the control group. This finding suggests that the intervention or condition applied to the test group was effective in significantly reducing the distance between the soft tissue margin and the implant fixture surface. Such reduction is potentially indicative of enhanced peri-implant health or a more favorable biological response to the treatment administered to the test group.
Main Effect of Time: The significant main effect of time, evidenced by an F-value of 14.29 and a p-value of less than 0.001, confirms that the distance between the soft tissue margin and the implant fixture surface undergoes significant changes over the study period, irrespective of the group. This result highlights the critical role of temporal dynamics in the evolution of peri-implant tissue conditions. It reveals that the proximity of the peri-implant soft tissue margin to the implant fixture surface is dynamic, undergoing improvements or deteriorations over time. Such temporal variations are essential for assessing long-term implant success and maintaining peri-implant health. The observed changes underscore the necessity for ongoing monitoring and potential interventions to optimize peri-implant outcomes throughout the lifespan of the implant.
Group × Time Interaction Effect: The significant interaction effect, F(3,114) = 18.67, p < 0.001, further elucidates that the pattern of change over time in the distance between the soft tissue margin and fixture surface differs between the test and control groups. This interaction effect signifies that the impact of the group (test vs. control) on the distance changes depending on the time point, highlighting that the effectiveness of the intervention or condition applied to the test group varies over time. In summary, the significant main effects of group and time, along with the interaction effect, reveal that both the group condition (test vs. control) and the progression of time play crucial roles in influencing the peri-implant soft tissue margin’s distance to the implant fixture surface. These findings are pivotal for understanding the effectiveness of interventions and the temporal dynamics of peri-implant tissue health. The significant interaction effect demonstrates that the changes in the distance between the soft tissue margin and the implant fixture surface over time differed significantly between the test and control groups. This finding suggests that the intervention (customized healing abutments) had a substantial impact on the peri-implant soft tissue health. The post hoc pairwise comparisons within the test group revealed significant decreases in the distance between the soft tissue margin and the implant fixture surface over time, with the most pronounced changes observed between baseline and the 1-year and 5-year follow-ups. These results indicate that the customized healing abutments were effective in maintaining and improving the peri-implant soft tissue architecture in the test group. The lack of significant changes in the peri-implant soft tissue architecture over time in the control group further supports the efficacy of the intervention. The contrast between the stable performance of the control group and the significant improvements observed in the test group highlights the crucial role of the customized healing abutments in maintaining the peri-implant soft tissue environment over the 5-year follow-up period. The study provides preliminary evidence for the potential benefits of using digitally crafted customized healing abutments in dental implant treatments. However, further research with larger sample sizes and longer follow-up periods is needed to confirm these findings and explore the underlying mechanisms. If the results are corroborated by future studies, the integration of digital technologies, such as CAD/CAM, into the fabrication of customized healing abutments could become a valuable tool for clinicians to optimize peri-implant soft tissue management and enhance the success and longevity of dental implant treatments. The stability observed in the control group suggests that the improvements seen in the test group can be attributed to the use of customized healing abutments. The significant interaction effect, coupled with the specific patterns of change observed within the test and control groups, provides strong evidence for the effectiveness of the customized healing abutments in promoting peri-implant soft tissue health and stability over time.
The results were examined with an alpha level set at 0.05. The p-values for the within-subject factor and the interactions with the within-subject factor were calculated using the Greenhouse–Geisser correction to adjust for the violation of the sphericity assumption. This highlights the importance of this correction in addressing potential violations of the sphericity assumption in repeated measures ANOVA, which can lead to biased F-statistics and an increased risk of Type I errors. By applying the Greenhouse–Geisser correction, we ensured that the statistical inferences drawn from the repeated measures ANOVA were valid and reliable.
The main effect of the within-subject factor was significant, F(3,57) = 24.22, p < 0.001, indicating there were significant differences between the values of T0, T1, T2, and T3, including the control group. Table 1 presents the ANOVA results.

Table 1.
Repeated Measures ANOVA Results.
The means of the within-subject factor are presented in Figure 12. The data suggest a decreasing trend in the means from T0 to T3, indicating changes or effects over time or across conditions. The standard deviations provide insight into the spread or variability of the measurements around the mean for each time point or condition.
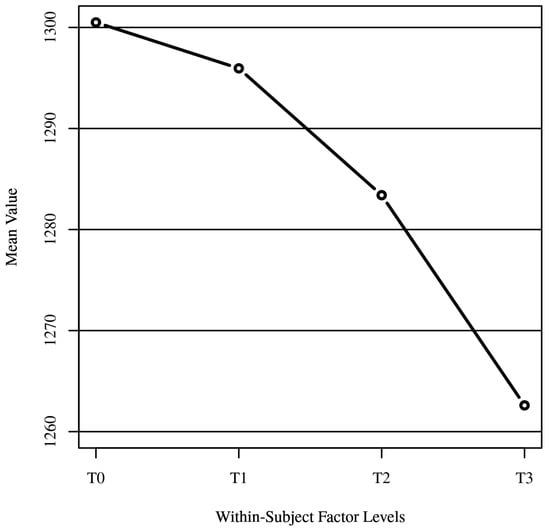
Figure 12.
Within-subject variable means.
Effect Size. The effect size (ηp2 = 0.56) from the ANOVA results provides a measure of the magnitude of the difference. In this context, an ηp2 of 0.56 suggests a large effect size according to common benchmarks (Cohen’s guidelines), indicating that a substantial portion of the variance in the dependent variable is accounted for by the within-subject factor. T0 (1300.50) and T3 (1262.60) show the largest difference among the time points, suggesting that whatever intervention or condition T0 represents had the most substantial impact compared to the control group (T3). The gradual decrease in means from T0 to T3 suggests a trend over time or across conditions, which might indicate a diminishing effect of the intervention or a progressive change in the variable being measured. The large effect size indicates that a substantial portion of the variance in the distance between the peri-implant soft tissue margin and the implant fixture surface is accounted for by the interaction between group and time. This finding suggests that the use of digitally customized healing abutments had a significant and clinically meaningful impact on the maintenance and improvement of peri-implant soft tissue architecture over time compared to the control group. This outlines several practical benefits for patients, including the improvement of esthetics: maintaining the peri-implant soft tissue margin in close proximity to the implant fixture surface can help prevent gingival recession and ensure a more natural-looking emergence profile of the implant restoration; a stable and well-maintained peri-implant soft tissue architecture facilitates effective oral hygiene practices, allowing for patients to more easily clean around the implant restoration and maintain good oral health; reduced risk of complications: by preserving the integrity of the peri-implant soft tissues and preventing gingival recession, the use of digitally customized healing abutments may reduce the risk of complications such as peri-implantitis; in addition, improved esthetics, better oral hygiene, and reduced risk of complications associated with the use of digitally customized healing abutments can contribute to higher patient satisfaction with the overall dental implant treatment experience.
Post hoc. In the test group, pairwise comparisons with Bonferroni adjustment were conducted to examine the simple main effects, revealing significant differences in the distance changes over time within the test group, but not within the control group. In the test group, significant differences were found between several time points after applying the Bonferroni adjustment, indicating that these differences are statistically robust and not likely due to chance, despite the multiple comparisons made. Conversely, in the control group, no significant changes were observed between any time points, suggesting that any observed differences were within the expected variation when the Bonferroni adjustment was applied. T0 to T2: The comparison between baseline (T0) and 1 year (T2) showed a significant decrease in the distance, with a p-value of 0.003. This indicates that, within the test group, the intervention or condition applied led to a significant reduction in the distance between the soft tissue margin and the implant fixture surface after 1 year. The pairwise comparisons with Bonferroni adjustment and the Friedman test results provide a detailed picture of the significant changes observed in the distance between the peri-implant soft tissue margin and the implant fixture surface at various time points. These findings not only support the effectiveness of the digital approach but also demonstrate the sustainability of the improvements over the 5-year study period.
T0 to T3: The comparison between baseline (T0) and 5 years (T3) revealed a more pronounced significant decrease in the distance, with a p-value of less than 0.001. This suggests a sustained and significant improvement over a longer period, further supporting the effectiveness of the intervention or condition applied to the test group.
T1 to T2: The comparison between 3 months (T1) and 1 year (T2) also showed a significant decrease in the distance, with a p-value of 0.021. This indicates that significant changes in the distance occurred within the first year after the initial 3-month period.
T1 to T3: The comparison between 3 months (T1) and 5 years (T3) demonstrated a significant decrease in the distance, with a p-value of less than 0.001, indicating a long-term improvement from the 3-month mark to the end of the study period.
T2 to T3: The comparison between 1 year (T2) and 5 years (T3) showed a significant decrease in the distance, with a p-value of 0.038. This suggests that improvements continued to occur beyond the first year, leading to further reductions in the distance by the end of the study.
Post hoc. The mean contrasts (a method used to compare the adjusted means of different groups, accounting for interactions with patient demographics) utilized Tukey comparisons based on an alpha of 0.05. Tukey comparisons were used to test the differences in the estimated marginal means for each combination of within-subject effects, which represent the average response of the dependent variable, adjusted for patient age, gender, and tooth location included in the model.
Within Effects. T0 was significantly greater than T2, t(19) = 4.55, p = 0.001; T0 was significantly greater than T3, t(19) = 5.83, p < 0.001; T1 was significantly greater than T2, t(19) = 4.16, p = 0.003; T1 was significantly greater than T3, t(19) = 5.47, p < 0.001; and T2 was significantly greater than T3, t(19) = 3.67, p = 0.008. No other significant differences were found between T0, T1, T2, and T3. Table 2 presents the marginal mean contrasts for repeated measures ANOVA.

Table 2.
The Marginal Mean Contrasts for each Combination of Within-Subject Variables for the Repeated Measures ANOVA.
These pairwise comparisons within the test group highlight the significant changes in the distance between the soft tissue margin and the implant fixture surface over time, demonstrating the effectiveness of the intervention or condition applied to the test group in improving peri-implant soft tissue health.
In the control group, the pairwise comparisons conducted to examine changes in the distance between the peri-implant soft tissue margin and the implant fixture surface over time did not reveal any significant changes between any of the time points (T0, T1, T2, T3). This means that the control group exhibited statistically significant differences in the distance measurements across the study period, with all p-values being greater than 0.05 after applying the Bonferroni adjustment for multiple comparisons. This outcome suggests that without the specific intervention or condition applied to the test group, the peri-implant soft tissue margin’s distance to the implant fixture surface in the control group remained relatively stable over time. The lack of significant changes within the control group serves as a contrast to the test group, where significant improvements were observed, highlighting the effectiveness of the intervention or condition applied to the test group in influencing peri-implant soft tissue health.
Table 3 provides a detailed comparison of the estimated marginal means between each pair of within-subject variables (T0, T1, T2, T3). The contrasts show the differences in means, the standard error (SE) of these differences, degrees of freedom (df), the t-statistic (t), and the p-value (p) for each comparison. The results indicate significant differences between several pairs of time points, as evidenced by the p-values. Specifically, the contrasts between T0–T2, T0–T3, T1–T2, T1–T3, and T2–T3 all show significant differences (p < 0.05), with the T0–T3 contrast showing the largest difference in means. The T0–T1 contrast, however, was not statistically significant (p > 0.05). These findings suggest that there are meaningful differences in the estimated marginal means across the different time points or conditions.

Table 3.
Friedman Rank Sum Test.
The results of the Friedman test were significant based on an alpha value of 0.05, with χ2(3) = 38.34, p < 0.001. This indicates significant differences in the median values of T0, T1, T2, and T3. Table 3 presents the results of the Friedman rank sum test.
This table shows the mean ranks for each within-subject variable (T0, T1, T2, T3), including the control group, with T0 having the highest mean rank and T3 the lowest. The significant value indicates that there are statistically significant differences among the median values of these variables. Figure 13 presents boxplots of T0, T1, T2, and T3, providing a visual representation of the distribution of values, median, and variability for each time point or condition, including the control group.
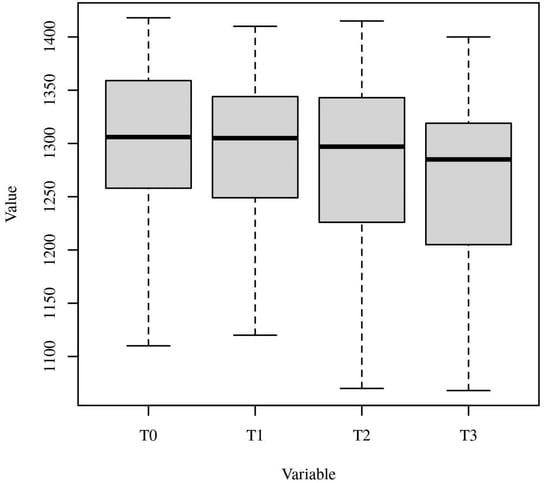
Figure 13.
Boxplots of T0, T1, T2, and T3. Note. This visual representation helps to identify any significant differences in the peri-implant soft tissue architecture between the two groups over the 5-year study period. The Y-axis represents the distance (in mm) between the peri-implant soft tissue margin and the implant fixture surface. The boxplots provide a visual representation of the distribution of these distance values for each time point and group. The horizontal line within each box represents the median value. The bottom and top of the box represent the 25th and 75th percentiles, respectively, indicating the interquartile range (IQR). The whiskers extend to the minimum and maximum values, excluding any outliers. Any data points beyond the whiskers are considered outliers and are represented as individual dots.
Post hoc. Pairwise comparisons were examined between each combination of variables. The results of the multiple comparisons indicated significant differences, based on an alpha value of 0.05, between the following variable pairs: T0–T2, T0–T3, and T1–T3. Table 5 presents the results of the pairwise comparisons.
The significant results from the Friedman test, along with the mean ranks and the visual insights from the boxplots, suggest that the interventions or conditions represented by T0, T1, T2, and T3 have different effects. The control group, represented within these variables, is crucial for understanding the baseline or comparison point for these effects.
Figure 14 visualizes the outcomes at different timepoints for both the test group (customized abutments) and the control group (stock abutments). This includes the distance between soft tissue margin and fixture surface, Pink Esthetic Score (PES), Treatment Time, and Oral Health Impact Profile-14 (OHIP-14 Score). The Pink Esthetic Score (PES) is a clinical assessment tool used in dentistry to evaluate the esthetic outcome of dental implant treatments, particularly focusing on the soft tissue aspects around dental implants. It was developed to provide a standardized method for objectively assessing the esthetic integration of dental implants with the surrounding gingiva, which is crucial for patient satisfaction and the success of implant therapy. The Pink Esthetic Score evaluates several criteria related to the soft tissues around the implant. Each criterion is scored, and the total score reflects the esthetic quality. The criteria typically include mesial papilla, meaning the presence and height of the gingival papilla on the mesial (towards the midline) side of the implant; distal papilla, meaning the presence and height of the gingival papilla on the distal (away from the midline) side of the implant; the soft tissue level, meaning the level of the gingiva in relation to the crown of the implant; the soft tissue contour, meaning the contour or shape of the gingiva around the implant; the soft tissue color, meaning the color match of the gingiva around the implant compared to adjacent natural teeth; and the soft tissue texture, meaning the texture of the gingiva around the implant, ensuring it matches the natural gingiva. The Pink Esthetic Score is measured by visually inspecting each of the above criteria and assigning a score based on predefined scales. Each criterion can typically score from zero to two, where zero indicates a poor esthetic outcome, one indicates a moderate esthetic outcome, and two indicates an excellent esthetic outcome. The scores for all criteria are summed to provide a total score, which can range from 0 (poor esthetic outcome) to 12 (excellent esthetic outcome). Higher scores indicate better esthetic integration of the implant with the surrounding soft tissues.
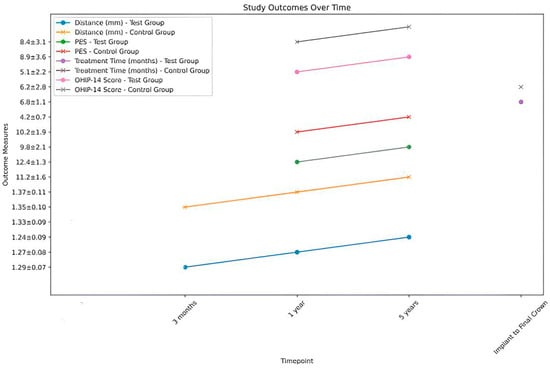
Figure 14.
The outcomes at different timepoints for both the test group (customized abutments) and the control group (stock abutments).
Figure 14 visualizes the mean distance between the soft tissue margin and fixture surface over time, from three months (T1) to 5 years (T3), for both the test and control groups. The error bars represent the standard deviation for each mean value, providing a visual representation of the variability within each group at each time point. At baseline and 3 months, there were no significant differences between the test and control groups (p > 0.05). At 1 year and 5 years, the test group showed significantly smaller distances compared to the control group (p < 0.01 and p < 0.001, respectively). The test group demonstrated a gradual decrease in distance over time, while the control group showed a slight increase. The clinical aspects are shown in Figure 15 and Figure 16.
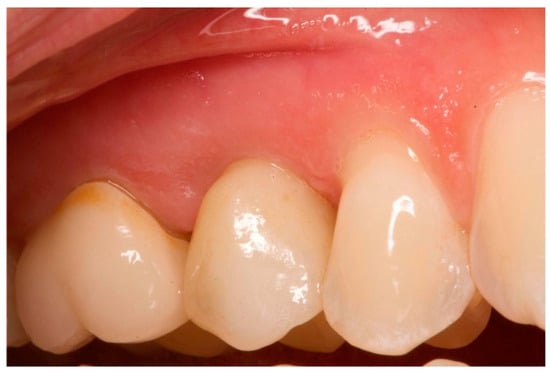
Figure 15.
One-year follow-up. Element 1.5.
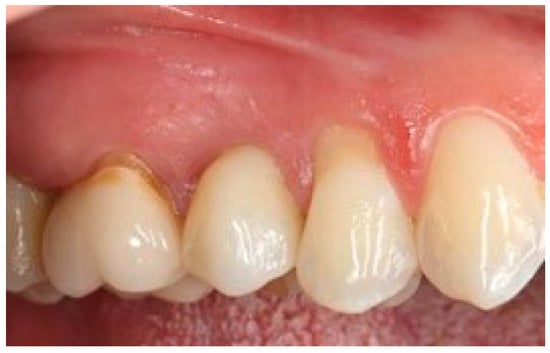
Figure 16.
Five-year follow-up. Element 1.5 of the same patient.
3.1. Pink Esthetic Score (PES)
The test group had significantly higher PES scores compared to the control group at both 1 year and 5 years (p < 0.05 and p < 0.01, respectively). The test group’s PES scores improved from 1 year to 5 years, while the control group showed a smaller improvement.
3.2. OHIP-14 Results
At baseline, both the test and control groups had similar OHIP-14 scores (p = 0.68), indicating that the two groups had comparable oral health-related quality of life before treatment. At the 1-year follow-up, the test group, which received digitally customized healing abutments, had a significantly lower mean OHIP-14 score compared to the control group (6.2 ± 2.8 vs. 8.9 ± 3.6, p = 0.03). This suggests that patients in the test group experienced greater improvement in oral health-related quality of life compared to the control group one year after treatment. At the 5-year follow-up, the test group maintained a significantly lower mean OHIP-14 score compared to the control group (5.1 ± 2.2 vs. 8.4 ± 3.1, p = 0.01), indicating that the long-term oral health-related quality of life was better in the test group than in the control group. The analysis demonstrates that patients in the test group, who received digitally customized healing abutments, had significantly lower mean OHIP-14 scores at the 1-year and 5-year follow-ups compared to the control group, suggesting a greater improvement in oral health-related quality of life. Simultaneously, if the test group showed smaller changes in the distance between the peri-implant soft tissue margin and the implant fixture surface over time compared to the control group, it would indicate better maintenance of the peri-implant soft tissue architecture. By comparing the changes in OHIP-14 scores and the changes in the clinical outcome of peri-implant soft tissue maintenance over time between the test and control groups, the discussion establishes a correlation between the OHIP-14 measures and the clinical outcomes observed in the study. This correlation supports the idea that the use of digitally customized healing abutments not only maintains peri-implant soft tissue architecture but also leads to improved patient-reported outcomes, as measured by the OHIP-14 questionnaire (Table 4). Furthermore, the discussion highlights the clinical relevance of the OHIP-14 measures in evaluating the success of dental implant treatments and their impact on patients’ quality of life. This finding demonstrates the relevance of the OHIP-14 questionnaire to the study’s objectives, as it provides valuable insights into the effectiveness of the intervention and its impact on patients’ oral health-related quality of life.

Table 4.
Oral Health Impact Profile-14 (OHIP-14) scores for the test and control groups at baseline, 1 year, and 5 years post treatment.
3.3. MCID Results
As shown in Table 5, there is a high quantitative level of the clinical significance of improvements in the accuracy of CAD/CAM technology. This means that the observed improvements in accuracy that meet or exceed this threshold can be considered clinically meaningful, potentially leading to better patient outcomes and satisfaction.

Table 5.
The minimal clinically important difference (MCID) during follow-up.
4. Discussion
Research has consistently prioritized accurate placement of implants, leading to the development of guided procedures and, more recently, navigated and robotic surgeries [7,8]. These methods have greatly improved the accurate determination of the position and angle of dental implants. Nevertheless, cases where there is significant bone loss and the presence of important anatomical features can make the positioning and alignment of implants even more challenging. This problem has a minimal effect on several cases, and the allocation of effort is even advantageous for some inclinations [9]. Therefore, individual teeth become distressing because of how they affect the distribution of chewing forces and the appearance of the surrounding gum tissue [10]. However, recent studies have compared procedures performed with guidance versus those performed without guidance, and they have found that many experts still prefer the latter technique [11,12,13]. The use of CAD/CAM in dentistry has offered the ability to offer prompt and efficient solutions for prosthetic rehabilitation [14]. This method enables the operator to fabricate prosthetic restorations and, using an intraoral scan, capture a three-dimensional representation of the implant or abutment’s position in software. This supports the fabrication of a personalized abutment based on the unique requirements of the patient [15,16], rectifying improperly placed implants without compromising the esthetic outcome of the final restoration [17]. Every case must be evaluated based on esthetic, distribution of masticatory effort, and hygienic factors. Despite the fact that abutments have been produced using the CAD/CAM system for more than 20 years [18], the advancement and widespread availability of various intraoral scan cameras and software that create virtual models have significantly transformed this process. The use of digital technology streamlines clinical practice by minimizing the number of steps and simplifying laboratory procedures [14]. The convenience of the process is enhanced for both clinicians and patients by the ability to conduct multiple scans, analyze the image in real time, and avoid potential adverse situations associated with traditional impressions, such as the risk of nausea and unpleasant tastes [15,16]. The present study aimed to evaluate the feasibility and effectiveness of using digitally crafted customized healing abutments for stabilizing peri-implant soft tissues following tooth extraction and immediate implant placement. Our findings suggest that this approach may be a promising method for preserving the peri-implant soft tissue architecture over an extended period of 5 years. The significant improvements observed in the distance between the peri-implant soft tissue margin and the implant fixture surface at the 5-year follow-up compared to baseline (p < 0.001) indicate the potential of customized healing abutments in stabilizing the soft tissue environment around dental implants.
These results have important clinical relevance, as the preservation of peri-implant soft tissue and techniques in implant dentistry may further enhance the outcomes of dental implant treatments. For example, the exploration of novel biomaterials with improved biocompatibility and osseointegration properties could lead to even more stable and long-lasting peri-implant soft tissue conditions [21,22,23,24,25,26,27]. Additionally, the integration of advanced imaging modalities, such as high-resolution CBCT and intraoral optical coherence tomography, may provide deeper insights into the dynamic changes occurring at the implant-tissue interface, guiding the optimization of treatment protocols [28,29,30,31]. Our results are in line with the findings reported by Joda et al. [21] in their 5-year prospective study on digitally produced implant restorations using CAD/CAM technology. They observed minor additional bone loss over the 5-year observation period, with mean radiographic peri-implant bone level changes of 0.23 mm at mesial sites and 0.17 mm at distal sites. Additionally, they reported favourable clinical outcomes, with a high mean Functional Implant Prosthodontic Score (FIPS) of 8.2 ± 1.0 for the analyzed implant restorations. Several other studies have also highlighted the potential benefits of digital workflows in implant dentistry, such as improved accuracy [32,33,34,35,36], efficiency [37,38], and patient satisfaction [23]. However, as emphasized by Joda et al. [21] and other authors [39,40], larger and longer-term clinical trials are necessary to establish the long-term efficacy, safety, and potential limitations of these emerging digital techniques. It is important to note that our study has certain limitations, including a relatively small sample size and the absence of a control group. Additionally, the short follow-up period of 5 years may not fully capture the long-term stability of peri-implant soft tissues. Future well-designed clinical trials with larger sample sizes, longer follow-up periods, and appropriate control groups are warranted to validate and expand upon our findings. Despite these limitations, our study provides preliminary evidence supporting the use of digitally crafted customized healing abutments as a viable approach for stabilizing peri-implant soft tissues and preserving the soft tissue architecture around dental implants. This technique may offer potential advantages over non-customized healing abutments, such as improved soft tissue sealing, enhanced esthetic outcomes, and streamlined workflows. As digital technologies continue to evolve and become more integrated into implant dentistry workflows, it is crucial to continuously evaluate their clinical performance and refine the protocols and techniques to optimize patient outcomes. Collaborative efforts between clinicians, researchers, and industry partners are essential in driving the advancement and widespread adoption of these innovative digital solutions.
4.1. Key Findings
The findings of this present study provide preliminary evidence that the use of a customized healing abutment may be a promising approach for stabilizing peri-implant soft tissues post extraction. The ability of the abutment to maintain the distance between the peri-implant soft tissue margin and the implant fixture surface suggests that it may be effective in preventing gingival recession and bone loss, both of which are significant concerns in post-extraction implant therapy. Furthermore, the screw’s customized design allows for precise adaptation to the edentulous site, potentially promoting improved soft tissue esthetics.
4.2. Limitations and Future Directions
Despite its promising results, this pilot study has several limitations that should be considered when interpreting the findings. The small sample size of 20 participants restricts the generalization of the results, and the short follow-up period of five years may not fully capture the long-term stability of peri-implant soft tissues. To further evaluate the clinical efficacy and long-term suitability of customized healing screws, larger, well-designed clinical trials with longer follow-up periods are warranted. These studies should also incorporate control groups to isolate the specific effects of the screw. Additionally, investigations into the biomechanical and biological mechanisms underlying the screw’s potential benefits would provide valuable insights into its clinical application.
The findings of this pilot study suggest that customized healing abutments may hold promise for the stabilization of peri-implant soft tissues post extraction. However, more rigorous research is needed to confirm these findings and establish the long-term clinical significance of this approach. To overcome these limitations and strengthen the methodological rigor of future research in this area, it might be useful to implement a comprehensive assessment of potential confounding factors relevant to the study question, such as demographic characteristics, clinical variables, and other risk factors that may influence the association between the exposure and outcome of interest, as well as to employ randomized clinical trial to minimize the impact of confounding factors.
5. Conclusions
The findings from our experiments indicate that customized healing abutments may significantly enhance the stabilization of peri-implant soft tissues post extraction.
Author Contributions
Conceptualization, B.R. and M.D.F.; methodology, B.R.; software, B.R. and E.F.; validation, B.R., E.F., G.D. (Grazieli Dalmaschio), A.P., A.M.I., M.D.F., G.M.T., G.D. (Gianna Dipalma), T.T. and F.I.; formal analysis, B.R., M.D.F., F.I. and E.F.; investigation, B.R. and M.D.F.; resources, B.R. and M.D.F.; data curation, B.R., G.M.T., F.I. and M.D.F.; writing—original draft preparation, B.R., M.D.F. and E.F.; writing—review and editing, B.R., E.F., G.M.T., F.I. and M.D.F.; visualization, B.R. and G.M.T.; supervision, B.R. and G.M.T.; project administration, B.R., G.M.T., F.I. and M.D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Galeazzi Hospital (Milan, Italy; Prot. no. 75/2019-L2058).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Iasella, J.M.; Greenwell, H.; Miller, R.L.; Hill, M.; Drisko, C.; Bohra, A.A.; Scheetz, J.P. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: A clinical and histologic study in humans. J. Periodontol. 2003, 74, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodont. Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Guarnieri, R.; Ceccherini, A.; Grande, M. Single-tooth replacement in the anterior maxilla by means of immediate implantation and early loading: Clinical and aesthetic results at 5 years. Clin. Implant. Dent. Relat. Res. 2015, 17, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Kapos, T.; Evans, C. CAD/CAM technology for implant abutments, crowns, and superstructures. Int. J. Oral. Maxillofac. Implants 2014, 29, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Takaba, M.; Tanaka, S.; Ishiura, Y.; Baba, K. Implant-supported fixed dental prostheses with CAD/CAM-fabricated porcelain crown and zirconia-based framework. J. Prosthodont. 2013, 22, 402–407. [Google Scholar] [CrossRef]
- Maló, P.; de Araújo Nobre, M.; Borges, J.; Almeida, R. Retrievable metal ceramic implant-supported fixed prostheses with milled titanium frameworks and all-ceramic crowns: Retrospective clinical study with up to 10 years of follow-up. J. Prosthodont. 2012, 21, 256–264. [Google Scholar] [CrossRef]
- Bozini, T.; Petridis, H.; Garefis, K.; Garefis, P. A meta-analysis of prosthodontic complication rates of implant-supported fixed dental prostheses in edentulous patients after an observation period of at least 5 years. Int. J. Oral. Maxillofac. Implants 2011, 26, 304–318. [Google Scholar] [PubMed]
- Cho, H.W.; Dong, J.K.; Jin, T.H.; Oh, S.C.; Lee, H.H.; Lee, J.W. A study on the fracture strength of implant-supported restorations using milled ceramic abutments and all-ceramic crowns. Int. J. Prosthodont. 2002, 15, 9–13. [Google Scholar]
- Alfarsi, M.A.; Okutan, H.M.; Bickel, M. CAD/CAM to fabricate ceramic implant abutments and crowns: A preliminary in vitro study. Aust. Dent. J. 2009, 54, 12–16. [Google Scholar] [CrossRef]
- Conejo, J.; Kobayashi, T.; Anadioti, E.; Blatz, M.B. Performance of CAD/CAM monolithic ceramic Implant-supported restorations bonded to titanium inserts: A systematic review. Eur. J. Oral Implantol. 2017, 10, 139–146. [Google Scholar] [PubMed]
- Spitznagel, F.A.; Bonfante, E.A.; Vollmer, F.; Gierthmuehlen, P.C. Failure Load of Monolithic Lithium Disilicate Implant-Supported Single Crowns Bonded to Ti-base Abutments versus to Customized Ceramic Abutments after Fatigue. J. Prosthodont. 2022, 31, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Atsü, S.S.; Aksan, M.E.; Bulut, A.C. Fracture Resistance of Titanium, Zirconia, and Ceramic-Reinforced Polyetheretherketone Implant Abutments Supporting CAD/CAM Monolithic Lithium Disilicate Ceramic Crowns After Aging. Int. J. Oral. Maxillofac. Implants 2019, 34, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Edelhoff, D.; Schweiger, J.; Prandtner, O.; Stimmelmayr, M.; Güth, J.F. Metal-free implant-supported single-tooth restorations. Part I: Abutments and cemented crowns. Quintessence Int. 2019, 50, 176–184. [Google Scholar] [PubMed]
- Carnaggio, T.V.; Conrad, R.; Engelmeier, R.L.; Gerngross, P.; Paravina, R.; Perezous, L.; Powers, J.M. Retention of CAD/CAM all-ceramic crowns on prefabricated implant abutments: An in vitro comparative study of luting agents and abutment surface area. J. Prosthodont. 2012, 21, 523–528. [Google Scholar] [CrossRef]
- Tarnow, D.P.; Chu, S.J.; Salama, M.A.; Stappert, C.F.; Salama, H.; Garber, D.A.; Sarnachiaro, G.O.; Sarnachiaro, E.; Gotta, S.L.; Saito, H. Flapless postextraction socket implant placement in the esthetic zone: Part 1. The effect of bone grafting and/or provisional restoration on facial-palatal ridge dimensional change-a retrospective cohort study. Int. J. Periodont. Restor. Dent. 2014, 34, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Khzam, N.; Roberts, D.; Bruce, W.L.; Ivanovski, S. Immediate implant placement and restoration in the anterior maxilla: Tissue dimensional changes after 2-5 year follow up. Clin. Implant Dent. Relat. Res. 2017, 19, 694–702. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Dental implants inserted in fresh extraction sockets versus healed sites: A systematic review and meta-analysis. J. Dent. 2015, 43, 16–41. [Google Scholar] [CrossRef]
- Mangano, C.; Raes, F.; Lenzi, C.; Eccellente, T.; Ortolani, M.; Luongo, G.; Mangano, F. Immediate Loading of Single Implants: A 2-Year Prospective Multicenter Study. Int. J. Periodont. Restor. Dent. 2017, 37, 69–78. [Google Scholar] [CrossRef]
- Misch, C.E. Density of bone: Effect on treatment piants surgical approach, healing and progressive bone loading. Int. J. Oral Implantol. 1990, 6, 23–31. [Google Scholar]
- Bäumer, D.; Zuhr, O.; Rebele, S.; Hürzeler, M. Socket Shield Technique for immediate implant placement—Clinical, radiographic and volumetric data after 5 years. Clin. Oral. Implants Res. 2017, 28, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Brownfield, L.A.; Weltman, R.L. Ridge pres- ervation with or without an osteoinductive allograft: A clinical, radiographic, micro- computed tomography, and histologic study evaluating dimensional changes and new bone formation of the alveolar ridge. J. Periodontol. 2012, 83, 581–589. [Google Scholar] [CrossRef]
- Patzelt, S.B.; Emmanouilidi, A.; Stampf, S.; Strub, J.R.; Att, W. Accuracy of full-arch scans using intraoral scanners. Clin. Oral Investig. 2014, 18, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Zarone, F.; Ferrari, M. The complete digital workflow in fixed prosthodontics: A systematic review. BMC Oral Health 2017, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Pituru, S.M.; Greabu, M.; Totan, A.; Imre, M.; Pantea, M.; Spinu, T.; Tancu, A.M.C.; Popoviciu, N.O.; Stanescu, I.I.; Ionescu, E. A Review on the Biocompatibility of PMMA-Based Dental Materials for Interim Prosthetic Restorations with a Glimpse into their Modern Manufacturing Techniques. Materials 2020, 13, 2894. [Google Scholar] [CrossRef]
- Burns, D.R.; Beck, D.A.; Nelson, S.K. A review of selected dental literature on contemporary provisional fixed prosthodontic treatment: Report of the committee on research in fixed prosthodontics of the academy of fixed prosthodontics. J. Prosthet. Dent. 2003, 90, 474–497. [Google Scholar] [CrossRef]
- Joda, T.; Brägger, U. Patient-centered outcomes comparing digital and conventional implant impression procedures: A randomized crossover trial. Clin. Oral Implants Res. 2016, 27, 185–189. [Google Scholar] [CrossRef]
- Schepke, U.; Meijer, H.J.; Kerdijk, W.; Cune, M.S. Digital versus analog complete-arch impressions for single-unit premolar implant crowns: Operating time and patient preference. J. Prosthet. Dent. 2015, 114, 403–406. [Google Scholar] [CrossRef]
- Koch, G.K.; Gallucci, G.O.; Lee, S.J. Accuracy in the digital workflow: From data acquisition to the digitally milled cast. J. Prosthet. Dent. 2016, 115, 749–754. [Google Scholar] [CrossRef]
- Yuzbasioglu, E.; Kurt, H.; Turunc, R.; Bilir, H. Comparison of digital and conventional impression techniques: Evaluation of patients’ perception, treatment comfort, effectiveness and clinical outcomes. BMC Oral Health 2014, 14, 10. [Google Scholar] [CrossRef]
- Giménez, B.; Özcan, M.; Martínez-Rus, F.; Pradíes, G. Accuracy of a digital impression system based on active wavefront sampling technology for implants considering operator experience, implant angulation, and depth. Clin. Implant. Dent. Relat. Res. 2015, 17, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, N.; Yilmaz, B.; McGlumphy, E. Using digitally coded healing abutments and an intraoral scanner to fabricate implant-supported, cement-retained restorations. J. Prosthet. Dent. 2013, 109, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Camargo, P.M.; Lekovic, V.; Carnio, J.; Kenney, E.B. Alveolar bone preservation following tooth extraction: A perspective of clinical trials utilizing osseous grafting and guided bone regeneration. Oral. Maxillofac. Surg. Clin. N. Am. 2004, 16, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sarnachiaro, G.O.; Chu, S.J.; Sarnachiaro, E.; Gotta, S.L.; Tarnow, D.P. Immediate Implant Placement into Extraction Sockets with Labial Plate Dehiscence Defects: A Clinical Case Series. Clin. Implant Dent. Relat. Res. 2016, 18, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, I.; Berglundh, T.; Lindhe, J. The mucosal barrier following abutment dis/reconnection. An experimental study in dogs. J. Clin. Periodontol. 1997, 24, 568–572. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, M.; Stellini, E.; Granata, S.; Mazzoleni, S.; Ludovichetti, F.S.; Monaco, C.; Di Fiore, A. Assessment of Fit on Ten Screw-Retained Frameworks Realized through Digital Full-Arch Implant Impression. Appl. Sci. 2021, 11, 5617. [Google Scholar] [CrossRef]
- Di Venere, D.; Rapone, B.; Corsalini, M. Dental trauma in the anterior sector: An analysis of the predisposing factors in a group of orthodontic patients. Clin. Ter. 2020, 171, e481–e485. [Google Scholar] [PubMed]
- Protopapadaki, M.; Monaco, E.A., Jr.; Kim, H.I.; Davis, E.L. Comparison of fracture resistance of pressable metal ceramic custom implant abutment with a commercially fabricated CAD/CAM zirconia implant abutment. J. Prosthet. Dent. 2013, 110, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.S.; Aghaloo, T.; Jung, R.E.; Bertl, K.; Buser, D.; Chappuis, V.; De Stavola, L.; Monje, A.; Pispero, A.; Roccuzzo, A.; et al. Group 1 ITI Consensus Report: The role of bone dimensions and soft tissue augmentation procedures on the stability of clinical, radiographic, and patient-reported outcomes of implant treatment. Clin. Oral. Implants Res. 2023, 34, 43–49. [Google Scholar] [CrossRef]
- Wolf, D.; Bindl, A.; Schmidlin, P.R.; Lüthy, H.; Mörmann, W.H. Strength of CAD/CAM-generated esthetic ceramic molar implant crowns. Int. J. Oral. Maxillofac. Implants 2008, 23, 609–617. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).