Abstract
Polyethylene (PE) liners are a common bearing surface of orthopaedic prostheses. Wear particles of ultra-high molecular weight PE (UHMWPE) contribute to periprosthetic osteolysis, a major cause of aseptic loosening. Vitamin E is added to some PE liners to prevent oxidative degradation. Osteocytes, an important cell type for controlling both bone mineralisation and bone resorption, have been shown to respond UHMWPE particles by upregulating pro-osteoclastogenic and osteocytic osteolysis. Here, we examined the effects of the vitamin E analogues α-tocopherol and γ-tocotrienol alone or in the context of UHMWPE particles on human osteocyte gene expression and mineralisation behaviour. Human osteoblasts differentiated to an osteocyte-like stage were exposed to UHMWPE wear particles in the presence or absence of either α-Tocopherol or γ-Tocotrienol. Both α-Tocopherol and γ-Tocotrienol induced antioxidant-related gene expression. UHMWPE particles independently upregulated antioxidant gene expression, suggesting an effect of wear particles on oxidative stress. Both vitamin E analogues strongly induced OPG mRNA expression and γ-Tocotrienol also inhibited RANKL mRNA expression, resulting in a significantly reduced RANKL:OPG mRNA ratio (p < 0.01) overall. UHMWPE particles reversed the suppressive effect of α-Tocopherol but not of γ-Tocotrienol on this pro-osteoclastogenic index. UHMWPE particles also upregulated osteocytic-osteolysis related gene expression. Vitamin E analogues alone or in combination with UHMWPE particles also resulted in upregulation of these genes. Consistent with this, both vitamin E analogues promoted calcium release from mineralised cultures of osteocyte-like cells. Our findings suggest that while vitamin E may suppress osteocyte support of osteoclastogenesis in the presence of UHMWPE particles, the antioxidant effect may induce osteocytic osteolysis, which could promote periprosthetic osteolysis. It will be important to conduct further studies of vitamin E to determine the long-term effects of its inclusion in prosthetic materials.
1. Introduction
Implant loosening is a major problem associated with articulating joint replacement surgery. A major cause of aseptic loosening is the generation of wear particles of implant materials leading to the development of peri-prosthetic osteolytic lesions. Ultra-high molecular weight polyethylene (UHMWPE) is a common liner used for the bearing surface and wear particles of this are directly associated with the production of osteolytic lesions, with high volumetric wear rates correlating with the greatest progression of lesion volume [1]. UHMWPE liner wear due to mechanical friction against the metal femoral head, may be exacerbated by oxidative degradation of the polyethylene (PE). UHMWPE wear particles are known to stimulate a variety of inflammatory pathways in a number of cell types with activation of a number of cellular processes, including macrophage recruitment and activation [2], osteoclastic bone resorption, inhibition of bone formation [3], as well as osteocytic perilacunar remodelling, also known as osteocytic osteolysis [4]. Contact with or engulfment of UHMWPE particles causes the release of pro-inflammatory cytokines as well as the generation of reactive oxygen species (ROS) in macrophages, and possibly also other cell types, including osteoclasts, osteoblasts and osteocytes [2].
The addition of Vitamin E analogues is a recent approach to improving the endurance of UHMWPE liners by preventing oxidation. The aim of imbuing PE liners with Vitamin E is to reduce the formation of free radicals within the PE, as well as improving fatigue properties that would normally occur after post-irradiation melting, without sacrificing fatigue strength [5]. Vitamin E, specifically α-tocopherol, can be blended with UHMWPE powder prior to radiation, which protects against oxidation within the PE chain but reduces the crosslinking efficiency [6,7,8]. Alternatively, UHMWPE is diffused with vitamin E following radiation, circumventing possible changes to the crosslinking efficiency [9]. However, diffusion of vitamin E would result in relatively less vitamin E covalently bound within the PE chain compared to its addition prior to radiation, potentially allowing unbound vitamin E to leach into the synovial fluid and surrounding tissues, including bone [10]. While the concentrations of vitamin E released from intact imbued UHMWPE liners in the short-term (up to 3.6 years) have been shown to be negligible [11], the concentrations released in the longer term are unknown. Furthermore, the intra- or peri-cellular concentrations experienced due to micron-sized particles of vitamin E-imbued UHMWPE may well be higher due to the high surface area:volume ratio and the attempted intracellular processing of such particulates.
Both tocopherols and tocotrienols play potentially important roles in regulating bone resorption [12,13]. α-Tocopherol has been shown to have varying effects on both bone formation and resorption, dependent on the dose and model system investigated [12]. For example, specific knockdown of the α-tocopherol transfer protein in mice, where vitamin E absorption is blocked, showed a significantly higher bone mass due to lower bone resorption and reduced osteoclast surface [14]. Tocotrienol is able to reduce oxidative damage in osteoblasts, promoting osteoblast differentiation and survival [15]. Human epidemiological studies have also investigated the relationship between vitamin E and bone metabolism, however, with mixed results. One study, in a US population aged 50 and older, described a negative correlation between the high serum concentration of tocopherol and the femoral neck BMD [16]. Similarly, a study by Wolf et al. showed a negative correlation between total vitamin E intake and femoral neck BMD in an age-adjusted regression analysis of women in the USA [17]. However, a longitudinal study on Swedish subjects over the age of 50 and monitored for 19 years, has shown an association between low intake and serum concentration of α-tocopherol, and an increased rate of fracture [18].
Osteocytes are the most abundant cell type in bone and are critical in controlling bone remodelling and maintaining skeletal integrity [19]. These cells are important in the control of bone resorption by osteoclasts, at least in adult bone by virtue of their expression of RANKL [20]. They are also capable of resorbing their perilacunar matrix by producing bone degrading enzymes [21], including matrix metallopeptidase 13 (MMP13) [22,23], Cathepsin K (CTSK) [24] and carbonic anhydrase II (CA2) [25]. Human bone-derived osteocytes cultured either in a 3-dimensional (3D) collagen gel matrix or in 2D differentiated cultures exposed to UHMWPE particles display both osteocytic osteolytic and pro-osteoclastogenic responses [4,26,27]. Examination of human bone biopsies from implant patients with aseptic loosening and with confirmed periprosthetic osteolysis, also showed significantly increased osteocyte lacunar area compared to those in primary THR biopsies [4,26]. A recent study of the short-term effects of UHMWPE particles on the oxidative stress response in human osteoblastic Saos2 cells reported induction by UHMWPE particles but not when these were derived from a vitamin E-blended material [28]. However, little is known regarding the effects of UHMWPE particles on the differentiated osteocyte in the added presence of vitamin E. In this study, we investigated the biological effects of two major analogues of vitamin E, α-Tocopherol and γ-Tocotrienol, in the presence or absence of UHMWPE particles, on human primary osteocyte-like cells in terms of oxidative stress and bone catabolic responses.
2. Materials and Methods
2.1. Patient Bone-Derived Cells
All patient-derived samples were obtained with written, informed consent and human research ethics approval (Royal Adelaide Hospital Human Research Ethics Committee approval no. 130114). Human primary osteoblasts (Normal Human Bone-derived Cells; NHBC) were derived from proximal femoral cancellous bone biopsies, taken from patients undergoing total hip replacement surgery, and cultured and passaged ex vivo, as described previously [23,26].
2.2. Osteocyte-like Cell Culture
NHBC isolated from 3 different donors were cultured for 28 days until the cells reached a mature osteocyte-like stage, as described previously [23,26,29]. The cells were then treated for a further 7 days with either α-Tocopherol or γ-Tocotrienol (Sigma Aldrich, St. Louis, MO, USA) at concentrations of 100 nM, 300 nM, 1 µM or untreated control with experimental quadruplicates. Similarly, osteocyte-like cells after 28 d of culture were overlaid with a collagen gel (Cellmatrix Type 1-A, Nitta, Tokyo, Japan) [4,27], containing UHMWPE particles (Ceridust 3615, Clariant Company, Muttenz, Switzerland) (100 µg/mL), with the addition of either α-Tocopherol or γ-Tocotrienol (Sigma-Aldrich, St. Louis, MO, USA) (1 µM), or particle free and vehicle controls, and cultured for a further 21 d. UHMWPE particles were prepared for experimental use, as previously described [27]. They were confirmed to be endotoxin free and were sized from 0.3–10 µM. Either α-Tocopherol or γ-Tocotrienol, 1 µM was added to the culture medium every 3 days.
2.3. Gene Expression
Total RNA was extracted using TRIzol (Life Technologies, Carlsbad, CA, USA) and complementary DNA was synthesised using iScript™ RT kit (Bio-Rad, Hercules, CA, USA), according to the manufacturer’s instructions. Real time RT-PCR was performed using SYBR Green Fluor qPCR Mastermix (Qiagen, Limburg, The Netherlands) on a CFX Connect (Bio-Rad). Gene expression was normalised to that of the housekeeping gene, HPRT1. Oligonucleotide sequences for the mRNA-specific amplification of MMP13, CA2, CTSK, RANKL, OPG and CSF1 are published [4]. Oligonucleotide sequences for HPRT1, SOD1, SOD2 and CAT are listed in Table 1. All primers were designed in-house to be mRNA specific and all were purchased from Sigma-Aldrich.

Table 1.
Sequences of sense (S) and anti-sense (AS)-specific oligonucleotide primers and predicted PCR product sizes. Primers were designed to be mRNA-specific.
2.4. In Vitro Mineralisation
Calcium deposition was visualised using the Alizarin Red stain, as described previously [30]. Briefly, quadruplicate wells per treatment were washed with PBS and then fixed with 10% neutral buffered formalin for 1 h at RT and then washed with PBS (3×). Each well was stained with aqueous 2% (w/v) Alizarin red solution (Sigma Aldrich) at pH 4.2, for 5 min at room temperature and then washed with distilled water to remove any unbound stain. Each well was imaged (Olympus SZ2-ILST, Tokyo, Japan) and then the bound stain was solubilised with 100 µL of 10% Cetylpyrridium chloride (CPC) (Sigma-Aldrich) in 10 mM Sodium phosphate monobasic monohydrate (pH 7.0) added to each well for 15 min at RT. The optical density was determined at 560 nm by spectrophotometry (Thermo Multiskan Ascent, Thermofisher Scientific, Waltham, MA, USA).
2.5. Statistical Analysis
Statistical differences within data sets were analysed using GraphPad Prism Version 9.0.0 software (GraphPad Prism, La Jolla, LA, USA). Differences in normalised gene expression to control values were analysed using non-parametric Kruskal–Wallis tests with Mann–Whitney post hoc tests. Differences in Alizarin Red mineralisation were tested using one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons tests. In all cases a value for p < 0.05 was considered significant.
3. Results and Discussion
3.1. Effects of UHMWPE and Vitamin E Analogues on the Oxidative Stress Response
In order to investigate the effects of vitamin E analogues and UHMWPE particles, we used a model where human primary osteoblasts isolated from THR implant recipients, the population at risk from developing periprosthetic osteolysis, were cultured for 28 days under pro-osteogenic conditions to generate mature, osteocyte-like cultures [4]. Once established, the cells were overlaid with a type I collagen gel containing UHMWPE particles, with the addition of either α-Tocopherol or γ-Tocotrienol and cultured for a further 21 days. The use of a collagen gel was considered a vital experimental design feature in order to facilitate direct cell contact with the hydrophobic UHMWPE particles, mimicking the observed co-proximity of these in patients affected by aseptic loosening [4].
Vitamin E is an attractive bone supplement due to its anti-inflammatory and antioxidant activities. The induction of antioxidant gene expression can be both a marker of oxidative stress and an indication of a physiological response to the causative reactive oxygen species (ROS). Therefore, we investigated the expression of key antioxidant genes, superoxide dismutases 1 and 2 (SOD1 and SOD2), which are produced in response to ROS and act by binding to superoxide radicals, metabolising them to oxygen and hydrogen peroxide [31]. The expression of catalase (CAT), which further converts hydrogen peroxide into water and oxygen [31], was also examined. Osteocyte-like cultures treated with either α-Tocopherol or γ-Tocotrienol demonstrated significantly upregulated expression of all three antioxidant genes over the 21 d period of exposure (Figure 1A–C). Moderate upregulation of these genes also occurred with exposure to UHMWPE particles alone, consistent with induction of oxidative stress. Importantly, both Vitamin E analogues stimulated further increased expression of these genes in the presence of UHMWPE particles. Previous studies have shown that human osteoblast-like cells cultured on intact UHMWPE results in induction of oxidative stress [32], and that PE liners and the resulting particles undergo oxidative degradation and can generate ROS [33,34]. Therefore, upregulation of antioxidant genes could be a protective mechanism by the osteocyte in response to ROS produced by the UHMWPE particles, to prevent DNA damage and potential cell death. Consistent with this protective mechanism, we have previously reported that UHMWPE particles do not induce apoptosis in human osteocyte-like cells [4]. A recent study [35] reported that tri-calcium phosphate particles also induced oxidative stress in the calvarial osteolysis model; however, this was associated with osteocyte death, suggesting a difference in response between materials in this respect.
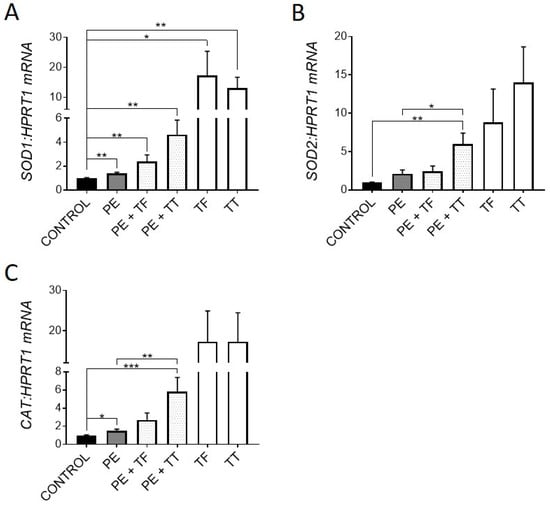
Figure 1.
Effect of vitamin E and UHMWPE particles on antioxidant gene expression. Human osteocyte-like cultures were overlaid with collagen gel containing UHMWPE particles (PE) and either α-Tocopherol (TF) or γ-Tocotrienol (TT), and cultured for a further 21 d. Relative gene expression was measured for antioxidant genes SOD1, SOD2 and Catalase (A–C). Data shown are means ± standard error of the mean (SEM) of quadruplicate experiments analysed in duplicate. Significant differences are denoted by * p < 0.05, ** p < 0.01, *** p < 0.001.
3.2. Effects of UHMWPE and Vitamin E on the Pro-Osteoclastogenic Response
We next investigated the expression of genes associated with osteoclastogenesis. It was previously shown that osteocytes respond to UHMWPE wear particles by upregulating the pro-osteoclastogenic RANKL:OPG mRNA ratio, as well as upregulating key osteoclastic regulatory genes, CSF1 and IL8 [4,27]. Here, osteocytes responded to treatment with either α-Tocopherol and γ-Tocotrienol by downregulating the RANKL:OPG mRNA ratio. In particular, treatment with γ-Tocotrienol suppressed RANKL and increased OPG mRNA expression in the primary human osteocyte cultures (Figure 2A,B), whereas α-Tocopherol, whilst having non-significant effects on either gene alone, also resulted in reduced RANKL:OPG mRNA expression overall.
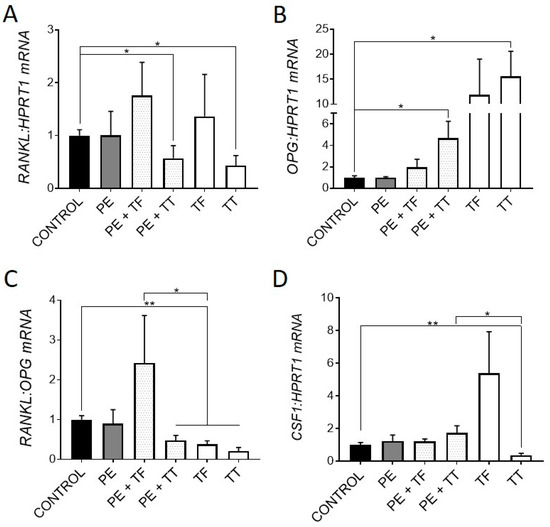
Figure 2.
Effect of vitamin E and UHMWPE particles on osteoclastogenic gene expression. Relative gene expression was measured for genes associated with osteoclastogenesis: RANKL, OPG, the pro-osteoclastogenic RANKL:OPG mRNA ratio and CSF1 mRNA expression (A–D). Data shown are means ± standard error of the mean (SEM) of quadruplicate experiments analysed in duplicate. Significant differences are denoted by * p < 0.05, ** p < 0.01.
The addition of α-Tocopherol had no effect on the UHMWPE response in terms of this ratio. However, γ-Tocotrienol significantly reduced the RANKL:OPG mRNA ratio in the presence of particles. Interestingly, γ-Tocotrienol but not α-Tocopherol alone significantly downregulated CSF1 expression, the protein product of which, CSF1/macrophage colony stimulating factor (M-CSF), is a key co-factor for RANKL-mediated osteoclastogenesis, although this effect was not seen in the presence of UHMWPE (Figure 2D). Overall, these findings suggest that both α-Tocopherol and γ-Tocotrienol exert an anti-osteoclastogenic effect on human osteocyte-like cells, in the presence or absence of UHMWPE particles. Consistent with this, previous studies have shown that mice treated with vitamin E, either α-Tocopherol or γ-Tocotrienol, have decreased bone resorption [13,36]. Furthermore, a study by Bichara et al. [37] showed Vitamin E-bound UHMWPE wear particles significantly decreased the amount of bone resorption in a mouse calvarial model of osteolysis when compared to conventional UHMWPE particles.
3.3. Effects of UHMWPE and Vitamin E on the Osteocytic Osteolysis Response
Consistent with previous reports [4,26], UHMWPE particles induced expression of genes associated with osteocytic osteolysis, including MMP13, CA2 (not significantly) and CTSK (p < 0.001) (Figure 3A–C).
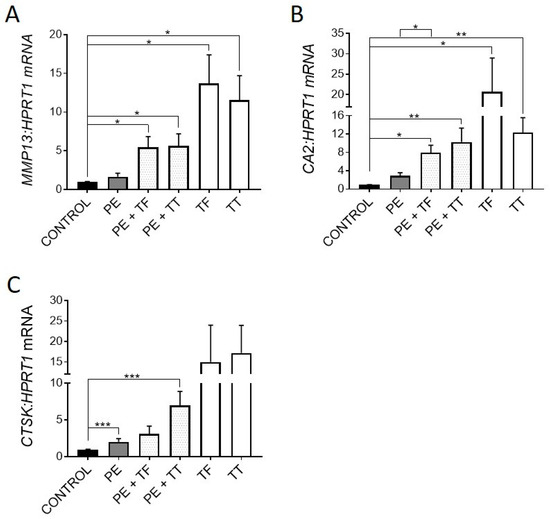
Figure 3.
Effect of vitamin E and UHMWPE particles on expression of genes associated with osteocytic osteolysis. (A) MMP13 mRNA expression, (B) CA2 mRNA expression, (C) CTSK mRNA expression. Data shown are means ± standard error of the mean (SEM) of quadruplicate experiments analysed in duplicate. Significant differences are denoted by * p < 0.05, ** p < 0.01, *** p < 0.001.
Treatment with either Vitamin E analogue upregulated the expression of all three genes, suggesting that Vitamin E could promote osteocytic osteolysis. Both α-Tocopherol and γ-Tocotrienol were able to further increase the expression of these genes in the presence of UHMWPE particles. As mentioned previously, UHMWPE particles are capable of producing ROS, which play a key role in osteoclastic differentiation and activity [38]. Therefore, here, osteocytes could be responding to ROS through one of two pathways. Firstly, the inhibition of ROS in human osteocytes by treatment with Vitamin E analogues alone and in the presence of UHMWPE particles may have caused the increase in expression of genes associated with osteocytic osteolysis [21,25], MMP13, CTSK and CA2. Secondly, UHMWPE particles may stimulate the production of ROS by the osteocyte, inducing oxidative stress, triggering a perilacunar remodelling response. This effect would explain why there was upregulation of antioxidant gene expression in response to UHMWPE. Therefore, removal of perilacunar bone could be one of the mechanisms used by osteocytes to relieve oxidative stress. Further investigation is required to determine if ROS produced by the osteocyte plays a role in regulating osteocytic osteolysis in vivo.
3.4. Effects of Vitamin E Analogues on Mineral Handling
To investigate the effects of α-Tocopherol and γ-Tocotrienol on osteocytic regulation of mineral handling [39], osteocyte-like cultures were exposed to either analogue (0, 100 nM, 300 nM and 1 µM) in the absence of UHMWPE particles. Calcium deposition was measured using the Alizarin Red stain, as described previously [30]. Consistent with the observed regulation of osteocytic osteolysis related genes, treatment with either Vitamin E analogue resulted in decreased mineralisation (Figure 4).
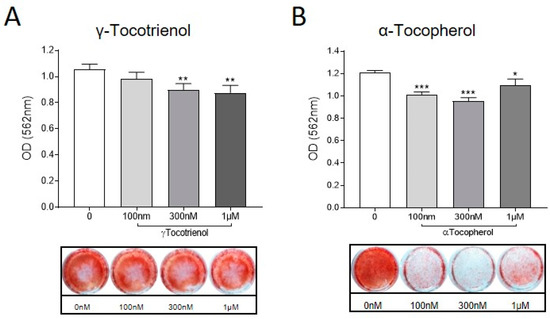
Figure 4.
Effect of vitamin E analogues on osteocyte mineral handling. Human osteocyte-like cultures were treated with either (A) γ-Tocotrienol or (B) α-Tocopherol (100 nM, 300 nM, 1 µM), or untreated (0) for a further 6 d. Cells were stained with Alizarin Red and then quantified using Cetylpyrridium chloride to elute the bound stain. Absorbance at 560 nM was measured by spectrophotometry. Data shown are means ± standard deviation of quadruplicate wells. Significant differences are denoted by * p < 0.05, ** p < 0.01, *** p < 0.001.
This suggests that vitamin E could contribute to the loss of bone that occurs during periprosthetic osteolysis specifically through promoting osteocytic removal of perilacunar bone.
This study has a number of limitations. In a human patient, aseptic loosening resulting from osteolysis typically takes years to manifest, and any in vitro model of this process cannot accurately recapitulate this. In order to specifically test the human osteocyte response we differentiated primary osteoblastic cells for a 4-week culture period and then measured responses to particles and vitamin E analogues after a further 3-weeks. Due to the hydrophobic nature of UHMWPE particles we chose to embed these in a type I collagen gel overlaid onto the differentiated cell layer. Any response to the UHMWPE requires direct contact either with osteocyte cell bodies, or more likely, with dendritic processes that grow into the gel over a period of days and weeks [26]. Thus, tuning the system to capture sufficient osteocyte-particle interactions within a given time frame to elicit a measurable response is challenging. In the current study we chose a single concentration of UHMWPE particles and a single assay time point, based on our previous studies [4,26], however we did not see the expected responses in terms of particle induction of RANKL:OPG expression, therefore, in the context of the osteoclastogenic response, this study only provided information on the responses of vitamin E analogues in the presence or absence of UHMWPE particles. However, we did observe direct effects of UHMWPE exposure on SOD1 and CAT expression, as well as on the osteocytic osteolysis gene CTSK, validating the system. In addition, the local bone or intracellular concentrations achieved in the case of vitamin E-imbued UHMWPE liner wear particles remain unknown, so the responses observed here to free vitamin E should be taken as proof of concept.
4. Conclusions
This study demonstrates the potential effects of α-Tocopherol and γ-Tocotrienol on the regulation of bone resorption by the human osteocyte. Our data suggest that UHMWPE particles induce oxidative stress in osteocytes over a relatively long culture period (21 d), which could be a potential trigger of perilacunar remodelling. Two distinct actions of the vitamin E analogues were observed: suppression of the pro-osteoclastic response, and stimulation of the osteocytic osteolysis response. Thus, while our findings are consistent with vitamin E suppressing the osteoclastic response to UHMWPE particles, it could potentially exacerbate the osteocytic osteolysis response, which in turn could contribute to periprosthetic osteolysis. The effects of vitamin E analogues on osteocytes requires further investigation to determine if their lytic effects have consequences in the long-term development of periprosthetic osteolysis and aseptic loosening.
Author Contributions
Conceptualization, G.J.A., A.E., R.T.O. and K.H.; methodology, G.J.A., R.T.O. and K.H.; formal analysis, R.T.O., K.H. and G.J.A.; investigation, R.T.O. and K.H.; resources, G.J.A., A.E. and A.O.; data curation, G.J.A. and R.T.O.; writing—original draft preparation, R.T.O., G.J.A. and D.M.F.; writing—review and editing, all authors; supervision, G.J.A., D.M.F. and L.B.S.; project administration, G.J.A.; funding acquisition, G.J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Health and Medical Research Council of Australia (NHMRC) Project Grant Scheme (ID 1041456) and the Royal Adelaide Hospital Special Purposes Fund. It was also supported in part by a Cyprus Research Promotion Foundation grant YGEIA/BIOS/0308(BIE) awarded to A.O. and A.E. R.T.O. was the recipient of a University of Adelaide post-graduate scholarship. G.J.A. was supported by a NHMRC Senior Research Fellowship (ID 1080806).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Human Research Ethics Committee of the Royal Adelaide Hospital (protocol code 130114, date of approval 18 January 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All relevant data are contained herein.
Acknowledgments
The authors thank the nursing and surgical staff of the Orthopaedic Trauma Service, RAH, for their help in consenting participants and collecting bone specimens.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Howie, D.W.; Neale, S.D.; Stamenkov, R.; McGee, M.A.; Taylor, D.J.; Findlay, D.M. Progression of acetabular periprosthetic osteolytic lesions measured with computed tomography. J. Bone Jt. Surg. 2007, 89, 1818–1825. [Google Scholar]
- Goodman, S.B.; Gallo, J. Periprosthetic osteolysis: Mechanisms, prevention and treatment. J. Clin. Med. 2019, 8, 2091. [Google Scholar]
- Goodman, S.; Aspenberg, P.; Song, Y.; Regula, D.; Lidgren, L. Polyethylene and titanium alloy particles reduce bone formation. Dose-dependence in bone harvest chamber experiments in rabbits. Acta Orthop. Scand. 1996, 67, 599–605. [Google Scholar]
- Ormsby, R.T.; Cantley, M.; Kogawa, M.; Solomon, L.B.; Haynes, D.R.; Findlay, D.M.; Atkins, G.J. Evidence that osteocyte perilacunar remodelling contributes to polyethylene wear particle induced osteolysis. Acta Biomater 2016, 33, 242–251. [Google Scholar]
- Costa, L.; Luda, M.P.; Trossarelli, L.; Brach del Prever, E.M.; Crova, M.; Gallinaro, P. Oxidation in orthopaedic uhmwpe sterilized by gamma-radiation and ethylene oxide. Biomaterials 1998, 19, 659–668. [Google Scholar]
- Oral, E.; Godleski Beckos, C.; Malhi, A.S.; Muratoglu, O.K. The effects of high dose irradiation on the cross-linking of vitamin e-blended ultrahigh molecular weight polyethylene. Biomaterials 2008, 29, 3557–3560. [Google Scholar]
- Oral, E.; Greenbaum, E.S.; Malhi, A.S.; Harris, W.H.; Muratoglu, O.K. Characterization of irradiated blends of alpha-tocopherol and uhmwpe. Biomaterials 2005, 26, 6657–6663. [Google Scholar]
- Parth, M.; Aust, N.; Lederer, K. Studies on the effect of electron beam radiation on the molecular structure of ultra-high molecular weight polyethylene under the influence of alpha-tocopherol with respect to its application in medical implants. J. Mater. Sci Mater. Med. 2002, 13, 917–921. [Google Scholar]
- Oral, E.; Wannomae, K.K.; Hawkins, N.; Harris, W.H.; Muratoglu, O.K. Alpha-tocopherol-doped irradiated uhmwpe for high fatigue resistance and low wear. Biomaterials 2004, 25, 5515–5522. [Google Scholar]
- Jarrett, B.T.; Cofske, J.; Rosenberg, A.E.; Oral, E.; Muratoglu, O.; Malchau, H. In vivo biological response to vitamin e and vitamin-e-doped polyethylene. J. Bone Jt. Surg. 2010, 92, 2672–2681. [Google Scholar]
- Currier, B.H.; Van Citters, D.W. A novel technique for assessing antioxidant concentration in retrieved uhmwpe. Clin. Orthop. Relat. Res. 2017, 475, 1356–1365. [Google Scholar]
- Chin, K.Y.; Ima-Nirwana, S. The effects of alpha-tocopherol on bone: A double-edged sword? Nutrients 2014, 6, 1424–1441. [Google Scholar]
- Chin, K.Y.; Ima-Nirwana, S. The biological effects of tocotrienol on bone: A review on evidence from rodent models. Drug Des. Dev. Ther. 2015, 9, 2049–2061. [Google Scholar]
- Fujita, K.; Iwasaki, M.; Ochi, H.; Fukuda, T.; Ma, C.; Miyamoto, T.; Takitani, K.; Negishi-Koga, T.; Sunamura, S.; Kodama, T.; et al. Vitamin e decreases bone mass by stimulating osteoclast fusion. Nat. Med. 2012, 18, 589–594. [Google Scholar]
- Fatokun, A.A.; Stone, T.W.; Smith, R.A. Responses of differentiated mc3t3-e1 osteoblast-like cells to reactive oxygen species. Eur. J. Pharmacol. 2008, 587, 35–41. [Google Scholar]
- Zhang, J.; Hu, X.; Zhang, J. Associations between serum vitamin e concentration and bone mineral density in the us elderly population. Osteoporos. Int. 2017, 28, 1245–1253. [Google Scholar]
- Wolf, R.L.; Cauley, J.A.; Pettinger, M.; Jackson, R.; Lacroix, A.; Leboff, M.S.; Lewis, C.E.; Nevitt, M.C.; Simon, J.A.; Stone, K.L.; et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: Results from the women’s health initiative. Am. J. Clin. Nutr. 2005, 82, 581–588. [Google Scholar]
- Michaelsson, K.; Wolk, A.; Byberg, L.; Arnlov, J.; Melhus, H. Intake and serum concentrations of alpha-tocopherol in relation to fractures in elderly women and men: 2 cohort studies. Am. J. Clin. Nutr. 2014, 99, 107–114. [Google Scholar]
- Prideaux, M.; Findlay, D.M.; Atkins, G.J. Osteocytes: The master cells in bone remodelling. Curr. Opin. Pharmacol. 2016, 28, 24–30. [Google Scholar]
- O’Brien, C.A.; Nakashima, T.; Takayanagi, H. Osteocyte control of osteoclastogenesis. Bone 2013, 54, 258–263. [Google Scholar]
- Tsourdi, E.; Jähn, K.; Rauner, M.; Busse, B.; Bonewald, L.F. Physiological and pathological osteocytic osteolysis. J. Musculoskelet. Neuronal Interact. 2018, 18, 292–303. [Google Scholar]
- Tang, S.Y.; Herber, R.P.; Ho, S.P.; Alliston, T. Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J. Bone Miner. Res. 2012, 27, 1936–1950. [Google Scholar]
- Ormsby, R.T.; Zelmer, A.R.; Yang, D.; Gunn, N.J.; Starczak, Y.; Kidd, S.P.; Nelson, R.; Solomon, L.B.; Atkins, G.J. Evidence for osteocyte-mediated bone-matrix degradation associated with periprosthetic joint infection (pji). Eur. Cells Mater. 2021, 41, 264–280. [Google Scholar]
- Qing, H.; Ardeshirpour, L.; Pajevic, P.D.; Dusevich, V.; Jahn, K.; Kato, S.; Wysolmerski, J.; Bonewald, L.F. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J. Bone Miner. Res. 2012, 27, 1018–1029. [Google Scholar]
- Kogawa, M.; Wijenayaka, A.R.; Ormsby, R.T.; Thomas, G.P.; Anderson, P.H.; Bonewald, L.F.; Findlay, D.M.; Atkins, G.J. Sclerostin regulates release of bone mineral by osteocytes by induction of carbonic anhydrase 2. J. Bone Miner. Res. 2013, 28, 2436–2448. [Google Scholar]
- Ormsby, R.T.; Solomon, L.B.; Yang, D.; Crotti, T.N.; Haynes, D.R.; Findlay, D.M.; Atkins, G.J. Osteocytes respond to particles of clinically-relevant conventional and cross-linked polyethylene and metal alloys by up-regulation of resorptive and inflammatory pathways. Acta Biomater. 2019, 87, 296–306. [Google Scholar]
- Atkins, G.J.; Welldon, K.J.; Holding, C.A.; Haynes, D.R.; Howie, D.W.; Findlay, D.M. The induction of a catabolic phenotype in human primary osteoblasts and osteocytes by polyethylene particles. Biomaterials 2009, 30, 3672–3681. [Google Scholar]
- Massaccesi, L.; Ragone, V.; Papini, N.; Goi, G.; Corsi Romanelli, M.M.; Galliera, E. Effects of vitamin e-stabilized ultra high molecular weight polyethylene on oxidative stress response and osteoimmunological response in human osteoblast. Front. Endocrinol. 2019, 10, 203. [Google Scholar]
- Yang, D.; Wijenayaka, A.R.; Solomon, L.B.; Pederson, S.M.; Findlay, D.M.; Kidd, S.P.; Atkins, G.J. Novel insights into staphylococcus aureus deep bone infections: The involvement of osteocytes. MBio 2018, 9, e00415–e00418. [Google Scholar]
- Kumarasinghe, D.D.; Sullivan, T.; Kuliwaba, J.S.; Fazzalari, N.L.; Atkins, G.J. Evidence for the dysregulated expression of twist1, tgfbeta1 and smad3 in differentiating osteoblasts from primary hip osteoarthritis patients. Osteoarthr. Cartil. 2012, 20, 1357–1366. [Google Scholar]
- Fattman, C.L.; Schaefer, L.M.; Oury, T.D. Extracellular superoxide dismutase in biology and medicine. Free Radic. Biol. Med. 2003, 35, 236–256. [Google Scholar]
- Gazzano, E.; Bracco, P.; Bistolfi, A.; Aldieri, E.; Ghigo, D.; Boffano, M.; Costa, L.; Brach Del Prever, E. Ultra high molecular weight polyethylene is cytotoxic and causes oxidative stress, even when modified. Int. J. Immunopathol. Pharmacol. 2011, 24, 61–67. [Google Scholar]
- Premnath, V.; Harris, W.H.; Jasty, M.; Merrill, E.W. Gamma sterilization of uhmwpe articular implants: An analysis of the oxidation problem. Ultra high molecular weight poly ethylene. Biomaterials 1996, 17, 1741–1753. [Google Scholar]
- Neuerburg, C.; Loer, T.; Mittlmeier, L.; Polan, C.; Farkas, Z.; Holdt, L.M.; Utzschneider, S.; Schwiesau, J.; Grupp, T.M.; Bocker, W.; et al. Impact of vitamin e-blended uhmwpe wear particles on the osseous microenvironment in polyethylene particle-induced osteolysis. Int. J. Mol. Med. 2016, 38, 1652–1660. [Google Scholar]
- Zhang, Y.; Yan, M.; Niu, W.; Mao, H.; Yang, P.; Xu, B.; Sun, Y. Tricalcium phosphate particles promote pyroptotic death of calvaria osteocytes through the ros/nlrp3/caspase-1 signaling axis in amouse osteolysis model. Int. Immunopharmacol. 2022, 107, 108699. [Google Scholar]
- Abdul-Majeed, S.; Mohamed, N.; Soelaiman, I.N. Effects of tocotrienol and lovastatin combination on osteoblast and osteoclast activity in estrogen-deficient osteoporosis. Evid.-Based Complementary Altern. Med. 2012, 2012, 960742. [Google Scholar]
- Bichara, D.A.; Malchau, E.; Sillesen, N.H.; Cakmak, S.; Nielsen, G.P.; Muratoglu, O.K. Vitamin e-diffused highly cross-linked uhmwpe particles induce less osteolysis compared to highly cross-linked virgin uhmwpe particles in vivo. J. Arthroplast. 2014, 29, 232–237. [Google Scholar]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar]
- Atkins, G.J.; Findlay, D.M. Osteocyte regulation of bone mineral: A little give and take. Osteoporos. Int. 2012, 23, 2067–2079. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).