Cell Fractionation and the Identification of Host Proteins Involved in Plant–Virus Interactions
Abstract
:1. Introduction
2. Cellular Organelles Are Involved in Virus Infection
3. Host Proteins Participate in Virus Infection
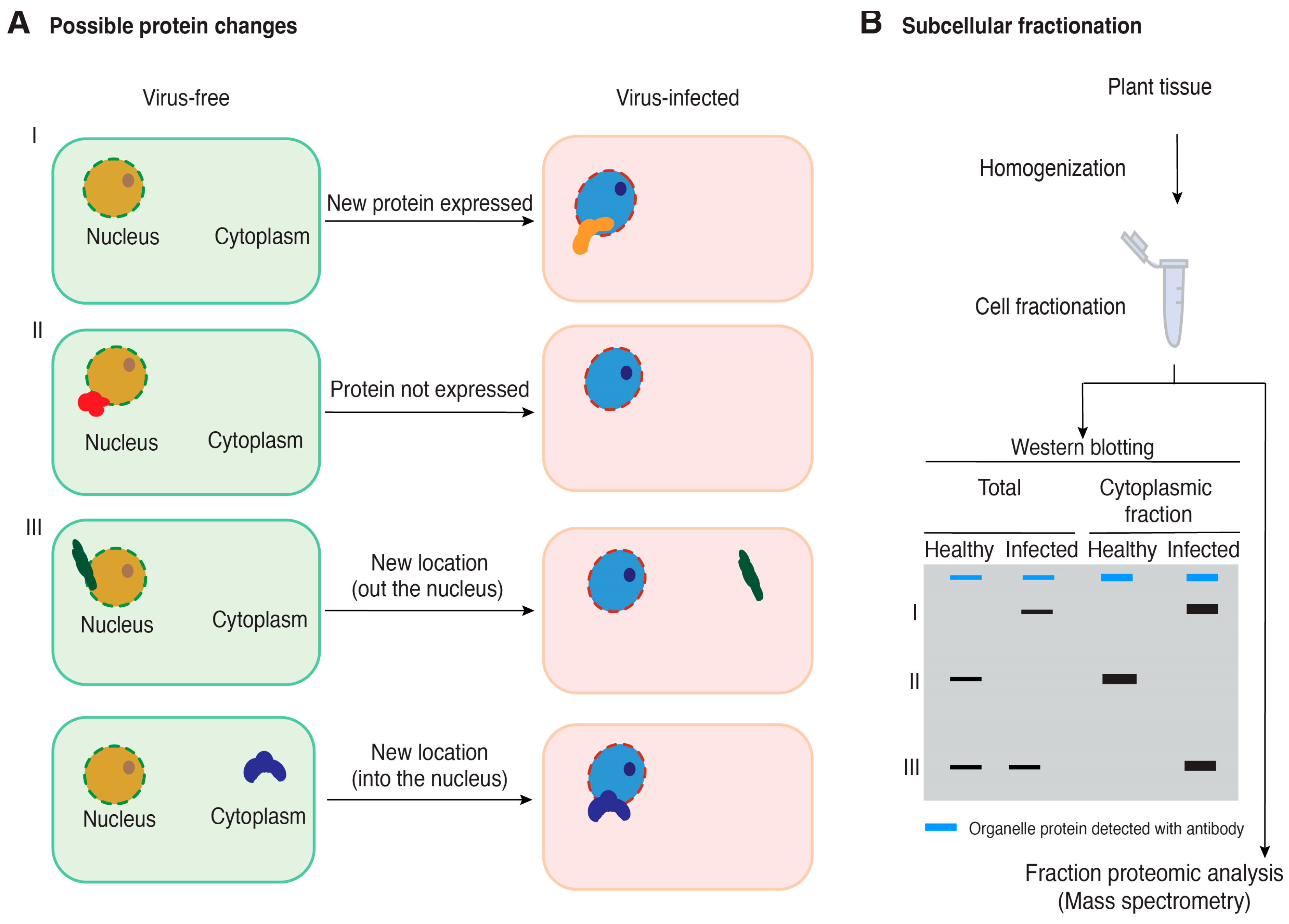
4. Changes in Accumulation and Subcellular Localization of Host Proteins
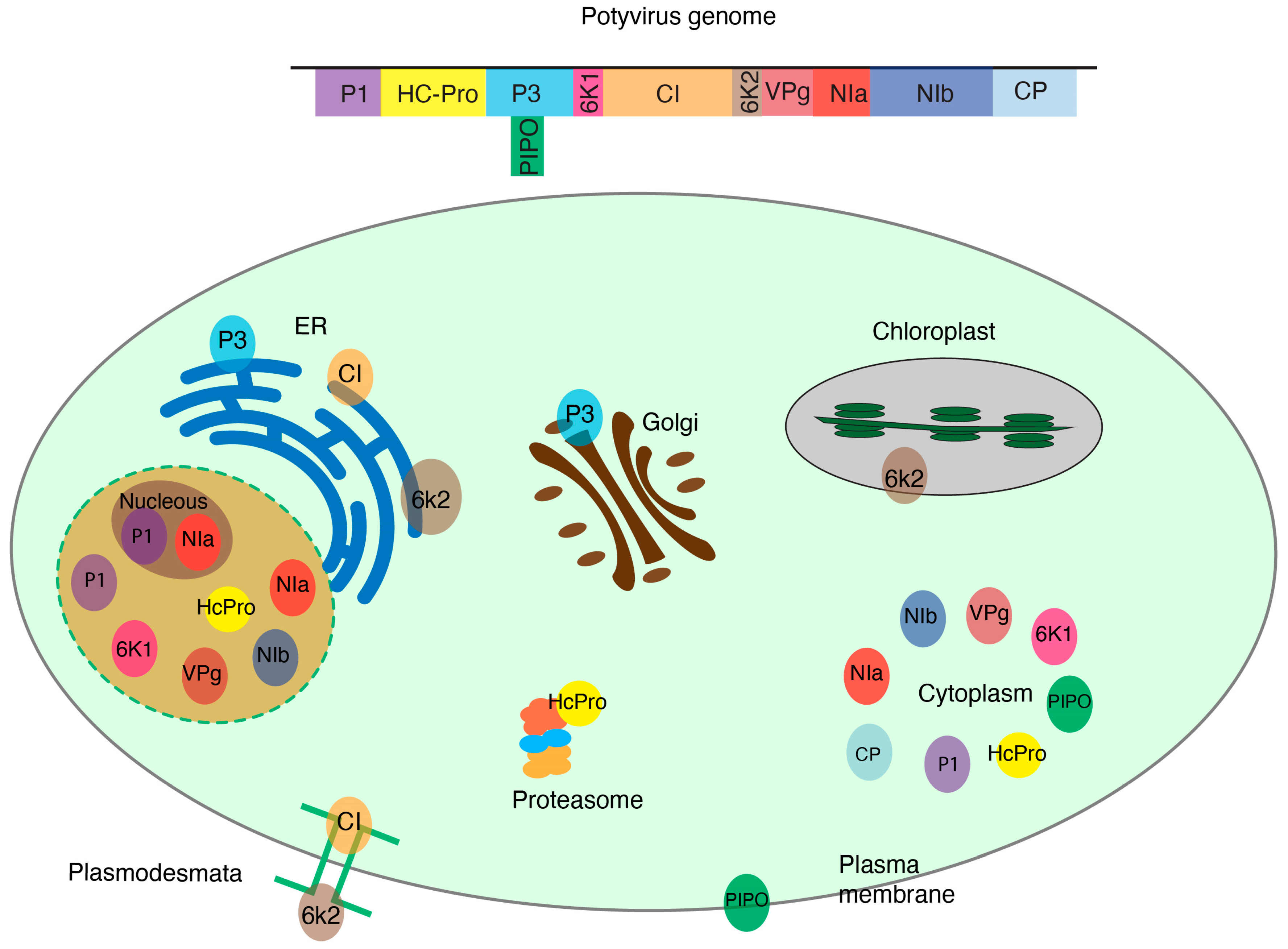
5. Potyviruses as a Model for Host–Viral Protein Interactions
6. Cellular Fractionation to Identify Host Proteins
| Host | Virus | Host Protein | Viral Protein | Technique | Ref. |
|---|---|---|---|---|---|
| Transgenic tobacco BY-2 cells | ToMV | Sar1, Sec61, and TOM1 | 130 KDa and 180 KDa replication proteins | Membrane flotation analysis and Sucrose gradient sedimentation analysis | [20] |
| Transgenic tobacco BY-2 cells | ToMV | Tm-1 | 130 K and 180 K | Differential centrifugation | [73] |
| Cucumis sativus | ToRSV | N/A | NTB | Membrane fractionation | [74] |
| Pea or lettuce plants | LMV | 20 s Proteasome | HCPro | 30% sucrose cushion and gel filtration column | [75] |
| N. benthamiana | CiLV-C | N/A | P29, P15, MP, and P24 | Bimolecular Fluorescence Complementation combined with ultracentrifugation | [76] |
| Tomato | TYLCV | HSP70 | CP | Sucrose density gradient | [77] |
| N. benthamiana | PVA | Ck2, CPIP, HSP70, and CHIP | NIb, VPg, and CP | Membrane fractionation | [78] |
| N. benthamiana | CMoV | SUMO1, SUMO2, and SCE1 | ORF4 | Cell wall fractionation | [79] |
7. The Process of Cellular Fractionation
| Organelle | Marker Proteins | Tissue Type | Homogeniza-tion Buffer | Centrifugation Speed, Condition | Fraction Obtained | Gradient Centrifugation | Purification | Final Obtained Fraction | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Membrane fraction | See other organelles | Cucumis sativus leaves | Homogenization buffer one | 30,000× g, 30 min | Crude membrane (P30) (pellet) | 20–45% sucrose gradient centrifugate at 143,000× g for 4 h | - | Membranes separated into 13 fractions | [74] |
| Cytoplasm | UDP-glucose pyrophosphorylase (UGPase) [83] | Tomato leaves | Nuclear extraction buffer | Filtrate centrifuged at 1300× g, 10 min | Cytoplasmic fraction concentrated 10 times by ultracentrifugation | 10–50% sucrose gradient centrifugation at 104,000× g for 20 h | - | 10 fractions obtained from the gradient | [77] |
| Rice cell culture | Enzyme buffer | 100,000× g, 1 h | Remove top lipid layer, take supernatant | - | Add trichloroacetic acid to supernatant; centrifuge at 20,000× g for 5 min | Wash the pellet with cold acetone at 20,000× g for 15 min and take the pellet | [84] | ||
| Vacuole | TIPs ( and isoforms), Epsilon subunit of tonoplast H+ ATPase (V-ATPase) [85] and [86] | Arabidopsis Rosette leaves | Protoplast solution | 80× g at 20 °C, 15 min | Pellet protoplast | 10% Ficoll buffer overlayed on 4% Ficoll and vacuole buffer; centrifugation at 50,000× g, 5 min at 10 °C | - | Vacuoles found on the 4% Ficoll buffer/vacuole buffer interface | [87] |
| Chloroplast | Plastocyanin (PC) light harvesting complex b (LHC) [83,85] | N. benthamiana leaves | Enzyme mixture | 300× g, 16 min | Resuspend the pellet | 40% and 80% Percoll gradient and centrifugate at 3000× g, 25 min; collect chloroplasts at the interface of 40%/80% Percoll | - | Resuspend chloroplast in resuspension buffer | [83] |
| ER | HDEL domain [85] | Castor Bean Endosperm | Homogenization buffer two | 1000× g, 15 min | supernatant | 20%, 30%, 40%, and 60% sucrose; centrifuge at 250,000× g, 22 h at 2 °C | Resuspend ER fraction between 20% and 30% and pellet via centrifugation at 250,000× g for 45 min | Resuspend ER pellet | [88] |
| Mitochondria | Voltage-dependent, anion-selective channel protein 1-5 (VDAC1-5) [89] | Citrus pulp | Extraction buffer for mitochondria | 3000× g, 10 min then centrifuge the supernatant at 12,000× g, 15 min | Resuspend the pellet in washing buffer | 18%, 22.5%, and 35% Percoll gradient; ultracentrifugation at 50,000× g, 1 h | Mitochondrial band enriched at 22.5–35% Percoll gradient interface, then diluted with washing buffer, and centrifuged at 1500× g | Resuspend, purified mitochondria pellet in small volume of washing buffer | [90] |
| Golgi | ADP-ribosylation factor 1 (ARF1) [91] | Wheat seedling | Extraction buffer for Golgi membranes | 3000× g, 20 min | supernatant | 25–40% sucrose gradient centrifuge at 100,000× g for 16 h | Ultracentrifuge fractions (1:10) at 100,000× g for 1 h | Resuspend the membrane pellet in 50 μL dilution buffer | [92] |
| Nucleus | Histone H3 [93] | Tomato leaves | Nuclear extraction buffer | 1300× g, 10 min | Pellet | 10–50% sucrose gradient centrifuge at 104,000× g, 20 h | - | 10 Nuclei fractions | [77] |
| Proteasomes | Regulatory Particle Triple-A ATPase subunit 2 (RPT2) and Regulatory Particle Non-ATPase 10 (RPN10) [94] | Arabidopsis seedlings | Extraction buffer for proteasomes | 30,000× g, 15 min | Supernatant | Precipitation with 2% and 10% PEG 8000 then re-clarifying via centrifugation at 30,000× g, 45 min | Anion exchange chromatography column | Precipitation with 10% PEG 8000 then size elution chromatography to obtain peak fraction. | [95] |
| Plasma membrane | P-type H″-ATPase [85] | Arabidopsis seedlings | Homogenizing medium | 2770× g, 10 min, take supernatant; ultracentrifugation at 231,000× g, 35 min | Pellet | - | Multiple ultracentrifugation | Resuspend the pellet in PM-suspension medium | [96] |
| Peroxisome | Catalase [97] | Arabidopsis rosette leaves | Grinding buffer | 5000× g, 1 min | Supernatant free from chloroplast and nuclei | 15–38% (v/v) Percoll gradient; centrifuge at 13,000× g, 12 min | 36% sucrose centrifuge at 39,000× g for 30 min | Leaf peroxisome fraction located at the bottom | [98] |
| Autophagosome | Autophagy-related protein 8 (ATG8) [99] | Tobacco BY-2 cell suspension culture | Lysis buffer | 17,000× g, 5 min | Pellet | 30% Percoll; centrifuge at 50,000× g, 1 h | Place density marker beads on 30% Percoll solution and centrifuge again | Fractionate into 30 fractions | [100] |
| Ribosome | Ribosomal Protein S6 (RPS6) [101] | Arabidopsis seedlings/leaves | Ribosome extraction buffer | 10,000× g, 15 min | Supernatant | Sucrose cushion, 149,000× g, 18 h | Resuspend ribosomal pellet in Staehelin A buffer; spin at 14,000× g for 15 min | Collect the supernatant | [102] |
| Extracellular vesicles | Tetraspanin 8 [103] Syntaxin PENETRATION1 (PEN1) [104] | Arabidopsis rosettes | Vesicle isolation buffer (VIB) | 700× g, 20 min at 2 °C | Supernatant | - | Centrifuge successively at 10,000× g for 60 min, 40,000× g for 60 min, and 100,000× g for 60 min and obtain the pellet each time | Pellet resuspended in VIB | [104] |
| Buffers | Components | Reference |
|---|---|---|
| Homogenization buffer one | 50 mM Tris–HCl (pH 7.4), 15 mM MgCl2, 10 mM KCl, 20% glycerol, 0.1% β-mercaptoethanol, 5 μg/mL leupeptin, and 2 μg/mL aprotinin. | [107] |
| Nuclear extraction buffer | 10 mM MES (pH 5.2), 250 mM sorbitol, 10 mM NaCl, 5 mM NaF, 5 mM EDTA, 10 mM MgCl2, 0.024% Triton X-100, 0.1% bovine serum albumin, 1 mM fresh DTT, and Complete Protease Inhibitor Mixture. | [77] |
| Enzyme buffer | 0.4 M Mannitol, 3.6 mM MES–KOH (pH 5.7), 2.0% (w/v) cellulase Onozuka RS, 0.5% (w/v) pectolyase Y-23, and 1.0% (w/v) Driselase. | [84] |
| Protoplast solution | 1% (w/v) Cellulase Onozuka R10, 1% (w/v) Macerozyme R10, 0.4 M mannitol, 25 mM CaCl2, 5 mM mercaptoethanol, and 10 mM 2-morpholinoethanesulfonic acid (MES)-KOH (pH 5.7). | [87] |
| Vacuole buffer | 0.45 M mannitol and 5 mM sodium phosphate 2 mM EDTA (pH 7.5). Keep on ice. The 200 mM sodium phosphate stock solution (pH 7.5) can be prepared by mixing 84 mL of 200 mM Na2HPO4 and 16 mL of 200 mM NaH2PO4. | [87] |
| Enzyme mixture | 1.5% (w/v) cellulase R-10, 0.5% (w/v) macerozyme R-10, 5 mM 2-morpholinoethanesulfonic acid (MES), 0.1% (w/v) BSA, 10 mM CaCl2, and 0.4 M mannitol (pH 5.8). | [83] |
| Chloroplast resuspension buffer | 0.3 M sorbitol, 20 mM Tricine-KOH (pH 7.6), 5 mM MgCl2, 2.5 mM EDTA. | [83] |
| Homogenization buffer two | 500 mM sucrose, 10 mM KCl, 1 mM EDTA, 1 mM MgCl2 2 mM dithiothreitol (DTT), 0.1 mM phenylmethyl-sulfonyl fluoride (PMSF), and 150 mM Tricine-KOH pH 7.5. | [88] |
| Extraction buffer for mitochondria | 0.4 M sorbitol, 0.2 M MOPS-Tris (pH 7.8), 7.5 mM EDTA, 1.5% (w/v) PVP-40, 0.1% [w/v] bovine serum albumin, and 2 mM DTT. | [90] |
| Washing buffer | 0.33 M sorbitol and 50 mM MOPS-Tris (pH 7.5). | [90] |
| Extraction buffer for Golgi membranes | 50 mM HEPES–KOH (pH 6.8), 0.4 M sucrose, 1 mM dithiothreitol (DTT), 5 mM MnCl2, and 5 mM MgCl2. | [92] |
| Extraction buffer for Proteasomes | 50 mM potassium phosphate (pH 6), 2 mM MgCl2, 5% (v/v) glycerol, and 5 mM 2-mercaptoethanol supplemented with 10 mM ATP, 5% polyvinylpyrrolidone, 0.6% sodium metabisulfite, and 2 mM phenylmethylsulfonyl fluoride. 0.8% plant protease inhibitor mixture is added just before use. | [95] |
| Homogenizing medium | 0.5 M sorbitol, 50 mM MOPS–KOH (pH 7.6), 5 mM EGTA, 5 mM EDTA, 1.5% (w/v) polyvinylpyrrolidone 40 (PVP-40, molecular weight 40,000), 0.5% (w/v) defatted-BSA, 2 mM phenylmethanesulfonyl fluoride (PMSF), 4 mM salicylhydroxamic acid (SHAM), and 2.5 mM 1,4-dithiothreitol (DTT). | [96] |
| Plasma membrane (PM)-suspension medium | 10 mM MOPSKOH (pH 7.3), 1 mM EGTA, 0.3 M sucrose, 1 mM DTT. Store the stock solution without DTT at 4 °C. | [96] |
| Grinding Buffer | 170 mM Tricine-KOH (pH 7.5), 1.0 M sucrose, 2 mM EDTA, 1% (w/v) BSA, 10 mM KCl, 1 mM MgCl2, 0.5% (w/v) PVP-40, and 5 mM DTT. | [98] |
| Lysis buffer | 50 mM HEPES–KOH (pH 7.5) buffer containing 1 mM EDTA, 10 μM leupeptin, 10 μM pepstatin A, 1 mM AEBSF, and 0.4 M sorbitol. Mix 50 mL of 0.1 M HEPES–KOH (pH 7.5); 20 mL of 2 M sorbitol; 29 mL of water; and 1 mL of 0.1 M EDTA–NaOH (pH 8.0). Store at 4 °C. Take 10–20 mL of lysis buffer and add 1/100 volume of 1 mM leupeptin, 1/100 volume of 1 mM pepstatin A, and 1/100 volume of 0.1 M AEBSF immediately before use. | [100] |
| Staehelin A buffer | 20 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 1 mM sodium molybdate, and 1 mM dithiothreitol. | [102] |
| Ribosome extraction buffer | 200 mM Tris–HCl (pH 7.5), 200 mM KCl, 25 mM EGTA, 36 mM MgCl2, 1 mM sodium molybdate, 1 mM dithiothreitol, 50 μg/mL cycloheximide, 50 μg/mL chloramphenicol, 80 mM β-glycerophosphate, 1% (v/v) Triton X-100, 1% (v/v) Brij 35, 1% (v/v) Tween 40, and 1% (v/v) NP40. | [102] |
| Vesicle isolation buffer VIB | 20 mM MES, 2 mM CaCl2, and 0.1 M NaCl (pH 6). | [104] |
8. Experimental Challenges of Cell Fractionation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, J.-S.; Ju, H.-J. The plant cellular systems for plant virus movement. Plant Pathol. J. 2017, 33, 213. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Peña, R.; Mounadi, K.E.; Garcia-Ruiz, H. Changes in subcellular localization of host proteins induced by plant viruses. Viruses 2021, 13, 677. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Huang, T.-S.; McNeil, J.; Laliberté, J.-F.; Hong, J.; Nelson, R.S.; Wang, A. Sequential recruitment of the endoplasmic reticulum and chloroplasts for plant potyvirus replication. J. Virol. 2010, 84, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, K.; Okuno, T. Hijacking of host cellular components as proviral factors by plant-infecting viruses. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 107, pp. 37–86. [Google Scholar]
- Das, P.P.; Lin, Q.; Wong, S.-M. Comparative proteomics of Tobacco mosaic virus-infected Nicotiana tabacum plants identified major host proteins involved in photosystems and plant defence. J. Proteom. 2019, 194, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Musidlak, O.; Nawrot, R.; Goździcka-Józefiak, A. Which plant proteins are involved in antiviral defense? Review on in vivo and in vitro activities of selected plant proteins against viruses. Int. J. Mol. Sci. 2017, 18, 2300. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.S.; Nakahara, K.S.; Masuta, C. Resistance induction based on the understanding of molecular interactions between plant viruses and host plants. Virol. J. 2021, 18, 176. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, H. Susceptibility genes to plant viruses. Viruses 2018, 10, 484. [Google Scholar] [CrossRef]
- Wang, L.; Tan, H.; Medina-Puche, L.; Wu, M.; Garnelo Gomez, B.; Gao, M.; Shi, C.; Jimenez-Gongora, T.; Fan, P.; Ding, X. Combinatorial interactions between viral proteins expand the potential functional landscape of the tomato yellow leaf curl virus proteome. PLoS Pathog. 2022, 18, e1010909. [Google Scholar] [CrossRef]
- Ivanov, K.I.; Eskelin, K.; Basic, M.; De, S.; Lohmus, A.; Varjosalo, M.; Makinen, K. Molecular insights into the function of the viral RNA silencing suppressor HCPro. Plant J. 2016, 85, 30–45. [Google Scholar] [CrossRef]
- Dufresne, P.J.; Thivierge, K.; Cotton, S.; Beauchemin, C.; Ide, C.; Ubalijoro, E.; Laliberte, J.F.; Fortin, M.G. Heat shock 70 protein interaction with Turnip mosaic virus RNA-dependent RNA polymerase within virus-induced membrane vesicles. Virology 2008, 374, 217–227. [Google Scholar] [CrossRef]
- Wu, L.; Han, Z.; Wang, S.; Wang, X.; Sun, A.; Zu, X.; Chen, Y. Comparative proteomic analysis of the plant-virus interaction in resistant and susceptible ecotypes of maize infected with sugarcane mosaic virus. J. Proteom. 2013, 89, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Rios, J.; Uetz, P. Global approaches to study protein–protein interactions among viruses and hosts. Future Microbiol. 2010, 5, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, G.; Lal, N.K.; Nagalakshmi, U.; Li, Y.; Zheng, W.; Huang, P.J.; Branon, T.C.; Ting, A.Y.; Walley, J.W.; et al. TurboID-based proximity labeling reveals that UBR7 is a regulator of N NLR immune receptor-mediated immunity. Nat. Commun. 2019, 10, 3252. [Google Scholar] [CrossRef] [PubMed]
- DeBlasio, S.L.; Chavez, J.D.; Alexander, M.M.; Ramsey, J.; Eng, J.K.; Mahoney, J.; Gray, S.M.; Bruce, J.E.; Cilia, M. Visualization of Host-Polerovirus Interaction Topologies Using Protein Interaction Reporter Technology. J. Virol. 2016, 90, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.; Rodrigo, G.; Aragones, V.; Ruiz, M.; Lodewijk, I.; Fernandez, U.; Elena, S.F.; Daros, J.A. Interaction network of tobacco etch potyvirus NIa protein with the host proteome during infection. BMC Genom. 2016, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- DeBlasio, S.L.; Johnson, R.; Mahoney, J.; Karasev, A.; Gray, S.M.; MacCoss, M.J.; Cilia, M. Insights into the polerovirus-plant interactome revealed by coimmunoprecipitation and mass spectrometry. Mol. Plant Microbe Interact. 2015, 28, 467–481. [Google Scholar] [CrossRef]
- Chkuaseli, T.; White, K.A. Complex and simple translational readthrough signals in pea enation mosaic virus 1 and potato leafroll virus, respectively. PLoS Pathog. 2022, 18, e1010888. [Google Scholar] [CrossRef] [PubMed]
- den Boon, J.A.; Ahlquist, P. Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories. Annu. Rev. Microbiol. 2010, 64, 241–256. [Google Scholar] [CrossRef]
- Nishikiori, M.; Dohi, K.; Mori, M.; Meshi, T.; Naito, S.; Ishikawa, M. Membrane-bound tomato mosaic virus replication proteins participate in RNA synthesis and are associated with host proteins in a pattern distinct from those that are not membrane bound. J. Virol. 2006, 80, 8459–8468. [Google Scholar] [CrossRef]
- Nishikiori, M.; Mori, M.; Dohi, K.; Okamura, H.; Katoh, E.; Naito, S.; Meshi, T.; Ishikawa, M. A host small GTP-binding protein ARL8 plays crucial roles in tobamovirus RNA replication. PLoS Pathog. 2011, 7, e1002409. [Google Scholar] [CrossRef]
- Heinlein, M.; Padgett, H.S.; Gens, J.S.; Pickard, B.G.; Casper, S.J.; Epel, B.L.; Beachy, R.N. Changing patterns of localization of the tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection. Plant Cell 1998, 10, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Reichel, C.; Beachy, R.N. Tobacco mosaic virus infection induces severe morphological changes of the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 1998, 95, 11169–11174. [Google Scholar] [CrossRef] [PubMed]
- Verchot, J. Wrapping membranes around plant virus infection. Curr. Opin. Virol. 2011, 1, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, A.G.; Atabekova, A.K.; Lezzhov, A.A.; Solovieva, A.D.; Chergintsev, D.A.; Morozov, S.Y. Distinct mechanisms of endomembrane reorganization determine dissimilar transport pathways in plant RNA viruses. Plants 2022, 11, 2403. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Chakraborty, S. Chloroplast: The Trojan horse in plant–virus interaction. Mol. Plant Pathol. 2018, 19, 504–518. [Google Scholar] [CrossRef]
- Angel, C.A.; Lutz, L.; Yang, X.; Rodriguez, A.; Adair, A.; Zhang, Y.; Leisner, S.M.; Nelson, R.S.; Schoelz, J.E. The P6 protein of Cauliflower mosaic virus interacts with CHUP1, a plant protein which moves chloroplasts on actin microfilaments. Virology 2013, 443, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Budziszewska, M.; Obrępalska-Stęplowska, A. The role of the chloroplast in the replication of positive-sense single-stranded plant RNA viruses. Front. Plant Sci. 2018, 9, 1776. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-F.; Huang, Y.-P.; Chen, L.-H.; Hsu, Y.-H.; Tsai, C.-H. Chloroplast phosphoglycerate kinase is involved in the targeting of Bamboo mosaic virus to chloroplasts in Nicotiana benthamiana plants. Plant Physiol. 2013, 163, 1598–1608. [Google Scholar] [CrossRef]
- Grangeon, R.; Agbeci, M.; Chen, J.; Grondin, G.; Zheng, H.; Laliberté, J.-F. Impact on the endoplasmic reticulum and Golgi apparatus of turnip mosaic virus infection. J. Virol. 2012, 86, 9255–9265. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, K.; Hu, H.; Xing, X.; Huang, X.; Gao, H. Extracellular vesicles: Their functions in plant–pathogen interactions. Mol. Plant Pathol. 2022, 23, 760–771. [Google Scholar] [CrossRef]
- Song, X.S.; Wang, Y.J.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Nogués, S.; Yu, J.Q. Effects of cucumber mosaic virus infection on electron transport and antioxidant system in chloroplasts and mitochondria of cucumber and tomato leaves. Physiol. Plant. 2009, 135, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ryabov, E.; Brown, J.; Taliansky, M. Involvement of the nucleolus in plant virus systemic infection. Biochem. Soc. Trans. 2004, 32, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; MacFarlane, S.; Kalinina, N.O.; Rakitina, D.V.; Ryabov, E.V.; Gillespie, T.; Haupt, S.; Brown, J.W.; Taliansky, M. Interaction of a plant virus-encoded protein with the major nucleolar protein fibrillarin is required for systemic virus infection. Proc. Natl. Acad. Sci. USA 2007, 104, 11115–11120. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ryabov, E.V.; Kalinina, N.O.; Rakitina, D.V.; Gillespie, T.; MacFarlane, S.; Haupt, S.; Brown, J.W.; Taliansky, M. Cajal bodies and the nucleolus are required for a plant virus systemic infection. EMBO J. 2007, 26, 2169–2179. [Google Scholar] [CrossRef]
- Hyodo, K.; Okuno, T. Host factors used by positive-strand RNA plant viruses for genome replication. J. Gen. Plant Pathol. 2014, 80, 123–135. [Google Scholar] [CrossRef]
- Nagy, P.D.; Pogany, J. The dependence of viral RNA replication on co-opted host factors. Nat. Rev. Microbiol. 2012, 10, 137–149. [Google Scholar] [CrossRef]
- Hipper, C.; Brault, V.; Ziegler-Graff, V.; Revers, F. Viral and cellular factors involved in phloem transport of plant viruses. Front. Plant Sci. 2013, 4, 154. [Google Scholar] [CrossRef]
- Gallois, J.L.; Charron, C.; Sanchez, F.; Pagny, G.; Houvenaghel, M.C.; Moretti, A.; Ponz, F.; Revers, F.; Caranta, C.; German-Retana, S. Single amino acid changes in the turnip mosaic virus viral genome-linked protein (VPg) confer virulence towards Arabidopsis thaliana mutants knocked out for eukaryotic initiation factors eIF(iso)4E and eIF(iso)4G. J. Gen. Virol. 2010, 91, 288–293. [Google Scholar] [CrossRef]
- Lellis, A.D.; Kasschau, K.D.; Whitham, S.A.; Carrington, J.C. Loss-of-susceptibility mutants of Arabidopsis thaliana reveal an essential role for eIF (iso) 4E during potyvirus infection. Curr. Biol. 2002, 12, 1046–1051. [Google Scholar] [CrossRef]
- Wang, X.; Kohalmi, S.E.; Svircev, A.; Wang, A.; Sanfaçon, H.; Tian, L. Silencing of the host factor eIF (iso) 4E gene confers plum pox virus resistance in plum. PLoS ONE 2013, 8, e50627. [Google Scholar] [CrossRef]
- Li, F.; Wang, A. RNA-targeted antiviral immunity: More than just RNA silencing. Trends Microbiol. 2019, 27, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, H. Host factors against plant viruses. Mol. Plant Pathol. 2019, 20, 1588–1601. [Google Scholar] [CrossRef] [PubMed]
- Kudchodkar, S.B.; Levine, B. Viruses and autophagy. Rev. Med. Virol. 2009, 19, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.U.; Kim, M.J.; Paek, K.H. Arabidopsis Pumilio protein APUM5 suppresses Cucumber mosaic virus infection via direct binding of viral RNAs. Proc. Natl. Acad. Sci. USA 2013, 110, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kasai, A.; Maoka, T.; Masuta, C.; Sano, T.; Nakahara, K.S. RNA silencing-related genes contribute to tolerance of infection with potato virus X and Y in a susceptible tomato plant. Virol. J. 2020, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, H.; Carbonell, A.; Hoyer, J.S.; Fahlgren, N.; Gilbert, K.B.; Takeda, A.; Giampetruzzi, A.; Garcia Ruiz, M.T.; McGinn, M.G.; Lowery, N.; et al. Roles and programming of Arabidopsis ARGONAUTE proteins during Turnip mosaic virus infection. PLoS Pathog. 2015, 11, e1004755. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, H.; Takeda, A.; Chapman, E.J.; Sullivan, C.M.; Fahlgren, N.; Brempelis, K.J.; Carrington, J.C. Arabidopsis RNA-dependent RNA polymerases and dicer-like proteins in antiviral defense and small interfering RNA biogenesis during Turnip Mosaic Virus infection. Plant Cell 2010, 22, 481–496. [Google Scholar] [CrossRef]
- Xie, Z.; Fan, B.; Chen, C.; Chen, Z. An important role of an inducible RNA-dependent RNA polymerase in plant antiviral defense. Proc. Natl. Acad. Sci. USA 2001, 98, 6516–6521. [Google Scholar] [CrossRef]
- Blake, J.A.; Lee, K.W.; Morris, T.J.; Elthon, T.E. Effects of turnip crinkle virus infection on the structure and function of mitochondria and expression of stress proteins in turnips. Physiol. Plant. 2007, 129, 698–706. [Google Scholar] [CrossRef]
- Kozieł, E.; Otulak-Kozieł, K.; Bujarski, J.J. Plant cell wall as a key player during resistant and susceptible plant-virus interactions. Front. Microbiol. 2021, 12, 656809. [Google Scholar] [CrossRef]
- Bucher, G.L.; Tarina, C.; Heinlein, M.; Di Serio, F.; Meins Jr, F.; Iglesias, V.A. Local expression of enzymatically active class I β-1, 3-glucanase enhances symptoms of TMV infection in tobacco. Plant J. 2001, 28, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, V.A.; Meins Jr, F. Movement of plant viruses is delayed in a β-1, 3-glucanase-deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. Plant J. 2000, 21, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Ershova, N.; Kamarova, K.; Sheshukova, E.; Antimonova, A.; Komarova, T. A novel cellular factor of Nicotiana benthamiana susceptibility to tobamovirus infection. Front. Plant Sci. 2023, 14, 1224958. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Zerbini, F.; French, R.; Rabenstein, F.; Stenger, D.; Valkonen, J. Family potyviridae. In Virus Taxonomy, Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1069–1089. [Google Scholar]
- Ivanov, K.I.; Eskelin, K.; Lohmus, A.; Makinen, K. Molecular and cellular mechanisms underlying potyvirus infection. J. Gen. Virol. 2014, 95, 1415–1429. [Google Scholar] [CrossRef]
- Pollari, M.; De, S.; Wang, A.; Makinen, K. The potyviral silencing suppressor HCPro recruits and employs host ARGONAUTE1 in pro-viral functions. PLoS Pathog. 2020, 16, e1008965. [Google Scholar] [CrossRef]
- Martínez, F.; Daròs, J.-A. Tobacco etch virus protein P1 traffics to the nucleolus and associates with the host 60S ribosomal subunits during infection. J. Virol. 2014, 88, 10725–10737. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Mäkinen, K. Insights into the functions of eIF4E-binding motif of VPg in potato virus A infection. Viruses 2020, 12, 197. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, A. The potyvirus silencing suppressor protein VPg mediates degradation of SGS3 via ubiquitination and autophagy pathways. J. Virol. 2017, 91, e01478-01416. [Google Scholar] [CrossRef]
- Cheng, G.; Dong, M.; Xu, Q.; Peng, L.; Yang, Z.; Wei, T.; Xu, J. Dissecting the molecular mechanism of the subcellular localization and cell-to-cell movement of the sugarcane mosaic virus P3N-PIPO. Sci. Rep. 2017, 7, 9868. [Google Scholar] [CrossRef]
- Chai, M.; Wu, X.; Liu, J.; Fang, Y.; Luan, Y.; Cui, X.; Zhou, X.; Wang, A.; Cheng, X. P3N-PIPO interacts with P3 via the shared N-terminal domain to recruit viral replication vesicles for cell-to-cell movement. J. Virol. 2020, 94, e01898-01819. [Google Scholar] [CrossRef]
- Shen, W.; Shi, Y.; Dai, Z.; Wang, A. The RNA-dependent RNA polymerase NIb of potyviruses plays multifunctional, contrasting roles during viral infection. Viruses 2020, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Sánchez, O.; Pérez-Rodríguez, P.; Delaye, L.; Tiessen, A. Plant proteins are smaller because they are encoded by fewer exons than animal proteins. Genom. Proteom. Bioinform. 2016, 14, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Chan, Z.; Qin, G.; Xu, X.; Li, B.; Tian, S. Proteome approach to characterize proteins induced by antagonist yeast and salicylic acid in peach fruit. J. Proteome Res. 2007, 6, 1677–1688. [Google Scholar] [CrossRef]
- Takac, T.; Vadovic, P.; Pechan, T.; Luptovciak, I.; Samajova, O.; Samaj, J. Comparative proteomic study of Arabidopsis mutants mpk4 and mpk6. Sci. Rep. 2016, 6, 28306. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, C.; Hepworth, S.R.; Ma, C.; Li, H.; Li, J.; Wang, S.-M.; Yin, H. SAUR15 interaction with BRI1 activates plasma membrane H+-ATPase to promote organ development of Arabidopsis. Plant Physiol. 2022, 189, 2454–2466. [Google Scholar] [CrossRef] [PubMed]
- Di Silvestre, D.; Passignani, G.; Rossi, R.; Ciuffo, M.; Turina, M.; Vigani, G.; Mauri, P.L. Presence of a Mitovirus Is Associated with Alteration of the Mitochondrial Proteome, as Revealed by Protein–Protein Interaction (PPI) and Co-Expression Network Models in Chenopodium quinoa Plants. Biology 2022, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Parsons, H.T.; Stevens, T.J.; McFarlane, H.E.; Vidal-Melgosa, S.; Griss, J.; Lawrence, N.; Butler, R.; Sousa, M.M.; Salemi, M.; Willats, W.G. Separating Golgi proteins from cis to trans reveals underlying properties of cisternal localization. Plant Cell 2019, 31, 2010–2034. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, C.; Laliberte, J.F. The poly(A) binding protein is internalized in virus-induced vesicles or redistributed to the nucleolus during turnip mosaic virus infection. J. Virol. 2007, 81, 10905–10913. [Google Scholar] [CrossRef]
- Spechenkova, N.; Samarskaya, V.O.; Kalinina, N.O.; Zavriev, S.K.; MacFarlane, S.; Love, A.J.; Taliansky, M. Plant Poly(ADP-Ribose) Polymerase 1 Is a Potential Mediator of Cross-Talk between the Cajal Body Protein Coilin and Salicylic Acid-Mediated Antiviral Defence. Viruses 2023, 15, 1282. [Google Scholar] [CrossRef]
- Wamaitha, M.J.; Nigam, D.; Maina, S.; Stomeo, F.; Wangai, A.; Njuguna, J.N.; Holton, T.A.; Wanjala, B.W.; Wamalwa, M.; Lucas, T.; et al. Metagenomic analysis of viruses associated with maize lethal necrosis in Kenya. Virol. J. 2018, 15, 90. [Google Scholar] [CrossRef]
- Ishibashi, K.; Ishikawa, M. The resistance protein Tm-1 inhibits formation of a Tomato mosaic virus replication protein-host membrane protein complex. J. Virol. 2013, 87, 7933–7939. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Sanfacon, H. Tomato ringspot virus proteins containing the nucleoside triphosphate binding domain are transmembrane proteins that associate with the endoplasmic reticulum and cofractionate with replication complexes. J. Virol. 2003, 77, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Ballut, L.; Drucker, M.; Pugniere, M.; Cambon, F.; Blanc, S.; Roquet, F.; Candresse, T.; Schmid, H.P.; Nicolas, P.; Gall, O.L.; et al. HcPro, a multifunctional protein encoded by a plant RNA virus, targets the 20S proteasome and affects its enzymic activities. J. Gen. Virol. 2005, 86, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Leastro, M.O.; Kitajima, E.W.; Silva, M.S.; Resende, R.O.; Freitas-Astúa, J. Dissecting the subcellular localization, intracellular trafficking, interactions, membrane association, and topology of citrus leprosis virus C proteins. Front. Plant Sci. 2018, 9, 1299. [Google Scholar] [CrossRef]
- Gorovits, R.; Moshe, A.; Kolot, M.; Sobol, I.; Czosnek, H. Progressive aggregation of Tomato yellow leaf curl virus coat protein in systemically infected tomato plants, susceptible and resistant to the virus. Virus Res. 2013, 171, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Lohmus, A.; Hafren, A.; Mäkinen, K. Coat protein regulation by CK2, CPIP, HSP70, and CHIP is required for potato virus A replication and coat protein accumulation. J. Virol. 2017, 91, e01316. [Google Scholar] [CrossRef]
- Jiang, J.; Kuo, Y.W.; Salem, N.; Erickson, A.; Falk, B.W. Carrot mottle virus ORF4 movement protein targets plasmodesmata by interacting with the host cell SUMOylation system. New Phytol. 2021, 231, 382–398. [Google Scholar] [CrossRef]
- Millar, A.H.; Taylor, N.L. The isolation of plant organelles and structures in the post-genomic era. In Isolation of Plant Organelles and Structures: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–11. [Google Scholar]
- Agrawal, G.K.; Bourguignon, J.; Rolland, N.; Ephritikhine, G.; Ferro, M.; Jaquinod, M.; Alexiou, K.G.; Chardot, T.; Chakraborty, N.; Jolivet, P. Plant organelle proteomics: Collaborating for optimal cell function. Mass Spectrom. Rev. 2011, 30, 772–853. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, M.; Gao, C.; Shen, J. Protein trafficking in plant cells: Tools and markers. Sci. China Life Sci. 2020, 63, 343–363. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, J.; Chen, B.; Cui, W.; Zhou, Z.; Song, X.; Chen, Z.; Zheng, H.; Lin, L.; Peng, J.; et al. Characterization of Proteins Involved in Chloroplast Targeting Disturbed by Rice Stripe Virus by Novel Protoplast-Chloroplast Proteomics. Int. J. Mol. Sci. 2019, 20, 253. [Google Scholar] [CrossRef]
- Lao, J.; Smith-Moritz, A.M.; Mortimer, J.C.; Heazlewood, J.L. Enrichment of the Plant Cytosolic Fraction. In Isolation of Plant Organelles and Structures; Springer: Berlin/Heidelberg, Germany, 2017; pp. 213–232. [Google Scholar]
- Jaquinod, M.; Villiers, F.; Kieffer-Jaquinod, S.; Hugouvieux, V.; Bruley, C.; Garin, J.; Bourguignon, J. A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture. Mol. Cell. Proteom. 2007, 6, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; LaMontagne, E.D.; Anderson, J.C.; Ekanayake, G.; Clarke, A.S.; Bond, L.N.; Salamango, D.J.; Cornish, P.V.; Peck, S.C.; Heese, A. EPSIN1 Modulates the Plasma Membrane Abundance of FLAGELLIN SENSING2 for Effective Immune Responses. Plant Physiol. 2020, 182, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Zouhar, J. Isolation of Vacuoles and the Tonoplast. In Isolation of Plant Organelles and Structures; Springer: Berlin/Heidelberg, Germany, 2017; pp. 113–118. [Google Scholar]
- Simon, W.J.; Maltman, D.J.; Slabas, A.R. Isolation and fractionation of the endoplasmic reticulum from castor bean (Ricinus communis) endosperm for proteomic analyses. In 2D PAGE: Sample Preparation and Fractionation; Springer: Berlin/Heidelberg, Germany, 2008; pp. 203–215. [Google Scholar]
- Belykh, E.S.; Velegzhaninov, I.O.; Garmash, E.V. Responses of genes of DNA repair, alternative oxidase, and pro-/antioxidant state in Arabidopsis thaliana with altered expression of AOX1a to gamma irradiation. Int. J. Radiat. Biol. 2022, 98, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chai, Y.; Yang, H.; Tian, Z.; Li, C.; Xu, R.; Shi, C.; Zhu, F.; Zeng, Y.; Deng, X. Isolation and comparative proteomic analysis of mitochondria from the pulp of ripening citrus fruit. Hortic. Res. 2021, 8, 31. [Google Scholar] [CrossRef]
- Hurný, A.; Cuesta, C.; Cavallari, N.; Ötvös, K.; Duclercq, J.; Dokládal, L.; Montesinos, J.C.; Gallemí, M.; Semerádová, H.; Rauter, T.; et al. SYNERGISTIC ON AUXIN AND CYTOKININ 1 positively regulates growth and attenuates soil pathogen resistance. Nat. Commun. 2020, 11, 2170. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Ebert, B.; Parsons, H.T.; Rautengarten, C.; Bacic, A.; Heazlewood, J.L. Enrichment of Golgi membranes from Triticum aestivum (wheat) seedlings. In Isolation of Plant Organelles and Structures; Springer: Berlin/Heidelberg, Germany, 2017; pp. 131–150. [Google Scholar]
- Jensen, G.S.; Fal, K.; Hamant, O.; Haswell, E.S. The RNA Polymerase-Associated Factor 1 Complex Is Required for Plant Touch Responses. J. Exp. Bot. 2016, 68, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, T.; Kawasaki, H.; Hirano, H. Identification of the 19S regulatory particle subunits from the rice 26S proteasome. Eur. J. Biochem. 2002, 269, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.S.; Gemperline, D.C.; Vierstra, R.D. Purification of 26S proteasomes and their subcomplexes from plants. In Isolation of Plant Organelles and Structures: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2017; pp. 301–334. [Google Scholar]
- Minami, A.; Takahashi, D.; Kawamura, Y.; Uemura, M. Isolation of plasma membrane and plasma membrane microdomains. In Isolation of Plant Organelles and Structures; Springer: Berlin/Heidelberg, Germany, 2017; pp. 199–212. [Google Scholar]
- Xu, Y.; Li, X.; Huang, J.; Peng, L.; Luo, D.; Zhang, Q.; Dan, Z.; Xiao, H.; Yang, F.; Hu, J. A simplified method to isolate rice mitochondria. Plant Methods 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Reumann, S.; Singhal, R. Isolation of Leaf Peroxisomes from Arabidopsis for Organelle Proteome Analyses. In Plant Proteomics: Methods and Protocols; Jorrin-Novo, J.V., Komatsu, S., Weckwerth, W., Wienkoop, S., Eds.; Humana Press: Totowa, NJ, USA, 2014; pp. 541–552. [Google Scholar]
- Bao, Y.; Mugume, Y.; Bassham, D. Biochemical methods to monitor autophagic responses in plants. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 588, pp. 497–513. [Google Scholar]
- Takatsuka, C.; Inoue-Aono, Y.; Moriyasu, Y. Isolation of autolysosomes from tobacco BY-2 Cells. In Isolation of Plant Organelles and Structures; Springer: Berlin/Heidelberg, Germany, 2017; pp. 151–161. [Google Scholar]
- Williams, A.J.; Werner-Fraczek, J.; Chang, I.-F.; Bailey-Serres, J. Regulated phosphorylation of 40S ribosomal protein S6 in root tips of maize. Plant Physiol. 2003, 132, 2086–2097. [Google Scholar] [CrossRef]
- Klang Årstrand, H.; Turkina, M.V. Isolation of cytosolic ribosomes. In Isolation of Plant Organelles and Structures; Springer: Berlin/Heidelberg, Germany, 2017; pp. 241–247. [Google Scholar]
- Chen, A.; He, B.; Jin, H. Isolation of extracellular vesicles from arabidopsis. Curr. Protoc. 2022, 2, e352. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Tuli, L.; Ressom, H.W. LC–MS based detection of differential protein expression. J. Proteom. Bioinform. 2009, 2, 416. [Google Scholar] [CrossRef] [PubMed]
- Vandenbogaert, M.; Li-Thiao-Té, S.; Kaltenbach, H.M.; Zhang, R.; Aittokallio, T.; Schwikowski, B. Alignment of LC-MS images, with applications to biomarker discovery and protein identification. Proteomics 2008, 8, 650–672. [Google Scholar] [CrossRef] [PubMed]
- Schaad, M.C.; Jensen, P.E.; Carrington, J.C. Formation of plant RNA virus replication complexes on membranes: Role of an endoplasmic reticulum-targeted viral protein. Embo J. 1997, 16, 4049–4059. [Google Scholar] [CrossRef] [PubMed]
- Jean Beltran, P.M.; Federspiel, J.D.; Sheng, X.; Cristea, I.M. Proteomics and integrative omic approaches for understanding host–pathogen interactions and infectious diseases. Mol. Syst. Biol. 2017, 13, 922. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.L.; Garrison, D.J.; May, J.P. Phase separation of a plant virus movement protein and cellular factors support virus-host interactions. PLoS Pathog. 2021, 17, e1009622. [Google Scholar] [CrossRef] [PubMed]
- Doucette, A.A.; Tran, J.C.; Wall, M.J.; Fitzsimmons, S. Intact proteome fractionation strategies compatible with mass spectrometry. Expert Rev. Proteom. 2011, 8, 787–800. [Google Scholar] [CrossRef]
- Low, T.Y.; Syafruddin, S.E.; Mohtar, M.A.; Vellaichamy, A.; A Rahman, N.S.; Pung, Y.-F.; Tan, C.S.H. Recent progress in mass spectrometry-based strategies for elucidating protein–protein interactions. Cell. Mol. Life Sci. 2021, 78, 5325–5339. [Google Scholar] [CrossRef]
- Li, X.; Wang, W.; Chen, J. From pathways to networks: Connecting dots by establishing protein–protein interaction networks in signaling pathways using affinity purification and mass spectrometry. Proteomics 2015, 15, 188–202. [Google Scholar] [CrossRef]
- Yakubu, R.R.; Nieves, E.; Weiss, L.M. The methods employed in mass spectrometric analysis of posttranslational modifications (PTMs) and protein–protein interactions (PPIs). In Advancements of Mass Spectrometry in Biomedical Research; Springer: Berlin/Heidelberg, Germany, 2019; pp. 169–198. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomaa, A.E.; El Mounadi, K.; Parperides, E.; Garcia-Ruiz, H. Cell Fractionation and the Identification of Host Proteins Involved in Plant–Virus Interactions. Pathogens 2024, 13, 53. https://doi.org/10.3390/pathogens13010053
Gomaa AE, El Mounadi K, Parperides E, Garcia-Ruiz H. Cell Fractionation and the Identification of Host Proteins Involved in Plant–Virus Interactions. Pathogens. 2024; 13(1):53. https://doi.org/10.3390/pathogens13010053
Chicago/Turabian StyleGomaa, Amany E., Kaoutar El Mounadi, Eric Parperides, and Hernan Garcia-Ruiz. 2024. "Cell Fractionation and the Identification of Host Proteins Involved in Plant–Virus Interactions" Pathogens 13, no. 1: 53. https://doi.org/10.3390/pathogens13010053







