Non-Specific Cross Protection of BCG Vaccination in Dairy Calves
Abstract
1. Introduction
2. Materials and Methods
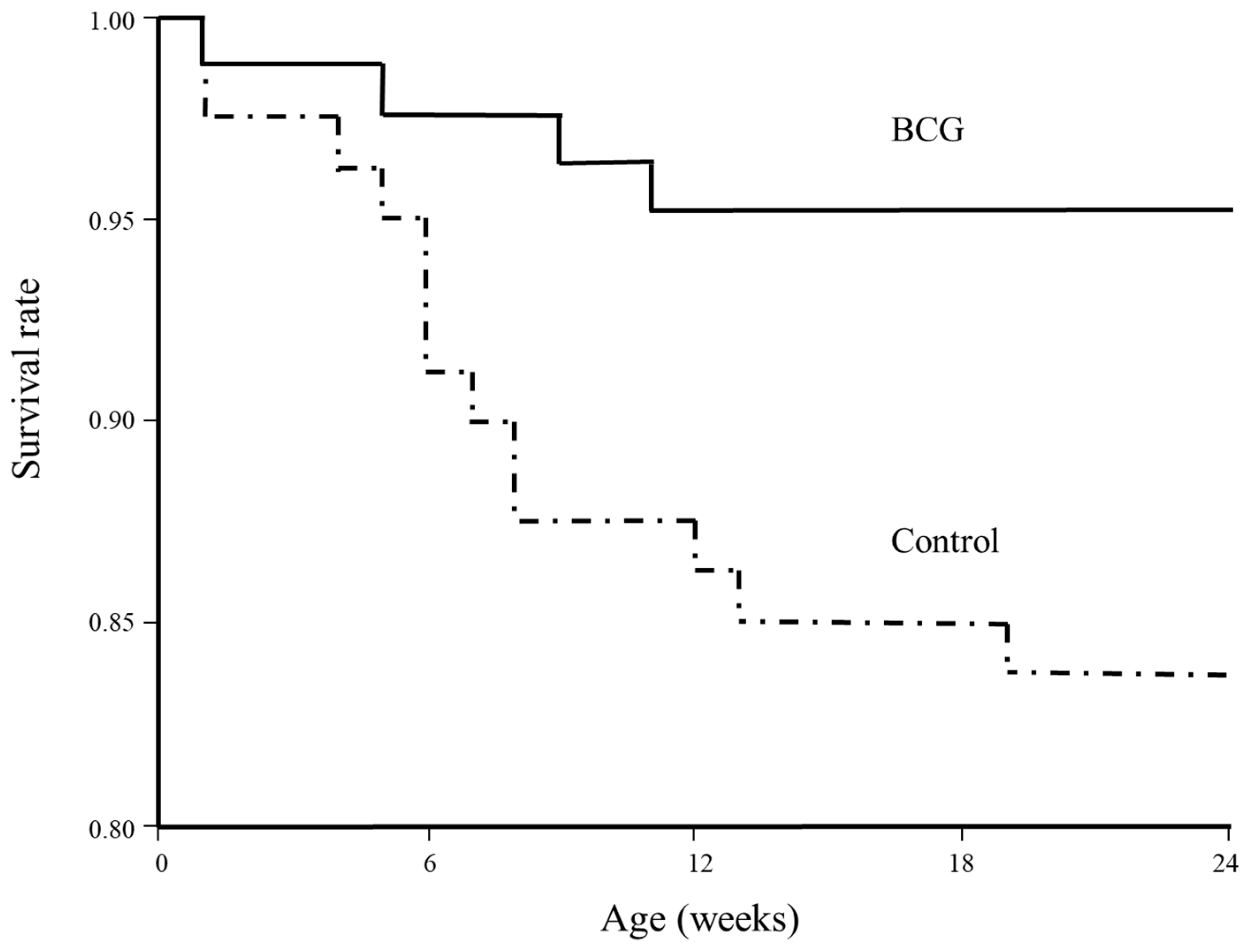
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCG | Bacillus Calmette-Guérin |
| bTB | Bovine tuberculosis |
| HF | Holstein Friesian |
| HFSR | Hybrid Holstein Friesian—Swedish Red |
| HUS | Hemolytic uremic syndrome |
| MTC | Mycobacterium tuberculosis complex |
| RTPCR | Real-Time PCR |
| STEC | Shiga toxin-producing Escherichia coli |
References
- Pérez-Morote, R.; Pontones-Rosa, C.; Gortázar-Schmidt, C.; Muñoz-Cardona, Á. Quantifying the Economic Impact of Bovine Tuberculosis on Livestock Farms in South-Western Spain. Animals 2020, 10, 2433. [Google Scholar] [CrossRef] [PubMed]
- Glanville, R. Australia’s colourful path to tuberculosis freedom. Ir. Vet. J. 2023, 76 (Suppl. 1), 15. [Google Scholar] [CrossRef]
- Singh, B.; Dhand, N.; Cadmus, S.; Dean, A.; Merle, C. Systematic review of bovine and zoonotic tuberculosis in the Western Pacific and the Southeast Asia regions of the World Health Organization. Front. Public Health 2024, 12, 1345328. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Aaby, P.; Behr, M.; Donald, P.; Kaufmann, S.; Netea, M.; Mandalakas, A. 100 years of Mycobacterium bovis bacille Calmette-Guérin. Lancet Infect. Dis. 2022, 22, E2–E12. [Google Scholar] [CrossRef]
- Covián, C.; Fernández-Fierro, A.; Retamal-Díaz, A.; Díaz, F.; Vasquez, A.; Lay, M.; Riedel, C.; González, P.; Bueno, S.; Kalergis, A. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019, 10, 2806. [Google Scholar] [CrossRef]
- Moorlag, S.; Arts, R.; van Crevel, R.; Netea, M. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478. [Google Scholar] [CrossRef]
- Schaltz-Buchholzer, F.; Berendsen, M.; Roth, A.; Jensen, K.; Bjerregaard-Andersen, M.; Kjær Sørensen, M.; Monteiro, I.; Aaby, P.; Stabell Benn, C. BCG skin reactions by 2 months of age are associated with better survival in infancy: A prospective observational study from Guinea-Bissau. BMJ Glob. Health 2020, 5, e002993. [Google Scholar] [CrossRef]
- Moorlag, S.; van Deuren, R.; van Werkhoven, C.; Jaeger, M.; Debisarun, P.; Taks, E.; Mourits, V.; Koeken, V.; de Bree, L.; Ten Doesschate, T.; et al. Safety and COVID-19 Symptoms in Individuals Recently Vaccinated with BCG: A Retrospective Cohort Study. Cell Rep. Med. 2020, 1, 100073. [Google Scholar] [CrossRef]
- Upton, C.; van Wijk, R.; Mockeliunas, L.; Simonsson, U.; McHarry, K.; van den Hoogen, G.; Muller, C.; von Delft, A.; van der Westhuizen, H.; van Crevel, R.; et al. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A double-blind, randomised, controlled, phase 3 trial. EClinicalMedicine 2022, 48, 101414. [Google Scholar] [CrossRef] [PubMed]
- OMSA. Guidelines for the Control of Mycobacterium Tuberculosis Complex in Livestock. In Beyond the Test and Slaughter; World Organisation for Animal Health: Paris, France, 2024. [Google Scholar] [CrossRef]
- Srinivasan, S.; Conlan, A.J.K.; Easterling, L.A.; Herrera, C.; Dandapat, P.; Veerasami, M.; Ameni, G.; Jindal, N.; Raj, G.D.; Wood, J.; et al. A Meta-Analysis of the Effect of Bacillus Calmette-Guérin Vaccination Against Bovine Tuberculosis: Is Perfect the Enemy of Good? Front. Veter. Sci. 2021, 8, 637580. [Google Scholar] [CrossRef]
- Guerra-Maupome, M.; Vang, D.X.; McGill, J.L. Aerosol vaccination with Bacille Calmette-Guerin induces a trained innate immune phenotype in calves. PLoS ONE 2019, 14, e0212751. [Google Scholar] [CrossRef]
- Díaz, F.; Guerra-Maupome, M.; McDonald, P.; Rivera-Pérez, D.; Kalergis, A.; McGill, J. A Recombinant BCG Vaccine Is Safe and Immunogenic in Neonatal Calves and Reduces the Clinical Disease Caused by the Respiratory Syncytial Virus. Front. Immunol. 2021, 12, 664212. [Google Scholar] [CrossRef] [PubMed]
- Retamal, P.; Ábalos, P.; Alegría-Morán, R.; Valdivieso, N.; Vordermeier, M.; Jones, G.; Saadi, K.; Perez Watt, C.; Salinas, C.; Ávila, C. Vaccination of Holstein heifers with Mycobacterium bovis BCG strain induces protection against bovine tuberculosis and higher milk production yields in a natural transmission setting. Transbound. Emerg. Dis. 2022, 69, 1419–1425. [Google Scholar] [CrossRef]
- Contreras, C.; Alegría-Moran, R.; Duchens, M.; Ábalos, P.; López, R.; Retamal, P. Specific and non-specific effects of Mycobacterium bovis BCG vaccination in dairy calves. Front. Veter. Sci. 2023, 10, 1278329. [Google Scholar] [CrossRef]
- Sun, S.; Aguirre-Gamboa, R.; de Bree, L.; Sanz, J.; Dumaine, A.; van der Velden, W.; Joosten, L.; Khader, S. BCG vaccination alters the epigenetic landscape of progenitor cells in human bone marrow to influence innate immune responses. Immunity 2024, 57, 2095–2107. [Google Scholar] [CrossRef]
- Chen, J.; Gao, L.; Wu, X.; Fan, Y.; Liu, M.; Peng, L.; Song, J.; Li, B.; Liu, A.; Bao, F. BCG-induced trained immunity: History, mechanisms and potential applications. J. Transl. Med. 2023, 21, 106. [Google Scholar] [CrossRef] [PubMed]
- Max, V.; Paredes, L.; Rivera, A.; Ternicier, C. National control and eradication program of bovine tuberculosis in Chile. Veter. Microbiol. 2011, 151, 188–191. [Google Scholar] [CrossRef]
- Valdivieso, N.; Retamal, P. Is it possible to control bovine tuberculosis without compensation? Reviewing ten years of the Chilean program and its progress. Ir. Veter. J. 2023, 76, 20. [Google Scholar] [CrossRef] [PubMed]
- Abalos, P.; Valdivieso, N.; Pérez de Val, B.; Vordermeier, M.; Benavides, M.; Alegría-Morán, R.; Saadi, K.; Wistuba, M.; Ortega, C.; Sánchez, N.; et al. Vaccination of Calves with the Mycobacterium bovis BCG Strain Induces Protection against Bovine Tuberculosis in Dairy Herds under a Natural Transmission Setting. Animals 2022, 12, 1083. [Google Scholar] [CrossRef]
- Maier, G.; Breitenbuecher, J.; Gomez, J.; Samah, F.; Fausak, E.; Van Noord, M. Vaccination for the Prevention of Neonatal Calf Diarrhea in Cow-Calf Operations: A Scoping Review. Veter. Anim. Sci. 2022, 15, 100238. [Google Scholar] [CrossRef]
- Probo, M.; Veronesi, M. Clinical Scoring Systems in the Newborn Calf: An Overview. Animals 2022, 12, 3013. [Google Scholar] [CrossRef]
- Jessop, E.; Li, L.; Renaud, D.; Verbrugghe, A.; Macnicol, J.; Gamsjäger, L.; Gomez, D. Neonatal Calf Diarrhea and Gastrointestinal Microbiota: Etiologic Agents and Microbiota Manipulation for Treatment and Prevention of Diarrhea. Veter. Sci. 2024, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. BioTechniques 1993, 15, 532–534. [Google Scholar] [PubMed]
- Bai, J.; Trinetta, V.; Shi, X.; Noll, L.; Magossi, G.; Zheng, W.; Porter, E.; Cernicchiaro, N.; Renter, D.; Nagaraja, T. A multiplex real-time PCR assay, based on invA and pagC genes, for the detection and quantification of Salmonella enterica from cattle lymph nodes. J. Microbiol. Methods 2018, 148, 110–116. [Google Scholar] [CrossRef]
- Guy, R.; Tremblay, D.; Beausoleil, L.; Harel, J.; Champagne, M. Quantification of E. coli O157 and STEC in feces of farm animals using direct multiplex real time PCR (RTPCR) and a modified most probable number assay comprised of immunomagnetic bead separation and RTPCR detection. J. Microbiol. Methods 2014, 99, 44–53. [Google Scholar] [CrossRef]
- Meng, W.; Chen, Z.; Jiang, Q.; Chen, J.; Guo, X.; Ma, Z.; Jia, K.; Li, S. A multiplex real-time fluorescence-based quantitative PCR assay for calf diarrhea viruses. Front. Microbiol. 2024, 14, 1327291. [Google Scholar] [CrossRef] [PubMed]
- Dykema, P.; Stokes, K.; Beckwith, N.; Mungin, J.; Xu, L.; Vickers, D.; Reising, M.; Bravo, D.; Thomsen, B.; Robbe-Austerman, S. Development and validation of a direct real-time PCR assay for Mycobacterium bovis and implementation into the United States national surveillance program. PeerJ 2016, 4, e1703v1. [Google Scholar] [CrossRef]
- Dohoo, R.; Martin, W.; Stryhn, H. Veterinary Epidemiologic Research, 2nd ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Jurczak, M.; Druszczynska, M. Beyond Tuberculosis: The Surprising Immunological Benefits of the Bacillus Calmette-Guérin (BCG) Vaccine in Infectious, Auto-Immune, and Inflammatory Diseases. Pathogens 2025, 14, 196. [Google Scholar] [CrossRef]
- Samuel, B.; Diaz, F.; Maina, T.; Corbett, R.; Tuggle, C.; McGill, J. Evidence of innate training in bovine γδ T cells following subcutaneous BCG administration. Front. Immunol. 2024, 15, 1423843. [Google Scholar] [CrossRef]
- Roche, S.; Genore, R.; Renaud, D.; Shock, D.; Bauman, C.; Croyle, S.; Barkema, H.; Dubuc, J.; Keefe, G.; Kelton, D. Describing mortality and euthanasia practices on Canadian dairy farms. J. Dairy Sci. 2020, 103, 3599–3605. [Google Scholar] [CrossRef]
- Calderón-Amor, J.; Gallo, C. Dairy Calf Welfare and Factors Associated with Diarrhea and Respiratory Disease Among Chilean Dairy Farms. Animals 2020, 10, 1115. [Google Scholar] [CrossRef]
- Ahmedin, U.; Assen, A. Calf morbidity, mortality, and management practices in dairy farms in Jimma City, Southwestern Ethiopia. BMC Veter. Res. 2023, 19, 249. [Google Scholar] [CrossRef]
- Compton, C.; Heuer, C.; Thomsen, P.; Carpenter, T.; Phyn, C.; McDougall, S. A systematic literature review and meta-analysis of mortality and culling in dairy cattle. J. Dairy Sci. 2017, 100, 1–16. [Google Scholar] [CrossRef]
- Cuttance, E.; Laven, R. Perinatal mortality risk factors in dairy calves. Veter. J. 2019, 253, 105394. [Google Scholar] [CrossRef]
- Chase, C.; Hurley, D.; Reber, A. Neonatal immune development in the calf and its impact on vaccine response. Veter Clin. N. Am. Food Anim. Pract. 2008, 24, 87–104. [Google Scholar] [CrossRef]
- Cho, Y.; Yoon, K.J. An overview of calf diarrhea–infectious etiology, diagnosis, and intervention. J. Veter. Sci. 2014, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, D.; Chui, L.; Zhou, T.; Feng, Y.; Cao, Y.; Zhi, S. A Comprehensive Review on Shiga Toxin Subtypes and Their Niche-Related Distribution Characteristics in Shiga-Toxin-Producing E. coli and Other Bacterial Hosts. Microorganisms 2024, 12, 687. [Google Scholar] [CrossRef] [PubMed]
- Fernández, D.; Sanz, M.; Parma, A.; Padola, N. Short communication: Characterization of Shiga toxin-producing Escherichia coli isolated from newborn, milk-fed, and growing calves in Argentina. J. Dairy Sci. 2012, 95, 5340–5343. [Google Scholar] [CrossRef]
- Ferreira, M.; Freitas, F.; Pinto, J.; Dias, M.; Moreira, C. Isolation, prevalence, and risk factors for infection by shiga toxin-producing Escherichia coli (STEC) in dairy cattle. Trop. Anim. Health Prod. 2014, 46, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Auvray, F.; Bièche-Terrier, C.; Um, M.; Dupouy, V.; Nzuzi, N.; David, L.; Allais, L.; Drouet, M.; Oswald, E.; Bibbal, D.; et al. Prevalence and characterization of the seven major serotypes of Shiga toxin-producing Escherichia coli (STEC) in veal calves slaughtered in France. Veter. Microbiol. 2023, 282, 109754. [Google Scholar] [CrossRef]
- Mainil, J.; Nakamura, K.; Ikeda, R.; Crombé, F.; Diderich, J.; Saulmont, M.; Piérard, D.; Thiry, D.; Hayashi, T. Emerging hybrid shigatoxigenic and enteropathogenic Escherichia coli serotype O80:H2 in humans and calves. Clin. Microbiol. Rev. 2025, 38, e0001125. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, M.; Vaseghi-Shanjani, M.; Afkhami, S.; Grondin, J.; Kang, A.; D’Agostino, M.; Yao, Y.; Jain, S.; Zganiacz, A.; Kroezen, Z.; et al. Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut-lung axis. Nat. Immunol. 2022, 23, 1687–1702. [Google Scholar] [CrossRef]
- Menge, C. The Role of Escherichia coli Shiga Toxins in STEC Colonization of Cattle. Toxins 2020, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Fakih, I.; Thiry, D.; Duprez, J.; Saulmont, M.; Iguchi, A.; Piérard, D.; Jouant, L.; Daube, G.; Ogura, Y.; Hayashi, T. Identification of Shiga toxin-producing (STEC) and enteropathogenic (EPEC) Escherichia coli in diarrhoeic calves and comparative genomics of O5 bovine and human STEC. Veter. Microbiol. 2017, 202, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Casaux, M.; Fraga, M.; Vignoli, R.; Bado, I.; Zunino, P.; Umpiérrez, A. Shiga Toxin-Producing Escherichia coli (STEC) Associated with Calf Mortality in Uruguay. Microorganisms 2023, 11, 1704. [Google Scholar] [CrossRef]
- Geletu, U.; Usmael, M.; Bari, F. Rotavirus in Calves and Its Zoonotic Importance. Veter. Med. Int. 2021, 2021, 6639701. [Google Scholar] [CrossRef]
- Bertoni, E.; Aduriz, M.; Bok, M.; Vega, C.; Saif, L.; Aguirre, D.; Cimino, R.; Miño, S.; Parreño, V. First report of group A rotavirus and bovine coronavirus associated with neonatal calf diarrhea in the northwest of Argentina. Trop. Anim. Health Prod. 2020, 52, 2761–2768. [Google Scholar] [CrossRef]
- Louge Uriarte, E.; Badaracco, A.; Spetter, M.; Miño, S.; Armendano, J.; Zeller, M.; Heylen, E.; Späth, E.; Leunda, M.; Moreira, A.; et al. Molecular Epidemiology of Rotavirus A in Calves: Evolutionary Analysis of a Bovine G8P[11] Strain and Spatio-Temporal Dynamics of G6 Lineages in the Americas. Viruses 2023, 15, 2115. [Google Scholar] [CrossRef]
- Dueñas, F.; Rivera, D.; Toledo, V.; Tardone, R.; Hervé-Claude, L.; Hamilton-West, C.; Switt, A. Short communication: Characterization of Salmonella phages from dairy calves on farms with history of diarrhea. J. Dairy Sci. 2017, 100, 2196–2200. [Google Scholar] [CrossRef]
- Stanford, K.; Johnson, R.; Alexander, T.; McAllister, T.; Reuter, T. Influence of Season and Feedlot Location on Prevalence and Virulence Factors of Seven Serogroups of Escherichia coli in Feces of Western-Canadian Slaughter Cattle. PLoS ONE 2016, 11, e0159866. [Google Scholar] [CrossRef]
- Lambertini, E.; Karns, J.; van Kessel, J.; Cao, H.; Schukken, Y.; Wolfgang, D.; Smith, J. Dynamics of Escherichia coli Virulence Factors in Dairy Herds and Farm Environments in a Longitudinal Study in the United States. Appl. Environ. Microbiol. 2015, 81, 4477–4488. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.; Gomes, M.; Campolina, J.; Campos, M.; Souza, E.; Diavão, J.; Silva, A.; Tomich, T.; Carvalho, W.; Lage, H.; et al. Impact of Heat Stress on Intake, Performance, Digestibility, and Health of Neonatal Dairy Calves. Animals 2025, 15, 1876. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.; Drillich, M.; Klein-Jöbstl, D. Invited review: Influence of climatic conditions on the development, performance, and health of calves. J. Dairy Sci. 2016, 99, 2438–2452. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.; Gong, D.; Williams, T.; Ogunsola, A.; Cavallo, K.; Arlehamn, C.S.L.; Acolatse, S.; Beamer, G.; Ferris, M.; Sassetti, C.; et al. Host genetic background is a barrier to broadly effective vaccine-mediated protection against tuberculosis. J. Clin. Investig. 2023, 133, e167762. [Google Scholar] [CrossRef]
| Target | Oligo Sequences (5′ to 3′) | Amplicon Size (bp) | Reference |
|---|---|---|---|
| invA | F′-CGTGTTTCCGTGCGTAATA R′-GCCATTGGCGAATTTATG Probe-FAM/ATTATGGAAGCGCTCGCATT/BHQ1 | 138 | [25] |
| stx1 | F-ATGTCAGAGGGATAGATCCA R-TATAGCTACTGTCACCAGACAAT Probe-FAM/CGCTTTGCTGATTTTTCACATGTTACC/BHQ1 | 185 | [26] |
| stx2 | F-AGTTCTGCGTTTTGTCACTGTC R-CGGAAGCACATTGCTGATT Probe-TET/CACTGTCTGAAACTGCTCCTGT/BHQ1 | 160 | [26] |
| nsp5 | F-AACGATCCACTCACCAGCTTT R-ATTGCTTGATGGTCGTGATTG Probe-FAM/TGAATCCATAGACACGCCAGC/BHQ1 | 105 | [27] |
| IS1081 | F-GAGGGCTACCGAGAGATCCT R-GACCAGGTCGCGGAAGAA Probe-FAM/TCCAGGTCACCTCCGCCGAG/BHQ1 | 84 | [28] |
| 3 Months N (%) | 6 Months N (%) | |
|---|---|---|
| Target sequences | ||
| invA | 1/149 (0.7) | 0/122 (0) |
| stx1 | 57/149 (36.3) | 22/122 (18) |
| stx2 | 113/149 (69.1) | 34/122 (27.9) |
| stx1/stx2 | 43/149 (28.9) | 17/122 (13.9) |
| nsp5 | 25/149 (16.8) | ND |
| IS1081 | 0/149 (0) | 0/122 (0) |
| Deaths | ||
| Diarrhea | 5/163 (3.1) | 2/148 (1.4) |
| Pneumonia | 10/163 (6.1) | 0/148 (0) |
| Total | 15/163 (9.2) | 2/148 (1.4) |
| Sequence | Explanatory Variable | OR | 95% CI | p-Value |
|---|---|---|---|---|
| stx 1 | Group: Control Season: Cold | 2.91 8.52 | 1.42–5.94 1.84–39.42 | 0.003 0.006 |
| Breed: HFSR | 0.64 | 0.31–1.30 | 0.214 | |
| stx2 | Group: Control Season: Cold | 1.19 2.13 | 0.59–2.42 0.81–5.61 | 0.629 0.126 |
| Breed: HFSR | 1.10 | 0.54–2.23 | 0.796 | |
| stx1/stx2 | Group: Control Season: Cold | 1.41 4.12 | 0.63–3.28 1.50–11.26 | 0.407 0.006 |
| Breed: HFSR | 1.05 | 0.46–2.38 | 0.906 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, R.; Villarroel, V.; Neira, V.; Aguayo, C.; Saadi, K.; Orozco, K.; Abalos, P.; Retamal, P. Non-Specific Cross Protection of BCG Vaccination in Dairy Calves. Dairy 2025, 6, 60. https://doi.org/10.3390/dairy6050060
López R, Villarroel V, Neira V, Aguayo C, Saadi K, Orozco K, Abalos P, Retamal P. Non-Specific Cross Protection of BCG Vaccination in Dairy Calves. Dairy. 2025; 6(5):60. https://doi.org/10.3390/dairy6050060
Chicago/Turabian StyleLópez, Renata, Valentina Villarroel, Víctor Neira, Carolina Aguayo, Karina Saadi, Katherinne Orozco, Pedro Abalos, and Patricio Retamal. 2025. "Non-Specific Cross Protection of BCG Vaccination in Dairy Calves" Dairy 6, no. 5: 60. https://doi.org/10.3390/dairy6050060
APA StyleLópez, R., Villarroel, V., Neira, V., Aguayo, C., Saadi, K., Orozco, K., Abalos, P., & Retamal, P. (2025). Non-Specific Cross Protection of BCG Vaccination in Dairy Calves. Dairy, 6(5), 60. https://doi.org/10.3390/dairy6050060






