A Combined Use of Custom-Made Partial Pelvic Replacement and Proximal Femur Megaprosthesis in the Treatment of Severe Bone Loss after Multiple Total Hip Arthroplasty Revisions
Abstract
1. Introduction
2. Case Presentation
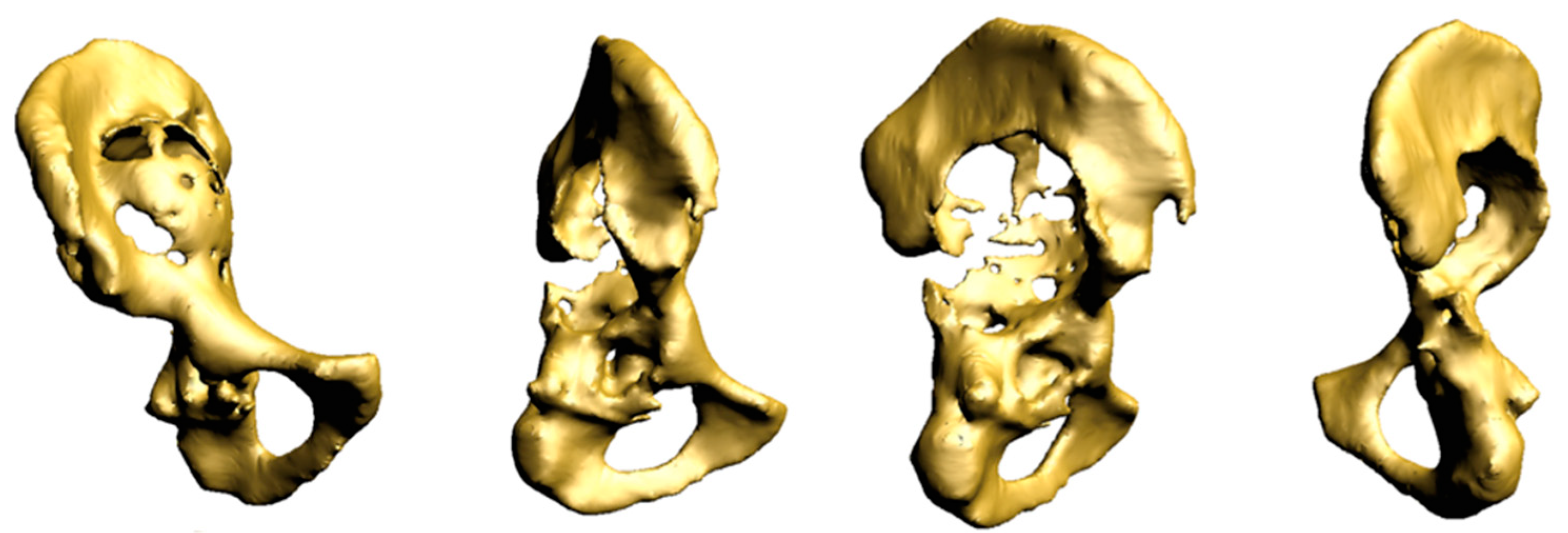
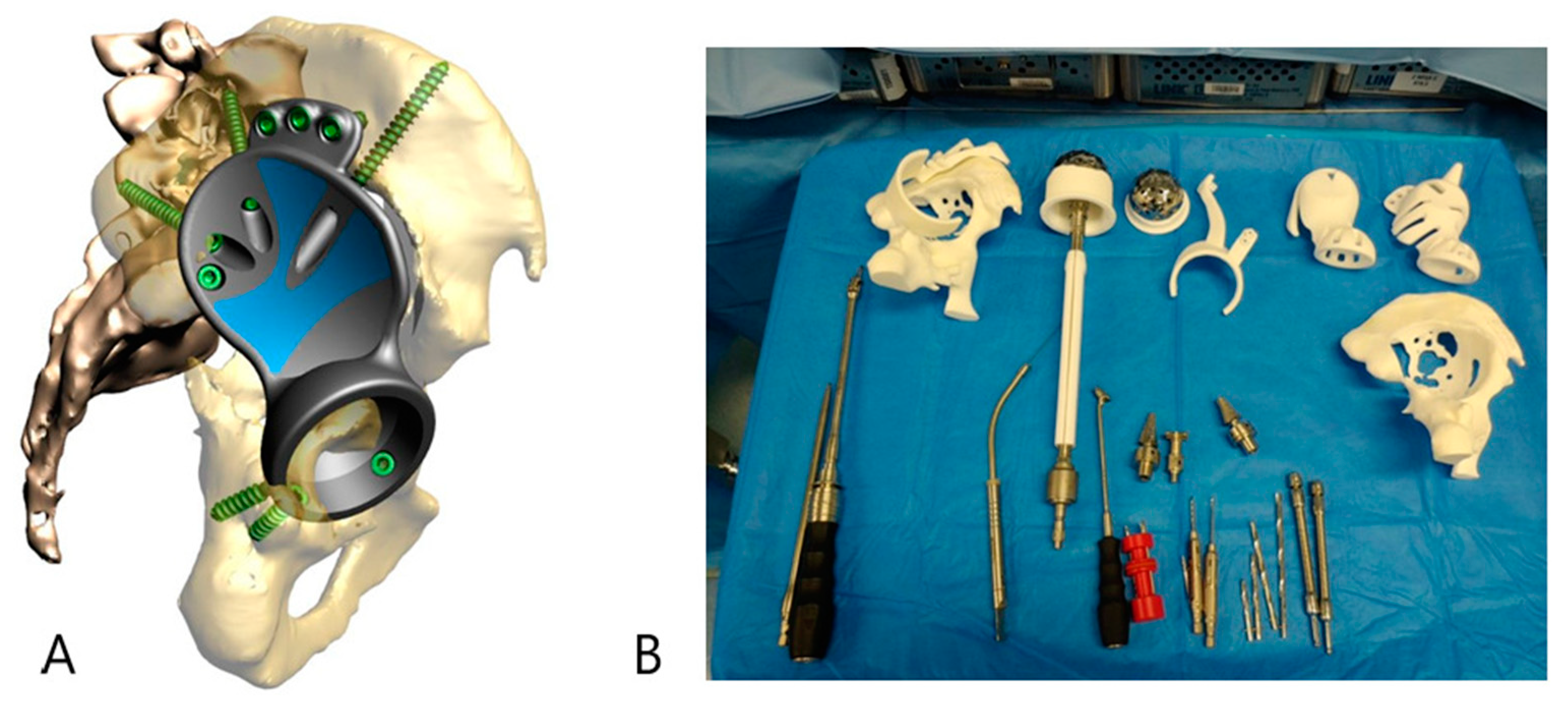
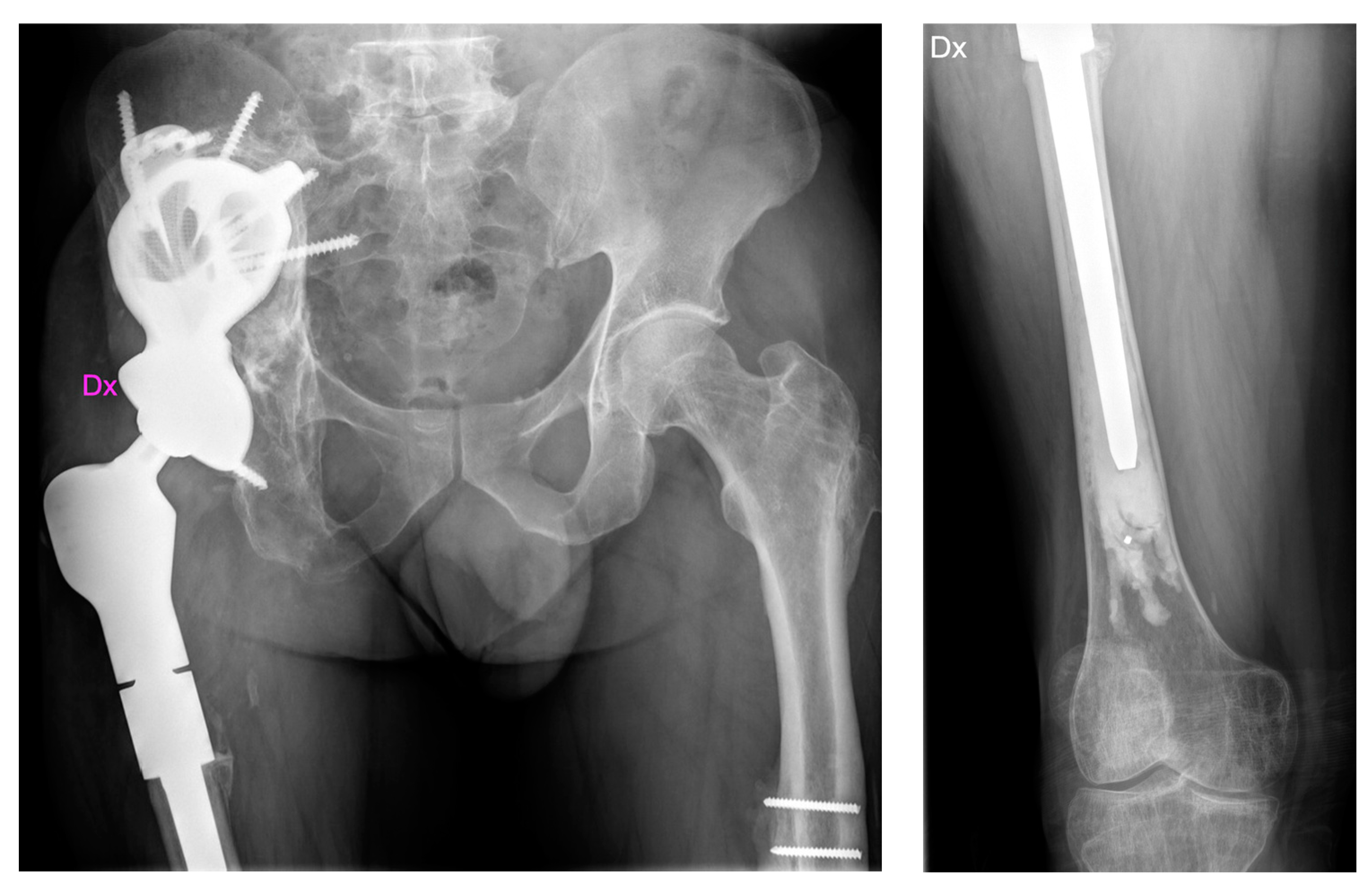
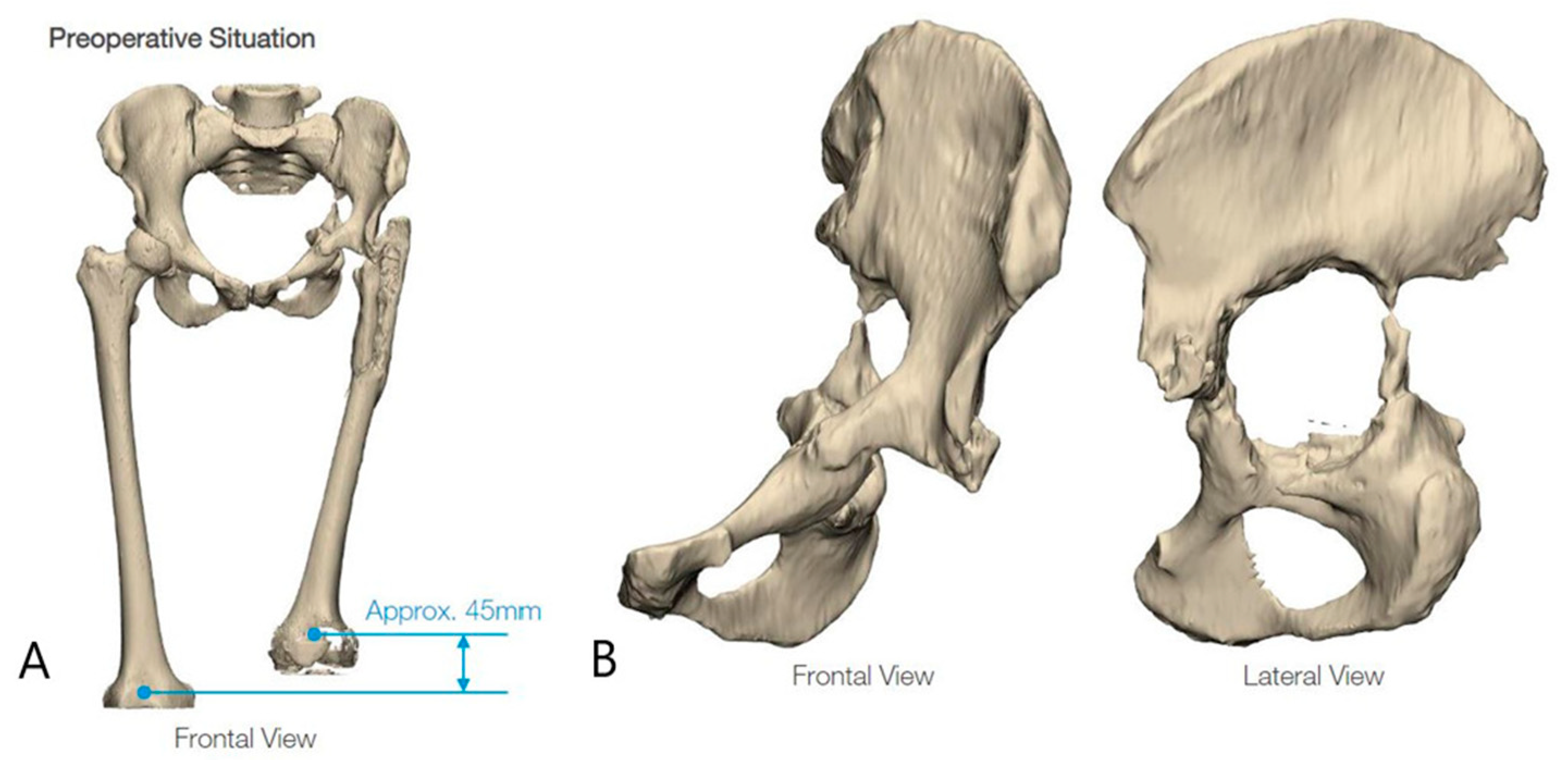
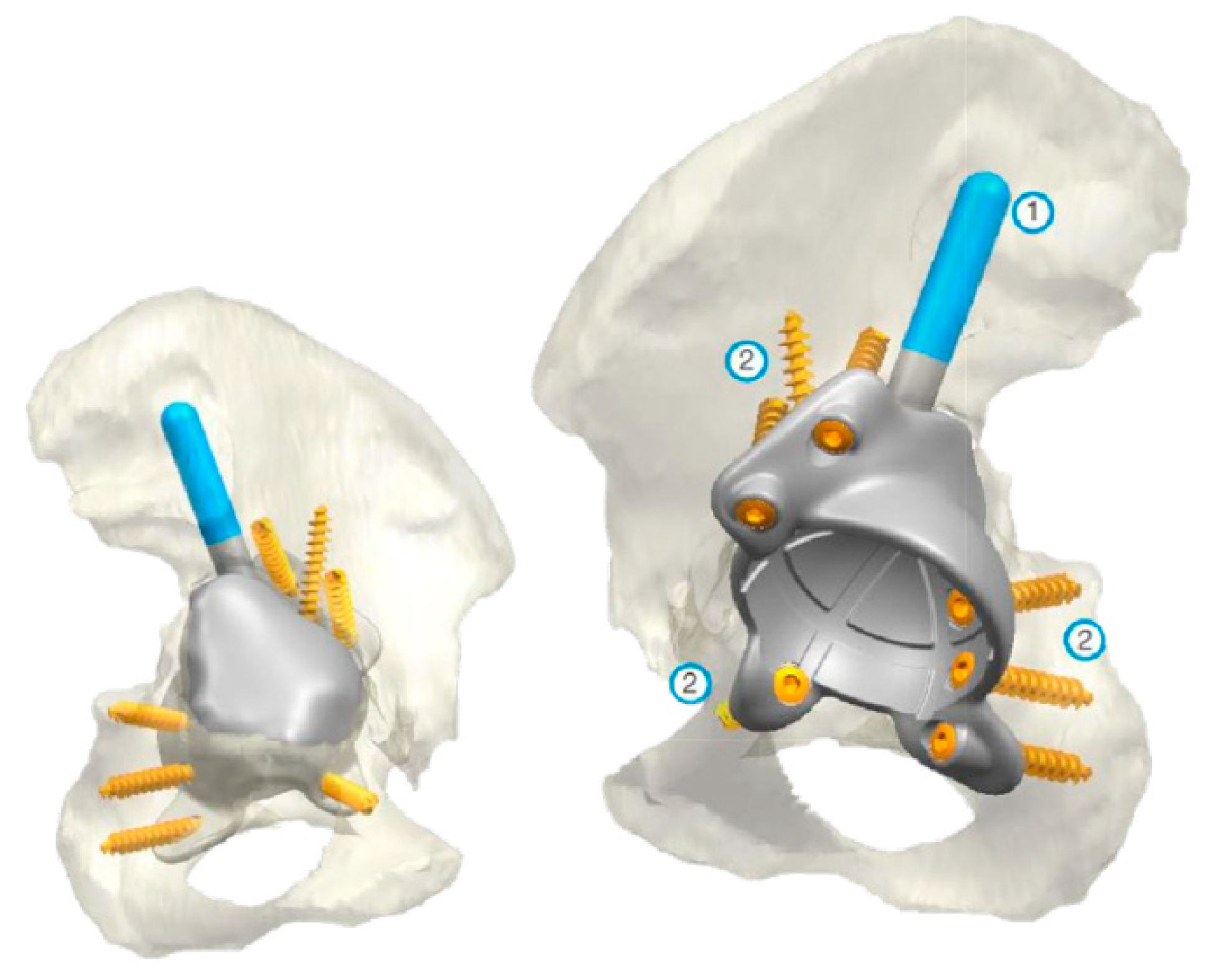
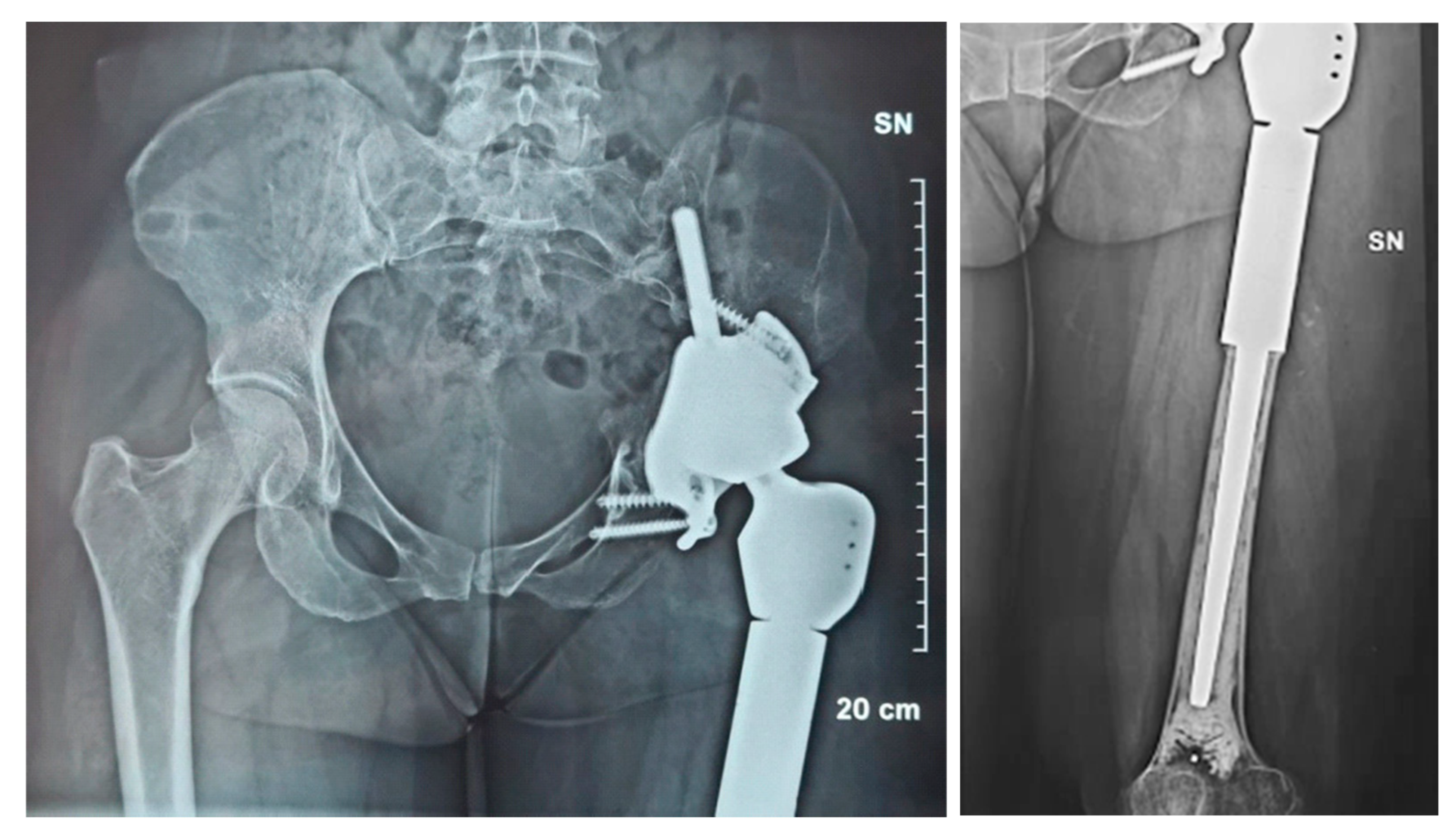
2.1. Case #1
2.2. Case #2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic joint infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef]
- Ong, K.L.; Lau, E.; Suggs, J.; Kurtz, S.M.; Manley, M.T. Risk of subsequent revision after primary and revision total joint arthroplasty. Clin. Orthop. Relat. Res. 2010, 468, 3070–3076. [Google Scholar] [CrossRef]
- Australian Orthopaedic Association National Joint Replacement Registry. Hip and Knee Arthroplasty Annual Report 2008. Available online: http://www.dmac.adelaide.edu.au/aoanjrr/documents/aoanjrrreport_2008.pdf (accessed on 28 April 2009).
- Garvin, K.L.; Hanssen, A.D. Infection after total hip arthroplasty. Past, present, and future. J. Bone Jt. Surg. 1995, 77, 1576–1588. [Google Scholar] [CrossRef]
- Charette, R.S.; Melnic, C.M. Two-Stage Revision Arthroplasty for the Treatment of Prosthetic Joint Infection. Curr. Rev. Musculoskelet. Med. 2018, 11, 332–340. [Google Scholar] [CrossRef]
- De Paolis, M.; Sambri, A.; Zucchini, R.; Frisoni, T.; Spazzoli, B.; Taddei, F.; Donati, D.M. Custom-made 3D-Printed Prosthesis in Periacetabular Resections Through a Novel Ileo-adductor Approach. Orthopedics 2022, 45, E110–E114. [Google Scholar] [CrossRef]
- Davis, D.L.; Morrison, J.J. Hip Arthroplasty Pseudotumors: Pathogenesis, Imaging, and Clinical Decision Making. J. Clin. Imaging Sci. 2016, 6, 17. [Google Scholar] [CrossRef]
- Tokgöz, M.A.; Sambri, A.; Rossi, G.; Bianchi, G.; Donati, D.M. Selective arterial embolization as neoadjuvant treatment in hip pseudotumors. Eklem. Hastalik. Cerrahisi. 2019, 30, 17–23. [Google Scholar] [CrossRef]
- Cordero-Ampuero, J. Girdlestone procedure: When and why. HIP Int. 2012, 22 (Suppl. S8), 36–39. [Google Scholar] [CrossRef]
- Emara, K.M. Pelvic support osteotomy in the treatment of patients with excision arthroplasty. Clin. Orthop. Relat. Res. 2008, 466, 708–713. [Google Scholar] [CrossRef]
- Sharma, H.; De Leeuw, J.; Rowley, D.I. Girdlestone resection arthroplasty following failed surgical procedures. Int. Orthop. 2005, 29, 92–95. [Google Scholar] [CrossRef]
- Bittar, E.S.; Petty, W. Girdlestone arthroplasty for infected total hip arthroplasty. Clin. Orthop. Relat. Res. 1982, 83–87. [Google Scholar] [CrossRef]
- Castellanos, J.; Flores, X.; Llusà, M.; Chiriboga, C.; Navarro, A. The Girdlestone pseudarthrosis in the treatment of infected hip replacements. Int. Orthop. 1998, 22, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Grauer, J.D.; Amstutz, H.C.; O’carroll, P.F.; Dorey, F.J. Resection arthroplasty of the hip. J. Bone Jt. Surg. 1989, 71, 669–678. [Google Scholar] [CrossRef]
- Logoluso, N.; Pedrini, F.A.; Morelli, I.; De Vecchi, E.; Romanò, C.L.; Pellegrini, A.V. Megaprostheses for the revision of infected hip arthroplasties with severe bone loss. BMC Surg. 2022, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Paprosky, W.G.; Perona, P.G.; Lawrence, J.M. Acetabular defect classification and surgical reconstruction in revision arthroplasty: A 6-year follow-up evaluation. J. Arthroplast. 1994, 9, 33–44. [Google Scholar] [CrossRef]
- Yu, R.; Hofstaetter, J.G.; Sullivan, T.; Costi, K.; Howie, D.W.; Solomon, L.B. Validity and reliability of the Paprosky acetabular defect classification. Clin. Orthop. Relat. Res. 2013, 471, 2259–2265. [Google Scholar] [CrossRef]
- D’Antonio, J.; Mccarthy, J.C.; Bargar, W.L.; Borden, L.S.; Cappelo, W.N.; Collis, D.K.; Steinberg, M.E.; Wedge, J.H. Classification of femoral abnormalities in total hip arthroplasty. Clin. Orthop. Relat. Res. 1993, 296, 133–139. [Google Scholar] [CrossRef]
- Ibrahim, D.A.; Fernando, N.D. Classifications in Brief: The Paprosky Classification of Femoral Bone Loss. Clin. Orthop. Relat. Res. 2017, 475, 917–921. [Google Scholar] [CrossRef]
- Wirtz, D.C.; Jaenisch, M.; Osterhaus, T.A.; Gathen, M.; Wimmer, M.; Randau, T.M.; Schildberg, F.A.; Rössler, P.P. Acetabular defects in revision hip arthroplasty: A therapy-oriented classification. Arch. Orthop. Trauma Surg. 2020, 140, 815–825. [Google Scholar] [CrossRef]
- Walter, S.G.; Thomas, T.S.; Kenndoff, D.; Thomas, W. Mid-term follow-up after all-size acetabular revision and proposal for a stability classification system. HIP Int. 2019, 30, 431–437. [Google Scholar] [CrossRef]
- Laaksonen, I.; Lorimer, M.; Gromov, K.; Rolfson, O.; Mäkelä, K.T.; Graves, S.E.; Malchau, H.; Mohaddes, M. Does the Risk of Rerevision Vary Between Porous Tantalum Cups and Other Cementless Designs After Revision Hip Arthroplasty? Clin. Orthop. Relat. Res. 2017, 475, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Pulido, L.; Rachala, S.R.; Cabanela, M.E. Cementless acetabular revision: Past, present, and future. Revision total hip arthroplasty: The acetabular side using cementless implants. Int. Orthop. 2011, 35, 289–298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mäkinen, T.J.; Fichman, S.G.; Watts, E.; Kuzyk, P.R.T.; Safir, O.A.; Gross, A.E. The role of cages in the management of severe acetabular bone defects at revision arthroplasty. Bone Jt. J. 2016, 98-B (Suppl. SA), 73–77. [Google Scholar] [CrossRef] [PubMed]
- García-Rey, E.; Fernández-Fernández, R.; Durán, D.; Madero, R. Reconstruction of the rotation center of the hip after oblong cups in revision total hip arthroplasty. J. Orthop. Traumatol. 2012, 14, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Volpin, A.; Konan, S.; Biz, C.; Tansey, R.J.; Haddad, F.S. Reconstruction of failed acetabular component in the presence of severe acetabular bone loss: A systematic review. Musculoskelet. Surg. 2018, 103, 1–13. [Google Scholar] [CrossRef]
- Sakellariou, V.I.; Babis, G.C. Management bone loss of the proximal femur in revision hip arthroplasty: Update on reconstructive options. World J. Orthop. 2014, 5, 614–622. [Google Scholar] [CrossRef]
- Döring, K.; Vertesich, K.; Martelanz, L.; Staats, K.; Böhler, C.; Hipfl, C.; Windhager, R.; Puchner, S. Proximal femoral reconstruction with modular megaprostheses in non-oncological patients. Int. Orthop. 2021, 45, 2531–2542. [Google Scholar] [CrossRef]
- Durastanti, G.; Belvedere, C.; Ruggeri, M.; Donati, D.M.; Spazzoli, B.; Leardini, A. A Pelvic Reconstruction Procedure for Custom-Made Prosthesis Design of Bone Tumor Surgical Treatments. Appl. Sci. 2022, 12, 1654. [Google Scholar] [CrossRef]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef]
- Getzlaf, M.A.; Lewallen, E.A.; Kremers, H.M.; Jones, D.L.; Bonin, C.A.; Dudakovic, A.; Thaler, R.; Cohen, R.C.; Lewallen, D.G.; van Wijnen, A.J. Multi-disciplinary antimicrobial strategies for improving orthopaedic implants to prevent prosthetic joint infections in hip and knee. J. Orthop. Res. 2015, 34, 177–186. [Google Scholar] [CrossRef]
- Kim, T.N.; Feng, Q.L.; Kim, J.O.; Wu, J.; Wang, H.; Chen, G.C.; Cui, F.Z. Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. J. Mater. Sci. Mater. Med. 1998, 9, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Gosheger, G.; Hardes, J.; Ahrens, H.; Streitburger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Kemper, F.H.; Winkelmann, W.; Von Eiff, C. Silver-coated megaendoprostheses in a rabbit model—An analysis of the infection rate and toxicological side effects. Biomaterials 2004, 25, 5547–5556. [Google Scholar] [CrossRef] [PubMed]
- Fröschen, F.S.; Randau, T.M.; Hischebeth, G.T.R.; Gravius, N.; Wirtz, D.C.; Gravius, S.; Walter, S.G. Outcome of repeated multi-stage arthroplasty with custom-made acetabular implants in patients with severe acetabular bone loss: A case series. HIP Int. 2020, 30 (Suppl. S1), 64–71. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Thapa, S.S.; Vaish, A. Non-neoplastic indications and outcomes of the proximal and distal femur megaprosthesis: A critical review. Knee Surg. Relat. Res. 2020, 32, 18. [Google Scholar] [CrossRef] [PubMed]
- Chiarlone, F.; Zanirato, A.; Cavagnaro, L.; Alessio-Mazzola, M.; Felli, L.; Burastero, G. Acetabular custom-made implants for severe acetabular bone defect in revision total hip arthroplasty: A systematic review of the literature. Arch. Orthop. Trauma Surg. 2020, 140, 415–424. [Google Scholar] [CrossRef]
- Cadossi, M.; Garcia, F.L.; Sambri, A.; Andreoli, I.; Dallari, D.; Pignatti, G. A 2- to 7-Year Follow-Up of a Modular Iliac Screw Cup in Major Acetabular Defects: Clinical, Radiographic and Survivorship Analysis with Comparison to the Literature. J. Arthroplast. 2016, 32, 207–213. [Google Scholar] [CrossRef]
- Gamradt, S.C.; Lieberman, J.R. Bone graft for revision hip arthroplasty: Biology and future applications. Clin. Orthop. Relat. Res. 2003, 417, 183–194. [Google Scholar] [CrossRef]
- Sheth, N.P.; Nelson, C.L.; Springer, B.D.; Fehring, T.K.; Paprosky, W.G. Acetabular bone loss in revision total hip arthroplasty: Evaluation and management. J. Am. Acad. Orthop. Surg. 2013, 21, 128–139. [Google Scholar] [CrossRef]
- Hothi, H.S.; Ilo, K.; Whittaker, R.K.; Eskelinen, A.; Skinner, J.A.; Hart, A.J. Corrosion of Metal Modular Cup Liners. J. Arthroplast. 2015, 30, 1652–1656. [Google Scholar] [CrossRef]
- Christie, M.J.; Barrington, S.A.; Brinson, M.F.; Ruhling, M.E.; DeBoer, D.K. Bridging massive acetabular defects with the triflange cup: 2- to 9-year results. Clin. Orthop. Relat. Res. 2001, 393, 216–227. [Google Scholar] [CrossRef]
- Joshi, A.B.; Lee, J.; Christensen, C. Results for a custom acetabular component for acetabular deficiency. J. Arthroplast. 2002, 17, 643–648. [Google Scholar] [CrossRef]
- Holt, G.E.; Dennis, D.A. Use of custom triflanged acetabular components in revision total hip arthroplasty. Clin. Orthop. Relat. Res. 2004, 429, 209–214. [Google Scholar] [CrossRef]
- DeBoer, D.K.; Christie, M.J.; Brinson, M.F.; Morrison, J.C. Revision total hip arthroplasty for pelvic discontinuity. J. Bone Jt. Surg. 2007, 89, 835–840. [Google Scholar] [CrossRef]
- Taunton, M.J.; Fehring, T.K.; Edwards, P.; Bernasek, T.; Holt, G.E.; Christie, M.J. Pelvic discontinuity treated with custom triflange component: A reliable option. Clin. Orthop. Relat. Res. 2012, 470, 428–434. [Google Scholar] [CrossRef]
- Colen, S.; Harake, R.; De Haan, J.; Mulier, M. A modified custom-made triflanged acetabular reconstruction ring (MCTARR) for revision hip arthroplasty with severe acetabular defects. Acta Orthop. Belg. 2013, 79, 71–75. [Google Scholar]
- Wind, M.A.; Swank, M.L.; Sorger, J.I. Short-term results of a custom triflange acetabular component for massive acetabular bone loss in revision THA. Orthopedics 2013, 36, e260–e265. [Google Scholar] [CrossRef]
- Friedrich, M.J.; Schmolders, J.; Michel, R.D.; Randau, T.M.; Wimmer, M.D.; Kohlhof, H.; Wirtz, D.C.; Gravius, S. Management of severe periacetabular bone loss combined with pelvic discontinuity in revision hip arthroplasty. Int. Orthop. 2014, 38, 2455–2461. [Google Scholar] [CrossRef]
- Berasi, C.C.; Berend, K.R.; Adams, J.B.; Ruh, E.L.; Lombardi, A.V. Are custom triflange acetabular components effective for reconstruction of catastrophic bone loss? Clin. Orthop. Relat. Res. 2014, 473, 528–535. [Google Scholar] [CrossRef]
- Barlow, B.T.; Oi, K.K.; Lee, Y.-Y.; Carli, A.V.; Choi, D.S.; Bostrom, M.P. Outcomes of Custom Flange Acetabular Components in Revision Total Hip Arthroplasty and Predictors of Failure. J. Arthroplast. 2015, 31, 1057–1064. [Google Scholar] [CrossRef]
- Mao, Y.; Xu, C.; Xu, J.; Li, H.; Liu, F.; Yu, D.; Zhu, Z. The use of customized cages in revision total hip arthroplasty for Paprosky type III acetabular bone defects. Int. Orthop. 2015, 39, 2023–2030. [Google Scholar] [CrossRef]
- Li, H.; Qu, X.; Mao, Y.; Dai, K.; Zhu, Z. Custom Acetabular Cages Offer Stable Fixation and Improved Hip Scores for Revision THA With Severe Bone Defects. Clin. Orthop. Relat. Res. 2015, 474, 731–740. [Google Scholar] [CrossRef]
- Baauw, M.; van Hooff, M.L.; Spruit, M. Current Construct Options for Revision of Large Acetabular Defects: A Systematic Review. JBJS Rev. 2016, 4, e2. [Google Scholar] [CrossRef] [PubMed]
- Citak, M.; Kochsiek, L.; Gehrke, T.; Haasper, C.; Suero, E.M.; Mau, H. Preliminary results of a 3D-printed acetabular component in the management of extensive defects. HIP Int. 2017, 28, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Gladnick, B.P.; Fehring, K.A.; Odum, S.M.; Christie, M.J.; DeBoer, D.K.; Fehring, T.K. Midterm Survivorship After Revision Total Hip Arthroplasty with a Custom Triflange Acetabular Component. J. Arthroplast. 2018, 33, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Berend, M.E.; Berend, K.R.; Lombardi, A.V.; Cates, H.; Faris, P. The patient-specific Triflange acetabular implant for revision total hip arthroplasty in patients with severe acetabular defects: Planning, implantation, and results. Bone Jt. J. 2018, 100-B (Suppl. SA), 50–54. [Google Scholar] [CrossRef]
- Kieser, D.C.; Ailabouni, R.; Kieser, S.C.J.; Wyatt, M.C.; Armour, P.C.; Coates, M.H.; Hooper, G.J. The use of an Ossis custom 3D-printed tri-flanged acetabular implant for major bone loss: Minimum 2-year follow-up. HIP Int. 2018, 28, 668–674. [Google Scholar] [CrossRef]
- Moore, K.D.; McClenny, M.D.; Wills, B.W. Custom Triflange Acetabular Components for Large Acetabular Defects: Minimum 10-Year Follow-up. Orthopedics 2018, 41, E316–E320. [Google Scholar] [CrossRef]
- Gruber, M.S.; Jesenko, M.; Burghuber, J.; Hochreiter, J.; Ritschl, P.; Ortmaier, R. Functional and radiological outcomes after treatment with custom-made acetabular components in patients with Paprosky type 3 acetabular defects: Short-term results. BMC Musculoskelet. Disord. 2020, 21, 835. [Google Scholar] [CrossRef]
- Walter, S.G.; Randau, T.M.; Gravius, N.; Gravius, S.; Fröschen, F.S. Monoflanged Custom-Made Acetabular Components Promote Biomechanical Restoration of Severe Acetabular Bone Defects by Metallic Defect Reconstruction. J. Arthroplast. 2019, 35, 831–835. [Google Scholar] [CrossRef]
- von Hertzberg-Boelch, S.P.; Wagenbrenner, M.; Arnholdt, J.; Frenzel, S.; Holzapfel, B.M.; Rudert, M. Custom Made Monoflange Acetabular Components for the Treatment of Paprosky Type III Defects. J. Pers. Med. 2021, 11, 283. [Google Scholar] [CrossRef]
- Fröschen, F.S.; Randau, T.M.; Gravius, N.; Wirtz, D.C.; Gravius, S.; Walter, S.G. Risk factors for implant failure of custom-made acetabular implants in patients with Paprosky III acetabular bone loss and combined pelvic discontinuity. Technol. Health Care 2022, 30, 703–711. [Google Scholar] [CrossRef]
- Augustyn, A.; Stołtny, T.; Rokicka, D.; Wróbel, M.; Pająk, J.; Werner, K.; Ochocki, K.; Strojek, K.; Koczy, B. Revision arthroplasty using a custom-made implant in the course of acetabular loosening of the J&J DePuy ASR replacement system—Case report. Medicine 2022, 101, e28475. [Google Scholar] [CrossRef] [PubMed]
- Winther, S.S.; Petersen, M.; Yilmaz, M.; Kaltoft, N.S.; Stürup, J.; Winther, N.S. Custom-made triflanged implants in reconstruction of severe acetabular bone loss with pelvic discontinuity after total hip arthroplasty consecutive cohort study: Two to 11 years of follow-up. Bone Jt Open 2022, 3, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Kumta, S.; Sze, K.; Wong, C. Use of a patient-specific CAD/CAM surgical jig in extremity bone tumor resection and custom prosthetic reconstruction. Comput. Aided Surg. 2012, 17, 284–293. [Google Scholar] [CrossRef]
- Merema, B.J.; Kraeima, J.; ten Duis, K.; Wendt, K.W.; Warta, R.; Vos, E.; Schepers, R.H.; Witjes, M.J.H.; Ijpma, F.F.A. The design, production and clinical application of 3D patient-specific implants with drilling guides for acetabular surgery. Injury 2017, 48, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Cartiaux, O.; Paul, L.; Francq, B.G.; Banse, X.; Docquier, P.-L. Improved accuracy with 3D planning and patient-specific instruments during simulated pelvic bone tumor surgery. Ann. Biomed. Eng. 2013, 42, 205–213. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Egawa, H.; Hamada, D.; Takao, S.; Nakano, S.; Yasui, N. Location of intrapelvic vessels around the acetabulum assessed by three-dimensional computed tomographic angiography: Prevention of vascular-related complications in total hip arthroplasty. J. Orthop. Sci. 2012, 17, 397–406. [Google Scholar] [CrossRef]
- Wyatt, M.C. Custom 3D-printed acetabular implants in hip surgery–Innovative breakthrough or expensive bespoke upgrade? HIP Int. 2015, 25, 375–379. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Engh, C.A.; Macalino, G.E.; Lauro, G.R. Outcome of revision hip arthroplasty done without cement. J. Bone Jt. Surg. 1994, 76, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Weeden, S.H.; Paprosky, W.G. Minimal 11-year follow-up of extensively porous-coated stems in femoral revision total hip arthroplasty. J. Arthroplast. 2002, 17 (Suppl. S1), 134–137. [Google Scholar] [CrossRef]
- Duncan, C.P.; Masterson, E.L.; Masri, B.A. Impaction allografting with cement for the management of femoral bone loss. Orthop. Clin. N. Am. 1998, 29, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Ornstein, E.; Linder, L.; Ranstam, J.; Lewold, S.; Eisler, T.; Torper, M. Femoral impaction bone grafting with the Exeter stem—The Swedish experience: Survivorship analysis of 1305 revisions performed between 1989 and 2002. J. Bone Jt. Surg. 2009, 91-B, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Regis, D.; Sandri, A.; Bonetti, I. Long-term results of femoral revision with the Wagner Self-Locking stem. Surg. Technol. Int. 2013, 23, 243–250. [Google Scholar] [PubMed]
- McInnis, D.P.; Horne, G.; Devane, P.A. Femoral revision with a fluted, tapered, modular stem: Seventy patients followed for a mean of 3.9 years. J. Arthroplast. 2006, 21, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Pluhar, G.E.; Heiner, J.P.; Manley, P.A.; Bogdanske, J.J.; Vanderby, R.; Markel, M.D. Comparison of three methods of gluteal muscle attachment to an allograft/endoprosthetic composite in a canine model. J. Orthop. Res. 2000, 18, 56–63. [Google Scholar] [CrossRef]
- Wang, J.-W.; Wang, C.-J. Proximal femoral allografts for bone deficiencies in revision hip arthroplasty: A medium-term follow-up study. J. Arthroplast. 2004, 19, 845–852. [Google Scholar] [CrossRef]
- Babis, G.C.; Sakellariou, V.I.; O’connor, M.I.; Hanssen, A.D.; Sim, F.H. Proximal femoral allograft-prosthesis composites in revision hip replacement: A 12-year follow-up study. J. Bone Jt. Surg. 2010, 92, 349–355. [Google Scholar] [CrossRef]
- Jeys, L.; Kulkarni, A.; Grimer, R.; Carter, S.; Tillman, R.; Abudu, A. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. Minerva Anestesiol. 2008, 90, 1265–1271. [Google Scholar] [CrossRef]
- Korim, M.T.; Esler, C.N.; Ashford, R.U. Systematic review of proximal femoral arthroplasty for non-neoplastic conditions. J. Arthroplast. 2014, 29, 2117–2121. [Google Scholar] [CrossRef]
- Malkani, A.L.; Settecerri, J.J.; Sim, F.H.; Chao, E.Y.; Wallrichs, S.L. Long-term results of proximal femoral replacement for non-neoplastic disorders. J. Bone Jt. Surg. Br. 1995, 77, 351–356. [Google Scholar] [CrossRef]
- Haentjens, P.; De Boeck, H.; Opdecam, P. Proximal femoral replacement prosthesis for salvage of failed hip arthroplasty:Complications in a 2–11 year follow-up study in 19 elderly patients. Acta Orthop. 1996, 67, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.R.; Parvizi, J.; Rapuri, V.; Wolf, C.F.; Hozack, W.J.; Sharkey, P.F.; Purtill, J.J. Proximal femoral replacement for the treatment of periprosthetic fractures. J. Bone Jt. Surg. 2005, 87, 1777–1781. [Google Scholar] [CrossRef]
- Parvizi, J.; Tarity, T.D.; Slenker, N.; Wade, F.; Trappler, R.; Hozack, W.J.; Sim, F.H. Proximal femoral replacement in patients with non-neoplastic conditions. J. Bone Jt. Surg. 2007, 89, 1036–1043. [Google Scholar] [CrossRef]
- Shih, S.-T.; Wang, J.-W.; Hsu, C.-C. Proximal femoral megaprosthesis for failed total hip arthroplasty. Chang. Gung. Med. J. 2007, 30, 73–80. [Google Scholar]
- Schoenfeld, A.J.; Leeson, M.C.; Vrabec, G.A.; Scaglione, J.; Stonestreet, M.J. Outcomes of modular proximal femoral replacement in the treatment of complex proximal femoral fractures: A case series. Int. J. Surg. 2008, 6, 140–146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hardes, J.; Budny, T.; Hauschild, G.; Balke, M.; Streitbürger, A.; Dieckmann, R.; Gosheger, G.; Ahrens, H. Proximal femur replacement in revision arthroplasty. Z. Orthop. Unfall. 2009, 147, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Fada, R.; Murphy, S.B.; Rasquinha, V.J.; Ranawat, C.S. Two-year to five-year follow-up of femoral defects in femoral revision treated with the link MP modular stem. J. Arthroplast. 2009, 24, 751–758. [Google Scholar] [CrossRef]
- Gebert, C.; Wessling, M.; Götze, C.; Gosheger, G.; Hardes, J. The Modular Universal Tumour and Revision System (MUTARS®) in endoprosthetic revision surgery. Int. Orthop. 2010, 34, 1261–1265. [Google Scholar] [CrossRef]
- Sewell, M.D.; Hanna, S.A.; Carrington, R.W.; Pollock, R.C.; Skinner, J.; Cannon, S.R.; Briggs, T.W.R. Modular proximal femoral replacement in salvage hip surgery for non-neoplastic conditions. Acta Orthop. Belg. 2010, 76, 493–502. [Google Scholar]
- Al-Taki, M.M.; Masri, B.A.; Duncan, C.P.; Garbuz, D.S. Quality of life following proximal femoral replacement using a modular system in revision THA. Clin. Orthop. Relat. Res. 2011, 469, 470–475. [Google Scholar] [CrossRef]
- McLean, A.L.; Patton, J.T.; Moran, M. Femoral replacement for salvage of periprosthetic fracture around a total hip replacement. Injury 2012, 43, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.J.F.; Matthews, J.J.; Price, A.; Stubbs, D.; Whitwell, D.; Gibbons, C.M.L.H. Modular endoprosthetic replacement for failed internal fixation of the proximal femur following trauma. Int. Orthop. 2011, 36, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.; Colombo, M.; Ripamonti, C.; Malagoli, E.; Mazza, E.; Fadigati, P.; Bucci, M. Megaprosthesis in large bone defects: Opportunity or chimaera? Injury 2014, 45, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Grammatopoulos, G.; Alvand, A.; Martin, H.; Whitwell, D.; Taylor, A.; Gibbons, C.L.M.H.; Gilg, M.M.; Gaston, C.L.; Jeys, L.; Abudu, A.; et al. Five-year outcome of proximal femoral endoprosthetic arthroplasty for non-tumour indications. Bone Jt. J. 2016, 98-B, 1463–1470. [Google Scholar] [CrossRef]
- Curtin, M.; Bryan, C.; Murphy, E.; Murphy, C.; Curtin, W. Early results of the LPS™ limb preservation system in the management of periprosthetic femoral fractures. J. Orthop. 2017, 14, 34–37. [Google Scholar] [CrossRef]
- Viste, A.; Perry, K.I.; Taunton, M.J.; Hanssen, A.D.; Abdel, M.P. Proximal femoral replacement in contemporary revision total hip arthroplasty for severe femoral bone loss: A review of outcomes. Bone Jt. J. 2017, 99-B, 325–329. [Google Scholar] [CrossRef]
- Khajuria, A.; Ward, J.; Cooper, G.; Stevenson, J.; Parry, M.; Jeys, L. Is endoprosthetic replacement of the proximal femur appropriate in the comorbid patient? HIP Int. 2017, 28, 68–73. [Google Scholar] [CrossRef]
- De Martino, I.; D’apolito, R.; Nocon, A.A.; Sculco, T.P.; Sculco, P.K.; Bostrom, M.P. Proximal femoral replacement in non-oncologic patients undergoing revision total hip arthroplasty. Int. Orthop. 2018, 43, 2227–2233. [Google Scholar] [CrossRef]
- Fenelon, C.; Murphy, E.P.; Kearns, S.R.; Curtin, W.; Murphy, C.G. Cemented Proximal Femoral Replacement for the Management of Non-Neoplastic Conditions: A Versatile Implant but Not Without Its Risks. J. Arthroplast. 2020, 35, 520–527. [Google Scholar] [CrossRef]
- Zanchini, F.; Piscopo, A.; Cipolloni, V.; Vitiello, R.; Piscopo, D.; Fusini, F.; Cacciapuoti, S.; Panni, A.S.; Pola, E. The major proximal femoral defects: Megaprosthesis in non oncological patients—A case series. Orthop. Rev. 2023, 15, 38432. [Google Scholar] [CrossRef]
- Fiore, M.; Sambri, A.; Zucchini, R.; Giannini, C.; Donati, D.M.; De Paolis, M. Silver-coated megaprosthesis in prevention and treatment of peri-prosthetic infections: A systematic review and meta-analysis about efficacy and toxicity in primary and revision surgery. Eur. J. Orthop. Surg. Traumatol. 2020, 31, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Hardes, J.; von Eiff, C.; Streitbuerger, A.; Balke, M.; Budny, T.; Henrichs, M.P.; Hauschild, G.; Ahrens, H. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J. Surg. Oncol. 2010, 101, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Streitbuerger, A.; Henrichs, M.P.; Hauschild, G.; Nottrott, M.; Guder, W.; Hardes, J. Silver-coated megaprostheses in the proximal femur in patients with sarcoma. Eur. J. Orthop. Surg. Traumatol. 2018, 29, 79–85. [Google Scholar] [CrossRef] [PubMed]


| Study | Number of Cases | Mean Age (Years) | Indications | Bone Defects | Implant Features | Silver Coating | Mean FU (Years) | Complication Rate (%) | Dislocation Rate (%) | PJI Rate (%) | Further Revision Rate (%) | Implant Survival (%) * | Last FU Functional Evaluation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Christie et al., 2001 [41] | 67 | 59 | Failed THA | AAOS III-IV | Ti triflanged | No | 4.4 | 28 | 18 | 0 | 9 | 100 | HHS 82 |
| Joshi et al., 2002 [42] | 27 | 68 | Failed THA | AAOS III | Ti triflanged | No | 4.8 | 22 | 4 | 7 | 14 | 100 | NR |
| Holt et al., 2004 [43] | 26 | 69 | NR | P. III B | Ti triflanged | No | 4.5 | 27 | 8 | 0 | 4 | 88 | HHS 78 |
| De Boer et al., 2007 [44] | 20 | 56 | Failed THA | AAOS IV | Ti triflanged | No | 10.25 | 40 | 30 | 0 | 30 | 100 | HHS 80 |
| Taunton et al., 2012 [45] | 57 | 61 | Failed THA | AAOS IV | Ti triflanged | No | 6.3 | 47 | 21 | 7 | 30 | 95 | HHS 75 |
| Colen et al., 2013 [46] | 6 | 69 | Failed THA | AAOS III-IV | Ti triflanged | No | 2.4 | 0 | 0 | 0 | 0 | 100 | HHS 61 |
| Wind et al., 2013 [47] | 19 | 58 | Aseptic loosening PJI Dislocation | P. IIIA-IIIb | Ti triflanged | No | 2.6 | 53 | 26 | 5 | 32 | 89 | HHS 63 |
| Friedrich et al., 2014 [48] | 18 | 68 | Aseptic loosening PJI | P. IIIB | Ti triflanged | No | 2.5 | 33 | 17 | 11 | 28 | 89 | HHS 69 |
| Berasi et al., 2015 [49] | 24 | 67 | Aseptic loosening PJI Dislocation | P. IIIB | Ti triflanged | No | 4.75 | 26 | 0 | 8 | 17 | 92 | HHS 65 |
| Barlow et al., 2015 [50] | 63 | 63 | Aseptic loosening PJI | P: IIIB | Ti triflanged | No | 4.3 | 27 | 0 | 3 | 27 | 86 | NR |
| Mao et al., 2015 [51] | 23 | 61 | Aseptic loosening PJI | P. IIIA-IIIB | Ti cage dome, hook flange or three-braid porous | No | 6.9 | 22 | 9 | 0 | 4 | 91 | HHS 81 |
| Li et al., 2016 [52] | 24 | 65 | Aseptic loosening PJI | P. IIIB | cage with iliac wing/braid, ischial flange or crest obturator hook | No | 5.6 | 17 | 4 | 4 | 8 | 100 | HHS 82 |
| Baauw et al., 2016 [53] | 9 | 66 | Aseptic loosening Girdlestone | P. IIIA/IIIB | Ti triflanged | No | NR | 33 | 8 | 0 | 0 | 100 | NR |
| Citak et al., 2018 [54] | 9 | 67 | PJI | P. IIIA-IIIB | Ti triflanged | No | 2.4 | 67 | 33 | 0 | 67 | 89 | HHS 59 |
| Gladnick et al., 2018 [55] | 73 | 60 | Aseptic loosening PJI Dislocation Periprosthetic fracture | P. IIIB | Ti triflanged | No | 7.5 | 37 | 10 | 11 | 36 | 90 | NR |
| Berend et al., 2018 [56] | 95 | 66 | Aseptic loosening PJI Dislocation Periprosthetic fracture Cage failure | P. IIC-IIIA-IIIB | Ti triflanged | No | 3.6 | 22 | 6 | 6 | 22 | 93 | HHS 75 |
| Kieser et al., 2018 [57] | 36 | 68 | Aseptic loosening PJI Dislocation Periprosthetic fracture Metallosis | P. IIA-IIIB | Ti triflanged | No | 3.2 | 11 | 3 | 3 | 3 | 97 | HHS 79 |
| Moore et al., 2018 [58] | 35 | 60 | Aseptic loosening Periprosthetic fracture | NR | Ti triflanged | No | 10 | 11 | 0 | 6 | 8 | 91 | HHS 90 |
| Gruber et al., 2020 [59] | 16 | 69 | Aseptic loosening Septic loosening Periprosthetic fracture | P. IIIA-IIIB | Ti triflanged | No | 1 | 33 | 12 | 0 | 6 | NR | HHS 53 |
| Walter et al., 2020 [60] | 58 | 69 | Aseptic loosening PJI Dislocation Girdlestone | P. IIIA-IIIB | Ti triflanged | No | 5 | 50 | 9 | 12 | 36 | 72 | HHS 60 |
| Von Hertzberg- Boelch et al., 2021 [61] | 114 | 69 | Aseptic loosening PJI | P. IIIA-IIIB | Monoflang ed | No | 2.9 | 56 | 21 | 3 | 50 | 60 | NR |
| Froschen et al., 2022 [62] | 4 | 68 | PJI | P. IIIA-IIIB | Monoflang ed | No | 2 | 50 | 25 | 50 | 5 | 50 | HHS 50 |
| Augustyn et al., 2022 [63] | 1 | 74 | Metallosis | P. IIIB | Ti triflanged | No | 1.2 | NR | NR | NR | NR | NR | HHS 81 |
| Winther et al., 2022 [64] | 39 | 69 | Aseptic loosening PJI | All pelvic discontinuity | Ti triflanged | No | 5 | 21 | 8 | 8 | 21 | NR | HHS 80 |
| Study | Numbe r of Cases | Mean Age (Years) | Indications | Implant Features | Silver Coating | Mean FU (Years) | Complication Rate (%) | Dislocation Rate (%) | PJI Rate (%) | Further Revision Rate (%) | Implant Survival (%) | Last Follow-Up Functional Evaluation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malkani et al., 1995 [81] | 30 | 61 | Aseptic loosening Periprosthetic fracture PJI | Cemented stem with custom-made proximal femur component | No | 11.1 | 70 | 37 | 10 | 53 | 64% at 12 years | HHS 76 |
| Haentjens et al., 1996 [82] | 16 | 78 | Aseptic loosening | Cemented stem with large stainless-steel proximal femoral component | No | 5 | 62 | 44 | 12 | 50 | NR | NR |
| Klein et al., 2005 [83] | 21 | 78 | Periprosthetic fracture | Cemented antibiotic-loaded stem with proximal porous coating | No | 3.2 | 38 | 9 | 9 | 9 | NR | HHS 71 |
| Parvizi et al., 2007 [84] | 43 | 74 | Aseptic loosening Periprosthetic fracture PJI Non-union Osteonecrosis | Cemented modular replacement system with porous coated proximal stem | No | 3 | 30 | 19 | 2 | 42 | 87% at 1 year 73% at 5 years | HHS 65 |
| Shih et al., 2007 [85] | 12 | 59 | Aseptic loosening Periprosthetic fracture PJI | Cemented antibiotic-loaded modular EPR | No | 5.7 | 116 | 42 | 33 | 42 | NR | HHS 83 |
| Shoenfeld et al., 2008 [86] | 19 | 76 | Proximal femur fracture Proximal femur non-union | Howmedica® Biomet® EPR | No | 3.7 | 26 | 16 | 5 | 16 | NR | MDA 14.3 |
| Hardes et al., 2009 [87] | 28 | 72 | Aseptic loosening Periprosthetic fracture PJI | Multiple systems | No | 3.8 | 28 | 14 | 7 | 29 | 812% at 5 years | HHS 66 |
| Rodriguez et al., 2009 [88] | 97 | NR | Proximal femur bone loss | Link® MP modular | No | 3.2 | 18 | 10 | 0 | 12 | NR | HHS 84 |
| Gebert et al., 2010 [89] | 45 | 62 | Aseptic loosening Periprosthetic fracture PJI | MUTARS Implantcast® | No | 3.2 | 18 | 2 | 11 | 18 | 85% at 10 years | HHS 78 |
| Sewell et al., 2010 [90] | 15 | 67 | Aseptic loosening Periprosthetic fracture PJI | METS Stanmore® | No | 5 | 27 | 13 | 13 | 13 | 87% at 5 years | HHS 69 |
| Al-Taki et al., 2011 [91] | 36 | 73 | Aseptic loosening Periprosthetic fracture PJI Dislocation | Cemented or cementless MRS Stryker® | No | 3.2 | 14 | 8 | 3 | 14 | NR | OHS 70 WOMAC 71 |
| McLean et al., 2012 [92] | 20 | 72 | Periprosthetic fracture | Cemented GMRS Stryker® | No | 4 | 30 | 15 | 10 | 20 | NR | TESS 68 |
| Dean et al., 2012 [93] | 8 | 67 | Failed internal fixation for proximal femur fracture | METS Stanmore® | No | 1.5 | 0 | 0 | 0 | NR | NR | HHS 71 |
| Calori et al., 2013 [94] | 11 | 68 | Aseptic loosening Periprosthetic fracture PJI Non-union | NR | Si | 1.5 | 9 | 9 | 0 | 9 | NR | NR |
| Grammatopoulos et al., 2016 [95] | 79 | 69 | Aseptic loosening Periprosthetic fracture PJI Non-union Pseudotumor | NR | No | 5 | 25 | 4 | 11 | NR | 87% at 5 years | NR |
| Curtin et al., 2017 [96] | 16 | 75 | Aseptic loosening Periprosthetic fracture Proximal femur bone loss | Cemented or cementless LPS DePuy® | No | 1.6 | 12 | 12 | 0 | 6 | 94% at 1.6 years | OHS 40 |
| Viste et al., 2017 [97] | 44 | 79 | Aseptic loosening Periprosthetic fracture PJI Dislocation | Cemented EPR | No | 6 | 27 | 14 | 2 | 4 | 86% at 5 years 66% at 10 years | HHS 68 |
| Khajuria et al., 2018 [98] | 37 | 80 | Aseptic loosening Periprosthetic fracture PJI Non-union Pediatric arthrodesis | METS Stanmore® | No | 2.7 | 8 | 3 | 5 | 5 | 97% at 1 year 95% at 5 years | OHS 31 |
| De Martino et al., 2019 [99] | 31 | 64 | Aseptic loosening Periprosthetic fracture PJI Non-union | GMRS Stryker® | No | 5 | 29 | 6 | 10 | 29 | 78% at 5 years | NR |
| Fenelon et al., 2020 [100] | 79 | 78 | Aseptic loosening Periprosthetic fracture PJI Non-union severe osteoarthritis fracture | GMRS Stryker® LPS DePuy® | No | 2.6 | 15 | 11 | 4 | 5 | 96% at 1 year 95% at 5 years | NR |
| Döring et al., 2021 [28] | 28 | 67 | Aseptic loosening Periprosthetic fracture PJI Non-union Dislocation Proximal femur fracture | KMFTR Howmedica® HMRS Howmedica® GMRS Stryker® | No | 7.3 | 64 | 28 | 0 | 36 | 68% at 1 year 46% at 5 years 38% at 10 years | NR |
| Logoluso et al., 2022 [15] | 21 | 68 | PJI | Cemented or cementless Mega C-System Link® Distally interlocked modular femoral reconstruction prosthesis REEF® | Si | 5.3 | 67 | 38 | 10 | 14 | 83% at 2 and 5 years | NR |
| Zanchini et al., 2023 [101] | 39 | 69 | Periprosthetic fractures Bone loss PJI | GMRS Stryker® | No | 5 | 18 | 5 | 8 | 10 | 100% at 5 years | MDA 7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, M.; Paolucci, A.; Zunarelli, R.; Bortoli, M.; Montanari, A.; Pace, A.; Di Prinzio, L.; Parisi, S.C.; De Cristofaro, R.; De Paolis, M.; et al. A Combined Use of Custom-Made Partial Pelvic Replacement and Proximal Femur Megaprosthesis in the Treatment of Severe Bone Loss after Multiple Total Hip Arthroplasty Revisions. Prosthesis 2023, 5, 1093-1110. https://doi.org/10.3390/prosthesis5040076
Fiore M, Paolucci A, Zunarelli R, Bortoli M, Montanari A, Pace A, Di Prinzio L, Parisi SC, De Cristofaro R, De Paolis M, et al. A Combined Use of Custom-Made Partial Pelvic Replacement and Proximal Femur Megaprosthesis in the Treatment of Severe Bone Loss after Multiple Total Hip Arthroplasty Revisions. Prosthesis. 2023; 5(4):1093-1110. https://doi.org/10.3390/prosthesis5040076
Chicago/Turabian StyleFiore, Michele, Azzurra Paolucci, Renato Zunarelli, Marta Bortoli, Andrea Montanari, Andrea Pace, Lorenzo Di Prinzio, Stefania Claudia Parisi, Roberto De Cristofaro, Massimiliano De Paolis, and et al. 2023. "A Combined Use of Custom-Made Partial Pelvic Replacement and Proximal Femur Megaprosthesis in the Treatment of Severe Bone Loss after Multiple Total Hip Arthroplasty Revisions" Prosthesis 5, no. 4: 1093-1110. https://doi.org/10.3390/prosthesis5040076
APA StyleFiore, M., Paolucci, A., Zunarelli, R., Bortoli, M., Montanari, A., Pace, A., Di Prinzio, L., Parisi, S. C., De Cristofaro, R., De Paolis, M., & Sambri, A. (2023). A Combined Use of Custom-Made Partial Pelvic Replacement and Proximal Femur Megaprosthesis in the Treatment of Severe Bone Loss after Multiple Total Hip Arthroplasty Revisions. Prosthesis, 5(4), 1093-1110. https://doi.org/10.3390/prosthesis5040076









