1. Introduction
Weaning represents one of the most physiologically and behaviourally stressful transitions in the early life of dairy calves, triggering significant stress responses that suppress immunity, growth performance, overall welfare, and long-term productivity [
1,
2]. This stress can significantly increase a calf’s susceptibility to diseases, like coccidiosis and bovine respiratory disease (BRD), and reduce resilience during early development [
3]. Moreover, abrupt weaning can lead to a suppression in the calf’s immune system, making them vulnerable to pathogens [
4]. Cannabidiol (CBD), a phytochemical derived from hemp (
Cannabis sativa), is increasingly recognized for its anxiolytic, anti-inflammatory, and immunomodulatory properties across species [
5]. Unlike tetrahydrocannabinol (THC), the principal psychoactive constituent in cannabis known for impairing mood, perception, and cognition, CBD is devoid of such properties and has shown promising results in stress reduction, modulating immune responses, and improving behavioural outcomes in both companion and farm animals [
6,
7].
Industrial hemp regulations (the legal
Cannabis sativa varieties with very low levels of delta 9-tetrahydrocannabinol (Δ9-THC), typically cultivated for purposes such as fibre production, seed, and cannabidiol extraction) are undergoing frequent updates. The inclusion of hemp-derived compounds, particularly CBD oil, in livestock nutrition has garnered interest for its potential therapeutic benefits without psychoactive consequences. As a result, in the EU’s Common Catalogue of Agricultural Plant Species, authorized hemp cultivars must adhere to a maximum delta 9-tetrahydrocannabinol level of 0.2% on a dry matter basis, maintaining compliance and safety for incorporation in feed chains [
8,
9,
10]. The endocannabinoid system (ECS) is a complex lipid-based signaling network composed of cannabinoid receptors (CB1 and CB2), endogenous ligands (endocannabinoids), transport proteins, and enzymes responsible for the synthesis and degradation of these ligands [
11,
12]. In mammals, the ECS plays a pivotal role in regulating stress responses, neuroimmune communication, energy metabolism, and inflammation [
13,
14]. Cannabinoid receptor 1 (CB1) is predominantly expressed in the central nervous system, modulating neurotransmitter release and behavioural responses. In contrast, cannabinoid receptor 2 (CB2) is mainly found in immune cells and peripheral tissues, mediating anti-inflammatory and immune-suppressive processes [
14,
15]. Recent studies have confirmed the presence of ECS components in bovine tissues, including adipose tissue, gastrointestinal tract, and immune cells, suggesting its functional relevance in ruminants [
16,
17].
Previous findings in companion species, such as horses and dogs, have identified CBD as a potential supplement for promoting well-being during stress events such as transportation or separation from owners [
18]. In steers and beef calves, preliminary trials have demonstrated that CBD oil supplementation during stressful events improves haematological stability, reduces inflammatory markers, and supports liver function [
19,
20,
21,
22]. However, data regarding its effects on livestock species, especially dairy calves, remains limited. The dual potential of hemp-based feeds to improve both health and product quality situates cannabinoid supplementation as an innovative strategy in dairy production systems. Nevertheless, the use of CBD in food-producing animals presents significant regulatory and food safety challenges. In many regions, CBD is not yet approved for livestock intended for human consumption due to limited data on residue levels, withdrawal times, and potential impacts on consumer health. Regulatory agencies require comprehensive toxicological assessments and residue studies to ensure that cannabinoids do not accumulate in edible tissues at levels that could pose risks. Additionally, the lack of standardized dosing protocols and quality control across CBD products complicates efforts to establish safe and consistent use in agricultural settings [
23,
24].
The pilot study investigates the effects of orally administered CBD oil in dairy calves undergoing the weaning process. The primary objectives were to assess whether CBD could (1) reduce stress markers such as serum cortisol, (2) stabilize haematological and biochemical parameters—including lymphocyte, neutrophil, monocyte counts, platelet indices, glucose, ALP, and ALT—and (3) enhance growth performance, measured through growth rates. We hypothesize that oral CBD supplementation would attenuate physiological stress responses, thereby promoting improved welfare and growth during weaning.
2. Materials and Methods
2.1. Ethical Approval
All experimental procedures complied with the provisions of Romanian Law no. 43/2014 [
25] and the Council Directive 2010/63/EU [
26] regarding the protection of animals used for scientific purposes. The experimental procedures and protocol were reviewed and approved by the Ethical Committee of the Research and Development Institute for Bovine, Balotesti, Romania (approval no. 0027).
2.2. Animals, Treatments, and Experimental Design
The study was conducted between March and June 2024 at the Experimental Farm of the Research and Development Institute for Bovine Balotesti, Romania. A total of 19 paternal siblings of Holstein dairy calves, descendants of two bulls, were selected and randomly assigned to two groups: the experimental group (CBD): n = 10 calves/group and the control group (CON): n = 9 calves/group. The study included a mix of male and female calves, the CBD group (5 male and 5 female), and the CON group (4 male and 5 female), assigned to the two groups based on age, sex, body weight, and genetic background (sire) to ensure homogenous distribution. Calves were weaned at 90 ± 5 days of age, at the average body weight of 97.7 ± 3.64 kg for the CBD group and 102.3 ± 4.52 kg for the CON group. Before enrolment, all calves underwent a health assessment by a trained veterinarian.
The experimental group received 5 mL of cannabidiol (CBD) oil/head/day in the first 2 days of the experiment (pre-weaning) and 10 mL of CBD oil/head/day for 3 consecutive days post-weaning as detailed in the experimental design protocol. The initial dose of 5 mL of CBD oil during the pre-weaning period was selected to allow gradual adaptation and minimize potential adverse effects in calves, given their immature metabolic systems, following the recommendations of the producers for human consumption. The dose was increased to 10 mL of CBD oil post-weaning to provide support during the heightened stress associated with weaning. CBD oil was administered
per os, once daily at 08:30 AM at two hours post morning milk administration, ensuring consistent absorption conditions. To ensure safe and stress-free administration throughout both phases, CBD oil was delivered using a smooth-tipped oral syringe, directly in the individual hutches. The syringe featured a blunt, rounded tip with no sharp edges and markings for precise dosing, minimizing the risk of oral mucosal injury and facilitating gentle, consistent delivery. No physical restraint was required, as calves were accustomed to human interaction and accepted the syringe calmly. The oil was dispensed slowly into the mouth while the calf suckled the syringe, followed by an additional 10–15 s of suckling to minimize leakage and ensure full ingestion. In the post-weaning phase, calves were relocated to group pens. For accurate individual dosing, each calf was briefly separated from the group during administration for a maximum of 3 to 5 min. The same oral syringe method was used, with the oil placed directly into the mouth to prevent spillage. No restraint was necessary; calves were familiar with the procedure and responded calmly when being approached by the technician, which reduced stress and handling time. The dosage was determined based on previously published pharmacokinetic studies [
27] and adjusted according to the body weight of dairy calves, in alignment with the manufacturer’s recommendations. The CBD oil was sourced from a certified supplier [
28]. The formulation contained a spectrum of phytocannabinoids, totaling a concentration of 1350 mg cannabinoid per 100 mL, as verified by the supplier’s analytical certificate (
Table 1).
The cannabinoid profile was confirmed through high-performance liquid chromatography (HPLC) coupled with high-resolution mass spectrometry (HRMS), according to certification tests 665–667/19 ML.
The CBD oil was confirmed to be free of chemical contaminants. Microbiological safety was assessed in accordance with pharmaceutical industry standards, as outlined in Bulletin 660I/02-12-2019 [
29]. The product tested negative for
Escherichia coli (
E. coli), Aerobic microorganisms, Fungi, including yeasts and molds.
2.3. Calf Feeding Protocol
During the study, calves were fed according to a structured protocol designed to support early rumen development and ensure a smooth transition to weaning. Each calf received 6 L of whole milk/day, divided into two feedings (6:30 AM and 6:00 PM) and administered via bucket. Solid feed (starter concentrate) was introduced at 8 days of age. The weaning process was done gradually, being initiated 7 days prior to the complete milk stoppage, beginning with a gradual reduction in milk volume (4 L per day, divided into two feedings), and on the last two days prior to weaning, the milk allowance was further reduced to 2 L per day (1 L/feeding), leading to complete milk withdrawal on Day 0. The milk offered to pre-weaned calves was sourced from the farm’s own dairy cows, immediately after milking. The diet was formulated to meet the energy, protein, vitamin, and mineral requirements appropriate for the developmental stage of the calves. Dietary formulation for calves during both pre-weaning and post-weaning phases was based on the nutritional requirements outlined by the National Research Council (2001) guidelines [
30]. The pre-weaning diet consisted of 6 kg of whole milk (with 4–4.3% fat and 3.6–3.7% protein), high-quality alfalfa hay ad libitum, concentrate ad libitum, and fresh water ad libitum. Starting from the day of weaning, the diet included alfalfa hay, concentrate, and fresh water, all provided ad libitum. The nutritive value of concentrates from pre-weaning and post-weaning periods is presented in
Table 2.
2.4. Calf Housing, Management, and Health
From birth, calves were housed individually in outdoor covered hutches to allow for controlled feeding, health monitoring, and reduced exposure to pathogens (Patura Comfort, 2.0 × 1.2 × 1.4 m the covered hutch, 1.5 × 1.2 × 1.0 m the individual paddock). The deep straw bedding (2–3 kg) was replaced weekly, and the hutches were cleaned and disinfected between calf series to maintain hygienic conditions. The calves remained in the same hutches until weaning. Post-weaning, all calves from both groups (CBD and CON) were transitioned to a shared pen group housing system, simulating conventional rearing conditions (allowance of 10–12 m/calf). This system consisted of an indoor free-range barn with straw bedding, allowing unrestricted movement and supporting natural behaviors. Calves also had free access to a securely fenced outdoor paddock throughout the day, which provided additional physical activity and environmental enrichment (16 m/head). Straw bedding was refreshed regularly to maintain hygiene and comfort. The feeding front was designed to minimize competition and ensure equal access for all individuals (0.5 m/calf). During the study, calves were closely monitored through daily clinical observations conducted by the farm veterinarian and the animal caretaker during feeding time. Health assessments focused on identifying signs of diarrhoea, respiratory illness, and other common calf diseases to ensure early detection of any clinical abnormalities. In addition, daily health checks included evaluations of appetite, restlessness, absence of coughing, and general appearance. During the trial, all caves were clinically healthy.
2.5. Sampling Protocol and Monitored Parameters
Blood samples were collected at three distinct time points: Day 0 (day of weaning), +2 days (two days post-weaning), and +5 days (five days post-weaning). Two samples per animal were collected aseptically via jugular venipuncture, approximately 2 h after the administration of CBD oil. These samples were analyzed for hematological parameters—lymphocytes (LYM), monocytes (MON), neutrophils (NEU), plateletcrit (PCT), mean platelet volume (MPV), and platelet distribution width count (PDWc), as well as serum biochemical biomarkers, including cortisol, glucose, alkaline phosphatase (ALP), and alanine aminotransferase (ALT). The body weight was recorded on day −7 before weaning (start of the study), day 0 as the day of weaning, day +7, day +14, and day +21 after weaning to evaluate growth performance across the weaning period. Average daily gain (ADG) was calculated between consecutive sampling intervals to assess post-weaning growth dynamics.
For haematological analysis, 2 mL of whole blood was drawn into Vacutest Kima IT tubes containing K3EDTA (tripotassium ethylenediaminetetraacetic acid) as an anticoagulant. For biochemical analysis, 6 mL of blood was collected into Vacutest Kima IT tubes containing a clot activator and separation gel. Haematology samples were stored at +4 °C and analysed within 2 h of collection to ensure cellular integrity. For biochemical parameters (glucose, ALP, ALT), samples were allowed to clot at room temperature (30–60 min), followed by centrifugation at 6000 rpm for 8 min to obtain serum. For cortisol analysis, blood samples were similarly clotted at room temperature for 30–60 min, then centrifuged at 3000 rpm (~1500× g) for 15 min at 20–22 °C. Serum was extracted using sterile pipettes and transferred into Eppendorf microtubes. All serum samples designated for biochemical analysis were stored at −20 °C until further examination. Haematological parameters were evaluated using an automated three-part differential haematology analyser—Abacus Junior Vet 5 (Diatron Group, Budapest, Hungary). The neutrophil-to-lymphocyte (NEU/LYM) ratio was calculated as a ratio between the neutrophil and lymphocyte counts measured in peripheral blood. Biochemical profiling was performed using a Spotchem EZ SP-4430 automated dry chemistry analyser (Arkray, Kyoto, Japan), utilizing manufacturer-specific tests and protocols. The biochemical markers measured included glucose, alkaline phosphatase (ALP), and alanine aminotransferase (ALT). Serum cortisol concentrations were determined via a commercially available enzyme-linked immunosorbent assay (ELISA) kit (DiaMetra, Perugia, Italy) with an intra-assay CV of <5.1% and an inter-assay CV of <11%, following the manufacturer’s standardized assay protocol.
2.6. Statistical Analysis
All data were processed using Minitab Statistical Software, Version 17 (Minitab Inc., State College, PA, USA). Descriptive statistics were presented as mean ± standard error of the mean (SEM). Outliers were identified using the IQR method, Grubbs’ test, and boxplot visualization, and were excluded to ensure the robust interpretation of the dataset. Data normality was assessed using the Ryan–Joiner test and visual inspection of histograms. Where deviations from normality were observed, logarithmic transformations (log base 10) were applied. Parametric analyses were performed using General Linear Models (GLM) for repeated measures, followed by Tukey’s post hoc test for pairwise comparisons. Statistical significance was set at p < 0.05, and trends were noted at p ≤ 0.10.
3. Results
The effect of CBD oil on BW in dairy calves is presented in
Table 3. At baseline (day −7), calves in the CBD group had slightly lower BW compared to the CON group; however, both groups followed similar growth trajectories throughout the 28-day trial. Significant differences between time points within each group confirmed a time-dependent increase in BW (
p = 0.000). There was no significant difference between groups (
p = 0.173), and the group × time interaction was also not significant (
p = 0.929), indicating that CBD supplementation did not affect the overall growth pattern. The observed changes in BW were primarily driven by time rather than treatment.
The effect of CBD oil on ADG revealed variations in growth performance metrics (
Table 4). The ADG of calves supplemented with CBD oil was higher than that of control calves at most time points, although differences were not statistically significant at individual intervals (
p ≥ 0.05). At day +7, the CBD group recorded an ADG of 971 ± 263 g compared to 936 ± 222 g in the CON group. At day +14, ADG was 743 ± 166 g in the CBD group vs. 489 ± 229 g in the CON group, with no significant difference. By day +21, ADG values were comparable between groups (CBD: 623 ± 228 g vs. CON: 666 ± 128 g). The main effect of the treatment group was not significant (
p = 0.116), nor was the effect of time (
p = 0.597). However, a significant group × time interaction was observed (
p = 0.006), indicating that the trajectory of growth over time differed between the CBD and the CON groups, in favour of the CBD-supplemented group.
Serum cortisol concentrations varied between treatment groups and across all assessed sampling times (
Table 5). At Day 0 and +2 days, calves supplemented with CBD oil exhibited significantly lower cortisol levels compared to the control group (Day 0: 43.08 ± 6.83 ng/mL vs. 92.3 ± 24.9 ng/mL; +2 days: 61.8 ± 12.2 ng/mL vs. 126.9 ± 10.2 ng/mL). By +5 Days, cortisol levels in the CBD group increased to 115.8 ± 20.9 ng/mL, surpassing those in the control group (97.1 ± 26.5 ng/mL), though this difference was not statistically significant. The main effect of the treatment group was statistically significant (
p = 0.043), indicating that CBD supplementation influenced overall cortisol concentrations. The effect of time was not significant (
p = 0.116), suggesting no consistent temporal trend across both groups. However, a trend-level group × time interaction was observed (
p = 0.067), implying that the pattern of cortisol changes over time differed between the CBD and the CON groups.
No differences were observed (
p ≥ 0.05) between groups, over time, or in the group × time interaction for LYM, MON, and NEU parameters assessed (
Table 6). For LYM, mean values remained stable across time in both groups, with no significant differences detected (
p = 0.192 for group effect;
p = 0.291 for time;
p = 0.686 for interaction). Similarly, MON counts showed no significant variation, although a trend toward lower monocyte levels was noted in the CBD group at Day 0 and +2 days compared to controls (
p = 0.082 for group effect). By +5 days, MON values in the CBD group increased slightly, while control values remained variable. NEU counts increased progressively in the CBD group from Day 0 to +5 days (2.28 ± 0.14 10
3/µL to 3.63 ± 0.33 10
3/µL), whereas control calves maintained higher baseline neutrophil levels throughout. However, none of these differences reached statistical significance (
p = 0.126 for group;
p = 0.826 for time;
p = 0.479 for interaction).
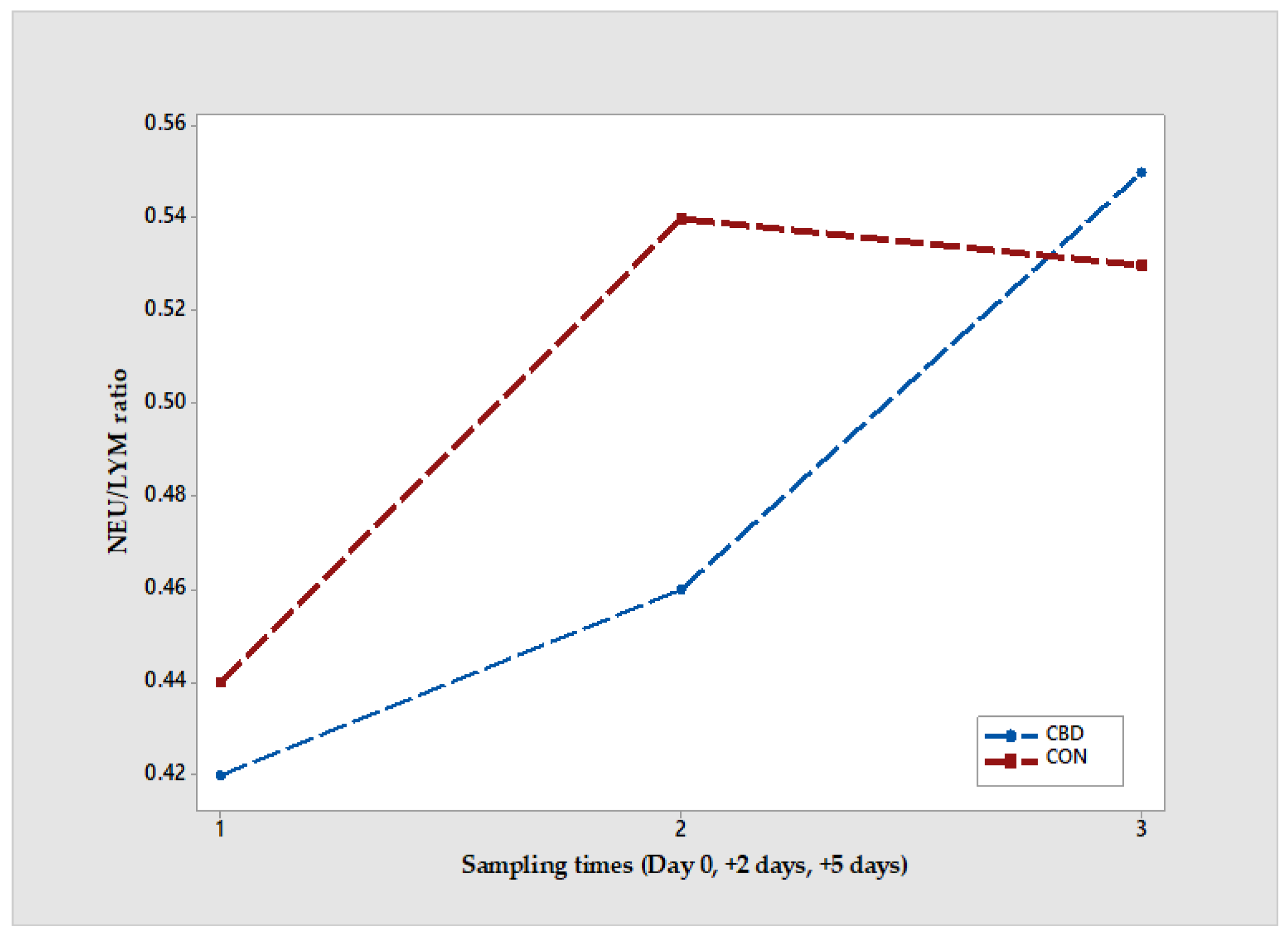
In the CBD-supplemented group, the NEU/LYM ratio (
Figure 1) remained at 0.55 across all assessed sampling times, with values of 0.42 at Day 0, 0.45 at +2 days, and 0.55 at +5 days. The ratios at Day 0 and +2 days were lower than those observed in the CON group. In contrast, the CON group exhibited a progressive increase in NEU/LYM ratio, rising from 0.44 at Day 0 to 0.53 at +5 days, with the highest value recorded at +2 days (0.54).
PCT values remained stable in the CBD group (0.21–0.23%), while the control group exhibited a transient increase at +2 days (
Table 7). Although the group effect approached significance (
p = 0.077), no significant changes were observed over time (
p = 0.667). MPV decreased significantly at +5 days in the CBD group (
p = 0.047 for time), mirroring a similar downward trend in the CON group. While the group effect was not significant (
p = 0.355), the interaction approached significance (
p = 0.079), indicating a potential group-specific temporal response. PDWc remained consistent in the CBD group across all time points, whereas the control group showed a slight decline from Day 0 to +5 days. No significant effects were detected for group (
p = 0.116) or time (
p = 0.108).
Glucose levels remained stable in the CBD group at Day 0 and +2 days (
Table 8); nevertheless, they decreased significantly at +5 days (97.00 ± 5.45 mg/dL;
p = 0.039 for time). A similar downward trend was observed in the CON group, though no significant group effect (
p = 0.348) or interaction (
p = 0.845) was detected. Alkaline phosphatase values fluctuated slightly, with a peak at +2 days in the CBD group (322.8 ± 27.8 IU/L), while the control group remained more stable. No significant effects were found for group (
p = 0.213), time (
p = 0.778), or interaction (
p = 0.847). Alanine aminotransferase levels increased progressively in both groups. The CBD group increased from 8.30 ± 0.49 IU/L at Day 0 to 11.30 ± 0.87 IU/L at +5 days, while the control group peaked at +2 days (13.33 ± 0.91 IU/L). Significant effects were observed for group (
p = 0.014) and time (
p < 0.001), indicating a treatment-related impact on hepatic enzyme activity. No significant interaction was found (
p = 0.358).
4. Discussion
This pilot study investigated the effects of CBD oil supplementation on body weight, stress biomarkers, and haematological profiles evolution in dairy calves over 28 days. Although calves in the CBD group exhibited slightly lower BW at baseline compared to the CON group, both groups demonstrated a consistent and comparable increase in BW throughout the trial. The significant effect of time confirms the expected growth trajectory in weaned calves, independent of treatment. Notably, no significant differences were observed between the CBD and the CON groups, and the lack of a group × time interaction suggests that CBD supplementation did not influence the rate or pattern of weight gain. The absence of treatment effects on BW may reflect the relatively short duration of supplementation, the dosage used, or the physiological resilience of calves during early growth phases. This outcome is consistent with the literature indicating limited impact of CBD on body weight or growth performance [
7]. Collectively, these findings suggest that while CBD may exert physiological effects, such as modulating stress or inflammatory responses, its influence on somatic growth under conventional rearing conditions appears minimal.
Similarly, calves supplemented with CBD exhibited higher average daily gains across all time points compared to the CON group, suggesting a potential growth-promoting effect. However, the absence of significant effects for treatment and time indicates that these differences were not statistically robust within the current samples. Notably, a significant group × time interaction was observed, implying that the trajectory of ADG over time differed between groups. This interaction may reflect a time-dependent response to CBD supplementation, potentially influenced by physiological adaptation or environmental factors. Given the small sample size, these preliminary findings should be considered exploratory. Further studies with larger cohorts and refined measurement protocols are warranted to validate the observed results and clarify the biological relevance of CBD supplementation in calf growth performance.
Weaned calves are known to exhibit elevated cortisol levels [
31], reflecting heightened stress during the weaning transition period. In the present pilot study, differences in serum cortisol concentrations between treatment groups suggest that CBD supplementation may modulate the stress response in dairy calves. At Day 0 and +2 days, calves receiving CBD exhibited significantly lower cortisol levels compared to the CON group, indicating a potential anti-stress effect of CBD oil. Although cortisol concentrations in the CBD group increased with +5 days, surpassing those in the CON group. This rise was not statistically significant and may reflect a post-supplementation rebound effect, whereby initial suppression or modulation of cortisol secretion—possibly influenced by CBD’s interaction with the hypothalamic–pituitary–adrenal (HPA) axis—is followed by a compensatory surge as homeostatic mechanisms attempt to restore baseline endocrine function. Such a rebound may be amplified by cumulative stressors post-weaning, including dietary transition, social restructuring, and environmental adaptation. CBD appeared to exert a transient anxiolytic effect during the immediate post-weaning period, reflected in reduced cortisol levels. These findings are consistent with prior research by Silvestri et al. [
32], which demonstrated dose-dependent anxiolytic and anti-nociceptive effects of CBD mediated via endocannabinoid pathways. Similarly, Henson et al. [
14] reported that CBD enhances endocannabinoid control of stress by modulating HPA axis activity and reducing cortisol secretion under acute stress conditions, supporting its role in short-term stress attenuation. However, following abrupt discontinuation, a rebound response may have occurred, with cortisol levels surpassing even baseline values. This phenomenon aligns with findings from preclinical models, where withdrawal from anxiolytic agents can lead to stress hypersensitivity and neuroendocrine dysregulation [
33]. In contrast, calves in the control group, not exposed to exogenous modulators, may have relied on intrinsic coping mechanisms to adapt to post-weaning stress. This could have facilitated a more stable and gradual adaptation to environmental challenges, resulting in lower cortisol levels at +5 days. Such adaptive resilience is supported by literature suggesting that unmedicated animals often develop compensatory behavioral and neuroendocrine strategies over time [
34,
35]. Furthermore, the learned helplessness behaviour adaptation typical to cattle might have influenced the cortisol response in calves, and, thus, in future trials we plan to prolong the CBD administration time, and to reduce the dose gradually as well, giving time to the calves to better cope with the withdrawal and stress adaptation. The significant main effect of treatment supports the overall influence of CBD on cortisol regulation. In contrast, the non-significant time effect suggests that temporal changes were not consistent across groups. A trend-level group × time interaction points to a differential temporal response, implying that CBD-treated calves may exhibit distinct patterns of adaptation to environmental or handling stressors over time.
Throughout the evaluation period, calves were closely monitored using both clinical observations and laboratory-based assessments, including haematological and biochemical analyses. All calves remained clinically healthy during the trial, with no reported cases of diarrhea, respiratory illness, or other common calf diseases. No significant deviations or pathological findings were observed in either group (CBD or CON). The calves were in good health, and the experimental conditions did not negatively impact their physiological status.
Immune cell profiles varied across time points, without a statistically significant difference. Lymphocyte counts remained relatively stable in both groups. This stability may reflect a contribution of CBD to immune homeostasis, potentially mediated by its anti-inflammatory properties previously demonstrated in human, canine, and rodent models [
18,
19,
36]. The CBD group consistently exhibited lower mean monocyte counts across all sampling points. Given the central role of monocytes in innate immunity and inflammatory regulation [
37], this pattern may suggest reduced inflammatory activation, consistent with CBD’s modulation of cytokine signaling pathways. A gradual rise in neutrophil counts among CBD-treated calves may indicate enhanced mobilization or altered stress responsiveness, potentially mediated by endocannabinoid regulation of immune cell trafficking. Neutrophils, as essential first responders in innate immune defense [
38], are sensitive to physiological stressors, and their temporal increase may reflect adaptive immune engagement. Although these changes did not reach statistical significance, the consistent directional trends across monocyte and neutrophil parameters suggest biologically relevant modulation of innate immune dynamics.
The neutrophil-to-lymphocyte ratio (NEU/LYM) in dairy calves is influenced by breed, age, health status, environmental conditions, and management practices. Elevated NEU/LYM ratios are commonly associated with physiological stressors such as weaning, transport, and illness [
39,
40]. In the CBD-supplemented group, NEU/LYM ratios remained at 0.45 throughout the experimental period (Day 0 and +2 days), suggesting a potential immunomodulatory effect of cannabidiol against weaning-induced stress. These findings are consistent with Austin [
21], who reported reduced monocyte activation and moderated stress biomarkers following CBD administration in beef calves during the weaning and transportation phase. These findings underscore the value of the NEU/LYM ratio as a reliable, non-invasive biomarker for evaluating stress and inflammatory responses in dairy calves during the weaning period, also supported by previous studies on neonatal calves [
41,
42].
CBD supplementation may enhance immune resilience and stabilize plateletcrit dynamics (PCT) during weaning stress. Although not statistically conclusive, the observed platelet-related trends may reflect subtle shifts in inflammatory status and platelet integrity associated with CBD administration. The significant reduction in mean platelet volume (MPV) at +5 days in the CBD group, alongside a near-significant group × time interaction, suggests a potential treatment-specific temporal modulation of platelet morphology. MPV is commonly regarded as a marker of platelet activation and systemic inflammation, and its decline may indicate reduced inflammatory burden or altered thrombopoietic activity in response to CBD. The consistent stability of PDWc and PCT values in the CBD group further supports the hypothesis of a homeostatic effect, contrasting with the greater variability observed in the control calves. These findings are consistent with emerging evidence that CBD may influence platelet function through endocannabinoid-mediated pathways, as proposed by Khayat and Lehmann [
43]. However, the absence of significant group effects and the modest sample size limit definitive conclusions.
The metabolic and hepatic responses observed in this pilot study provide early insights into the physiological impact of CBD supplementation in weaned dairy calves. Glucose concentrations remained stable in the CBD group during the initial phases, but declined significantly at +5 days, mirroring a similar trend in the CON group. This time-dependent decrease likely reflects post-weaning metabolic adaptation rather than a direct effect of CBD, as no significant group effect or interaction was detected.
Previous studies have shown that stress and dietary transitions during weaning can disrupt glucose homeostasis, with transient hypoglycaemia reported in calves exposed to environmental or nutritional stress. Conversely, stress-induced hyperglycaemia is typically mediated by cortisol-driven hepatic gluconeogenesis and glycogenolysis during weaning transitions [
44]. The modulation of cortisol observed in CBD-treated calves may, therefore, contribute indirectly to metabolic stabilization, although further investigation is needed to confirm this relationship.
Alkaline phosphatase (ALP) and alanine aminotransferase (ALT) are considered sensitive biomarkers of liver function [
45]. ALP levels fluctuated modestly, peaking at +2 days in the CBD group, yet remained within physiological ranges. The absence of significant effects suggests that CBD did not markedly influence bone or hepatic ALP activity during the study period. In contrast, ALT levels increased significantly over time in both groups, with a notable group effect, indicating a potential hepatic response to CBD supplementation.
While ALT elevations may reflect hepatic enzyme induction or mild hepatocellular stress, values remained within reference ranges, and no clinical signs of liver dysfunction were observed. These findings align with previous research by Ewing et al. [
46], who demonstrated that CBD can modulate liver enzyme expression and protect against oxidative hepatic damage in mammalian models. However, its impact on hepatic enzyme activity in ruminants remains poorly characterized, and the observed changes warrant cautious interpretation. Further studies incorporating oxidative stress markers, histopathological evaluation, and dose–response analysis are warranted to clarify the hepatic safety profile of CBD in bovine species
We acknowledge that the small sample size and short study duration represent important limitations that constrain the generalizability of our findings. While the results offer preliminary insights into the physiological effects of CBD supplementation in calves, they should be interpreted with caution. The limited number of animals reduces statistical power and increases susceptibility to individual variability, while the short observation period may not capture long-term outcomes or adaptive responses. Future studies with larger cohorts, extended monitoring, and behavioural assessments are essential to validate these findings and to better understand the sustained impact of CBD oil on animal health and welfare. Additionally, integrating genotype and microbiome profiling may further elucidate the biological mechanisms underpinning individual response variability.