Abstract
Cheese products worldwide have gained protected designation of origin status in many instances, yet this food group also has the highest reported fraud rates. Quesillo Caquetá is the first Colombian cheese to acquire a protected designation of origin, but still there is a lack of information regarding its composition. In this study, a compositional analysis was performed to establish a set of characteristic parameters to aid the identification of the authenticity of Quesillo Caquetá. Physicochemical analysis, mineral composition determination, carbohydrate, fatty acid, and peptide profiles were conducted on 29 samples of Quesillo Caquetá made with milk from the northern, southern, and central regions of the province of Caquetá. The results revealed 7 minerals, 3 carbohydrates, 19 fatty acids, and 45 peptides (21 peptides from bovine αs1-casein and 24 peptides from bovine β-casein). This suggests that Quesillo Caquetá is a significant source of sodium, calcium, phosphorus, and monounsaturated fatty acids such as oleic acid, omega-3, and omega-6, as well as some peptides that match sequences with antihypertensive, immunomodulatory, antioxidant, and antimicrobial activity reported in the literature. The specificity of the fatty acid and peptide profiles can become a valuable tool for identifying the authenticity of Quesillo Caquetá against possible imitations in the market.
1. Introduction
Quesillo Caquetá is the first Colombian cheese with a protected designation of origin (PDO), which certifies that a product is produced in a specific place or geographical area with raw materials and conditions that distinguish it from similar products from other places. Quesillo Caquetá is a pasta filata cheese produced in Caquetá, the Andean-Amazon region of southern Colombia, using bovine milk traditionally from Holsteins and Criollo Caqueteño cattle. In various Latin American countries, there is special interest in the manufacture and marketing of pasta filata cheeses due to their high consumption and utilization, especially in the preparation of fast foods [1]. Quesillo Caquetá can be classified as a semi-hard cheese with a medium–high fat content, and its characteristic properties are its acidic flavor, high stretchability, and melting capacity. In addition, it does not have a rind, and its color is white-yellowish (CIE lab scale data: L* (80–75), a* (−0.12–−1.63), and b* (25–29)) [2]. Quesillo Caquetá is a valuable source of nutritional compounds, but there has not yet been an exhaustive chemical study conducted on the characteristic features of this traditional product. The production of Quesillo Caquetá has increased in recent years, with the province of Caquetá producing about 82 tons/day [3]. Moreover, its market price has increased by 56.1% over the last five years [4], thus making it a more attractive product and one susceptible to imitations.
Cheese is among the food groups with the most protected designations of origin [5], but it also attracts the highest fraud rate [6]. Several authors have described different physicochemical analysis, mass spectrometry, and DNA-based techniques to determine the authenticity of PDO products such as cheese [6,7,8].
Fatty acid determination has recently been used as a tool for cheese authentication purposes [9,10]. In another study, the fatty acid profile was determined in several Greek PDO cheeses to form a database for fraud detection [11]. Sheep ricotta cheese was also authenticated from related products through the fatty acid profile [12]. Additionally, the fatty acid profile was used as a chemical biomarker to evaluate the production origin of Serra da Estrela PDO cheeses [13].
Similarly, due to peptide specificity, peptide profiling has also been successfully used to determine the authenticity of diverse types of cheeses [14,15,16]. Determining peptide masses results in unique patterns due to substrate–enzyme combinations, which can then be compared with peptide sequence databases [17]. In addition, techniques such as SDS-PAGE electrophoresis and RC-HPLC analysis have been used to identify the peptide profile in cheeses such as buffalo mozzarella to assess their authenticity [18]. In the same way, other authors reviewed proteomic methods to identify proteins and peptides in PDO cheeses as a useful tool to avoid misleading consumers and to enhance the value of these traditional products [7].
In this regard, this study focuses on the milk origin in the characterization of Quesillo Caquetá by assessing its mineral composition and carbohydrate, fatty acid, and peptide profile. The aim is to establish a set of characteristic parameters that will serve as a reference when verifying the authenticity of Quesillo Caquetá and promote its protection as a distinctive product of the region.
2. Materials and Methods
2.1. Samples of Quesillo Caquetá
In total, 29 samples of Quesillo Caquetá were manufactured with raw bovine milk from three regions of Caquetá: 9 samples were made with milk from the northern region, 9 samples from the southern region, and 11 samples from the central region. A total of 10 L of milk was used to manufacture each cheese sample. The manufacturing process for Quesillo Caquetá is shown in Figure 1. The process began with the reception of milk collected from several individual dairy farms, followed by a physicochemical analysis and impurity filtration. The fat content in the milk was not standardized, and the acidity was adjusted to 4% (lactic acid), adding acid whey at 90% (lactic acid). The acidity was measured by volumetric titration with 0.1 N sodium hydroxide and phenolphthalein as a pH indicator according to color change. The acidification of the milk was achieved to obtain the characteristic sour taste of Quesillo Caquetá and its elastic texture. The milk was heated to 37.5 °C, and 8 mL/100 L of enzymatic coagulant Chy-Max Plus (50 IMCU/mL) (Chy-Max Plus, Chr. Hansen A/S, Hørsholm, Denmark) was added. The curd was cut, and the mass was drained for 15 min. The curd mass was placed in a cooking and spinning vat at 70 °C, and 2% salt, 5% fermented whey, and 0.5% sodium citrate were added according to the weight of the mass in each batch. The use of sodium citrate is permitted under local legislation, and it is used as an emulsifying salt for the stretching step [19]. Once the stretched paste was formed, it was deposited in steel molds for 6 h, stored in a room at a temperature between 0 and 4 °C, and packaged in polyethylene film bags. Quesillo Caquetá is usually commercialized after a brief ripening period of less than one week.
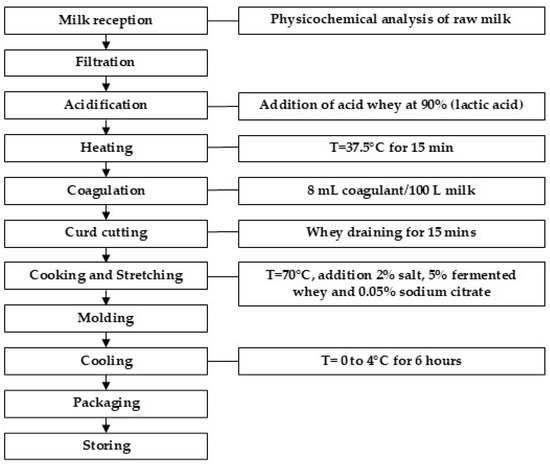
Figure 1.
Flow chart of manufacturing process of Quesillo Caquetá.
2.2. Physicochemical Analysis
The physicochemical analyses performed on the milk tested the following components: protein (Kjeldahl Method ISO 8968-1) [20], fat (Gerber Method AOAC 933.05 21th 2019) [21], moisture (Method 926.08 AOAC 21th 2019) [22], and ash (ISO Method 5545) [23]. These analyses were performed in triplicate.
The physicochemical analyses performed on the Quesillo Caquetá samples tested the following components: protein (Kjeldahl method ISO 8968-1), fat (Soxhlet method 920.29 AOAC 21th 2019) [24], moisture (Method 926.08 AOAC 21th 2019), and ash (Method ISO 5545).
2.3. Determination of Minerals
The determination of minerals Ca, Mg, Na, K, P, S, and F in the Quesillo Caquetá samples was performed according to the method described by Álvarez et al. [25]. A ContrAA700 high-resolution continuous-source atomic absorption spectrometer (HR-CS AAS) from Analytik Jena (Analytik Jena GmbH + Co. KG, Jena, Germany) was used. The samples were digested in closed Teflon (TFM) vessels using a Milestone Ethos 1 Microwave Digestion Labstation (Milestone, Shelton, CT, USA) with HNO3 and H2O2 (PA, Panreac Química, S.A., Barcelona, Spain). After digestion, the samples were diluted with ultrapure deionized water (18.2 MW·cm resistivity at 25 °C). The apparatus was equipped with a graphite furnace and a xenon short-arc lamp with a wavelength between 185 nm and 900 nm and a transversely heated graphite tube with a platform used for the determination of P and F operating in a “hot spot” mode as the radiation source. An acetylene/air flame was used with an inlet pressure of 0.7 MPa for the determination of Mg, Na, K, and S, and an acetylene flame was used for Ca. Calibration was performed using aqueous standards from commercial single-element stock solutions (Panreac Química, S.A., Barcelona, Spain). Measurements were performed at the wavelength for Ca (422.7 nm), Mg (285.2 nm), Na (589.0 nm), K (766.5 nm), and S (180.7 nm) and the non-resonance line for P (213.6 nm) and F (227.4 nm).
2.4. Analysis of Carbohydrates
The determination of carbohydrates in the cheese samples was conducted via gas chromatography according to the method described by Corzo et al. [26] with some modifications: 4 g of each cheese was taken and homogenized in 20 mL of distilled water and 5 mL of internal standard (β-phenyl-glucoside) with a concentration of 1 mg/mL. The samples were mixed in a vortex shaker for 2 min and filtered in a vacuum funnel with Whatman No1 paper. A total of 5 mL of the filtrate was then taken and diluted in 25 mL of methanol, left to stand for 1 h, and filtered once more under vacuum with Whatman No 42 paper; then the filtrate was taken to the rotary evaporator at T = 38 to 40 °C for 1 h. Derivatization was performed by adding 300 μL of hydroxylamine chloride in pyridoxine and an oven at 70 °C for 30 min to facilitate oxime formation. Then 300 μL of hexamethyldisiloxane (HDMS) and 30 μL of trifluoroacetic acid (TFA) were added, and the filtrate was incubated again at 50 °C for 30 min. The samples were cooled and then centrifuged at 10,000 rpm for 2 min, and 2 μL was transferred to vials. The analysis was performed on a gas chromatography system with a flame ion detector (GC-FID), Model 7820A, coupled with a 7693 automatic injector (Agilent Technologies Ing., Palo Alto, CA, USA). A DB-5HT silica capillary column (30 m × 0.32 mm × 0.10 μm) (J&W Scientific, Folson, CA, USA) was used. Sample injection was achieved in a 1:20 split mode, and the carrier gas used was nitrogen at a flow rate of 1 mL/min. The oven temperature started at 150 °C with a temperature ramp of 10 °C/min until reaching 380 °C, and the detector temperature was 280 °C. Data analysis and peak integration were performed using the Agilent ChemStation software program (Agilent, Washington, DC, USA). Galactose, glucose, and lactose concentrations were calculated using the areas under the peaks of the chromatograms and the response factors of the galactose, glucose, and lactose standards previously injected at known concentrations from 0.025 to 2 mg/mL, in addition to the β-phenyl-glucoside used as an internal standard.
2.5. Determination of Fatty Acids
The profile of fatty acid methyl esters (FAMEs) was determined using a gas chromatographer—FID Agilent 7820A (Agilent Technologies Ing., Palo Alto, CA, USA). For the analysis, 100 mg of sample was taken, and derivatization was performed using a mixture of 0.5 M sodium methoxide in methanol and acetyl chloride in methanol (1:10 v/v), according to the procedure described by Lee et al. [27] with the following modifications: The quantitative determination of fatty acids was performed using C 13:0 as an internal standard prior to methylation (1 mg internal standard in the total starting sample). The fatty acids were extracted with hexane, and the samples were transferred to vials for detection. The method started with an oven temperature of 50 °C and a ramp of 25 °C/min to 175 °C and then 4 °C/min until 230 °C was reached. The column used was GC-26 (60 m × 250 μm × 0.25 μm), and the carrier gas was helium with a flow rate of 1 mL/min. The injector temperature was 250 °C with a 40:1 split and an injection volume of 1 μL, and the detector temperature was 260 °C. The identification of the fatty acids was achieved using the EZ Chrom Elite control software tool by comparing the retention times of the peaks in the chromatogram with those found with the fatty acid methyl ester standard No. 37 Supelco Ref CRM47885 + PUFA No. 2 Animal Source Ref 47015-U Sigma + PUFA No. 3 Menhaden oil Ref 47085-U Sigma.
2.6. Determination of Proteins and Peptides
2.6.1. Preparation of Water-Soluble Extracts
The preparation of water-soluble extracts (WSEs) was performed following the method reported by [28]. As the composition of the milk samples (protein, fat, and moisture content) and the Quesillo samples (protein, fat, lactose, and moisture content) was similar among the regions, each of the 29 Quesillo samples was crushed in a blade grinder, grouped by region, and mixed. One pooled sample of 10 g was taken from each region (northern, southern, and central) and added to Falcon tubes with 20 mL of ultrapure water type II MilliQ (Merck-millipore Corp., Darmstadt, Germany), homogenized using ultraturrax equipment (KA-Werke GmbH & CO, Staufen, Germany) for 3 min, placed in a water bath at T = 40 °C for 1 h, and then centrifuged at 3800× g for 30 min at 4 °C. The tube was placed on ice to solidify and remove the lipid fraction; the supernatant was filtered through glass wool and Whatman No 40 filter paper. The soluble extracts were freeze-dried and stored at T = −20 °C until analysis.
2.6.2. SDS-PAGE Electrophoresis
Protein separation by molecular weight was performed through SDS-PAGE (Sodium Dodecyl Sulfate–Polyacrylamide Gel) electrophoresis, considering the protein concentrations of the solubilized extracts. Electrophoresis was performed on all 3 WSE samples (from the northern, southern, and central regions) and on the samples of crushed freeze-dried cheese dissolved in distilled water at a concentration of 1 mg protein/mL in a loading buffer prepared with Tris-HCl (0.5 M, pH 6.8), anionic sodium dodecyl sulfate (SDS, 1.6% w/v), glycerol (8% v/v), bromophenol blue (0.002% w/v), and β-mercaptoethanol (2% v/v), and the samples were heated in a thermoshaker at 95 °C with moderate agitation for 5 min. A run buffer was also prepared with 475 mL milli-Q water and 25 mL of the commercial solution XTMES 20X (1,3-propanediol, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl) sodium salt of monododecyl sulfuric acid ester, 1:1 ratio). Criterion XT 12% Bis-Tris polyacrylamide commercial gel (Bio-Rad, Richmond, CA, USA) and a molecular weight marker (Precision Plus Protein Unstained Std, Bio-Rad) were used. Electrophoresis was performed at 100 V for 5 min, and then, the voltage increased to 150 V for 50 min. Finally, the gel was covered with commercial Instant Blue revealing solution with soft agitation for 24 h for detection.
2.6.3. Liquid Chromatography Coupled to Tandem Mass Spectrometry (LC-MS/MS)
The 3 WSEs (northern, southern, and central regions) were analyzed via liquid chromatography coupled to tandem mass spectrometry (HPLC-MS/MS) on an Agilent 1100 HPLC System chromatograph (Agilent Technologies, Waldbronn, Germany) coupled to an Esquire 3000 ion trap spectrometer (Bruker Daltonik GMBH, Bremen, Germany). A Gemini NX-C18 column (150 × 2 mm id, 3 µM) (Phenomenex) was used; solvent A consisted of a mixture of water and formic acid (0.1%) (1000:0.37), and solvent B was a mixture of acetonitrile and formic acid (0.1%) with a flow rate of 0.2 mL/min. Mass spectra were identified in the range 200–2100 m/z using helium as the carrier gas, and a fragmentation ramp between 0.3 and 2.0 V was used. Peptide sequence identification was performed with the Data Analysis software, Sequence Editor, and Biotools from Bruker Daltoniks and MASCOT (Bruker Daltonik GMBH, Bremen, Germany) using our own databases with the sequences of bovine milk proteins and from diverse sources and variants [29].
The identified peptide sequences were aligned according to the precursor protein to calculate the percentage of coverage; the peptide profile, considering the signal intensities and peptide overlapping, was plotted using the Bioware Peptigram Program developed by Manguy et al. [30]. The bioactive functions of the peptides were referenced using the MBPDB dairy peptide database (Milk Bioactive Peptide Database, OR, USA) [31]. To identify common peptides between each of the samples, a Venn diagram was created [32].
2.7. Statistical Analysis
A descriptive analysis followed by an analysis of variance (ANOVA) was performed to determine differences among regions in the physicochemical composition of the milk and the physicochemical composition, mineral composition, and carbohydrate and fatty acid profile of the Quesillo Caquetá samples. Tukey’s mean comparison test (α = 0.05) was also performed to determine the differences between regions. A multivariate analysis was performed using Partial Least Squares (PLS) to reduce the dimensionality of the data and analyze the relationships between the predictor variables (milk composition variables) and the dependent or response variables (Quesillo composition variables) and to construct a tri-plot graph. The dataset used for PLS is available in the Supplementary Materials (Table S1). In addition, these results were compared through a Pearson correlation analysis to evaluate the correlations among milk and cheese composition variables. Statistical analysis was conducted using InfoStat software version 2020 [33].
3. Results
3.1. Physicochemical Analysis of Milk and Quesillo Caquetá
The composition of the milk from the northern, central, and southern regions is presented in Table 1. The ANOVA and Tukey’s comparative test revealed a similar composition among the milk samples from the northern, central, and southern regions; there were no significant differences (p > 0.05) between the samples for protein, fat, and moisture. On the contrary, a significant difference was observed for ash (p < 0.05). Differences in the ash or mineral content of milk may be due to factors such as the nutritional status of the cow, lactation stage, genetic, and environmental conditions [34,35,36].

Table 1.
Physicochemical analysis of milk and Quesillo Caquetá.
The physicochemical variables of Quesillo Caquetá also showed a similar composition among the samples from the northern, central, and southern regions (Table 1). There were no significant differences (p > 0.05) between cheeses for the variables protein, fat, lactose, and moisture. Similarly to the milk samples, there was also a significant difference in ash content (p < 0.05), with cheese made from milk from the northern region having the highest ash content, which is directly related to the ash content of the milk.
The mean protein content of Quesillo Caquetá was similar to that of fresh Quesillo cheese and double cream cheese (19 to 21%) [2,37,38] and Oaxaca cheese (21.75%) [39]. The protein content was higher than that in melted cheese (13.86 to 16.98%) [40], mozzarella cheese (18.23%) [10], panela cheese (14.4%) [41], and coalho cheese (17.91 to 18.77%) [42].
The average fat content was 25.36%; this value was close to that for other fresh cheeses such as Quesillo cheese (25.88%) [37], mozzarella cheese (25.85 to 27.25%) [43], porungo cheese (24.73%) [44], and kashkaval cheese (25.5%) [45]. However, this value was higher than that of fresh cheese (21.13%) [38] and Oaxaca cheese (21.75%) [39].
The mean moisture content was 48.25%, similar to Quesillo Huilense (49.33–60%) [46] and mozzarella cheese (48.59–50.51%) [43] and higher than that of melted cheese (42%) [40] and porungo cheese (46.3%) [44]. The average ash content was 4.15%, a value similar to that reported for mozzarella cheese (3.62 to 4.2%) [47] and higher than that of fresh cheese (3.4%) [38] and Oaxaca cheese (3.2 to 3.7%) [48]. The ash content is closely correlated to the mineral composition of the cheese, particularly calcium, phosphorus, potassium, and sodium, which determines the structural integrity and moisture retention of the cheese [49]. This physicochemical composition of Quesillo Caquetá is in accordance with that reported to the competent authorities in Colombia for granting the protected designation of origin [50], but it is not sufficient to determine its authenticity, as it is similar to that of other types of Quesillo.
Figure 2 shows the tri-plot obtained using Partial Least Squares (PLS), where all response and predictor variables were standardized before the analysis. The addition of Factors 1 and 2 explains 84.2% of the variability in the data. The variables with the greatest influence in Factor 1 were Quesillo protein, milk protein, milk fat, and Quesillo fat. There is a high positive correlation between Quesillo protein and milk protein (r = 0.93, p < 0.0001), milk fat and Quesillo fat (r = 0.58, p < 0.0009), milk protein and cheese fat (r = 0.59, p < 0.0007), milk fat and cheese protein (r = 0.88, p < 0.0001), and cheese protein and cheese fat (r = 0.66, p < 0.0001), and these data are consistent with those presented by Amenu et al. [51] for Cheddar cheese, as well as a correlation between Quesillo ash and milk ash like that reported by Amalfitano et al. [52]. Regarding the origin of the samples, there are no defined groupings of the data analyzed, which indicates that there are no marked differences in the characteristics of the milk according to the region. Variations in milk and cheese variables are therefore due to differences between individual farms rather than regions. This result is consistent with the ANOVA, in which only milk and Quesillo ash showed differences among regions.
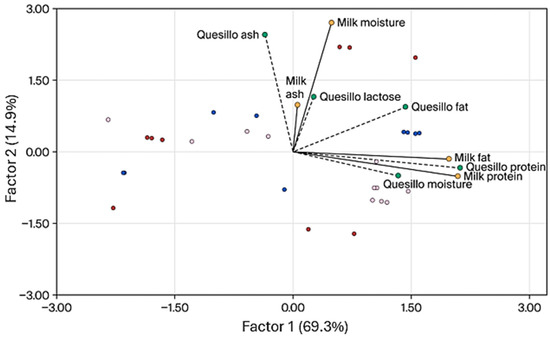
Figure 2.
The tri-plot obtained using PLS to ordinate twenty-nine samples through the variables associated with milk composition (represented in yellow) and the variables of Quesillo composition (represented in green). The blue dots correspond to the data of the latent variables of the northern region, the red dots correspond to the data of the southern region, and the pink dots correspond to the central region.
3.2. Mineral Composition
The mineral content is presented in Table 2. The ANOVA revealed no significant differences (p > 0.05) for the minerals Ca, Mg, P, and F with respect to the origin of the milk (northern, southern, and central regions) and significant differences for the minerals Na, K, and S. These differences may be due to variations in salting or the addition of emulsifying salts during the cooking and stretching stage to improve their texture and characteristic flavor. The minerals with the highest concentration in Quesillo Caquetá were Na, Ca, and P, which agrees with those reported for mozzarella, provolone, and pasta filata cheeses [10,53]. Traces of fluorine were also found in Quesillo Caquetá, a scarce mineral in this type of product.

Table 2.
Mineral composition of Quesillo Caquetá.
The Na, Ca, Mg, P, K, and S contents in Quesillo Caquetá were higher than those reported for mozzarella and pasta filata cheese [10]. The addition of fermented whey may be responsible for the high mineral content and sourness of cheeses [54], one of the main sensory attributes of Quesillo Caquetá.
3.3. Carbohydrate Profile
The concentrations of galactose, glucose, and lactose of the Quesillo Caquetá samples are shown in Table 3. The ANOVA revealed no significant differences (p > 0.05) for galactose and lactose according to the origin of the milk (northern, southern, and central regions) and significant differences for glucose (p < 0.05). These differences in glucose content in the samples may be due to the degree of transformation of lactose into galactose, glucose, and subsequently lactic acid via lactic acid bacteria [55,56]. The lactose content was close to that presented for Oaxaca cheese (0.1 to 0.3%) [48] and higher than that reported for stretched cheese (0.17%) [57] and mozzarella cheese (0.0%) [58]. A high lactose content in the samples of Quesillo Caquetá can be related to the use of whey in the stretching stage of the manufacture, where heating facilitates the concentration of lactose, as the main component of whey after water. The variation in lactose content can also be attributed to factors such as fat content and salt addition in cheeses [59].

Table 3.
Carbohydrate fraction in Quesillo Caquetá.
The mean content of galactose was lower than that reported for mozzarella cheese (0.3%) [58], stretched cheese (0.95%) [57], and soft cheese (0.72 to 0.83%) [60]. Similarly, the mean glucose content was also lower than reported for mozzarella cheese (0.03%) [61], stretched cheese (0.02%) [57], and soft cheese (0.1 to 0.15%) [60]. The low glucose content may be caused by the higher assimilation of this monosaccharide than galactose by microorganisms and their participation in the Maillard reaction, promoted through heat treatment [26]. Galactose, glucose, and lactose chromatograms are available in the Supplementary Materials (Figure S1).
3.4. Fatty Acid Profile
A total of 19 fatty acids were determined in the Quesillo Caquetá samples (Table 4). The ANOVA showed no significant differences (p > 0.05) for the following fatty acids: caproic (C6:0), caprylic (C8:0), capric (C10:0), lauric (C12:0), myristic (C14:0), palmitic (C16:0), margaric (C17:0), cis-vacenic (C18:1n7c), linoleic (C18:2n6c), α-Linolenic acid (C18:3n3), arachidic (C20:0), and DPA Clupanodonic (C22:5n3) acids. A significant difference (p < 0.05) was shown for the following fatty acids: butyric (C4:0), pentadecyl (C15:0), palmitoleic (C16: 1n7), stearic (C18:0), oleic (C18:1n9c), and lignoceric (C24:0) acids. The fatty acids with the highest presence in Quesillo Caquetá were (in descending order) palmitic acid (C16:0) (90.15 mg/g), oleic acid (C18:1n9c) (78.56 mg/g), stearic acid (C18:0) (42.28 mg/g), myristic acid (C14:0) (31.93 mg/g), and lauric acid (C12:0) (7.99 mg/g). Oleic acid is one of the monounsaturated fatty acids with anti-atherogenic and even anticarcinogenic properties [62]. These five fatty acids were also the most exhibited in mozzarella, provolone, pasta filata, and parmigiano cheese [53] and similarly in kashkaval and provolone cheese [63]. The milk used in the Quesillo Caquetá production process has a notable level of fat, which may be a significant source of fatty acids.

Table 4.
Quesillo Caquetá fatty acid profile.
The essential fatty acids omega-3 (α-Linolenic acid, C18:3n3) and omega-6 (linoleic acid, C18:2n6c) were also found in Quesillo Caquetá samples, with mean values of 1.59 mg/g and 2.7 mg/g, respectively, and their levels were higher than those reported for mozzarella cheese and pasta filata cheese [10]. These two fatty acids have beneficial effects on human health, as described in [64]. Short-chain saturated fatty acids (SCFAs) were also found, particularly butyric acid, which has demonstrated anti-inflammatory effects and the inhibition of breast and colorectal cancer progression [65]. The fatty acids reported, particularly the concentrations of omega-3 and omega-6, are distinctive features that may serve as a reference for identifying Quesillo Caquetá.
3.5. Protein Profile
The electrophoresis results obtained via SDS-PAGE are shown in Figure 3. The samples were freeze-dried after manufacture and analyzed 3 months later; the separation of proteins in the three samples each of cheese WSEs and crushed freeze-dried cheese was similar (Lanes 2 to 7). Most of the casein milk proteins were identified as intact bands, according to the low proteolysis observed in this type of cheese with a short ripening time. The bands with the highest intensity for the Quesillo Caquetá samples correspond to caseins αs2, αs1, and β-casein, with molecular weights between 23 and 37 kDa, followed by p-κ-casein with a molecular weight around 14 kDa, consistent with the rennet coagulation. This electrophoretic profile with intact proteins is in accordance with other fresh pasta filata cheeses such as mozzarella cheese [66], Oaxaca cheese [67], coalho cheese [68], fresh minas cheese [69], and white cheese [70,71]. Lanes 8 (milk standard) and 9 (casein standard) presented similar bands and values for αs2, αs1, and β-casein. In addition, β-Lactoglobulin and α-Lactalbumin were identified, with molecular weights under 20 kDa, similar to those presented by other authors [72,73,74].
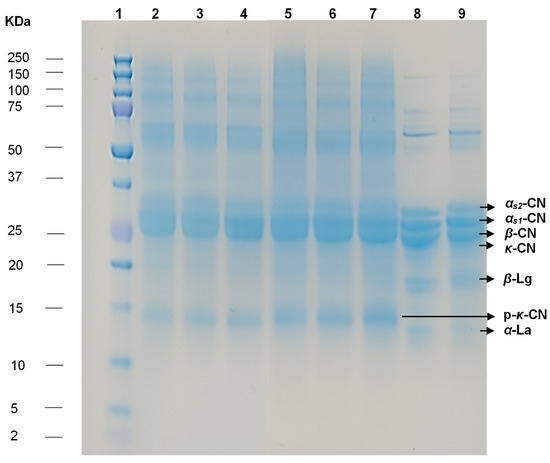
Figure 3.
Electrophoretic pattern obtained by SDS-PAGE of WSEs and crushed freeze-dried cheese. Lane 1: Molecular weight marker. Lane 2: WSE of Quesillo Caquetá sample (northern region). Lane 3: WSE of Quesillo Caquetá sample (central region). Lane 4: WSE of Quesillo Caquetá sample (southern region). Lane 5: Crushed freeze-dried Quesillo Caquetá sample (northern region). Lane 6: Crushed freeze-dried Quesillo Caquetá sample (central region). Lane 7: Crushed freeze-dried Quesillo Caquetá sample (southern region). Lane 8: Milk standard. Lane 9: Casein standard. CN: Casein; Lg: Lactoglobulin; La: Lactalbumin.
3.6. Peptide Profile
Three samples of Quesillo Caquetá (northern, southern, and central regions) were analyzed for their peptide profile, and the peptide alignment for β- and αs1-casein is represented by a Peptigram graph (Figure 4). The height of the green bars refers to the count of peptides overlapping at that position in the protein structure, and the intensity of the green color corresponds to the addition of the peptide intensities of overlapping peptides. Figure 4a corresponds to the profile of β-casein-derived peptides, and Figure 4b corresponds to the profile of αs1-casein-derived peptides. The peptide sequences derived from αs1-casein are shown in Table 5. A total of 21 peptides were found in the water-soluble fractions from Quesillo Caquetá samples, and their start and end numbers in the sequence and the respective intensities are given. The peptide with the highest intensity was APFPEVF, which has been reported to exhibit angiotensin-converting enzyme (ACE) inhibitory activity [75,76]. Several peptide sequences have previously shown different bioactivities. VLNENLL has thus been reported to have antimicrobial activity [77], VAPFPE regulates cholesterol by inhibiting its solubility [78], and ENLLRF also has potential antihypertensive properties due to the inhibitory activity of ACE [79]. Table 6 presents 19 peptides derived from bovine β-casein consistently found in the samples. Peptide YQEPVLLGPVRGPFPI has been reported to exhibit antimicrobial activity [80], DKIHPF to have ACE inhibitory activity [81], LYQEPVLGPVRGPFPIIV an immunomodulatory effect [82], and RELEEL an antioxidant effect [83]. Apart from YQEPVLLGPVRGPFPI, these sequences have been previously shown to resist in vivo digestion [84], so they are of high significance as they emphasize the beneficial properties for the organism associated with the consumption of this distinctive product.
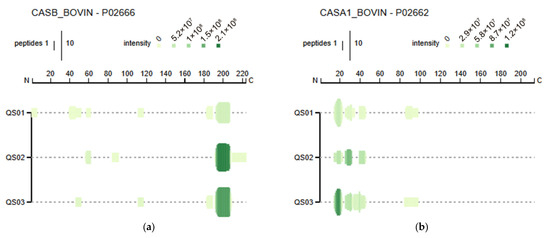
Figure 4.
Peptide profile identified via LC-MS/MS corresponding to β-casein (CASB_BOVIN-P02666) (a) and αs1-casein (CASA1-BOVINE-P02662) (b) peptides in WSE (n = 3), represented as Peptigram graphs. QS01: Quesillo Caquetá sample (north). QS02: Quesillo Caquetá sample (south). QS03: Quesillo Caquetá sample (central). Each vertical bar corresponds to one amino acid identified as part of a peptide sequence.

Table 5.
Peptide sequences in Quesillo Caquetá water-soluble extract (n = 3) identified via LC-MS/MS from β-casein mature sequence (Uniprot ID P02666).

Table 6.
Peptide sequences in Quesillo Caquetá water-soluble extract (n = 3) identified via LC-MS/MS from αs1-casein mature sequence (Uniprot ID P02662).
According to the Venn diagram (Figure 5), eleven common peptides (YQEPVLGPVRGPFPI, EPVLGPVRGPFPI, QEPVLGPVRGPFPI, YQEPVLGPVRGPFP, PVLGPVRGPFPI, VAPFPEV, VAPFPE, APFPEVF, FPEVF, VLNENLL, and ENLLRF) were found among the Quesillo Caquetá samples made with milk from the three regions: northern, southern, and central. In addition, the highest number of common peptides (16) was found among the Quesillo Caquetá samples from the northern and central regions.
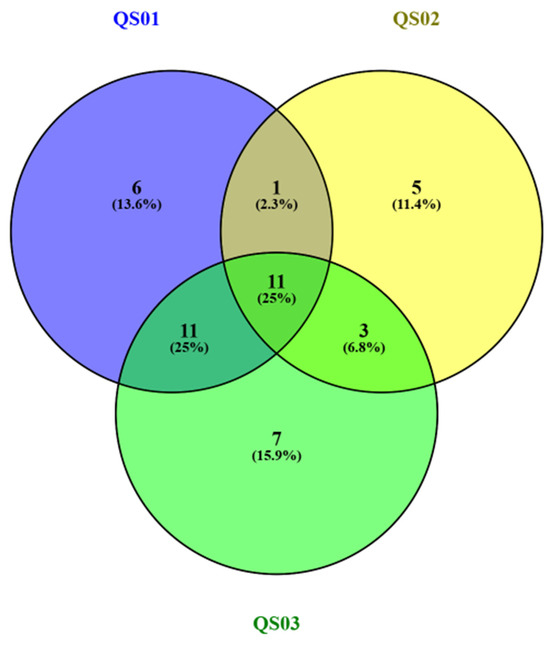
Figure 5.
Venn diagram of common and unique peptides found in Quesillo Caquetá samples from the northern, southern, and central regions. QS01: Quesillo Caquetá sample (north). QS02: Quesillo Caquetá sample (south). QS03: Quesillo Caquetá sample (central).
The profile and peptide sequences showing 45 representative peptides from αs1- and β-casein—especially YQEPVLGPVRGPFPI, EPVLGPVRGPFPI, QEPVLGPVRGPFPI, YQEPVLGPVRGPFP, PVLGPVRGPFPI, VAPFPEV, VAPFPE, APFPEVF, FPEVF, VLNENLL, and ENLLRF found in Quesillos made with milk from the northern, southern, and central regions—might be used as a characteristic pattern to identify the originality of Quesillo Caquetá, similarly to previous reports for buffalo mozzarella cheese [18] or Parmigiano Reggiano cheese with a number peptides identified and considered its ‘fingerprint’ [85].
4. Conclusions
The milk from the northern, southern, and central regions presented similar characteristics in terms of their physicochemical composition. Similarly, Quesillo Caquetá showed resemblances in its physicochemical composition, mineral content, carbohydrates, and fatty acids. Nevertheless, the peptide profile allowed us to discriminate the samples and find 11 common peptides among the Quesillo Caquetá manufactured with milk from the northern, southern, and central regions. The mineral composition exhibited a high content of sodium, calcium, and phosphorus. The fatty acid profile identified components such as oleic acid (C18:1n9c) and traces of omega-3 (α-Linolenic, C18:3n3) and omega-6 (linoleic, C18:2n6c) fatty acids. The peptide profile revealed the occurrence of peptides with previously reported antihypertensive, immunomodulatory, antioxidant, and antimicrobial activity, which would merit further studies, as well as the release of potential valuable peptides during gastrointestinal digestion. The concentrations of omega-3 and omega-6 and the presence of the peptides YQEPVLGPVRGPFPI, EPVLGPVRGPFPI, QEPVLGPVRGPFPI, YQEPVLGPVRGPFP, PVLGPVRGPFPI, VAPFPEV, VAPFPE, APFPEVF, FPEVF, VLNENLL, and ENLLRF in Quesillo Caquetá may become baseline indicators for identifying its authenticity as a cheese with a protected designation of origin against imitations in the market, thereby enhancing its protection and continued recognition as a traditional product of the region.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/dairy6050052/s1, Figure S1: Galactose, glucose, and lactose chromatograms; Table S1: Results of physicochemical analysis of milk and Quesillo Caquetá. Table S2. Pearson correlations/probability.
Author Contributions
Conceptualization, A.G.-Z., S.E. and A.H.; methodology, A.G.-Z., A.H., M.V., B.M. and I.R.; software, A.G.-Z., A.H., M.V., B.M. and I.R.; validation, A.G.-Z. and A.H.; formal analysis, A.G.-Z., S.E. and A.H.; investigation, A.G.-Z., A.H., M.V., B.M. and I.R.; resources, A.G.-Z.; data curation, S.E., A.H., M.V., B.M. and I.R.; writing—original draft preparation, A.G.-Z.; writing—review and editing, A.G.-Z., S.E., A.H., M.V., B.M. and I.R.; visualization, A.G.-Z. and A.H.; supervision, S.E., A.H., M.V., B.M. and I.R.; project administration, A.G.-Z.; funding acquisition, A.G.-Z., M.V., B.M. and I.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Ciencia y Tecnología Minciencias Colombia, grant number Bicentenario 2019, the doctoral program in engineering, “Universidad Tecnológica de Pereira y Vicerrectoría de Investigaciones, Innovación y Extensión”, “Universidad de la Amazonia y Vicerrectoría de Investigación e Innovación”, and project 202370E209 funded by CSIC.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The main author gives thanks to the following: Doctoral program in engineering, “Universidad Tecnológica de Pereira a través de la Vicerrectoría de Investigaciones, Innovación y Extensión” for the support for publication as part of the doctoral project, “Universidad de la Amazonia, Vicerrectoría de Investigación e Innovación y Centro de Investigaciones Amazónicas CIMAZ-MACAGUAL”, “Comité Departamental de Ganaderos del Caquetá”, the Institute of Food Science Research CIAL (CSIC-UAM), and the analytical technique services unit of ICTAN for their support in the experimental analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ramírez-Navas, J.S.; Osorio-Londoño, M.; Rodríguez De Stouvenel, A. El Quesillo: Un Queso Colombiano de Pasta Hilada. Tecnol. Láctea Latinoam. 2010, 60, 63–67. [Google Scholar]
- Ramírez-Navas, J.S.; Rodríguez-Stouvenel, A. Characterization of Colombian Quesillo Cheese by Spectrocolorimetry. Vitae—Food Sci. Technol. Eng. 2012, 19, 178–185. [Google Scholar] [CrossRef]
- Torrijos Rivera, R. Cifras de Contexto Ganadero Caquetá 2024; Ed. Comité Departamental de Ganaderos del Caquetá: Florencia, Caquetá, Colombia, 2024; Available online: https://udlaedu-my.sharepoint.com/:b:/g/personal/and_grajales_udla_edu_co/EUmRgUGCy9NJltWjT5Zoa88B_y4ONWY4C9Pf7EpcJSrDPQ?e=y4KYKM (accessed on 25 August 2025).
- Colombian Ministry of Agriculture and Rural Development. Precios Derivados En Planta Resolucion 017-2002, Colombian Ministry of Agriculture and Rural Development: Bogotá, Colombia, 2024.
- Dias, C.; Mendes, L. Protected Designation of Origin (PDO), Protected Geographical Indication (PGI) and Traditional Speciality Guaranteed (TSG): A Bibiliometric Analysis. Food Res. Int. 2018, 103, 492–508. [Google Scholar] [CrossRef]
- Cardin, M.; Cardazzo, B.; Mounier, J.; Novelli, E.; Coton, M.; Coton, E. Authenticity and Typicity of Traditional Cheeses: A Review on Geographical Origin Authentication Methods. Foods 2022, 11, 3379. [Google Scholar] [CrossRef]
- Bouroutzika, E.; Proikakis, S.; Anagnostopoulos, A.K.; Katsafadou, A.I.; Fthenakis, G.C.; Tsangaris, G.T. Proteomics Analysis in Dairy Products: Cheese, a Review. Appl. Sci. 2021, 11, 7622. [Google Scholar] [CrossRef]
- Valletta, M.; Ragucci, S.; Landi, N.; Di Maro, A.; Pedone, P.V.; Russo, R.; Chambery, A. Mass Spectrometry-Based Protein and Peptide Profiling for Food Frauds, Traceability and Authenticity Assessment. Food Chem. 2021, 365, 130456. [Google Scholar] [CrossRef] [PubMed]
- Białek, A.; Białek, M.; Lepionka, T.; Czerwonka, M.; Czauderna, M. Chemometric Analysis of Fatty Acids Profile of Ripening Chesses. Molecules 2020, 25, 1814. [Google Scholar] [CrossRef] [PubMed]
- Manuelian, C.L.; Currò, S.; Penasa, M.; Cassandro, M.; De Marchi, M. Characterization of Major and Trace Minerals, Fatty Acid Composition, and Cholesterol Content of Protected Designation of Origin Cheeses. J. Dairy Sci. 2017, 100, 3384–3395. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsiplakou, E.; Pappa, E.C.; Pappas, A.C.; Mavrommatis, A.; Sotirakoglou, K.; Georgiou, C.A.; Zervas, G. Fatty Acid Profile and Physicochemical Properties of Greek Protected Designation of Origin Cheeses, Implications for Authentication. Eur. Food Res. Technol. 2020, 246, 1741–1753. [Google Scholar] [CrossRef]
- Biancolillo, A.; Reale, S.; Foschi, M.; Bertini, E.; Antonelli, L.; D’Archivio, A.A. Characterization and Authentication of “Ricotta” Whey Cheeses through GC-FID Analysis of Fatty Acid Profile and Chemometrics. Molecules 2022, 27, 7401. [Google Scholar] [CrossRef]
- Reis Lima, M.J.; Bahri, H.; Sá Morais, J.; Veloso, A.C.A.; Fontes, L.; Lemos, E.T.; Peres, A.M. Assessing Serra Da Estrela PDO Cheeses’ Origin-Production Date Using Fatty Acids Profiles. J. Food Meas. Charact. 2019, 13, 2988–2997. [Google Scholar] [CrossRef]
- Rocchetti, G.; Michelini, S.; Pizzamiglio, V.; Masoero, F.; Lucini, L. A Combined Metabolomics and Peptidomics Approach to Discriminate Anomalous Rind Inclusion Levels in Parmigiano Reggiano PDO Grated Hard Cheese from Different Ripening Stages. Food Res. Int. 2021, 149, 110654. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, J.S.; Barros, M.; Fernandes, P.; Pires, P.; Bardsley, R. Principal Component Analysis of Proteolytic Profiles as Markers of Authenticity of PDO Cheeses. Food Chem. 2013, 136, 1526–1532. [Google Scholar] [CrossRef]
- Fontenele, M.A.; Bastos, M.d.S.R.; dos Santos, K.M.O.; Bemquerer, M.P.; do Egito, A.S. Peptide Profile of Coalho Cheese: A Contribution for Protected Designation of Origin (PDO). Food Chem. 2017, 219, 382–390. [Google Scholar] [CrossRef]
- Creydt, M.; Fischer, M. Omics Approaches for Food Authentication. Electrophoresis 2018, 39, 1569–1581. [Google Scholar] [CrossRef]
- Gonçalves, B.H.R.F.; Silva, G.D.J.; Pontes, S.F.O.; Fontan, R.D.C.I.; Egito, A.S.D.; Ferrão, S.P.B. Evaluation of the Peptide Profile with a View to Authenticating Buffalo Mozzarella Cheese. Int. J. Food Sci. Technol. 2016, 51, 1586–1593. [Google Scholar] [CrossRef]
- Ministerio de Salud de Colombia Resolución 1804 de 1989. Características Productos Lácteos, Quesos. Ministerio de Salud de Colombia: Bogotá, Colombia. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/Resolucion-1804-de-1989.pdf (accessed on 27 August 2025).
- ISO 9868-1; Milk and Milk Products—Determination of Nitrogen Content. Part 1: Kjeldahl Principle and Crude Protein Calculation. The International Organization for Standardization: Geneva, Switzerland, 2014.
- AOAC 933.05; Gerber Method for Fat Determination in Milk. Association of Official Analytical Chemists: Washington, DC, USA, 2019.
- AOAC 926.08; Method for Moisture in Cheese. Association of Official Analytical Chemists: Washington, DC, USA, 2019.
- ISO 5545; Rennet Caseins and Caseinates—Determination of Ash. The International Organization for Standardization: Geneva, Switzerland, 2014.
- AOAC 920.39; Method for Determining the Fat Crude or Ether Extract. Association of Official Analytical Chemists: Washington, DC, USA, 2019.
- Alvarez, M.D.; Fuentes, R.; Guerrero, G.; Canet, W. Characterization of Commercial Spanish Hummus Formulation: Nutritional Composition, Rheology, and Structure. Int. J. Food Prop. 2017, 20, 845–863. [Google Scholar] [CrossRef]
- Corzo, N.; Villamiel, M.; Arias, M.; Jiménez-Perez, S.; Morales, F.J. The Maillard Reaction during the Ripening of Manchego Cheese. Food Chem. 2000, 71, 255–258. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Tweed, J.K.S.; Kim, E.J.; Scollan, N.D. Beef, Chicken and Lamb Fatty Acid Analysis—A Simplified Direct Bimethylation Procedure Using Freeze-Dried Material. Meat Sci. 2012, 92, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ruiz, J.Á.; Ramos, M.; Recio, I. Angiotensin-Converting Enzyme-Inhibitory Peptides in Manchego Cheeses Manufactured with Different Starter Cultures. Int. Dairy J. 2002, 12, 697–706. [Google Scholar] [CrossRef]
- Miralles, B.; del Barrio, R.; Cueva, C.; Recio, I.; Amigo, L. Dynamic Gastric Digestion of a Commercial Whey Protein Concentrate. J. Sci. Food Agric. 2018, 98, 1873–1879. [Google Scholar] [CrossRef]
- Manguy, J.; Jehl, P.; Dillon, E.T.; Davey, N.E.; Shields, D.C.; Holton, T.A. Peptigram: A Web-Based Application for Peptidomics Data Visualization. J. Proteome Res. 2017, 16, 712–719. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk Bioactive Peptide Database: A Comprehensive Database of Milk Protein-Derived Bioactive Peptides and Novel Visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, J.C. Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 22 June 2025).
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, C.W. InfoStat Software Estadístico; Universidad Nacional de Córdoba, Argentina. 2020. Available online: https://www.infostat.com.ar/index.php?mod=page%26id=34 (accessed on 25 August 2025).
- Qin, N.; Faludi, G.; Beauclercq, S.; Pitt, J.; Desnica, N.; Pétursdóttir, Á.; Newton, E.E.; Angelidis, A.; Givens, I.; Juniper, D.; et al. Macromineral and Trace Element Concentrations and Their Seasonal Variation in Milk from Organic and Conventional Dairy Herds. Food Chem. 2021, 359, 129865. [Google Scholar] [CrossRef] [PubMed]
- Stocco, G.; Summer, A.; Malacarne, M.; Cecchinato, A.; Bittante, G. Detailed Macro- and Micromineral Profile of Milk: Effects of Herd Productivity, Parity, and Stage of Lactation of Cows of 6 Dairy and Dual-Purpose Breeds. J. Dairy Sci. 2019, 102, 9727–9739. [Google Scholar] [CrossRef] [PubMed]
- Denholm, S.J.; Sneddon, A.A.; McNeilly, T.N.; Bashir, S.; Mitchell, M.C.; Wall, E. Phenotypic and Genetic Analysis of Milk and Serum Element Concentrations in Dairy Cows. J. Dairy Sci. 2019, 102, 11180–11192. [Google Scholar] [CrossRef]
- Londoño-Ospina, M. Aprovechamiento Del Suero Ácido de Queso Doble Crema Para La Elaboración de Quesillo Utilizando Tres Métodos de Complementación de Acidez Con Tres Ácidos Orgánicos. Perspect. En Nutr. Humana 2006, 16, 11–20. [Google Scholar] [CrossRef]
- Pulido, R.; Pinzón, D.M.; Tarazona Díaz, M.P. Nutritional, Microbiological and Sensorial Characterization of Fresh Cheese. Nutr. Clin. Diet. Hosp. 2018, 38, 74–79. [Google Scholar] [CrossRef]
- Sandoval-Copado, J.; Orozco-Villafuerte, J.; Pedrero-Fuehrer, D.; Colín-Cruz, M.A. Sensory Profile Development of Oaxaca Cheese and Relationship with Physicochemical Parameters. J. Dairy Sci. 2016, 99, 7075–7084. [Google Scholar] [CrossRef]
- Arteaga-Márquez, M.R.; Hernández-Hernández, H.L.; Peñate-Quiroz, C.D. Elaboration of a Processed Cheese Spread Obtained from Costeño Cheese. Inf. Tecnol. 2020, 31, 187–194. [Google Scholar] [CrossRef]
- Ochoa-Flores, A.A.; Hernández-Becerra, J.A.; Velázquez-Martínez, J.R.; Piña-Gutiérrez, J.M.; Hernández-Castellano, L.E.; Toro-Mujica, P.; Chay-Canul, A.J.; Vargas-Bello-Pérez, E. Chemical and Fatty Acid Composition of Manchego Type and Panela Cheeses Manufactured from Either Hair Sheep Milk or Cow Milk. J. Dairy Sci. 2021, 104, 7457–7465. [Google Scholar] [CrossRef]
- De Oliveira, I.L.S.; do Nascimento Rangel, A.H.; Madruga, R.C.; de Lima Júnior, D.M.; da Silva Gomes, R.D.; Sales, D.C.; De Oliveira, J.P.F.; da Silva Bezerra, J. Physicochemical Composition, Yield and Sensory Acceptance of Coalho Cheese Obtained from Zebu’s Cow Milk. Rev. Mex. Cienc. Pecu. 2021, 12, 337–352. [Google Scholar] [CrossRef]
- Jana, A.H.; Mandal, P.K. Manufacturying and Quality of Mozzarella Cheese: A Review. Int. J. Dairy Sci. 2011, 6, 199–226. [Google Scholar] [CrossRef]
- Nogueira Silva, N.F.; Siqueira De Aguiar, K.; Pimentel Filho, N.D.J.; De Paula Ferreira, I.E.; Lanzoni Troiani, C.A.; Artigiani Lima Tribst, A.; Fernandes De Carvalho, A. Milk Quality, Production Process and Physicochemical Characteristics of Porungo, an Artisanal Cheese from the State of Sao Paulo, Brazil. J. Dairy Res. 2020, 87, 480–483. [Google Scholar] [CrossRef]
- Satric, A.; Miloradovic, Z.; Mirkovic, M.; Mirkovic, N.; Miocinovic, J. Quality Characteristics of ‘Pasta-Filata’ Serbian Kačkavalj Cheese and Regulatory Compliance Assessment. Mljekarstvo 2023, 73, 38–49. [Google Scholar] [CrossRef]
- Cortes Macias, E.T.; Peña Gomez, N.; Amorocho Cruz, C.M.; Gutierrez Guzman, N. Evolución de Parámetros Fisicoquímicos de Quesillo Huilense En Almacenamiento Refrigerado. Biotecnol. Sect. Agropecu. Agroind. 2016, 14, 110. [Google Scholar] [CrossRef]
- Smith, J.R.; Carr, A.J.; Golding, M.; Reid, D. Mozzarella Cheese—A Review of the Structural Development During Processing. Food Biophys. 2018, 13, 1–10. [Google Scholar] [CrossRef]
- Ramírez-López, C.; Vélez-Ruiz, J.F. Quesos Frescos: Propiedades, Métodos de Determinación y Factores Que Afectan Su Calidad. Temas Sel. Ing. Aliment. 2012, 6, 131–148. [Google Scholar]
- Bähler, B.; Kunz, A.; Hinrichs, J. Hot Brining of Pasta Filata Cheese: Effect of Sodium and Calcium Chloride on Composition, Yield, and Hardness. Dairy Sci. Technol. 2016, 96, 703–714. [Google Scholar] [CrossRef]
- Superintendencia de Industria y Comercio. Resolución 0068463 de 2011 Denominación de Orígen Queso Caquetá; Superintendencia de Industria y Comercio: Bogotá, Colombia, 2011. [Google Scholar]
- Amenu, B.; Deeth, H. The Impact of Milk Composition on Cheddar Cheese Manufacture. Artic. Aust. J. Dairy Technol. 2007, 62, 171–184. [Google Scholar]
- Amalfitano, N.; Patel, N.; Haddi, M.-L.; Benabid, H.; Pazzola, M.; Vacca, G.M.; Tagliapietra, F.; Schiavon, S.; Bittante, G. Detailed Mineral Profile of Milk, Whey, and Cheese from Cows, Buffaloes, Goats, Ewes and Dromedary Camels, and Efficiency of Recovery of Minerals in Their Cheese. J. Dairy Sci. 2024, 107, 8887–8907. [Google Scholar] [CrossRef] [PubMed]
- Manuelian, C.L.; Pozza, M.; Franzoi, M.; Righi, F.; Schmutz, U.; De Marchi, M. Comparison of Chemical Composition of Organic and Conventional Italian Cheeses from Parallel Production. J. Dairy Sci. 2023, 106, 6646–6654. [Google Scholar] [CrossRef]
- Smith, S.; Smith, T.J.; Drake, M.A. Short Communication: Flavor and Flavor Stability of Cheese, Rennet, and Acid Wheys. J. Dairy Sci. 2016, 99, 3434–3444. [Google Scholar] [CrossRef]
- Fox, P.F.; Guineee, T.P.; Cogann, T.M.; Mcsweeney, P.L.H. Overview of Cheese Manufacture. In Fundamentals of Cheese Science Second Edition; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Wu, Q.; Cheung, C.K.W.; Shah, N.P. Towards Galactose Accumulation in Dairy Foods Fermented by Conventional Starter Cultures: Challenges and Strategies. Trends Food Sci. Technol. 2015, 41, 24–36. [Google Scholar] [CrossRef]
- Cebeci, A.; Yalcin, B.; Esra Gunes, F.; Yaman, M. Determination of Carbohydrate Amounts of Various Cheese Types Presented to Sale in the Market. Int. J. Food Sci. Nutr. 2020, 5, 30–35. [Google Scholar]
- Ahmed, M.E.; Hammam, A.R.A.; Ali, A.E.F.; Alsaleem, K.A.; Elfaruk, M.S.; Kamel, D.G.; Moneeb, A.H.M. Measurement of Carbohydrates and Organic Acids in Varieties of Cheese Using High-Performance Liquid Chromatography. Food Sci. Nutr. 2023, 11, 2081–2085. [Google Scholar] [CrossRef]
- McCarthy, C.M.; Wilkinson, M.G.; Kelly, P.M.; Guinee, T.P. Effect of Salt and Fat Reduction on the Composition, Lactose Metabolism, Water Activity and Microbiology of Cheddar Cheese. Dairy Sci. Technol. 2015, 95, 587–611. [Google Scholar] [CrossRef]
- Vénica, C.I.; Wolf, V.I.; Bergamini, C.V.; Perotti, M.C. Effect of the Incorporation of β-Galactosidase in the GOS Production during Manufacture of Soft Cheese. Food Res. Int. 2020, 137, 109654. [Google Scholar] [CrossRef]
- Portnoi, P.A.; MacDonald, A. The Lactose and Galactose Content of Cheese Suitable for Galactosaemia: New Analysis. In JIMD Reports; Springer: Berlin/Heidelberg, Germany, 2016; Volume 29, pp. 85–87. [Google Scholar]
- Hanus, O.; Samkova, E.; Křížova, L.; Hasoňova, L.; Kala, R. Role of Fatty Acids in Milk Fat and the Influence of Selected Factors on Their Variability—A Review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Khalifa, S.A.; Gan, R.Y.; Shah, N.; Ayyash, M. Fatty Acids, Lipid Quality Parameters, and Amino Acid Profiles of Unripened and Ripened Cheeses Produced from Different Milk Sources. J. Food Compos. Anal. 2023, 123, 105588. [Google Scholar] [CrossRef]
- Renes, E.; Gómez-Cortés, P.; de la Fuente, M.A.; Linares, D.M.; Tornadijo, M.E.; Fresno, J.M. CLA-Producing Adjunct Cultures Improve the Nutritional Value of Sheep Cheese Fat. Food Res. Int. 2019, 116, 819–826. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Juárez, M.; de la Fuente, M.A. Milk Fatty Acids and Potential Health Benefits: An Updated Vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar] [CrossRef]
- Metzger, L.E.; Barbano, D.M.; Rudan, M.A.; Kindstedt, P.S.; Guo, M.R. Whiteness Change during Heating and Cooling of Mozzarella Cheese. J. Dairy Sci. 2000, 83, 1–10. [Google Scholar] [CrossRef]
- Escobedo-Avellaneda, Z.; Espiricueta-Candelaria, R.S.; Calvo-Segura, S.; Welti-Chanes, J.; Chuck-Hernández, C. Changes Induced by High Hydrostatic Pressure in Acidified and Non-Acidified Milk during Oaxaca Cheese Production. Int. J. Food Sci. Technol. 2021, 56, 4639–4649. [Google Scholar] [CrossRef]
- Silva, R.A.; Bezerra, V.S.; Pimentel, M.D.C.B.; Porto, A.L.F.; Cavalcanti, M.T.H.; Filho, J.L.L. Proteomic and Peptidomic Profiling of Brazilian Artisanal “Coalho” Cheese. J. Sci. Food Agric. 2016, 96, 4337–4344. [Google Scholar] [CrossRef] [PubMed]
- Magenis, R.B.; Prudêncio, E.S.; Molognoni, L.; Daguer, H. A Control Method to Inspect the Compositional Authenticity of Minas Frescal Cheese by Gel Electrophoresis. J. Agric. Food Chem. 2014, 62, 8333–8339. [Google Scholar] [CrossRef]
- Özcan Yardım, D.; Durak, M.Z. Identification of Antihypertensive Bioactive Peptides in the Herby and White Cheeses Produced from Different Milk Types. Eur. Food Res. Technol. 2023, 249, 2265–2272. [Google Scholar] [CrossRef]
- Öztürk, H.İ.; Konak Göktepe, Ç.; Akın, N. Proteolysis Pattern and Functional Peptides in Artisanal Tulum Cheeses Produced from Mut Province in Turkey. LWT 2021, 149, 111642. [Google Scholar] [CrossRef]
- Miralles, B. Detección de Caseinato y Suero En Leche y Productos Lácteos Mediante Técnicas Electroforéticas, Cromatográficas y Espectroscópicas; Universidad Complutense de Madrid: Madrid, Spain, 2001; ISBN 8466920145. [Google Scholar]
- Barac, M.; Pesic, M.; Zilic, S.; Smiljanic, M.; Ignjatovic, I.S.; Vucic, T.; Kostic, A.; Milincic, D. The Influence of Milk Type on the Proteolysis and Antioxidant Capacity of White-Brined Cheese Manufactured from High-Heat-Treated Milk Pretreated with Chymosin. Foods 2019, 8, 128. [Google Scholar] [CrossRef]
- Cruz-Huerta, E.; García-Nebot, M.J.; Miralles, B.; Recio, I.; Amigo, L. Caseinophosphopeptides Released after Tryptic Hydrolysis versus Simulated Gastrointestinal Digestion of a Casein-Derived by-Product. Food Chem. 2015, 168, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Mirzapour-Kouhdasht, A.; Garcia-Vaquero, M. Cardioprotective Peptides from Milk Processing and Dairy Products: From Bioactivity to Final Products Including Commercialization and Legislation. Foods 2022, 11, 1270. [Google Scholar] [CrossRef]
- Atanasova, J.; Dalgalarrondo, M.; Iliev, I.; Moncheva, P.; Todorov, S.D.; Ivanova, I.V. Formation of Free Amino Acids and Bioactive Peptides During the Ripening of Bulgarian White Brined Cheeses. Probiotics Antimicrob. Proteins 2021, 13, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Norberg, S.; O’Connor, P.M.; Stanton, C.; Ross, R.P.; Hill, C.; Fitzgerald, G.F.; Cotter, P.D. Altering the Composition of Caseicins A and B as a Means of Determining the Contribution of Specific Residues to Antimicrobial Activity. Appl. Environ. Microbiol. 2011, 77, 2496–2501. [Google Scholar] [CrossRef]
- Jiang, X.; Pan, D.; Zhang, T.; Liu, C.; Zhang, J.; Su, M.; Wu, Z.; Zeng, X.; Sun, Y.; Guo, Y. Novel Milk Casein–Derived Peptides Decrease Cholesterol Micellar Solubility and Cholesterol Intestinal Absorption in Caco-2 Cells. J. Dairy Sci. 2020, 103, 3924–3936. [Google Scholar] [CrossRef]
- Quirós, A.; Hernández-Ledesma, B.; Ramos, M.; Amigo, L.; Recio, I. Angiotensin-Converting Enzyme Inhibitory Activity of Peptides Derived from Caprine Kefir. J. Dairy Sci. 2005, 88, 3480–3487. [Google Scholar] [CrossRef] [PubMed]
- Birkemo, G.A.; O’Sullivan, O.; Ross, R.P.; Hill, C. Antimicrobial Activity of Two Peptides Casecidin 15 and 17, Found Naturally in Bovine Colostrum. J. Appl. Microbiol. 2009, 106, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Ferranti, P.; Smacchi, E.; Goffredi, F.; Addeo, F. Production of Angiotensin-I-Converting-Enzyme-Inhibitory Peptides in Fermented Milks Started by Lactobacillus delbrueckii Subsp. Bulgaricus SS1 and Lactococcus lactis Subsp. Cremoris FT4. Appl. Environ. Microbiol. 2000, 66, 3898–3904. [Google Scholar] [CrossRef]
- Coste, M.; Rochet, V.; Léonil, J.; Mollé, D.; Bouhallab, S.; Tomé, D. Identification of C-Terminal Peptides of Bovine β-Casein That Enhance Proliferation of Rat Lymphocytes. Immunol. Lett. 1992, 33, 41–46. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, M.; Zhao, B.; Yang, F. Isolation of Antioxidant Peptides from Yak Casein Hydrolysate. RSC Adv. 2020, 10, 19844–19851. [Google Scholar] [CrossRef]
- Sanchón, J.; Fernández-Tomé, S.; Miralles, B.; Hernández-Ledesma, B.; Tomé, D.; Gaudichon, C.; Recio, I. Protein Degradation and Peptide Release from Milk Proteins in Human Jejunum. Comparison with in Vitro Gastrointestinal Simulation. Food Chem. 2018, 239, 486–494. [Google Scholar] [CrossRef]
- Sforza, S.; Cavatorta, V.; Lambertini, F.; Galaverna, G.; Dossena, A.; Marchelli, R. Cheese Peptidomics: A Detailed Study on the Evolution of the Oligopeptide Fraction in Parmigiano-Reggiano Cheese from Curd to 24 Months of Aging. J. Dairy Sci. 2012, 95, 3514–3526. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).