Abstract
Total Mixed Ration (TMR) particle size significantly impacts dairy cow health and productivity. This study investigated the effects of TMR particle size tertiles on rumen pH, dry matter intake (DMI), and milk characteristics in Simmental cows by continuous pH monitoring (Moonsyst Ltd., Kilkenny, Republic of Ireland) and particle separation by 19, 8, 4 mm sieves and pad using the Wasserbauer particle separator, along with regular milk and DMI measurements. Data were analyzed by IBM SPSS 26.0 with ANOVA, Pearson correlations and statistically significant differences between tertiles by post hoc Tukey HSD test were performed (p < 0.05). Tertiles by frequency analysis were used to categorize particle size proportions into three groups, each containing an equal number of observations. Principal component analysis (PCA) and heatmaps by SRplot were generated. Moderate particle size distributions (second tertiles of 19 mm, 8 mm, 4 mm sieves, and pad as the fraction of TMR particles that pass through the all sieves and are collected in the bottom pan) optimized rumen pH stability, reducing time below 6.2 (SARA risk) or above 6.8, and correlated with milk β-hydroxybutyrate (BHB), oleic acid, and acetone levels. Moreover, milk production was maximized with a combination of coarser (19 mm and 8 mm, third tertiles) and finer (4 mm, first tertile) particles, milk fat peaked in both the finest pad fraction (third tertile) and coarsest larger sieves (first tertiles), and milk protein in the first tertiles of 19 mm and 8 mm sieves. Similarly, DMI positively correlated with coarser particles, but sometimes negatively with milk quality. In addition, PCA showed fine particle groups clustering with higher milk fat-to-protein ratios, somatic cell counts, and urea. In conclusion, mid-range TMR particle sizes (second tertiles) consistently provided the most benefits across ruminal, metabolic, and production parameters, underscoring TMR structure as a crucial precision feeding tool.
1. Introduction
Dairy farming holds global significance as it plays a crucial role in food security, providing essential nutrients through products like milk, cheese, and yogurt. It supports economic growth by creating jobs in farming, processing, and distribution sectors. Additionally, dairy production contributes to the livelihoods of millions of farmers worldwide [1].
Total Mixed Ration (TMR) feeding offers several advantages that contribute to improved animal health, performance, and nutrient utilization. Firstly, one of the primary benefits is consistent nutrient delivery, as TMR ensures animals receive a balanced and homogeneous diet. This uniformity prevents nutrient deficiencies or excesses that may occur with separate feeding of forages and concentrates [2,3]. Secondly, TMR reduces feed sorting behavior, which is common in non-TMR systems where animals selectively consume more palatable components. By mixing all dietary ingredients, TMR minimizes selective feeding and ensures animals consume the formulated ration as intended [2]. Furthermore, research has shown that TMR enhances nutrient digestibility by increasing crude protein availability and improving overall absorption and utilization [3]. In addition, another major advantage is improved rumen stability, as TMR provides a steady flow of nutrients that support optimal rumen fermentation [4,5]. Consequently, this stability contributes to healthier microbial populations, more efficient digestion, and reduced production of metabolites such as ammonia and methane [6,7]. Additionally, a better understanding of the influence of forage-to-concentrate ratio (F:C), forage particle length (FPL), and their interactions on the intake of physically effective fiber (peNDF), ruminal environment, and digestive efficiency is crucial for optimizing dairy cow nutrition [8,9]. The peNDF content in the diet is primarily influenced by these variables, which impact chewing activity and the stabilization of ruminal [9,10]. These variations are associated with enhanced milk fat concentration [11] and elevated milk protein yield [12]. Mathematical models predict chewing time and ruminal pH based on dietary characteristics, thereby informing recommendations for minimum forage NDF levels in total mixed rations (TMR) with different particle sizes, particularly as evaluated using the Penn State Particle Separator (PSPS) with 8 mm and 19 mm sieves [8,9,13,14].
To optimize peNDF in TMR, rations should be formulated using a variety of fiber sources, including high-quality forages and fibrous by-products, to meet both structural and nutritional requirements [15]. The use of feed additives like enzymes or essential oils may enhance fiber digestibility, allowing for slight reductions in peNDF levels without negatively affecting rumen health [16]. Finally, routine monitoring of TMR composition alongside animal performance indicators enables timely adjustments to peNDF content, ensuring optimal rumen function and sustained milk production [17].
Physically effective neutral detergent fiber (peNDF) plays a crucial role in maintaining rumen health and optimizing dairy cow performance. peNDF stimulates chewing and saliva production, which helps buffer rumen pH and prevent acidosis—an increasingly common challenge in high-yield dairy systems [17]. Adequate levels of peNDF in total mixed rations (TMR) support proper rumen function by promoting the digestion of fiber and the production of volatile fatty acids (VFAs), which are a key energy source for dairy cows [16]. Additionally, peNDF helps maintain the physical structure of the rumen mat, aiding in the separation of digesta and enhancing microbial fermentation efficiency [15].
Balanced peNDF levels in the diet not only support rumen stability but also have a direct impact on milk production and quality. Optimizing rumen fermentation through appropriate peNDF levels leads to better nutrient absorption, which translates into improved milk yield [17]. Research indicates that sufficient fiber digestion, promoted by peNDF, is linked to increased milk fat content due to the production of fat precursors during fermentation [16]. Furthermore, maintaining adequate peNDF reduces the risk of metabolic disorders, such as subacute ruminal acidosis (SARA), which can impair both milk production and overall cow health [18].
Particle size distribution (PSD) plays a vital role in the efficiency of rumen fermentation and overall dairy cow performance. The Penn State Particle Separator (PSPS) is commonly used to evaluate the proportion of long, medium, and short particles in total mixed rations (TMR), which directly affects how well the rumen processes feed [2]. Proper PSD ensures optimal rumen function by supporting the physical structure necessary for microbial fermentation and nutrient digestion.
However, while PSD analysis is essential, it should be integrated into a broader nutritional and management strategy. Innovations such as membrane processing technologies in the dairy industry can further improve nutrient recovery and reduce waste, contributing to sustainability [19]. Moreover, attention to dietary supplements, including vitamins and minerals, is especially important during high-demand periods like the transition phase, where overall cow health and productivity are at greater risk [20].
The relationship between physically effective neutral detergent fiber (peNDF) and total mixed ration (TMR) particle size is fundamental to maintaining optimal rumen health and dairy cow performance. Adequate particle size within TMR systems is essential for stimulating the chewing activity necessary to promote saliva production and maintain proper rumen pH buffering, as reduced particle size compromises fiber effectiveness regardless of chemical composition [21,22]. Research demonstrates that when alfalfa particle size is reduced below critical thresholds, significant decreases in chewing activity and digestibility occur, directly impacting cow performance and rumen function [23]. The practical challenge lies in balancing adequate particle size to ensure effective fiber function while maintaining TMR mixing consistency and palatability, as the cumulative effects of all dietary components determine overall ration effectiveness [24]. Successful TMR management requires careful attention to particle size distribution to optimize both the physical and nutritional benefits of dietary fiber in dairy cattle nutrition to prevent metabolic disorders.
SARA is a prevalent health and production issue in ruminants, particularly dairy cattle. It results in substantial economic losses due to decreased dry matter intake and milk yield, as well as reduced overall profitability [25]. This also increases culling and mortality rates. European field studies estimate SARA incidence in cows to be between 11% and 19% during early lactation and between 18% and 20% during mid-lactation or peak dietary dry matter intake [26]. SARA is associated with several adverse health outcomes, including damage to the ruminal epithelium and reduced efficiency of fiber degradation [27]. Typical clinical signs include diarrhea, suboptimal body condition, weight loss, and reduced dry matter intake and milk production [28]. Research indicates that diets containing 50% to 65% grain can induce subacute ruminal acidosis in ruminants [29]. Dietary fiber content and structure significantly contribute to SARA development [30]. Even with adequate quantitative fiber levels, overly long or unpalatable particles can cause selective feeding, leading to insufficient consumption of physically effective fiber [31]. In healthy cows, the ruminal microbiota ferments starch into volatile fatty acids, glucose, and lactic acid [32]. These fermentation products are rapidly absorbed into the bloodstream via the ruminal papillae, serving as metabolic precursors for milk synthesis. However, in acidic ruminal conditions, the accumulation of volatile fatty acids and lactic acid leads to a decrease in ruminal pH, contributing to acidosis onset [33]. Increasing the proportion of concentrate feed in dairy cow rations leads to a higher abundance of Gram-positive bacteria, characterized by a relatively thick peptidoglycan layer. Diets rich in fast fermentable nutrients, such as certain TMR formulations, are associated with a significant increase in volatile fatty acid concentrations [34]. This low pH environment inhibits the growth of cellulolytic bacteria and protozoa, while favoring amylolytic bacteria [35], increasing propionate production and decreasing acetate production [26]. The borderline pH value for SARA onset is less than 6.2 [36]. While acute acidosis typically leads to more severe milk yield depression, SARA is characterized by prolonged periods of reduced ruminal pH, which can also decrease milk production. SARA can lower milk fat content [27,37] and alter milk fatty acid composition, decreasing unsaturated fatty acids and increasing saturated fatty acids [38]. This can negatively affect the nutritional quality of the milk. While some studies suggest SARA herds may have higher milk yield with lower fat concentrations and no evident symptoms of ruminal disorder or systemic inflammation [37], it is important to consider the broader implications of SARA on overall herd health and milk quality.
Ketosis represents the most common metabolic disorder in high-producing dairy cows within the initial 6–8 weeks of lactation. The condition is primarily characterized by an excessive accumulation of ketone bodies in the bodily fluids of affected cows [39]. Determining the prevalence of subclinical ketosis (SCK) is valuable for routine monitoring and assessing the health and performance of transition cows. Early detection through on-site blood testing, followed by appropriate treatment and management, can help minimize the negative effects of SCK [40]. Ketone bodies consist of β-hydroxybutyric acid (βHBA), acetoacetic acid, and acetone, with βHBA representing the majority, approximately 70%, of the total ketone body concentration in the blood [41,42].
Ketosis in dairy cows is characterized by clinical signs such as decreased appetite, a notable preference for forage compared to concentrate feeds, and a distinct acetone odor in both breath and urine. These symptoms are generally associated with reduced milk production, an increased occurrence of concurrent diseases (including mastitis, metritis, and displaced abomasum), and diminished reproductive performance [43,44]. Ketosis is characterized by an increased milk fat concentration exceeding 5%, whereas the milk protein content typically declines to below 2.9% [45]. Subclinical ketosis (SCK) is characterized by an increased fat-to-protein ratio in milk, usually surpassing 1.4:1 [46]. The prevention of ketosis depends on sustaining cows in optimal body condition during the periparturient period, ideally achieving a score of approximately 3.5 on a five-point body condition scale [47,48]. Additionally, it is crucial to meticulously balance the energy-to-protein ratio in the diet during the initial two months of lactation.
The main goal of this study is to examine the effects of TMR particle size tertiles on rumen pH, dry matter intake (DMI), and milk characteristics in Simmental cows by continuous pH monitoring and particle separation.
2. Materials and Methods
2.1. Experimental Site and Animal Management
The experiment was conducted in collaboration with PD Kozárovce, an agricultural cooperative located in the Tekov region of western Slovakia (District of Levice), situated on the right bank of the Hron River at an altitude of 185 m above sea level (GPS coordinates: 48.31501313969128, 18.509875019112915).
Since 1999, the Kozárovce farm has held the status of a breeding farm for Slovak Simmental cattle, a significant dual-purpose breed with a long-standing tradition in Slovakia. The breed is valued for its balanced performance in milk and meat production and is classified within the Simmental cattle type. It was officially recognized as an autochthonous breed in 1958 [49,50,51]. As of 2023, the farm housed 276 lactating cows.
From the herd, 10 Slovak Simmental dairy cows were randomly selected for the study (average lactation number: 2.87 ± 0.88). Before calving, rumen boluses (Moonsyst Ltd., Kilkenny, Republic of Ireland) were administered via applicator by a veterinarian on 14 April 2023. The main observation period spanned from May to September 2023 (average days in milk: 99.03 ± 70.31). The rumen boluses recorded pH every 10 min throughout this period.
The cows were housed in a loose housing system within a large-capacity barn equipped with freestalls featuring rubber mattresses and the Zeobedding mineral additive (Zeocem, SK, Bystré, Slovak Republic). Milking was performed twice daily using a 24-stall herringbone milking parlor (AGROMilk, Komárno, Slovak Republic).
2.2. Feeding and Diet Composition
Milk yield and composition were monitored monthly by the Breeding Services of the Slovak Republic, s. e., Bratislava, Slovak Republic, as part of the official performance recording scheme. Cows had ad libitum access to water from open surface drinkers.
Feeding was carried out once daily at 5:00 a.m., using a GILIOLI DESILMIX, Ghedi, Italy, horizontal mixer wagon with augers and a loading cutter. The total mixed ration (TMR) was continuously pushed up throughout the day, and cows had free access to feed (ad libitum). All cows were fed a single, homogenous TMR diet from a feed table (Table 1). The differences in TMR structure between samples were based on variability under consistent mixing conditions. Throughout the trial, the diet formulation and mixing techniques were consistent. Natural differences in the feed components and small, inevitable changes in the mixing process were the causes of the observed particle size variability.

Table 1.
Nutritional composition of 1 kg of TMR for dairy cows.
The TMR formulation and feed mix were developed in cooperation with Ing. Roman Mokráň (VVS SK Ltd., Bánovce nad Bebravou, Slovakia) by Milk progress, Ltd., Červenka, Czech Republic, ver. 2023. The composition of the TMR per cow per day was as follows: 24.0 kg corn silage, 12.0 kg alfalfa silage, 0.5 kg wheat straw, 5.5 kg compound feed, 6.5 kg corn cob mix (CCM), 0.5 kg molasses, 0.1 kg mineral supplement (containing 97 g/kg Ca and 72 g/kg Na), 3.0 kg added water. For the calculation of TMR nutritional content were components analyzed by standard analytical laboratory methods by Nutrivet, Ltd., Pohořelice, Czech Republic and VVS Verměřovice, Ltd., Verměřovice, Czech Republic, according to [52].
The component composition of the compound feed mixture formulated for lactating cows is presented as follows (per 100 kg of feed mix): 37.0 kg rapeseed meal (extracted), 19.0 kg soybean meal (extracted), 18.5 kg barley, 18.5 kg oats, 5.7 kg vitamin–mineral supplement (see Table 2 for detailed composition), 1.3 kg feeding salt.

Table 2.
Nutritional composition of 1 kg of vitamin-mineral supplement.
2.3. Data Collection and Measurements
2.3.1. TMR Physical Structure
The physical structure of the total mixed ration (TMR) was evaluated twice a month throughout the trial period. Representative TMR samples (n = 3) were collected immediately after feed delivery and analyzed using the Wasserbauer particle separator (Wasserbauer GmbH, Waldneukirchen, Austria), following the method described by [53]. The system consisted of three sieves with mesh sizes of 19 mm, 8 mm, and 4 mm, along with a bottom pan (pad) for the finest particles. At each sampling, the percentage distribution of particles retained on each sieve and on the pan was recorded to evaluate forage physical effectiveness and feed structure uniformity. Sieving of TMR was performed with a total of 80 horizontal shaking movements, rotating the sieve stack 90° after every 10 movements while maintaining the same forward–backward motion. These structural characteristics were monitored at 2-week intervals (Table 3).

Table 3.
Structural parameters of TMR in % during experiment.
2.3.2. Rumen pH Monitoring
Using rumen boluses administered through the esophagus into the rumen, the rumen pH and temperature were recorded continuously every 10 min (6 data points per hour, totaling 144 data points per cow per day). Rumen boluses measurements were 130 mm × 32 mm, weight 180 g. Bolus pH accuracy was ±0.2 pH, communication range of 200–500 m (Moonsyst Ltd., Kilkenny, Republic of Ireland). The communication between the boluses and central main receiver by IoT technology was realized [54]. The data were uploaded every hour to the Mooncloud and downloaded in .csv format. From this data, the following variables were calculated: RpH7a—7-day average rumen pH, measured 3 days before TMR structure analysis and 3 days after that; RpHd—rumen pH on the test day of TMR structure analysis.
2.3.3. Milk Production and Composition
Monthly measurements of milk yield and milk quality parameters were obtained in accordance with the official performance recording system of the Breeding Services of the Slovak Republic, s. e., using the Afimilk milk meter during milking in milking parlor individually for each dairy cow. Milk samples were analyzed by infrared spectrometry [55] and optical fluorescence [56] to determine the following variables: M—milk yield (kg/day), F—milk fat (g/100 g), P—milk protein (g/100 g), L—lactose (g/100 g), U—milk urea (mg/dL), OA—oleic acid (g/100 g), AC—acetone (mg/L), BHB—β-hydroxybutyrate in milk (mg/L), SC—somatic cell count (thousands/mL). From these variables, the following ratios were calculated: F/P—fat-to-protein ratio, F/S—fat-to-dry matter ratio. F/SNF—fat-to-solids non-fat ratio, F/L—fat-to-lactose ratio.
2.3.4. Dry Matter Intake
In addition, dry matter intake (DMI) was determined daily by subtracting feed refusals at the end of the feeding day from the initial feed offered, corrected for dry matter content (kg/cow/day).
2.4. Statistical Analysis
Before statistical analysis, TMR structure data measured on the date of sampling were statistically associated with individual cow performance and physiological parameters. Parameters included the average rumen pH on the TMR analysis date, the 7-day average rumen pH (3 days before and 3 days after the TMR analysis date), then individual milk production and quality data collected during the control days by the Breeding Servies of the Slovak Republic, s. e.
All statistical analyses were performed using IBM SPSS Statistics 26.0 (IBM Corp., Armonk, NY, USA).
For statistical analysis, raw ruminal pH data were processed to mitigate the effect of water intake. Observations of ruminal pH potentially influenced by drinking, identified by a ruminal temperature decrease exceeding 0.5% compared to the previous measurement, were excluded. This exclusion was realized by a custom Excel function (=IF(AND(currentRT < previousRT;currentRT < followingRT); “LOW”; “”)) to mark and subsequently remove these affected pH values. This method ensured that only temporary, localized drops in temperature associated with drinking were removed, preserving the overall ruminal pH profile for analysis.
The TMR particle size data were categorized into tertiles (three equal groups) based on the number of observations on sieves and pad, using the Frequency Cut Points function in SPSS.
Descriptive statistics (means and standard deviations) were generated for all measured parameters. The effect of TMR particle size tertiles on the various response variables was assessed using a one-way Analysis of Variance (ANOVA). Post hoc comparisons between specific tertile groups, within each sieve and the pad fractions, were subsequently conducted using Tukey’s HSD test, with statistical significance at p < 0.05.
To investigate the relationships between milk production parameters, rumen health indicators, TMR structure, and dry matter intake, Pearson correlation coefficients (r) were calculated. The statistical significance of these correlations was evaluated at a one-tailed p < 0.05 level.
Furthermore, a comprehensive Principal Component Analysis (PCA) was performed to explore the underlying structure and primary sources of variation within the multivariate dataset. To visually represent the relationships between variables and TMR tertiles, a heatmap with hierarchical clustering was generated using SRplot software, ver. 2023 [57]. This visual approach aided in identifying patterns and groupings among the diverse parameters in relation to feed physical characteristics. PCA was used to reduce the dimensionality of the dataset for the identification of the main sources of variation in the data and visualize the relationships among variables and TMR groups. The PCA was performed on a correlation matrix of all measured variables. The correlation relationships among variables that are overlaid in the PCA biplot are an inherent feature of this visualization generated directly by SRplot software, ver. 2023 [57].
3. Results and Discussion
3.1. Associations Between TMR Structure and Metabolic Indicators
The impact of total mixed ration (TMR) structure on milk composition and metabolic parameters is summarized in Table 4. The concentration of oleic acid (cis-9 C18:1) was statistically highest in the second tertile of the 4 mm sieve and the first tertiles of the 19 mm and 8 mm sieves. In contrast, the significantly lowest concentrations of oleic acid were observed in the third tertile of the 19 mm, 8 mm, and their combined sieve fractions.

Table 4.
Milk Production, Quality, and Metabolite Responses to TMR Particle Size Tertiles.
In the present study, the recalculated oleic acid thresholds indicative of NEB ranged from 0.78 to 0.90 g/100 g milk fat, suggesting good metabolic status under the experimental conditions. According to [58], the composition of milk fatty acids—especially long-chain fatty acids like oleic acid—can reflect the energy status of dairy cows, particularly during early lactation. Elevated levels of oleic acid in milk have been associated with metabolic stress and negative energy balance (NEB), serving as predictive indicators of hyperketonemia [59]. For example, ref. [60] suggested that a milk C18:1 cis-9 concentration ≥24 g/100 g of total fatty acids during the second week of lactation indicates elevated plasma NEFA (≥0.6 mmol/L), thus signaling NEB.
In the current study, BHB levels exceeding 15.62 mg/L were found only in the second tertile of the 4 mm sieve. In contrast, the significantly lowest acetone levels were noted in the second tertile of the pad, and the lowest BHB levels in the first tertile of the 4 mm sieve. TMR structure, mainly the distribution of particle size, influences nutrient intake and digestibility. In this context, milk acetone levels—a biomarker of ketosis—were elevated only in the first tertile of the pad fraction. Ketosis is a metabolic disorder from impaired energy utilization post-partum, characterized by increased ketone body production due to intensified lipolysis. Acetone concentrations above 0.30 mmol/L (17.42 mg/L), along with elevated β-hydroxybutyrate (BHB) levels (>0.15 mmol/L or 15.62 mg/L), are indicative of ketosis and are associated with decreased milk yield and altered milk composition [39,61].
3.2. Effects of TMR Structure on Milk Production and Quality
Furthermore, feed composition significantly affects milk production and quality. Optimizing TMR structure enhances nutrient utilization, improves rumen function, and supports microbial safety. The significantly highest milk yields were associated with the third tertiles of the 19 mm and 8 mm sieves (and their sum) and the first tertile of the 4 mm sieve. The average milk yield across the dataset was 10,596 kg per lactation, or approximately 34.9 kg/day, aligning with estimates by [62]. In contrast, the significantly lowest milk production was observed in the third tertile of the 4 mm sieve and the first tertiles of the 19 mm and 8 mm sieves (and their sum).
In the case of milk fat content, the significantly highest concentrations were found in the third tertile of the pad, as well as in the first tertiles of the 19 mm, 8 mm, and 4 mm sieves. Conversely, the significantly lowest milk fat content was detected in the first tertile of the pad and in the third tertiles of the 19 mm, 8 mm, and their combined sieves. These findings are consistent with Simmental cow averages (fat: 3.71%, protein: 3.42%) as reported by [62,63], where milk fat levels between 3.86% and 4.66% were observed.
Previous research has shown that decreasing forage particle size improves dry matter intake (DMI) and digestibility [64], although it may reduce NDF digestibility and milk fat content. However, reducing particle size limits sorting behavior, which can stabilize rumen fermentation and support milk fat synthesis [65].
Milk protein concentrations were significantly highest in the first tertiles of the 19 mm, 8 mm, and their combined sieve fractions. In contrast, the significantly lowest protein concentrations were consistently found in the second tertile across all sieves except the pad. These values are in agreement with reports by [63,66], who observed milk protein contents from 3.22% to 4.09%. Milk protein synthesis is supported by a stable rumen environment and adequate NDF levels, as noted by [67]; however, ref. [68] found that reduced forage particle size did not significantly alter protein content.
Rumen fermentation relies on proper TMR structure and NDF content to maintain chewing activity and saliva production. These, in turn, buffer rumen pH and support VFA production—especially acetate and butyrate—which are precursors for milk fat synthesis [69].
The fat-to-protein ratio was significantly narrowest in the first tertile of the pad and the third tertile of the 19 mm, 8 mm, and their combined sieves. On the other hand, the significantly widest ratio was found in the third tertile of the pad and the first tertiles of the 8 mm and 19 mm sieves. A similar trend was observed in fat-to-milk dry matter and fat-to-solids non-fat ratios. Principal component analysis (PCA) further confirmed these relationships: variables such as Fat, F/S, F/SNF, F/L, and oleic acid clustered strongly along PC1, indicating their shared contribution to variance among TMR groups (Figure 1).
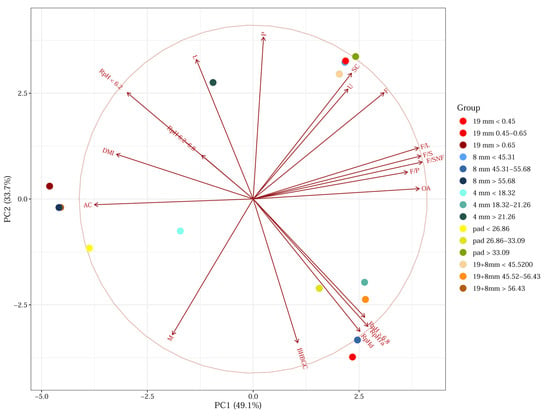
Figure 1.
Principal Component Analysis of TMR Structure Tertiles and Their Relationship with Milk Production and Rumen Indicators. Abbreviations: 19 mm—ratio of particles caught on 19 mm sieve, 8 mm—ratio of particles caught on 8 mm sieve, 4 mm—ratio of particles caught on 4 mm sieve, Pad—ratio of particles caught on the pad, OA—oleic acid in milk, F/P—milk fat-to-protein ratio, F/S—milk fat-to-milk dry matter ratio, F/SNF—milk fat-to-milk non-fatty dry matter ratio, F/L—milk fat-to-milk lactose ratio, AC—milk acetone, BHBGC—betahydroxy butyrate in milk, M—milk production, F—milk fat, P—milk proteins, L—milk lactose, SC—somatic cells count in milk, U—milk urea, RpH7a—rumen pH 7 days average (3 days before TMR analysis, 3 days after TMR analysis), RpHd—rumen pH on the day of TMR structure analysis, <6.2—rumen pH frequency spent under the risk of SARA, 6.2–6.8—rumen pH frequency spent in the optimal pH, >6.8—rumen pH frequency spent in the risk of ketosis, DMI—dry matter intake of TMR. The length of each line (vector) indicates the strength of the variable’s contribution to the principal components. Longer lines mean the variable is better represented in the plot. The distance between the points (groups) reflects their similarity; closer points are more similar in their overall variable profiles.
Additionally, PCA biplots revealed that TMR tertile groups based on particle size clustered in distinct regions, reflecting shared profiles. Groups with a higher proportion of soft particles (e.g., first tertiles of 8 mm and 19 + 8 mm sieves; third tertile of the pad) were located in the upper right quadrant of the PCA plot, correlating positively with F, UREA, and F/S. These groups also showed lower DMI and acetone (Ac1) values but higher F/S, F/SNF, F/L, and somatic cell (SC) values.
The somatic cell count (SC) was significantly lowest in the first tertile of the pad and the third tertiles of the 8 mm and 19 + 8 mm sieves, as shown in the heat map (Figure 2). Pad fractions were positively correlated with F/P (r = 0.608, p < 0.001), fat (r = 0.403, p = 0.003), and UREA (r = 0.380, p = 0.005), and negatively with the 19 mm (r = −0.905, p < 0.001) and 8 mm sieve fractions (r = −0.921, p < 0.001), due to their structural complementarity. Conversely, the significantly highest SC was found in the first tertiles of the 19 + 8 mm sieves and the third tertile of the pad. For Simmental cows, SC typically ranges from 330.57 to 410.98 [66], and is negatively associated with upper sieve fractions, NDF, and geometric mean particle size [70]. These results are consistent with previous observations in Holstein cows, where varying corn silage particle lengths affected SC nonlinearly [71].
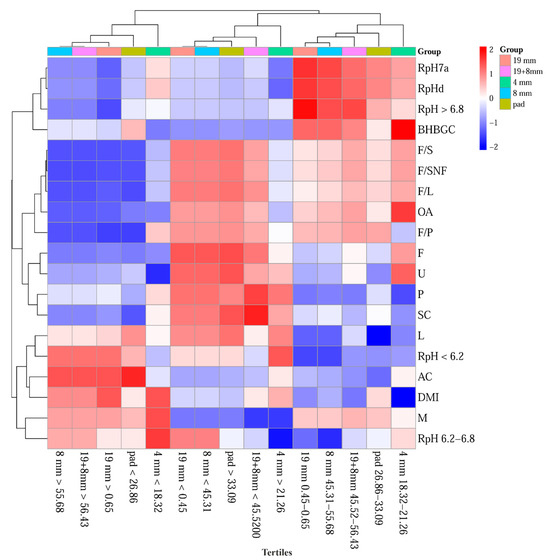
Figure 2.
Heatmap and Hierarchical Clustering of TMR Structure Tertiles on Milk Production and Rumen Indicators. Abbreviations: 19 mm—ratio of particles caught on 19 mm sieve, 8 mm—ratio of particles caught on 8 mm sieve, 4 mm—ratio of particles caught on 4 mm sieve, Pad—ratio of particles caught on the pad, OA—oleic acid in milk, F/P—milk fat-to-protein ratio, F/S—milk fat-to-milk dry matter ratio, F/SNF—milk fat-to-milk non-fatty dry matter ratio, F/L—milk fat-to-milk lactose ratio, AC—milk acetone, BHBGC—betahydroxy butyrate in milk, M—milk production, F—milk fat, P—milk proteins, L—milk lactose, SC—somatic cells count in milk, U—milk urea, RpH7a—rumen pH 7 days average (3 days before TMR analysis, 3 days after TMR analysis), RpHd—rumen pH on the day of TMR structure analysis, <6.2—rumen pH frequency spent under the risk of SARA, 6.2–6.8—rumen pH frequency spent in the optimal pH, >6.8—rumen pH frequency spent in the risk of ketosis, DMI—dry matter intake of TMR.
Finally, a linear trend in milk urea content across tertiles was observed. The significantly highest urea concentrations were found in the third tertile of the pad, the second tertile of the 4 mm sieve, and the first tertile of the 19 mm sieve. Conversely, the significantly lowest levels occurred in the first tertile of the 4 mm sieve, second tertile of the pad, and third tertile of the 8 mm sieve. Correlation analysis further supported this, with moderate negative associations between urea content and the 19 mm (r = −0.396, p = 0.003) and 8 mm sieve fractions (r = −0.413, p = 0.002). A decrease in particle length has previously been associated with increased milk urea [71]. Moreover, milk yield was negatively correlated with SC (r = −0.295, p = 0.026), reinforcing the relationship between udder health and productivity. In contrast, the correlation between the SC and TMR particle size was statistically non-significant (p > 0.05). However, the negative correlation between the tertiles on the 19 and 8 mm sieves and SC (r = −0.111 and r = −0.118), as well as the positive correlation between the tertiles on the 4 mm sieve and pad and SC (r = 0.034 and r = 0.157), was observed.
3.3. Influence of TMR Particle Size on Rumen pH and Dry Matter Intake
Overall, a statistically significant effect of total mixed ration (TMR) structure—based on sieve and pad distribution—on both 7-day rumen pH and rumen pH during the control days was observed (Table 5). Specifically, the significantly highest rumen pH values were consistently found in the second tertile interval across the 19 mm, 8 mm, and 4 mm sieves, as well as on the pad. Similarly, for the combined fraction of the 19 mm and 8 mm sieves, the second tertile interval (45.5–56.4%) was again associated with the significantly highest pH values. These findings suggest that mid-range particle distributions (second tertiles) consistently support optimal ruminal pH across all physical fractions.

Table 5.
TMR Particle Size Tertile Effects on Rumen Health Indicators and Dry Matter Intake.
Moreover, the TMR structures corresponding to the second tertiles on the 19 mm, 8 mm, 4 mm, and 19 + 8 mm sieves, and the pad, clustered together in the principal component analysis (PCA). These clusters were located on the right side of PC1 and lower on the PC2 axis, showing positive correlations with BHB (milk β-hydroxybutyrate concentration), rumen pH measured on the day of sampling, the 7-day average pH, and the duration of time that rumen pH remained above the critical threshold of 6.8 (Figure 1). This supports the theory that excessively long particles—such as those found in the third tertiles of the 19 mm and 8 mm sieves, as well as their combined fraction—may be selectively avoided by cows, leading to suboptimal rumen fermentation patterns.
Comparable results were reported by [2], who found that coarse particle size distributions induced feed sorting behavior, particularly when cows were fed inadequately structured TMRs containing very long particles. This behavior can impair rumen function and reduce salivary buffering capacity, as long particles are critical for proper rumen motility and stimulating saliva production. Similarly, ref. [72] reported prolonged eating and rumination times in cows fed TMRs with higher physically effective NDF (peNDF) and greater pad fractions.
It is important to note that a rumen pH below 5.8 can lead to subacute ruminal acidosis (SARA), a condition associated with increased production of volatile fatty acids (VFAs) and lipopolysaccharides, potentially triggering inflammatory responses detrimental to cow health [73,74]. Conversely, a rumen pH exceeding 6.5 may compromise fiber digestion efficiency by inhibiting cellulolytic bacterial activity, which could result in reduced milk yield [75].
With respect to pH distribution frequency, the lowest pH values (below 6.2) were most frequently observed in the coarsest TMR fractions (third tertiles), except for the pad. In contrast, the first tertiles (comprising the finest particles) exhibited the highest frequencies within the pH range of 6.2 to 6.8 across all sieves and the pad, except for the 19 + 8 mm combined fraction. Notably, the highest frequency of values exceeding the threshold of 6.8 occurred in the second tertiles, further reinforcing their association with favorable ruminal conditions.
In contrast, dry matter intake (DMI) displayed an opposing orientation in the PCA biplot, suggesting a negative association with the aforementioned favorable pH-related variables. This interpretation is corroborated by the correlation matrix, which revealed a weak but negative correlation between DMI and the F/S ratio (r = –0.203, p = 0.088). More significantly, a strong and statistically significant negative correlation was observed between DMI and milk BHBGC (r = –0.619, p < 0.001), implying that higher DMI is linked to lower ketone body concentrations—a key indicator of improved energy balance in dairy cows.
Interestingly, the third tertile fractions exhibited a moderate positive correlation with DMI, particularly in the 19 mm (r = 0.271, p = 0.034) and 8 mm sieves (r = 0.212, p = 0.078). Despite this, heatmap visualizations (Figure 2) often associate the first tertiles of these sieves with lower DMI values. Furthermore, the first tertile fractions (19 mm, 8 mm, and the combined 19 + 8 mm) were linked to reduced DMI and acetate-to-concentrate (AC) ratios, but elevated F/S, F/SNF, F/L, F/P, F, P, and somatic cell (SC) values.
Haselmann et al. (2019) [68] demonstrated that reducing TMR particle size can enhance DMI. However, conflicting findings were presented by [76], who investigated the implementation of compact TMR—characterized by reduced particle size and optimized dry matter through water addition and extended mixing. Their study reported decreases in DMI, eating time, feed sorting, and antagonistic social interactions, but found no significant impact on milk yield, rumen pH, or nutrient digestibility compared to conventional TMR.
In overall, an intermediate level of coarse particles provides the ideal amount of physical stimulation to the rumen wall. Physically effective fiber is the portion of feed that promotes chewing, which in turn increases saliva production. The bicarbonate and phosphate buffers present in saliva help neutralize the acids formed during the fermentation of organic matter in the rumen [77].
The presence of a sufficient, but not excessive, amount of coarse particles facilitates the formation of a stable fiber mat or raft in the rumen [78]. This mat traps small, fermentable particles, ensuring they are retained in the rumen for a longer period [77]. The extended retention allows for more gradual fermentation, which prevents a rapid drop in pH [79]. It also facilitates cud chewing (rumination), which further increases saliva production and buffering [80].
A medium particle size distribution secure balance between fast carbohydrate fermentation and slower fiber degradation [81]. Fine TMRs, with little physical structure, lead to rapid fermentation of highly digestible carbohydrates, producing a large volume of volatile fatty acids (VFAs) in a short time [82]. This overwhelms the rumen’s buffering capacity and causes the pH to plummet, increasing the risk of acidosis [83]. On the other hand, an excessively coarse TMR may not be effectively mixed, leading to pockets of poorly fermented material and reduced overall digestibility, which can also disrupt pH stability [2]. The medium particle size group avoids both of these extremes, promoting a more balanced and stable fermentation process [81].
4. Conclusions
This study evaluated how total mixed ration (TMR) particle size distribution influences milk yield, composition, rumen pH stability, and metabolic health in Simmental dairy cows. Overall, the results confirm that TMR structure—specifically the balance of fine, medium, and coarse particles—is a key driver of both performance and metabolic stability.
First, moderate particle size distributions (second tertiles of 19 mm, 8 mm, 4 mm sieves, and the pad) most effectively stabilized rumen pH by limiting time below 6.2 (SARA risk) and above 6.8 (ketosis risk). As a result, this balance created favorable fermentation conditions and was positively linked with healthy levels of β-hydroxybutyrate (BHB), oleic acid, and acetone—key metabolic biomarkers.
Second, milk yield was optimized by a combination of coarser particles on large sieves (third tertiles of 19 mm and 8 mm) and finer particles on small sieves (first tertile of 4 mm). In addition, milk fat content peaked in the pad (third tertile) and in finer fractions of large sieves, while milk protein was highest in the first tertiles of 19 mm and 8 mm sieves. Together, these findings suggest that different milk components respond to distinct particle ranges.
Third, although dry matter intake (DMI) increased with coarser particles, it was negatively related to some milk quality traits and metabolic indicators. This indicates that overly long particles encourage selective feeding and reduce fermentation efficiency. Supporting this, principal component analysis clustered fine particle fractions with higher fat-to-protein ratios, somatic cell counts, and urea concentrations.
Taken together, mid-range particle sizes (second tertiles) delivered the most consistent benefits across production, metabolic, and ruminal parameters. Therefore, TMR particle size distribution can be viewed as a practical precision feeding tool that enhances milk yield and quality, supports metabolic health, and improves feed efficiency.
Dairy producers should strategically manage the TMR structure to align feed structural parameters with cow physiology, reduce metabolic stress, and maximize productivity. Future research should investigate the long-term effects of particle size management on reproduction, longevity, and farm economics.
Author Contributions
Conceptualization, O.H., B.G., M.Š. and M.J.; methodology, O.H. and M.J.; software, O.H.; validation, M.K., S.D. and M.D.; formal analysis, L.Z.; investigation, O.H. and M.J.; resources, O.H. and M.Š.; data curation, O.H. and M.R.; writing—original draft preparation, O.H. and M.J.; writing—review and editing, M.Š. and L.Z.; visualization, O.H. and M.K.; supervision, S.D. and M.D.; project administration, M.J.; funding acquisition, M.J., M.R., O.H. and B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was supported by the Scientific Grant Agency of The Ministry of Education, Science, Research and Sport of the Slovak Republic and the Slovak Academy of Sciences, project no. 1/0321/23 (Effective management of the ruminant nutrition using a modern monitoring of the internal environment).
Institutional Review Board Statement
The conditions of animal care, manipulations, and use adhered to the guidance of the Ethics Committee of the Slovak University of Agriculture in Nitra, Protocol No. 48/2013. According to the State Veterinary and Food Administration of the Slovak Republic, the given study has been evaluated as an inexperienced agricultural practice that does not fall under the legislation of Government Regulation of the Slovak Republic 377/2012 of 14 November 2012, laying down requirements for the protection of animals used for scientific or educational purposes.
Data Availability Statement
The datasets presented in this article are not readily available because the data are part of an ongoing larger research project. Requests to access the datasets should be directed to the corresponding author.
Acknowledgments
This publication was supported by the Scientific Grant Agency of The Ministry of Education, Science, Research, and Sport of the Slovak Republic and the Slovak Academy of Sciences, project no. 1/0321/23 (Effective management of the ruminant nutrition using a modern monitoring of the internal environment). Appreciation for cooperation: P. Repiský (PD Kozárovce—chairman), M. Szabo), L. Ivičič, L. Chadimová, and other staff of PD Kozárovce, R. Mokráň (VVS SK Ltd.), M. Palko, and P. Gesler.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AC | Acetone |
| ANOVA | Analysis of Variance |
| BHB | β-hydroxybutyrate |
| DMI | Dry Matter Intake |
| F | Milk Fat |
| F/L | Fat-to-Lactose Ratio |
| F/P | Fat-to-Protein Ratio |
| F/S | Fat-to-Dry Matter Ratio |
| F/SNF | Fat-to-Solids Non-Fat Ratio |
| L | Lactose |
| M | Milk Yield |
| NEB | Negative Energy Balance |
| OA | Oleic Acid |
| PCA | Principal Component Analysis |
| peNDF | Physically Effective Neutral Detergent Fiber |
| PSD | Particle Size Distribution |
| PSPS | Penn State Particle Separator |
| RpH7a | Rumen pH 7 days average (3 days before TMR analysis, 3 days after TMR analysis) |
| RpHd | Rumen pH on the day of TMR structure analysis |
| SARA | Subclinical ruminal acidosis |
| SC | Somatic Cell Cunt |
| SCK | Subclinical ketosis |
| TMR | Total Mixed Ration |
| U | Milk Urea |
| VFAs | Volatile Fatty Acids |
References
- Berthiller, G.D.C. Review of Dairy Production, Processing, and Strategies for Milk Marketing Development. Int. J. Agric. Life Sci. 2024, 10, 455–458. [Google Scholar] [CrossRef]
- Spina, A.A.; Iommelli, P.; Morello, A.R.; Britti, D.; Pelle, N.; Poerio, G.; Morittu, V.M. Particle Size Distribution and Feed Sorting of Hay-Based and Silage-Based Total Mixed Ration of Calabrian Dairy Herds. Dairy 2024, 5, 106–117. [Google Scholar] [CrossRef]
- Ali, A.; Harahap, A.E.; Juliantoni, J. Evaluation of Nutrient and Digestibility of Agricultural Waste Total Mixed Ration Silage as Ruminant Feed. Bul. Peternak. 2023, 47, 237–241. [Google Scholar] [CrossRef]
- Repetto, J.L.; Ciancio, E.; Castro, G.; Santana, Á.; Cajarville, C. Performance and Rumen Fermentation in Finishing Steers Fed a Total Mixed Ration Supplemented with a Blend of Essential Oils, Tannins, and Bioflavonoids or Monensin. Animals 2025, 15, 594. [Google Scholar] [CrossRef]
- Li, W.; Ye, B.; Wu, B.; Yi, X.; Li, X.; A, R.; Cui, X.; Zhou, Z.; Cheng, Y.; Zhu, X.; et al. Effect of Total Mixed Ration on Growth Performance, Rumen Fermentation, Nutrient Digestion, and Rumen Microbiome in Angus Beef Cattle during the Growing and Fattening Phases. Fermentation 2024, 10, 205. [Google Scholar] [CrossRef]
- Foggi, G.; Terranova, M.S.; Daghio, M.; Amelchanka, S.L.; Conte, G.; Ineichen, S.; Agnolucci, M.; Viti, C.; Mantino, A.; Buccioni, A.; et al. Evaluation of Ruminal Methane and Ammonia Formation and Microbiota Composition as Affected by Supplements Based on Mixtures of Tannins and Essential Oils Using Rusitec. J. Anim. Sci. Biotechnol. 2024, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.G.; Halvorson, J.; MacAdam, J.W.; Kronberg, S.L.; Hendrickson, J.R. Forages Containing Plant Secondary Compounds Can Improve Digestibility, Rumen Ammonia and Rumen Metabolites of Pasture: An in Vitro Continuous Culture Case Study. J. Anim. Sci. 2023, 101, 159–160. [Google Scholar] [CrossRef]
- Li, C.; Beauchemin, K.A.; Yang, W. Feeding Diets Varying in Forage Proportion and Particle Length to Lactating Dairy Cows: I. Effects on Ruminal pH and Fermentation, Microbial Protein Synthesis, Digestibility, and Milk Production. J. Dairy Sci. 2020, 103, 4340–4354. [Google Scholar] [CrossRef]
- Shi, R.; Dong, S.; Mao, J.; Wang, J.; Cao, Z.; Wang, Y.; Li, S.; Zhao, G. Dietary Neutral Detergent Fiber Levels Impacting Dairy Cows’ Feeding Behavior, Rumen Fermentation, and Production Performance during the Period of Peak-Lactation. Animals 2023, 13, 2876. [Google Scholar] [CrossRef]
- Humer, E.; Petri, R.M.; Aschenbach, J.R.; Bradford, B.J.; Penner, G.B.; Tafaj, M.; Südekum, K.-H.; Zebeli, Q. Invited Review: Practical Feeding Management Recommendations to Mitigate the Risk of Subacute Ruminal Acidosis in Dairy Cattle. J. Dairy Sci. 2018, 101, 872–888. [Google Scholar] [CrossRef] [PubMed]
- Woolpert, M.E.; Dann, H.M.; Cotanch, K.W.; Melilli, C.; Chase, L.E.; Grant, R.J.; Barbano, D.M. Management Practices, Physically Effective Fiber, and Ether Extract Are Related to Bulk Tank Milk de Novo Fatty Acid Concentration on Holstein Dairy Farms. J. Dairy Sci. 2017, 100, 5097–5106. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, A.J.; Heinrichs, B.S.; Cavallini, D.; Fustini, M.; Formigoni, A. Limiting Total Mixed Ration Availability Alters Eating and Rumination Patterns of Lactating Dairy Cows. JDS Commun. 2021, 2, 186–190. [Google Scholar] [CrossRef]
- Goulart, R.S.; Vieira, R.A.M.; Daniel, J.L.P.; Amaral, R.C.; Santos, V.P.; Toledo Filho, S.G.; Cabezas-Garcia, E.H.; Tedeschi, L.O.; Nussio, L.G. Effects of Source and Concentration of Neutral Detergent Fiber from Roughage in Beef Cattle Diets: Comparison of Methods to Measure the Effectiveness of Fiber. J. Anim. Sci. 2020, 98, skaa108. [Google Scholar] [CrossRef]
- Hossain, E. Forage Particle Size: It s Implications on Behavior, Performance, Health and Welfare of Dairy Cows. Online J. Anim. Feed. Res. 2021, 11, 72–81. [Google Scholar] [CrossRef]
- Lee, M.; Seo, S.; Tedeschi, L.O. PSXI-23 Development of Sub-Models to Estimate Protein Requirements and Supply of Lactating Dairy Cows Using Machine Learning Algorithms. J. Anim. Sci. 2024, 102, 764–765. [Google Scholar] [CrossRef]
- Bossche, T.V.D.; Goossens, K.; Ampe, B.; Tamassia, L.M.; Boever, J.L.D.; Vandaele, L. Effect of Supplementing an α-Amylase Enzyme or a Blend of Essential Oil Components on the Performance, Nutrient Digestibility and Nitrogen Balance of Dairy Cows. J. Dairy Sci. 2024, 107, 4509–4523. [Google Scholar] [CrossRef]
- Terefe, G.; Walelegne, M. Effect of Feeds and Hygienic Practices on Milk Production and Its Nutritional and Microbiological Quality. CABI Rev. 2024, 19, 0017. [Google Scholar] [CrossRef]
- Verhoef, W.; Zuidhof, S.; Ross, J.A.; Beaugrand, K.; Olson, M. Evaluation of a Novel Dipotassium Phosphate Bolus for Treatment of Metabolic Disorders in Dairy Cattle. Front. Vet. Sci. 2023, 10, 1274183. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, P.K.; Singla, P. Advances in Non-Thermal Membrane Processing for Nutrient Recovery in the Dairy Industry. In Non-Thermal Processing of Functional Foods; CRC Press: Boca Raton, FL, USA, 2024; pp. 223–245. [Google Scholar] [CrossRef]
- Bhimte, A. Role of Vitamin and Minerals Supplementation in Periparturient Dairy Cows. Int. J. Curr. Res. Acad. Rev. 2024, 12, 23–30. [Google Scholar] [CrossRef]
- Kononoff, P.J.; Heinrichs, A.J. The Effect of Reducing Alfalfa Haylage Particle Size on Cows in Early Lactation. J. Dairy Sci. 2003, 86, 1445–1457. [Google Scholar] [CrossRef]
- Dijkstra, J.; Ellis, J.L.; Kebreab, E.; Strathe, A.B.; López, S.; France, J.; Bannink, A. Ruminal pH Regulation and Nutritional Consequences of Low pH. Anim. Feed. Sci. Technol. 2012, 172, 22–33. [Google Scholar] [CrossRef]
- Yansari, A.T.; Valizadeh, R.; Naserian, A.; Christensen, D.A.; Yu, P.; Shahroodi, F.E. Effects of Alfalfa Particle Size and Specific Gravity on Chewing Activity, Digestibility, and Performance of Holstein Dairy Cows. J. Dairy Sci. 2004, 87, 3912–3924. [Google Scholar] [CrossRef]
- Lippke, H.; Ellis, W.C.; Jacobs, B.F. Recovery of Indigestible Fiber from Feces of Sheep and Cattle on Forage Diets1. J. Dairy Sci. 1986, 69, 403–412. [Google Scholar] [CrossRef]
- Danscher, A.M.; Li, S.; Andersen, P.H.; Khafipour, E.; Kristensen, N.B.; Plaizier, J.C. Indicators of Induced Subacute Ruminal Acidosis (SARA) in Danish Holstein Cows. Acta Vet. Scand. 2015, 57, 39. [Google Scholar] [CrossRef] [PubMed]
- Kovács, L.; Szenci, O.; Baumgartner, W.; Hejel, M.; Rózsa, L. Subacute Ruminal Acidosis in Dairy Cows-Physiological Background, Risk Factors and Diagnostic Methods. Vet. Stanica 2020, 51, 5–17. [Google Scholar] [CrossRef]
- Mirzad, A.N.; Haidary, M.H.; Sohail, M.N.; Sahab, M.N.; Alizada, H.; Monis, A.; Monir, T.M.; Upendra, H.A. Effects of Subacute Ruminal Acidosis (SARA) on Epidemiological and Clinicopathological Parameters of Dairy Cattle. Asian J. Dairy Food Res. 2021, 40, 260–266. [Google Scholar] [CrossRef]
- Morar, D.; Văduva, C.; Morar, A.; Imre, M.; Tulcan, C.; Imre, K. Paraclinical Changes Occurring in Dairy Cows with Spontaneous Subacute Ruminal Acidosis under Field Conditions. Animals 2022, 12, 2466. [Google Scholar] [CrossRef]
- Llonch, L.; Castillejos, L.; Ferret, A. Increasing the Content of Physically Effective Fiber in High-Concentrate Diets Fed to Beef Heifers Affects Intake, Sorting Behavior, Time Spent Ruminating, and Rumen pH. J. Anim. Sci. 2020, 98, skaa192. [Google Scholar] [CrossRef]
- Russo, V.M.; Leury, B.J.; Kennedy, E.; Hannah, M.C.; Auldist, M.J.; Morris, G.L.; Wales, W.J. Prior Forage Type Influences Ruminal Responses to a Wheat Grain Challenge in Lactating Dairy Cows. Animals 2021, 11, 3188. [Google Scholar] [CrossRef]
- Khiaosa-ard, R.; Pourazad, P.; Aditya, S.; Humer, E.; Zebeli, Q. Factors Related to Variation in the Susceptibility to Subacute Ruminal Acidosis in Early Lactating Simmental Cows Fed the Same Grain-Rich Diet. Anim. Feed. Sci. Technol. 2018, 238, 111–122. [Google Scholar] [CrossRef]
- Hernández, R.; Chaib De Mares, M.; Jimenez, H.; Reyes, A.; Caro-Quintero, A. Functional and Phylogenetic Characterization of Bacteria in Bovine Rumen Using Fractionation of Ruminal Fluid. Front. Microbiol. 2022, 13, 813002. [Google Scholar] [CrossRef]
- Minami, N.S.; Sousa, R.S.; Oliveira, F.L.C.; Dias, M.R.B.; Cassiano, D.A.; Mori, C.S.; Minervino, A.H.H.; Ortolani, E.L. Subacute Ruminal Acidosis in Zebu Cattle: Clinical and Behavioral Aspects. Animals 2020, 11, 21. [Google Scholar] [CrossRef]
- Maskaľová, I.; Vajda, V.; Bujňák, L. 2,6-Diaminopimelic Acid as a Biological Marker of Rumen Synthesis and Fermentation Capacities in the Transition Period and Early Lactation of Dairy Cows. Acta Vet. Brno 2014, 83, 355–361. [Google Scholar] [CrossRef][Green Version]
- Zeng, J.; Lv, J.; Duan, H.; Yang, S.; Wu, J.; Yan, Z.; Zhang, R.; Hu, J.; Zhang, Y. Subacute Ruminal Acidosis as a Potential Factor That Induces Endometrium Injury in Sheep. Int. J. Mol. Sci. 2023, 24, 1192. [Google Scholar] [CrossRef]
- Khorrami, B.; Khiaosa-ard, R.; Zebeli, Q. Models to Predict the Risk of Subacute Ruminal Acidosis in Dairy Cows Based on Dietary and Cow Factors: A Meta-Analysis. J. Dairy Sci. 2021, 104, 7761–7780. [Google Scholar] [CrossRef]
- Trevisi, E.; Minuti, A.; Cogrossi, S.; Grossi, P.; Ahmed, S.; Bani, P. Can a Single Rumen Sample Really Diagnose SARA in Commercial Farms? Anim. Prod. Sci. 2014, 54, 1268. [Google Scholar] [CrossRef]
- Kara, K. Milk Urea Nitrogen and Milk Fatty Acid Compositions in Dairy Cows with Subacute Ruminal Acidosis. Veterinární Medicína 2020, 65, 336–345. [Google Scholar] [CrossRef]
- Melendez, P.; Serrano, M.V. Update on Ketosis in Dairy Cattle with Major Emphasis on Subclinical Ketosis and Abdominal Adiposity. Vet. Med. Sci. 2024, 10, e1525. [Google Scholar] [CrossRef]
- Krempaský, M.; Maskaľová, I.; Bujňák, L.; Vajda, V. Ketone Bodies in Blood of Dairy Cows: Prevalence and Monitoring of Subclinical Ketosis. Acta Vet. Brno 2014, 83, 411–416. [Google Scholar] [CrossRef]
- Guliński, P. Ketone Bodies–Causes and Effects of Their Increased Presence in Cows’ Body Fluids: A Review. Vet. World 2021, 14, 1492–1503. [Google Scholar] [CrossRef]
- Zhang, G.; Ametaj, B.N. Ketosis an Old Story Under a New Approach. Dairy 2020, 1, 42–60. [Google Scholar] [CrossRef]
- van Erp-van der Kooij, E.; Derix, J.; van Gorp, S.; Timmermans, A.; Krijnen, C.; Fodor, I.; Dingboom, L. Breath Analysis for Early Detection of Rising Ketone Bodies in Postpartum Dairy Cows Classified as at Risk of Ketosis. Ruminants 2023, 3, 39–54. [Google Scholar] [CrossRef]
- Trebukhov, A.V.; Elenschleger, A.A. Clinical and Biochemical Aspects of Acetonemia (Ketosis) of Dairy Cows. IOP Conf. Ser. Earth Environ. Sci. 2019, 341, 012152. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, B.; Xu, C.; Zhang, H.; Xia, C. Effects of Ketosis in Dairy Cows on Blood Biochemical Parameters, Milk Yield and Composition, and Digestive Capacity. J. Vet. Res. 2019, 63, 555–560. [Google Scholar] [CrossRef]
- Deniz, A.; Aksoy, K.; Metin, M. Transition Period and Subclinical Ketosis in Dairy Cattle: Association with Milk Production, Metabolic and Reproductive Disorders and Economic Aspects. Med. Weter. 2020, 76, 495–502. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, P.; Sun, Y.; Zhang, Y. Effects of Body Condition Score Changes During Peripartum on the Postpartum Health and Production Performance of Primiparous Dairy Cows. Animals 2019, 9, 1159. [Google Scholar] [CrossRef]
- Lean, I.J. Non-Infectious Diseases: Ketosis. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2022; pp. 405–413. ISBN 978-0-12-818767-8. [Google Scholar]
- Bujko, J.; Candrák, J.; Žitný, J.; Kasarda, R. Changes in Production and Reproduction Traits in Population of the Slovak Spotted Cattle. Acta Fytotech. Zootech. 2020, 23, 161–166. [Google Scholar] [CrossRef]
- Kasarda, R.; Trakovická, A.; Moravčíková, N.; Šidlová, V.; Kadlečí, O. Research on Diversity, Utilization and Production Quality of Local Breeds in Slovakia. Poljoprivreda 2015, 21, 11–15. [Google Scholar] [CrossRef]
- Strapák, P.; Tančin, V.; Vavrišínová, K.; Grafenau, P.; Bulla, J.; Chrenek, P.; Šimko, M.; Juráček, M.; Polák, P.; Ryba, Š.; et al. Chov Hovädzieho Dobytka, 1st ed.; Slovenská Poľnohospodárska Univerzita: Nitra, Slovakia, 2013; ISBN 978-80-552-0994-4. [Google Scholar]
- AOAC International. Official Methods of Analysis, 22nd ed.; AOAC International: Rockville, MD, USA, 2023. [Google Scholar]
- Heinrichs, J.; Jones, C.M. Penn State Particle Separator. Penn State Extension, 2022. updated 19 December 2022. 2025. Available online: https://extension.psu.edu/penn-state-particle-separator (accessed on 5 September 2025).
- Gesler, P. Chapter 10: Rumen Bolus Technology at Commercial Farms. In Practical Precision Livestock Farming; Brill|Wageningen Academic: Leiden, The Netherlands, 2022; pp. 165–173. ISBN 978-90-8686-382-2. [Google Scholar]
- Slovenský Normalizačný Ústav STN 570536: Stanovenie Zloženia Mlieka Infračerveným Absorpčným Analyzátorom [Determination of Milk Composition Using an Infrared Absorption Analyzer] 1995. Bratislava: Úrad pre Normalizáciu, Metrológiu a Skúšobníctvo SR. Available online: https://eshop.normservis.sk/norma/stn-570536-1.4.1995.html (accessed on 5 September 2025).
- Slovenský Normalizačný Ústav STN EN ISO 13366-2: Mlieko. Stanovenie Počtu Somatických Buniek. Časť 2: Návod Na Obsluhu Zariadenia Na Elektronické Počítanie Častíc Fluorescenčnou Optickou Metódou [Milk. Enumeration of Somatic Cells. Part 2: Guidance on the Operation of Fluoro-Opto-Electronic Particle Counters] 2007. Bratislava: Slovenský Ústav Technickej Normalizácie, Bratislava, Slovensko, 1–13. Available online: https://eshop.normservis.sk/norma/stneniso-13366-2-2007-opravaac-1.10.2007.html (accessed on 5 September 2025).
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A Free Online Platform for Data Visualization and Graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Vranković, L.; Aladrović, J.; Octenjak, D.; Bijelić, D.; Cvetnić, L.; Stojević, Z. Milk Fatty Acid Composition as an Indicator of Energy Status in Holstein Dairy Cows. Arch. Anim. Breed. 2017, 60, 205–212. [Google Scholar] [CrossRef]
- Reus, A.; Mansfeld, R. Predicting Metabolic Health Status Using Milk Fatty Acid Concentrations in Cows—A Review. Milk Sci. Int.-Milchwiss. 2020, 73, 7–15. [Google Scholar]
- Jorjong, S.; van Knegsel, A.T.M.; Verwaeren, J.; Lahoz, M.V.; Bruckmaier, R.M.; De Baets, B.; Kemp, B.; Fievez, V. Milk Fatty Acids as Possible Biomarkers to Early Diagnose Elevated Concentrations of Blood Plasma Nonesterified Fatty Acids in Dairy Cows. J. Dairy Sci. 2014, 97, 7054–7064. [Google Scholar] [CrossRef]
- Karlikova, G.; Sermyagin, A.; Lashneva, I. Assessment of the Metabolic State of Metabolism in Holstein Cows Using Milk Biomarkers. Bull. KSAU 2024, 10, 96–104. [Google Scholar] [CrossRef]
- Koç, A.; Öner, M. A Research on Fertility, Herd Life, Milk Production and Milk Quality Characteristics of Simmental (Fleckvieh) Cows: 1. Reproduction, Herd Life and Milk Production Characteristics. Turk. J. Agric.-Food Sci. Technol. 2023, 11, 2339–2346. [Google Scholar] [CrossRef]
- Rabus, T.; Oehm, A.W.; Knubben-Schweizer, G.; Hoedemaker, M.; Müller, K.; Zablotski, Y. Relationship of Body Condition and Milk Parameters during Lactation in Simmental Cows in Bavaria, Germany. Prev. Vet. Med. 2023, 220, 106042. [Google Scholar] [CrossRef]
- Nasrollahi, S.M.; Imani, M.; Zebeli, Q. A Meta-Analysis and Meta-Regression of the Effect of Forage Particle Size, Level, Source, and Preservation Method on Feed Intake, Nutrient Digestibility, and Performance in Dairy Cows. J. Dairy Sci. 2015, 98, 8926–8939. [Google Scholar] [CrossRef]
- Nasrollahi, S.M.; Ghorbani, G.R.; Khorvash, M.; Yang, W.Z. Effects of Grain Source and Marginal Change in Lucerne Hay Particle Size on Feed Sorting, Eating Behaviour, Chewing Activity, and Milk Production in Mid-lactation Holstein Dairy Cows. J. Anim. Physiol. Anim. Nutr. 2014, 98, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Khastayeva, A.Z.; Zhamurova, V.S.; Mamayeva, L.A.; Kozhabergenov, A.T.; Karimov, N.Z.; Muratbekova, K.M. Qualitative Indicators of Milk of Simmental and Holstein Cows in Different Seasons of Lactation. Veter World 2021, 14, 956–963. [Google Scholar] [CrossRef]
- Kurepin, A.A. Feed Consumption and Nutrient Utilization with Different Ratio of Structural Carbohydrates in the Diet of Cows. Zootech. Sci. Belarus 2022, 57, 267–276. [Google Scholar] [CrossRef]
- Haselmann, A.; Zehetgruber, K.; Fuerst-Waltl, B.; Zollitsch, W.; Knaus, W.; Zebeli, Q. Feeding Forages with Reduced Particle Size in a Total Mixed Ration Improves Feed Intake, Total-Tract Digestibility, and Performance of Organic Dairy Cows. J. Dairy Sci. 2019, 102, 8839–8849. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Fox, P.; Cogan, T. From Farm to Table; Oxford University Press (OUP): Oxford, Oxfordshire, UK, 2024. [Google Scholar] [CrossRef]
- Evangelista, C.; Jasinski, F.P.; Basiricò, L.; Turriziani, G.; Bernabucci, U. Particle Size Distribution of Total Mixed Rations Fed to Italian Mediterranean Buffaloes Measured by the PSPS: Impact on Milk Quality and Digestibility. Ital. J. Anim. Sci. 2024, 23, 1535–1550. [Google Scholar] [CrossRef]
- Zebeli, Q.; Ametaj, B.N.; Junck, B.; Drochner, W. Maize Silage Particle Length Modulates Feeding Patterns and Milk Composition in Loose-Housed Lactating Holstein Cows. Livest. Sci. 2009, 124, 33–40. [Google Scholar] [CrossRef]
- Kapusniaková, M.; Juráček, M.; Hanušovský, O.; Rolinec, M.; Gálik, B.; Džima, M.; Duchoň, A.; Vavrišínová, K.; Madajová, V.; Šimko, M. Nutrition of Dairy Cows: How Starch and Fiber Influence Their Overall Activity. Acta Fytotech. Zootech. 2024, 27, 104–109. [Google Scholar] [CrossRef]
- Hou, G.; You, J.; Zhuang, Y.; Gao, D.; Xu, Y.; Jiang, W.; Li, S.; Zhao, X.; Chen, T.; Zhang, S.; et al. Disorders of Acid-Base Balance Promote Rumen Lipopolysaccharide Biosynthesis in Dairy Cows by Modulating the Microbiome. Front. Microbiol. 2024, 15, 1492476. [Google Scholar] [CrossRef]
- Kitkas, G.C.; Valergakis, G.E.; Kritsepi-Konstantinou, M.; Gelasakis, A.I.; Katsoulos, P.D.; Kalaitzakis, E.; Panousis, N.K. Association between Ruminal pH and Rumen Fatty Acids Concentrations of Holstein Cows during the First Half of Lactation. Ruminants 2022, 2, 382–389. [Google Scholar] [CrossRef]
- Fernandes, T.; Manuel, C.; Vahmani, P.; Alves, S.P.; Dugan, M.; Bessa, R.J. 400 Effect of pH on in Vitro Ruminal Metabolism of Trans-10 18:1 and Trans-11 18:1. J. Anim. Sci. 2024, 102, 378–379. [Google Scholar] [CrossRef]
- Kronqvist, C.; Petters, F.; Robertsson, U.; Lindberg, M. Evaluation of Production Parameters, Feed Sorting Behaviour and Social Interactions in Dairy Cows: Comparison of Two Total Mixed Rations with Different Particle Size and Water Content. Livest. Sci. 2021, 251, 104662. [Google Scholar] [CrossRef]
- Allen, M.S. Relationship Between Fermentation Acid Production in the Rumen and the Requirement for Physically Effective Fiber. J. Dairy Sci. 1997, 80, 1447–1462. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei-Aghsaghali, A.; Maheri-Sis, N. Importance of “Physically Effective Fibre” in Ruminant Nutrition: A Review. Ann. Biol. Res. 2011, 2, 262–270. [Google Scholar]
- Zhang, Z.; Li, F.; Li, F.; Wang, Z.; Guo, L.; Weng, X.; Sun, X.; He, Z.; Meng, X.; Liang, Z.; et al. Influence of Dietary Forage Neutral Detergent Fiber on Ruminal Fermentation, Chewing Activity, Nutrient Digestion, and Ruminal Microbiota of Hu Sheep. Animals 2025, 15, 314. [Google Scholar] [CrossRef]
- Banakar, P.; Anand Kumar, N.; Shashank, C. Physically Effective Fibre in Ruminant Nutrition: A Review. J. Pharmacogn. Phytochem. 2018, 7, 303–308. [Google Scholar]
- Zebeli, Q.; Mansmann, D.; Ametaj, B.N.; Steingass, H.; Drochner, W. A Model to Optimise the Requirements of Lactating Dairy Cows for Physically Effective Neutral Detergent Fibre. Arch. Anim. Nutr. 2010, 64, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, X.; Zou, Y.; Yang, Z.; Li, S.; Cao, Z. Changes in Feed Intake, Nutrient Digestion, Plasma Metabolites, and Oxidative Stress Parameters in Dairy Cows with Subacute Ruminal Acidosis and Its Regulation with Pelleted Beet Pulp. J. Anim. Sci. Biotechnol. 2013, 4, 31. [Google Scholar] [CrossRef]
- Oetzel, G.R. Diagnosis and Management of Subacute Ruminal Acidosis in Dairy Herds. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 463–480. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).