Abstract
This study evaluated two techniques for encapsulating Lactiplantibacillus plantarum using bovine lactoferrin and sodium alginate. The first method involved a layer-by-layer (LbL) coating of lactoferrin and alginate directly onto individual cells, using three layers of these electrolytes. The second method focused on encapsulating the probiotics in calcium alginate miniaturized beads, followed by a lactoferrin coating (AAcL). Encapsulation efficiency was measured at 52.7% for the LbL method and 32.6% for AAcL. Encapsulation was confirmed through zeta potential changes and scanning electron microscopy (SEM) micrographs. After freeze drying, the LbL technique showed a 2.67 log CFU decrease in survival rates, whereas the AAcL method resulted in a 3.77 log CFU decline. Nonencapsulated probiotics experienced a reduction of 5.8 log CFU. In storage at −20 °C, the LbL method led to a 32% decrease in survival after 30 days and 41% after 90 days, while the AAcL method showed a decline of 15% after 30 days and 28% after 90 days. Both techniques preserved 75% of the initial L. plantarum population under simulated gastrointestinal conditions. Overall, these methods effectively protected the probiotic from environmental stress.
1. Introduction
The consumption of probiotics has gained significant attention over recent decades due to their potential to improve health and assist in treating certain diseases when used alongside other therapies [1]. The World Health Organization (WHO) defines probiotics as “live microbes that confer a health benefit to their host when administered in adequate amounts” [2]. Probiotics help maintain a balanced intestinal microbiota, which supports energy metabolism, protects against harmful pathogens, aids immune function, and preserves intestinal mucosa integrity [3,4].
Probiotics are often recommended when beneficial intestinal bacteria levels decrease, a condition known as dysbiosis, which can lead to various health issues [1,3,5]. In particular, Lactiplantibacillus plantarum is widely used as a probiotic in both the food industry and clinical settings [6,7].
Systematic meta-analyses show that L. plantarum supplementation significantly improves periodontal health, reduces abdominal pain, and offers cardiovascular benefits, such as lowering total cholesterol and low-density lipoprotein (LDL) cholesterol levels [6,8]. Additionally, studies indicate that it can decrease body weight, body mass index (BMI), and abdominal fat, while lowering inflammatory markers like interleukin-6 (IL-6) and high-sensitivity C-reactive protein (hs-CRP) [6,9,10,11]. Evidence suggests that L. plantarum may aid metabolic health by stabilizing insulin levels and reducing inflammation [6,7,8]. Moreover, L. plantarum has been applied to address conditions such as celiac disease [12], irritable bowel disease [13], and cognitive impairment [14,15]. However, it is essential to consider the efficacy of specific probiotic strains, as different strains may produce varying effects [6,8,11,14,15].
In recent years, there has been a significant rise in advanced research on functional foods that contain probiotics. This trend reflects the growing variety of new products in the market, as well as a wealth of research documented across various fields [16,17]. To ensure probiotics are metabolically active and effectively released in the colon, a concentration of over 106 CFU per mL or gram of the product is required for optimal physiological function in the human body [1,5,16]. However, there are significant barriers to making probiotic microorganisms and their metabolites available in food products. One of the main challenges in using probiotics as functional food products is ensuring their viability and stability, as they are sensitive to environmental factors such as temperature, pH, and exposure to oxygen [16,17,18]. These factors can significantly reduce both the viability and shelf life of probiotics. Additionally, food processing methods, including heating, freezing, thawing, high shear action, and the complexity of food matrices, can further impact the survival of these microorganisms [18,19]. Furthermore, probiotics must survive the journey through the gastrointestinal tract. Choosing appropriate strains is essential; they must be recognized as safe (e.g., GRAS or QPS) and able to withstand gastrointestinal conditions while proving their benefits [19]. Not all strains within one genus behave similarly, complicating standardization. Another challenge is probiotic compatibility with food matrices, as acidic juices, baked goods, and sausages may create harsh conditions for bacteria, necessitating careful formulation for stability and function. Lastly, consumer acceptance plays a crucial role, as sensory attributes like flavor and texture can impact product success if added microorganisms negatively alter them [17,18,19].
Various delivery systems for food probiotics have been developed to address these challenges, including microencapsulation [16,17]. Microencapsulation of L. plantarum using various edible matrices improves its survivability during simulated food processing and gastrointestinal conditions [20,21,22]. This technique also helps prevent undesirable aromas and preserves the original taste of liquid food products [16,17,18,23]. However, applying encapsulated probiotics to actual foods presents challenges due to the relatively large size of bacterial cells [18]. The most common method for microencapsulating L. plantarum is alginate gelation using calcium [24,25,26]. Unfortunately, this method often results in larger capsules, typically exceeding 300 μm, which can negatively impact the sensory qualities of food, frequently resulting in a gritty or chalky texture [27]. In contrast, Martin et al. [28] recommend that capsule sizes not exceed 80 μm to maintain optimal sensory quality.
Another method for encapsulating probiotics is spray drying, which is cost-effective and scalable. However, the high temperatures can reduce probiotic viability [29]. Freeze drying effectively preserves viability but is time-consuming and energy-intensive, mainly for preserving probiotic strains. Emulsion techniques enable the production of microcapsules with controlled sizes. However, solvents used in this method might not be appropriate for all applications. Recently, encapsulation studies have increased with innovative techniques to create miniaturized capsules incorporating probiotics without affecting food sensory properties. For instance, processes convert alginate-probiotic suspensions into aerosols, crosslinking with calcium and coating with a protective layer [30]. Compared to the one-layer strategy, the double layer may better protect L. plantarum against pH, oxygen, and temperature [31]. Another technique under study is layer-by-layer (LbL) encapsulation, allowing individualized encapsulation of bacterial cells [32]. LbL assembly creates multilayered shells around probiotics, offering superior protection against harsh gastrointestinal environmental conditions.
Hugues-Ayala et al. [30] developed alginate microbeads encapsulating Lactobacillus rhamnosus GG utilizing an innovative airbrush encapsulation technique. This process creates an aerosol suspension by mixing an alginate solution with a microbial suspension, which is then directed into a calcium solution bath to form the microbeads. Notably, over 60% of these capsules have 45 to 100 μm diameters. The microbeads were coated with proteins derived from buttermilk to enhance their effectiveness. This protective protein layer significantly fortifies the alginate microcapsules, safeguarding the probiotics against the acidic conditions of the stomach and bile salts in the intestines, ultimately improving their survival rates [30,31,32]. Alginate provides several benefits in coating encapsulation techniques, notably for its biocompatibility and safety, straightforward ionic gelation, and versatility and compatibility with other polymers. These qualities make it a preferred material for food applications [27].
Bovine lactoferrin (bLF), a multifunctional iron-binding glycoprotein found in mammalian secretions, may be a protein that can coat alginate microbeads. It has antimicrobial, anti-inflammatory, and immunomodulatory properties. Lf promotes the growth of lactobacilli, which can enhance the effects of encapsulated bioactive ingredients, such as probiotics or functional peptides. In addition, it improves the targeted release of the active ingredient toward the intestine, where the more neutral pH destabilizes the coating layers, allowing release. The opposite charges of alginate and lactoferrin allow a strong electrostatic interaction, facilitating the formation of stable layers [33].
Bovine lactoferrin (bLF) promotes the growth of Lactobacillus acidophilus, L. acidophilus CH-2, L. rhamnosus, L. rhamnosus ATCC 7469, Bifidobacterium breve, and B. infantis, as well as B. bifidum, Pediococcus pentosaceus, L. rhamnosus, and L. paracasei [34,35]. The presence of proteins in the bacterial membrane that bind to bLf can enhance its utilization as a source of iron, thereby promoting growth [35,36,37]. The prebiotic effect relies on the physiological environment, the type and concentration of lactoferrin used, the specific probiotic strain, and environmental conditions such as temperature, pH, and oxygen availability [34,35,36,37,38]. For instance, it has been observed that holo-bLf (iron-saturated) can stimulate the growth of L. acidophilus, whereas apo-bLf (iron-free) exhibits variable effects that depend on the strain and conditions [24,39]. Furthermore, lactoferrin can enhance the adhesion capacity of specific probiotic strains to the intestinal mucosa, which is vital for their colonization and protective effects [34,40]. Conversely, bLF can inhibit the growth of certain probiotics, particularly when the concentration of bLF reaches 32 mg/mL [36]. The inhibition is more potent when hydrolysis of lactoferrin (Liu et al.) [36]. Additionally, bLF inhibits the growth of pathogenic bacteria, particularly in its apo form. This has been demonstrated in several bacteria of the genus Escherichia, such as E. coli O157, and in Staphylococcus aureus by sequestering the iron necessary for their growth. Furthermore, it inhibits the formation of pathogen biofilms by altering their cell membranes and interacting with molecules involved in adhesion, like lipopolysaccharides (LPS) and fimbriae, preventing the adhesion sites required for pathogens to adhere to host epithelial cells [40,41].
Lactoferrin serves not only as a functional ingredient but also as an intelligent platform for encapsulating and protecting bioactive compounds. Its ability to create controlled and targeted release systems makes it highly appealing for pharmaceutical, nutraceutical, and cosmetic applications. It promotes interaction with anionic molecules, withstands moderate heat processes, and protects the encapsulated contents while extending shelf life [42,43]. bLF exhibits amphiphilic, biodegradable, bioadhesive, and self-assembling properties, enabling it to form supramolecular structures capable of encapsulating various compounds, such as hydrophobic drugs, antibiotics, lipophilic nutrients, and RNA/DNA [42,44,45]. These characteristics establish it as a natural carrier of bioactive compounds, including probiotics, comparable to proteins like casein or β-lactoglobulin but with the added value of its intrinsic biological activity [43,46,47].
Another method that uses coating strategies is layer-by-layer (LbL) encapsulation. This technique involves the sequential coating of probiotic cells with multiple layers of oppositely charged materials, typically polyelectrolytes such as proteins, polysaccharides, or synthetic polymers [48,49]. Each layer adheres to the preceding one through electrostatic interactions, hydrogen bonding, or hydrophobic interactions [49]. This methodology results in a nanocoating or microcapsule surrounding each bacterial cell, composed of alternating layers of charged biopolymers. Notably, LbL encapsulation adds only a few nanometers to the size of the cells, resulting in a negligible impact on their overall dimensions [48,50]. This encapsulation technique has received limited investigation when using the electrolyte bovine lactoferrin (bLf), particularly in combination with hyaluronate for drug encapsulation [51].
Therefore, the purpose of this study was to examine the effects of layer-by-layer encapsulation of Lactiplantibacillus plantarum using alginate and lactoferrin and to compare it with airbrush encapsulation, resulting in alginate microparticles loaded with L. plantarum and coated with lactoferrin.
2. Materials and Methods
2.1. Materials
Food-grade sodium alginate was obtained from Ingredient Solutions Inc. (Waldo, Maine, USA). Lactoferrin was purchased from Creative Enzymes (Shirley, Long Island, NY, USA) and Culture Media was acquired from Difco (Becton, Dickinson & Co., Sparks, MD, USA). Unless otherwise noted, the other reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Bacteria and Culture Conditions
L. plantarum subsp. plantarum was purchased from the American Type Culture Collection (ATCC, 14917). Freeze-dried bacteria were inoculated into de Man, Rogosa, and Sharpe broth (MRS) and added with 0.5% L-cysteine (MRC-Cys) according to the supplier’s instructions. Subsequently, bacteria were stored at −20 °C in cryo-culture vials containing MRC-Cys broth with 20% sterile glycerol.
2.3. SDS-PAGE Analysis of Lactoferrin
The purity of lactoferrin was confirmed under denaturing and reduced conditions using 10% polyacrylamide gels (SDS-PAGE), according to Laemmli [52]. Samples containing 25 µg of commercial lactoferrin were loaded onto duplicated gels and run at 80 V using an SE250 Mighty Small II Mini Vertical Protein Electrophoresis Unit (Hoffer, San Francisco, CA, USA) and subsequently stained with Coomassie brilliant blue R-250. The protein migration patterns were compared with the broad range of molecular weight standards from Bio-Rad and analyzed using the ImageLab program (Bio-Rad, Hercules, CA, USA) of the Molecular Imager Gel Doc XR+ (Bio-Rad, Hercules, CA, USA).
2.4. Growth Kinetics of L. plantarum in the Presence of Lactoferrin
The effect of lactoferrin (Lf) on the growth of L. plantarum was determined by the micro-dilution 96-well microtiter plate technique using Lf concentrations from 4 mg·mL−1 to 0.04 μg/mL. Experiments were conducted in a laminar flow hood (Class II, Labconco, MI, USA). A total of 100 μL of MRC-Cys broth was placed in sterile, clear polystyrene 96-well culture plates with lids (Falcon #353072), as well as lactoferrin in phosphate buffer saline (PBS; 20 mM phosphate buffer, 0.15 M NaCl), pH 7.2. The solution was diluted serially (4 mg·mL−1 to 0.5 μg·mL−1) using a multichannel pipette. For inoculum preparation, bacteria were precultured in MRC-Cys broth at 37 °C for 24 h, harvested by centrifugation, washed two times with PBS, pH 7.2, and adjusted with the same buffer at an OD of 0.70 at 600 nm (~1.5 × 109 CFU·mL−1 mL−1). A total of 100 µL of this inoculum was placed aseptically into each well. Growth kinetics were determined at 37 °C for 24 h in an Anthos Zenyth 340st spectrophotometer (Alcobendas, Madrid, Spain). Sample readings were taken every 30 min at 620 nm with constant shaking every 20 s to promote bacterial growth. MRC-Cys broth without inoculum and MRC-Cys broth with inoculum, without lactoferrin, were used as controls. The analysis was conducted using 12 observations for each treatment. The DMFit program (https://combasebrowser.errc.ars.usda.gov/DMFit.aspx, accessed on 19 April 2025) was used to establish differences in the growth of bacteria.
2.5. Zeta Potential Measurement
The zeta potential (ζ-potential) refers to the electrical potential difference between the surface of a charged particle and its surrounding medium. This measurement is a crucial indicator of colloidal stability and electrostatic interactions within suspensions. A notable change in the sign of the zeta potential (transitioning from negative to positive or vice versa) signifies that a new layer has been effectively deposited [48,50].
The zeta potential values of L. plantarum, lactoferrin, and alginate were determined at 25 °C by dynamic light scattering (DLS) using a Zetasizer Nano-ZP90 analyzer (Malvern Instruments Ltd., Worcestershire, UK). Suspension of 103 cells/mL of L. plantarum and solutions of 1 mg·mL−1 and 0.5 mg·mL−1 of lactoferrin and alginate were individually prepared using phosphate buffer (PB, 10 mM), pH 6.0, as diluent, and the ζ-potential was estimated from the mobility measurements. The mobility was converted into zeta potential using the Smoluchowski relation. Results are the average of six independent measurements and their standard deviations.
2.6. Layer-by-Layer Assembly Process
Layer-by-layer encapsulation was conducted according to Priya et al. [48] with modifications. Before assembly, L. plantarum was grown in MRC-Cys broth in anaerobiosis at 37 °C until the cells reached the logarithmic phase. Cells were collected after 24 h by centrifugation (Sorvall ST40R, Thermo Fisher Scientific, Dreieich, Germany) at 2675× g for 15 min at 4 °C, washed two times with 10 mM phosphate buffer (PB), pH 6.0, and harvested as above. Afterward, cells with a final concentration of approximately 11.2 log CFU were resuspended in PB solution and used immediately for encapsulation. Since L. plantarum is negatively charged, the deposition of the first layer was conducted using Lf (2 mg·mL−1 in PB, pH 6.0). A mixture of 1:1 bacterium/electrolyte was used for this purpose. Lf was allowed to interact with bacteria for 15 min under gentle stirring. Subsequently, the mixture was centrifuged at 2675× g for 15 min at 4 °C to remove the excess Lf, and the cells were washed twice in PB solution, pH 6.0, as described above. The supernatant was removed and the second layer was made by suspending bacteria in 5 mL of alginate solution (1 mg·mL−1 in PB, pH 6.0). The alginate was allowed to interact with cells under similar conditions to lactoferrin. Centrifugation and washing procedures were repeated. Then, the supernatant was discarded and the third layer was made by suspending the cells in 5 mL of lactoferrin (2 mg·mL−1 in PB, pH 6.0) solution under similar conditions. As shown in Figure 1A, the bacteria were individually coated with three layers of polyelectrolytes, with the last layer being positively charged to counteract its removal in the stomach’s acidic environment. After each layer formation step, the zeta potential of bacteria was measured as described previously. At the layer assembly pH of 6.0, lactoferrin is positively charged (PI: 8.7) and alginate is negatively charged (PKa: 3.4–4.6). In addition, the optimum growth pH for L. plantarum is between 5.7 and 6.5 [53].
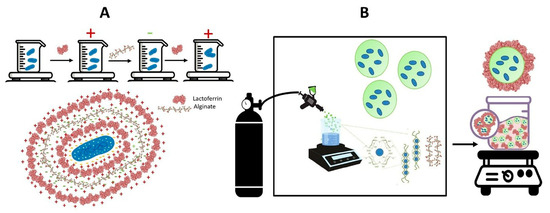
Figure 1.
Methods for encapsulating Lactiplantibacillus plantarum using bovine lactoferrin and alginate include (A) layer-by-layer encapsulation and (B) calcium alginate coated with lactoferrin through airbrush encapsulation.
Single-cell encapsulated probiotics were lyophilized at −54 °C and 0.1 mbar for 48 h (Virtis Benchtop, Model 2312, Gardiner, NY, USA) and stored at 4 °C until analysis. Five independent batches of layer-by-layer encapsulated probiotics were prepared.
2.7. Encapsulation of Lactiplantibacillus plantarum in Alginate Microbeads Coated with Lactoferrin: An Airbrush Approach
L.-plantarum-loaded calcium alginate microbeads were prepared according to Hugues-Ayala et al. [30] via an airbrush system and subsequently coated with lactoferrin (Figure 1B). A mini airbrush with a capacity of 20 mL was connected to a compressed air cylinder (Praxair Technology Inc, Alc., Azcapotzalco, CDMX, Mexico). The airbrush was positioned at a spray angle of 22.5° above the horizontal. The airbrush features include a 0.3 mm nozzle size, an air pressure of 1 bar, and a spray flow rate of 22.5 L/min.
A 1:1 suspension of 1.5% alginate and L. plantarum (11.2 log CFU·mL in phosphate buffer) was loaded into the airbrush and then extruded into a shaking bath at 70 rpm. This bath contained a 0.3 M CaCl2 solution mixed with 1% Tween 20. The spray time was 2 s, with 5 s intervals between discharges.
After cross-linking in the CaCl2–Tween solution, the resultant microbeads were allowed to harden in the shaking bath for 30 min. Subsequently, they were washed three times with double-distilled water to remove the unreacted calcium. For coating alginate particles with lactoferrin, aliquots of microbeads (0.5 g) were prepared by placing them in microtubes and immersing them in a suspension of 30 g/L lactoferrin. The tubes were gently shaken in an orbital platform shaker, model NK-0S-20 (Noke lab, Chongqing, CN), for 20 min. The coated microbeads were collected by vacuum filtration with a pore size filter of 0.22 μm and washed three times with double-distilled water to remove unbound lactoferrin. Afterward, the lactoferrin-coated microbeads were lyophilized at −54 °C and 0.1 mbar for 48 h (Virtis Benchtop, Model 2312, Gardiner, NY, USA) and stored at 4 °C until analysis. Five independent batches of lactoferrin-coated microbeads were prepared.
The zeta potential of the alginate microbeads coated with lactoferrin was determined at 25 °C using a Zeta Sizer instrument (Nano-ZS90, Malvern, UK) with individual solutions of 1 mg·mL−1 and 0.5 mg·mL−1 diluted in deionized water. Experiments were conducted in triplicate.
2.8. Sizes and Morphology of Encapsulated Probiotics
The particle size of LbL-encapsulated probiotics was measured at a scattering angle of 90° and a constant temperature of 25 °C, utilizing a Zetasizer Nano-ZS90 (Malvern Instruments Ltd., Worcestershire, UK) through dynamic light scattering (DLS). The size of the lactoferrin-coated alginate microbeads was assessed using a particle size analyzer (Mastersizer Micro V 2.19, Malvern, UK). Data collection was conducted with the Mastersizer Micro Ver. 2.19 software (Malvern, UK). All analyses were carried out in triplicate.
The morphology of the probiotic vehicles was acquired by scanning electron microscopy (SEM) in a JEOL instrument (JSM-5900LV, Tokyo, JPN) operating at 10 kV electron acceleration. Dried samples were fixed on aluminum cylinders using graphite tape and coated with a thin gold layer (60 Ǻ) before observation.
2.9. Cell Entrapment Efficiency
The cell entrapment efficiency (CE) was determined by calculating the difference between the viable cell count before and after each immobilization process [30]. Each milligram of the carriers (either layer-by-layer single-cell assembled or alginate-coated microbeads) was individually dispersed in 10 mL of 1% sodium citrate (w/v) and shaken for 15 min at 25 °C. The survival of the released bacteria was determined by performing 10-fold serial dilutions and plating on MRS-cys agar plates. After 48 h of incubation at 37 °C, the total viable cells were calculated and reported as log CFU.
CE was calculated using the following equation:
CE = (N/N0) × 100
N0 is the initial number of free viable cells (CFU·mL−1) used for immobilization and N is the number of viable cells released in CFU·mL−1.
2.10. Survival of Immobilized Lactiplantibacillus plantarum After Lyophilization
To preserve the viability of L. plantarum for long periods, both probiotic carriers (layer-by-layer encapsulation or alginate-coated microbeads) were individually freeze-dried. To determine the survival of L. plantarum after freeze drying, 0.5 g of the probiotic-loaded vehicles was collected and separately dispersed in a sterile 1% (w/v) sodium citrate solution. Viable bacteria were determined using 10-fold serial dilutions and plating on MRC-Cys agar plates. Free lyophilized bacteria were used as controls. Total viable bacteria after lyophilization (TL) were calculated by following the equation:
TL = (NL·ML)
NL (CFU·g−1) is the number of viable cells released from 0.5 g of microbeads and ML is the dry weight of lyophilized microbeads [54,55]. The experiment was conducted in triplicate.
2.11. Survival of L. plantarum During Storage
For storage stability analysis, probiotic-loaded vehicles were stored at −20 °C in parafilm-sealed tubes wrapped in aluminum foil. The viability of L. plantarum after 30 and 90 days of storage was determined by 10-fold serial dilution and plating on MRC-Cys agar. Free lyophilized bacteria were used as controls.
2.12. Survival of L. plantarum Under Simulated Gastrointestinal Conditions
The viability of L. plantarum under simulated gastrointestinal (GI) conditions was determined according to Mendoza-Madrigal et al. [54] in a laminar flow chamber. Two bioreactors maintained at 37 °C with controlled shaking (50 rpm) were connected by a peristaltic pump to simulate the stomach and intestine, respectively. The sterile simulated gastric juice (GJ) was prepared in a 0.02 M phosphate buffer containing 9 g/L of NaCl, 3 g/L of pepsin, and 4 g/L of gastric mucin. The pH of the solution was adjusted to 2.0 ± 0.2 using 1 M HCl [55]. The sterile simulated intestinal juice (IJ) was formulated in 0.02 M phosphate buffer containing 10 g·L−1 of bovine pancreas trypsin, 1 g·L−1 of pancreatin, 4 g·L−1 of mucin, and 0.3 g·100 mL−1 bile salts. The pH was adjusted to 6.8 ± 0.1 by using 1 M NaOH.
For the survival analysis, 0.5 g of probiotic-loaded vehicles were individually mixed with 10 mL of ultra-pasteurized milk to create two milk–cell suspensions (MCS1 for LbL encapsulation and MRC2 for coated microbeads). Each MCS was individually suspended in 20 mL of GJ for 90 min of incubation in the first bioreactor. Afterward, the gastric content was moved to the second bioreactor containing 20 mL of IJ and incubated for 150 min. Throughout the experiment, 1 mL aliquot samples were taken from each bioreactor every 30 min to assess the survival of L. plantarum. This was determined by making 10-fold dilutions and then plating the samples on MRC-Cys agar plates. The results were reported in log CFU·mL−1.
2.13. Statistical Analysis
All experiments were conducted in triplicate. A one-way analysis of variance (ANOVA) was used to assess the encapsulation efficiency, survival under storage conditions, and the survival data of L. plantarum in simulated gastrointestinal conditions. All data were analyzed using the NCSS statistical package (Version 2019). The Tukey–Kramer test was employed to compare the means. Statistical significance was defined as a probability level of 0.05.
3. Results and Discussion
3.1. Lactoferrin Analysis
The purity of bovine lactoferrin (bLf) used in this study was proved by 10% SDS-PAGE gel (Figure 2A). A majority band at the same position as bLf (79.8 ± 0.6 kDa) confirms its purity [56].
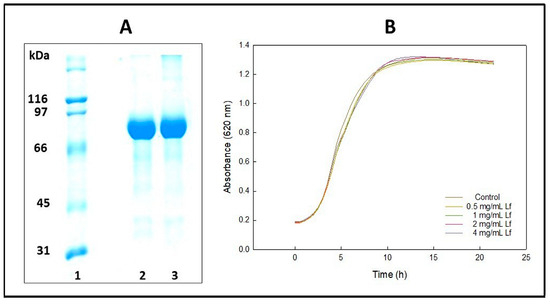
Figure 2.
Bovine lactoferrin characterization. (A) Electrophoresis of lactoferrin under denaturing and reducing conditions. Lane 1, molecular mass standards. Lanes 2 and 3, bovine lactoferrin. (B) The effect of lactoferrin on the growth of Lactiplantibacillus plantarum was assessed at concentrations ranging from 4 mg/mL to 0.5 mg/mL.
Figure 2B displays the growth curves of L. plantarum when exposed to lactoferrin at concentrations ranging from 0.5 mg·mL−1 to 4 mg·mL−1. There was a slight delay in the onset of the exponential phase, although this delay was not statistically significant (p > 0.05), as shown in Table 1. While variations in the growth rates of the bacteria were observed, no significant differences were found in the growth levels at which the stationary phase was reached. In conclusion, the protein did not inhibit the growth of the probiotic. These results support the idea that lactoferrin will not affect the survival of L. plantarum encapsulated either by layer-by-layer encapsulation with bLf and alginate or by alginate coated with bLf.

Table 1.
Growth parameters of Lactiplantibacillus plantarum in the presence of lactoferrin.
Bovine lactoferrin has been found to inhibit various pathogens like Escherichia coli, Salmonella typhimurium, Enterococcus faecalis, and Staphylococcus aureus. However, it does not affect the growth of certain strains of Lactobacillus, such as Lactobacillus rhamnosus ATCC 7469, L. reuteri ATCC 23272, L. acidophilus BCRC, and L. fermentum ATCC 11739 [57].
Oda et al. [58] reported that bLf either enhances the growth of different species of Lactobacillus or does not affect it, while Petschow et al. [59] stated that bLF exhibited prebiotic activity, independent of receptor binding capacity and iron saturation level. Moreover, Xu et al. [60] developed a genetically engineered L. plantarum that produces porcine lactoferrin without affecting the growth of this probiotic. This lactoferrin resistance of probiotics has been attributed to several factors, such as lower expression of lactoferrin binding proteins on the cell surface of probiotics compared to the surface of pathogens [36,61].
3.2. Zeta Potential Assays
Zeta potential (ζ-potential) was used to determine the surface electrical charge of free and immobilized L. plantarum. This property characterizes the electrochemical potential at the shear plane of the double layer surrounding a bacterium in solution [62]. Free L. plantarum shows a ζ-potential of-10.9 ± 0.9 mV. The net surface charge of most bacteria is usually negative due to the presence of ionized groups on the cell wall, such as teichuronic and teichoic acids in Gram-positive bacteria [62].
The changes in the ζ-potential surface of L. plantarum after the deposition of each polyelectrolyte layer in the LbL procedure were monitored. As shown in Figure 3A, the adsorption of subsequent layers displays alternate negative and positive ζ potential. Adding the first layer of bLf modified the ζ-potential of the bacteria’s surface by raising it to 5.13 ± 0.48 mV. The deposition of the next alginate layer caused a further change in the zeta potential, reaching values of -43.4 9 ± 1.3 mV. When the last layer of bLf was added, positive zeta potential values (+19.5 ± 1.0 mV) were again reached. These results indicate the success of the layer-by-layer procedure [48].
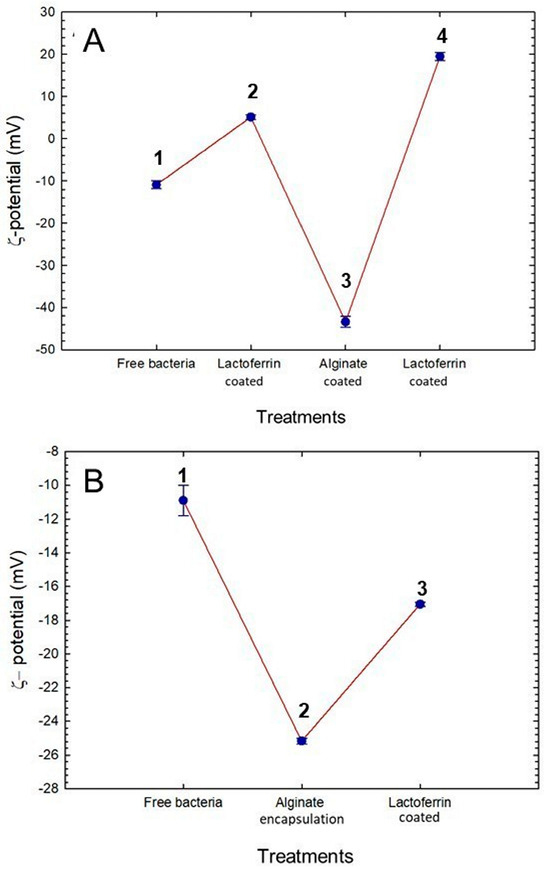
Figure 3.
Changes in ζ-potential during immobilization of Lactiplantibacillus plantarum. (A) Changes in layer-by-layer encapsulation. A1, z-potential of free bacteria; A2; A3, and A4, changes in z-potential after addition of lactoferrin, alginate, and lactoferrin layers, respectively. (B) Changes in alginate microbeads coated with lactoferrin through airbrush encapsulation. B1, z-potential of free bacteria; B2, and B3, changes in ζ-potential after encapsulation with alginate and coated with lactoferrin, respectively.
The zeta potential of bLf-coated alginate microbeads was also measured (see Figure 3B). When L. plantarum was encapsulated with alginate, the ζ-potential increased from −10.9 ± 0.9 mV (free bacteria) to −25.16 ± 0.17 mV. This increase results from the nonreacted mannuronic acid carboxyl groups of alginates, giving the microcapsules a negative surface charge [63]. The coating process with bLf caused an additional reduction in the surface charge to −17.05 ± 0.12 mV, indicating the presence of a bLf layer around the particles.
Monitoring changes in zeta potential is an effective method for characterizing both encapsulation techniques. Observing the alteration in zeta potential during a layer-by-layer (LbL) encapsulation process is crucial for verifying the successful deposition of each electrolyte layer [48]. Conversely, the variation in zeta potential of an alginate capsule after it has been coated with a protein primarily reflects the electrostatic interaction between the alginate and the protein, in addition to indicating the effectiveness of the surface coating [30].
Positive or neutral charge on the probiotic surface, like that of layer-by-layer encapsulations, may enhance the adhesion of L. plantarum to enterocytes, given that enterocyte surfaces are primarily negative [64,65]. Electrostatic repulsion from negative charges can hinder adherence. In the case of alginate Lf-coated microcapsules, the probiotic is expected to be released in the intestine. These probiotics carry a negative charge, making it challenging to adhere to enterocytes [64,65]. However, they possess specific adhesion factors that can counteract the repulsion from negative charges. These interactions rely not just on electrostatic forces but also on biochemical mechanisms, including adhesion proteins, components of the extracellular matrix, and low-energy interactions like hydrogen bonds and Van der Waals forces [64,65]. Notably, L. plantarum produces specific surface proteins called adhesins, such as mucin-binding proteins (Mub), mucus adhesion-promoting protein A (MapA), and Lp_1643, which binds to glycoproteins. These adhesins facilitate the adhesion of the probiotic to the intestine [66].
3.3. Sizes and Morphology of Encapsulated Probiotics
The size of the particles and their polydispersity index (PDI) are key physical properties for evaluating the suitability of adding encapsulated probiotics in food formulations, as well as their cohesiveness and fluidity during storage conditions [67]. Figure 4A shows that the particle size of free L. plantarum was 1.42 ± 0.49 μm, while, after layer-by-layer encapsulation, the particle sizes rose to 3.9 ± 0.56 μm. The size increase indicates that probiotics have been successfully encapsulated in the lactoferrin–alginate–lactoferrin matrix [68,69]. The free bacteria exhibited a polydispersity index of 0.132, whereas the encapsulated bacteria showed a polydispersity index of 0.236. In both scenarios, monodisperse populations were present, with no additional populations of different sizes detected. This indicates that the bacteria did not aggregate.
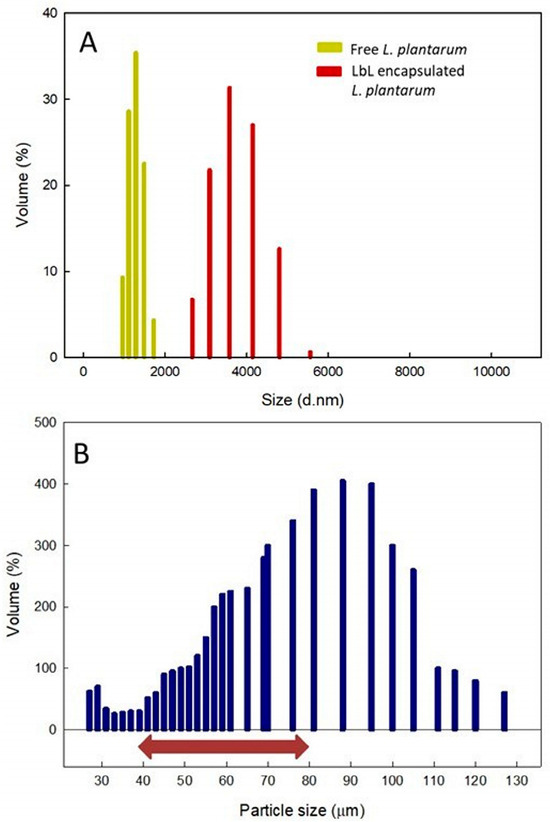
Figure 4.
Particle size distribution of free and encapsulated Lactiplantibacillus plantarum. (A) Size comparison of free L. plantarum (yellow) versus L. plantarum encapsulated using layer-by-layer methods (red). (B) L. plantarum encapsulated within lactoferrin-coated alginate microcapsules. The red arrow highlights the optimal capsule size range for integrating probiotics into food.
Figure 4B displays the size distribution of alginate microbeads coated with lactoferrin containing L. plantarum. Although the microbeads showed a broad spectrum of sizes, only a small percentage (25.72%) measured under 44 μm. The survival of the encapsulated bacteria diminishes as capsule size decreases, mainly due to the creation of more fragile wall matrices [70,71]. The remaining particles (83.02%) had diameters varying from 45 to 255 μm, a range linked to improved survival rates [29]. Nonetheless, other research indicates that particles larger than 80 μm can produce a gritty texture and an unpleasant taste [28]. Consequently, utilizing the AAcL technique outlined in this study, around 51% of the capsules produced would be within an optimal size range.
The morphology of probiotics vehicles was examined using scanning electron microscopy. Figure 5A,B display the micrographs of LbL encapsulates. Instead of microcapsules, the images revealed the presence of amorphous aggregates. This indicates that the encapsulation matrices were added to the bacteria individually, encapsulating them separately. The LbL approach is based on a surface modification procedure, which covers the probiotic surface to protect bacteria from challenging environments by adsorbing oppositely charged electrolytes, providing probiotics with new characteristics [50]. The SEM micrographs of alginate microbeads coated with bLf showed a quasi-spheroidal morphology with some depositions on the microcapsule surface, corresponding to lactoferrin (Figure 5C,D).
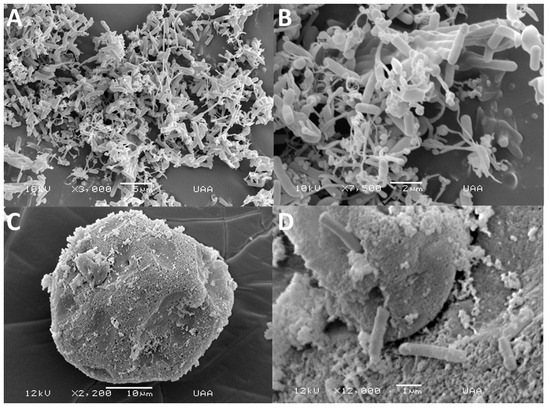
Figure 5.
Scanning electron microscopy images of layer-by-layer assembled Lactiplantibacillus plantarum (A,B) combined with bovine lactoferrin and alginate, as well as alginate microbeads that were loaded with L. plantarum and coated with bovine lactoferrin (C,D).
3.4. Cell Viability After Immobilization and Lyophilization
Table 2 illustrates the effect of the LbL process and alginate-coated encapsulation on the viability of L. plantarum cells. A 2.9-log CFU decrease in bacteria viability was observed when immobilized using the LbL-assembled process. In comparison, bacteria immobilized on alginate microbeads coated with lactoferrin showed a 3.3-log CFU decline in viability compared to the initial concentration. A possible explanation for this behavior is that the LbL method produces a consistent coating that tightly adheres to the cell surface, ensuring individualized protection for the probiotic during oxidative stress and dehydration typically encountered during freeze drying [72]. Conversely, coated microbeads encapsulate multiple cells within a larger matrix, which may not afford the same level of protection for each cell or customized protection for probiotic bacteria cells [31,69].

Table 2.
Viability of Lactiplantibacillus plantarum after immobilization and lyophilization.
The results reported here differ from those of Yucel Falco et al. [73] and Liu et al. [49]. Yucel Falco et al. [73] observed a 2-log CFU decrease in cell viability when immobilizing L. acidophilus using a layer-by-layer assembly process with four total layers of β-glucan and chitosan. Liu et al. [33] found a reduced viability of only 0.47 log CFU when immobilizing L. plantarum 550 with five total layers of zein and pectin. The lower cell viability in our study may be attributed to the limited loading capacity associated with the LbL assembly process using alginate and lactoferrin.
When examining lactoferrin and zein for encapsulating L. plantarum, each presents unique advantages based on its biochemical properties. Zein, which is highly hydrophobic, forms dense films that withstand the acidic environment of the stomach, thereby providing enhanced protection to probiotics like L. plantarum during gastric transit. However, zein needs alcohol–water mixtures or organic solvents for adequate dispersion, which can potentially harm probiotic cells [74]. In contrast, lactoferrin is a water-soluble protein that facilitates layer-by-layer (LbL) and coating encapsulation techniques in mild aqueous conditions, thus preserving the viability of probiotics [75]. Lactoferrin’s ability to enhance mucosal adhesion could improve the retention and colonization of probiotics within the gastrointestinal tract. Moreover, lactoferrin possesses immunomodulatory and prebiotic qualities, which can synergistically enhance gut health and the efficacy of probiotics [37,59]. Additionally, its varied bioactivity and good human tolerance make lactoferrin appealing for probiotic encapsulation [42]. Researchers are exploring alginate–lactoferrin coatings in the food industry due to their biocompatibility, safety, and synergistic effectiveness in extending the shelf life of perishable goods. These coatings help inhibit microbial growth on food surfaces, improving safety and longevity, making them suitable for pharmaceutical, cosmetic, and food-grade uses [33,34,35,36,42,47].
However, lactoferrin demonstrated lower protective capability in this study than other polycations, potentially due to its layer-forming properties. One possible solution to mitigate this limitation is to modify lactoferrin with polyethylene glycol or functionalize it with other molecules to increase positive charge concentration [76,77,78]. Nonetheless, additional research is required to confirm this strategy.
There was a significant difference (p < 0.05) in the viability of probiotics immobilized by the LbL assembly process (8.3 ± 0.30; 74%) compared to those encapsulated with alginate and coated with lactoferrin (7.4 ± 0.28; 66%). Both methods demonstrated low survival rates following the immobilization of L. plantarum. This issue may be linked to the low encapsulation efficiency observed in both approaches (52.7% for LbL and 32.6% for AAcL), suggesting losses during immobilization. In the case of layer-by-layer (LbL) encapsulation, adding each layer is a labor-intensive process that requires continuous centrifugation and washing, during which some bacteria may be lost, hindering effective encapsulation [15,47,48,49].
Similarly, in the alginate-coating method with lactoferrin, the device designed for making alginate capsules through airbrush encapsulation may have caused some bacterial losses during the process [30]. However, Hugues Ayala et al. [30] encapsulated Lactobacillus rhamnosus GG in alginate microcapsules coated with buttermilk proteins and noted a decrease in bacterial survival of 1.62 log after the encapsulation process. In this study, alginate was coated with lactoferrin, and a reduction in survival of 3.8 log-CFU was observed. These differences may be due to the type of species encapsulated. Another reason may be that the lactoferrin coating was insufficient to coat the alginate capsules’ pores (see Figure 5C).
As shown in Table 2, the survival rate of L. plantarum encapsulated using the LbL method after freeze drying was higher (5.6 ± 0.21; 67.5%) compared to the probiotic encapsulated using the alginate method coated with lactoferrin (3.6 ± 0.30; 48.7%). Priya et al. [48] reported survival rates of 92% log for L. acidophilus that was freeze-dried after being encapsulated through layer-by-layer self-assembly using polyelectrolytes such as chitosan (CHI) and carboxymethyl cellulose (CMC). Similarly, Hugues Ayala et al. [30] observed survival rates of 96% log for L. rhamnosus GG after freeze drying while using alginate coated with buttermilk proteins using airbrush encapsulation.
The relatively low survival rates observed in this study for both methods could be linked to the matrices and the encapsulated specific probiotic species [16]. Although lactoferrin is a charged protein capable of acting as a coating or aiding in layer formation in conjunction with other electrolytes, its nonpolymeric nature may hinder coating efficiency and the creation of stable layers.
Due to its globular structure, Lf is less capable of forming dense or flexible layers than linear polyelectrolytes [43,73]. One potential strategy to enhance its encapsulation efficiency and effectiveness in creating layers or coatings is to conjugate Lf with polyethylene glycol (PEG) [76]. PEGylation can improve the stability and solubility of Lf, thus enhancing its performance in LbL or coating systems [46,78,79]. Alternatively, introducing additional amine groups to the Lf molecule may increase its positive charge, strengthening its interaction with anionic polymers such as alginate [76].
Although the survival rates are low, both immobilization methods successfully safeguarded the probiotics. This is evidenced by the lyophilization of free bacteria, which led to a 10-log CFU decline in viability (Table 2).
The amount of L. plantarum needed to produce health benefits varies based on the specific strain and the intended effect. For instance, a daily intake of 109 colony-CFU is recommended to alleviate irritable bowel syndrome [80]. Therefore, it is crucial to continue developing and improving single-cell encapsulation techniques [29,47,48,49,72].
3.5. Stability During Storage
After lyophilization, the layer-by-layer assembled probiotic and the probiotic loaded in alginate-coated microbeads were stored at −20 °C. Table 3 shows the decrease in cell viability at 30 and 90 days of storage. Cell viability decreased as the storage time increased under all the tested conditions. However, the survival rate of the probiotic was significantly lower (p < 0.05) in the LbL-assembled probiotic. After 30 and 90 days of storage, the survival rate of the single-cell LbL-assembled probiotic decreased by 32% and 41%, respectively. In comparison, the probiotic loaded in alginate-coated microbeads showed a survival decline of 15% after 30 days and 28% after 90 days. However, in an analysis of the final survival rate of L. plantarum after lyophilization and a subsequent three-month storage period at -20 °C, it was found that the overall survival rate was significantly higher for the bacteria encapsulated using the LbL technique (59%) compared to those encapsulated in alginate coated with lactoferrin (47%).

Table 3.
Viability of Lactiplantibacillus plantarum after storage at −20 °C.
Various biological, physical, and chemical factors affect the preservation of L. plantarum at -20 °C. Different probiotic strains of L. plantarum exhibit varying stress tolerance levels, particularly to cold and freezing conditions [81]. Cells encapsulated during the late exponential or early stationary phase survive better than those harvested during the logarithmic phase [16,29].
Slow freezing can lead to the formation of large ice crystals, which can damage cell membranes. Additionally, prolonged storage may result in a gradual loss of cell viability. Cryoprotectants such as glycerol, skim milk, glucose, trehalose, soy polysaccharides, prebiotics, or sucrose can be added to counteract these issues [82,83,84]. These substances help stabilize cell membranes and proteins during freezing by preventing large ice crystal formation [82].
Excess moisture can also cause cellular damage, making freeze drying a recommended method before storage [30]. High osmotic pressure can stress the cells, so buffering agents are recommended [49]. Furthermore, reactive oxygen species (ROS) can cause damage during freezing and storage; however, antioxidants such as ascorbic acid and glutathione can help reduce this oxidative stress [16]. All these factors are crucial for future research to enhance the probiotics’ encapsulated survival through the methods used in this study.
3.6. Survival Under In Vitro Simulated Gastrointestinal Conditions
The viability of L. plantarum decreased considerably when the carrier vehicles were exposed to simulated in vitro gastrointestinal conditions (Figure 6). After 90 min of incubation in simulated gastric juice, a 1.7 log decrease in viable counts was observed for both the probiotic assembled in LbL and that encapsulated in protein-coated alginate. At the end of incubation in the simulated intestinal juice, a total reduction of 2.2 log in viable counts was observed. These results contrast with those obtained by Liu et al. [49], who used the LbL technique to assemble L. plantarum 550 into zein and pectin layers. The loss of viability using zein and pectin depended on the number of layers added and, in some of their treatments, it was only 1.28 log. The researchers attributed the protective effect against acidic conditions to the buffering capacity of zein. In contrast, it has been observed that lactoferrin can be hydrolyzed under acidic gastrointestinal conditions and that it can be degraded by intestinal proteases [85]. These effects may have decreased the protection of the probiotic. However, further studies should be carried out to prove it.
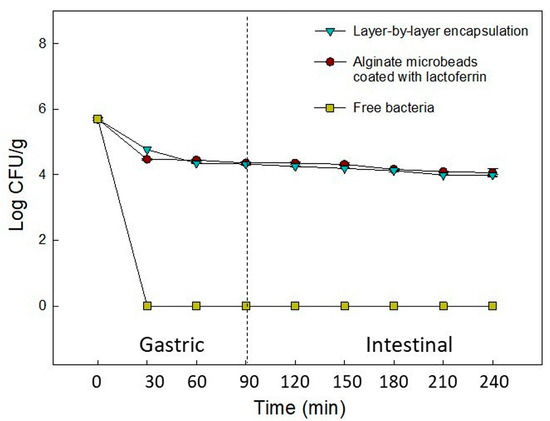
Figure 6.
Viability of Lactiplantibacillus plantarum microencapsulated by layer-by-layer encapsulation and alginate-coated lactoferrin via airbrush encapsulation, subjected to a sequential simulated gastrointestinal condition.
4. Conclusions
Lactoferrin and alginate were used as coating materials for the encapsulation of L. plantarum through a layer-by-layer (LBL) technique and for calcium alginate coated with lactoferrin via airbrush encapsulation. The LBL system offered better protection for the probiotic than the alginate-coated system after lyophilization and during storage at −20 °C, although both systems performed similarly under simulated gastrointestinal conditions. Both methods effectively maintained the viability of the bacteria after the in vitro test that simulated gastrointestinal tract conditions, unlike the free bacteria that underwent the same treatment.
The protective effect of both encapsulation methods was evident when comparing the impact of different testing conditions on free-living L. plantarum cells. However, final counts remained below the recommended 10⁹ CFU per daily dose for clinical efficacy. This shortfall was mainly due to the low encapsulation efficiency of both methods, which affected the cell counts after freeze drying and influenced the results of the subsequent tests.
Moreover, the number of layers used in the layer-by-layer (LBL) system and the lactoferrin coating on the alginate microcapsules may not have adequately protected the probiotics against the tested stresses. In addition, the globular shape of Lf restricts its ability to form dense or flexible layers compared to linear polyelectrolytes. This limitation may impact the survival of probiotics if they do not have sufficient coating. One potential solution is to alter the lactoferrin molecule through pegylation or by introducing amino groups to enhance charge density. Therefore, ongoing research is needed to develop improved strategies for improving the encapsulation efficiency of both systems.
Future studies should consider increasing the number of layers in the LBL system and enhancing the alginate coating with lactoferrin. Additionally, new experiments should explore combinations of different molecules, such as polysaccharide–protein, protein–protein, and polysaccharide–protein, to identify synergistic protective effects for the bacteria. Improving these encapsulation methods could lead to more effective probiotic incorporation into foods in the future.
Author Contributions
Conceptualization, G.R.C.M. and C.C.-W.; Methodology, N.I.D.-N. and S.G.L.-F.; Formal Analysis, R.R.B.-Q., C.C.-W. and N.I.D.-N.; Investigation, N.I.D.-N., S.G.L.-F., R.R.B.-Q. and C.C.-W.; Resources, G.R.C.M., C.C.-W. and R.R.B.-Q.; Data Curation, C.C.-W., R.R.B.-Q. and G.R.C.M.; Writing—Original Draft Preparation, S.G.L.-F. and G.R.C.M.; Writing—Review and Editing, G.R.C.M.; Visualization, G.R.C.M. and C.C.-W.; Supervision, C.C.-W., R.R.B.-Q., S.G.L.-F. and G.R.C.M.; Project Administration, G.R.C.M.; Funding Acquisition, G.R.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
The Secretary of Science, Humanities, Technology and Innovation of Mexico, SECIHTI, project CB-169358, supported this work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not aplicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Secretary of Science, Humanities, Technology and Innovation of Mexico, SECIHTI, for the financial support for this research under project CB-169358, and the scholarship awarded for MSc studies. The authors thank the Institutional Analytical Platform of CIAD, project PAI-10363.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Sarita, B.; Samadhan, D.; Hassan, M.Z.; Kovaleva, E.G. A comprehensive review of probiotics and human health-current prospective and applications. Front. Microbiol. 2025, 15, 1487641. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; FAO/WHO: Cordoba, Argentina, 2001. [Google Scholar]
- Gul, S.; Durante-Mangoni, E. Unraveling the puzzle: Health benefits of probiotics—A comprehensive Review. J. Clin. Med. 2024, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic species in the modulation of gut microbiota: An overview. BioMed. Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef]
- Vera-Santander, V.E.; Hernández-Figueroa, R.H.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Health benefits of consuming foods with bacterial probiotics, postbiotics, and their metabolites: A review. Molecules 2023, 28, 1230. [Google Scholar] [CrossRef]
- Aljohani, A.; Rashwan, N.; Vasani, S.; Alkhawashki, A.; Wu, T.T.; Lu, X.; Castillo, D.A.; Xiao, J. The Health benefits of probiotic Lactiplantibacillus plantarum: A systematic review and Meta-Analysis. Probiotics Antimicrob. Proteins 2024. [Google Scholar] [CrossRef] [PubMed]
- Arasu, M.V.; Al-Dhabi, N.A.; Ilavenil, S.; Choi, K.C.; Srigopalram, S. In vitro importance of probiotic Lactobacillus plantarum related to medical field. Saudi J. Biol. Sci. 2016, 23, S6–S10. [Google Scholar] [CrossRef]
- Hofeld, B.C.; Puppala, V.K.; Tyagi, S.; Ahn, K.W.; Anger, A.; Jia, S.; Salzman, N.H.; Hessner, M.J.; Widlansky, M.E. Lactobacillus plantarum 299v probiotic supplementation in men with stable coronary artery disease suppresses systemic inflammation. Sci. Rep. 2021, 11, 3972. [Google Scholar] [CrossRef]
- Li, C.P.; Chen, C.C.; Hsiao, Y.; Kao, C.H.; Chen, C.C.; Yang, H.J.; Tsai, R.Y. The role of Lactobacillus plantarum in reducing obesity and inflammation: A Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 7608. [Google Scholar] [CrossRef]
- Sudha, M.R.; Ahire, J.J.; Jayanthi, N.; Tripathi, A.; Nanal, S. Effect of multi-strain probiotic (UB0316) in weight management in overweight/obese adults: A 12-week double blind, randomized, placebo-controlled study. Benef. Microbes 2019, 10, 855–866. [Google Scholar] [CrossRef]
- Rahayu, E.S.; Mariyatun, M.; Putri Manurung, N.E.; Hasan, P.N.; Therdtatha, P.; Mishima, R.; Komalasari, H.; Mahfuzah, N.A.; Pamungkaningtyas, F.H.; Yoga, W.K.; et al. Effect of probiotic Lactobacillus plantarum Dad-13 powder consumption on the gut microbiota and intestinal health of overweight adults. World J. Gastroenterol. 2021, 27, 107–128. [Google Scholar] [CrossRef]
- Håkansson, Å.; Andrén Aronsson, C.; Brundin, C.; Oscarsson, E.; Molin, G.; Agardh, D. Effects of Lactobacillus plantarum and Lactobacillus paracasei on the peripheral immune response in children with Celiac Disease Autoimmunity: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2019, 11, 1925. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ma, C.; Zhao, F.; Chen, P.; Liu, Y.; Sun, Z.; Cui, L.; Kwok, L.Y.; Zhang, H. Adjunctive treatment with probiotics partially alleviates symptoms and reduces inflammation in patients with irritable bowel syndrome. Eur. J. Nutr. 2021, 60, 2553–2565. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Park, S.; Paik, J.W.; Chae, S.W.; Kim, D.H.; Jeong, D.G.; Ha, E.; Kim, M.; Hong, G.; Park, S.H.; et al. Efficacy and safety of Lactobacillus Plantarum C29-Fermented soybean (DW2009) in individuals with mild cognitive impairment: A 12-Week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo-controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Dalbhagat, C.G.; Mishra, H.N. Emerging technologies and coating materials for improved Probiotication in food products: A review. Food Bioprocess Technol. 2022, 15, 998–1039. [Google Scholar] [CrossRef]
- Cassani, L.; Gomez-Zavaglia, A.; Simal-Gandara, J. Technological strategies ensuring the safe arrival of beneficial microorganisms to the gut: From food processing and storage to their passage through the gastrointestinal tract. Food Res. Int. 2020, 129, 108852. [Google Scholar] [CrossRef]
- Penha Rodrigues Pereira, E.; Silva da Graça, J.; Manfrinato Ferreira, B.; Fasura Balthazar, C.; Xavier-Santos, D.; França Bezerril, F.; Magnani, M.; Sant’Ana, A.S. What are the main obstacles to turning foods healthier through probiotics incorporation? A review of functionalization of foods by probiotics and bioactive metabolites. Food Res. Int. 2024, 176, 113785. [Google Scholar] [CrossRef]
- Tyutkov, N.; Zhernyakova, A.; Birchenko, A.; Eminova, E.; Nadtochii, L.; Baranenko, D. Probiotics viability in frozen food products. Food Biosci. 2022, 50, 101996. [Google Scholar] [CrossRef]
- Guo, Q.; Li, S.; Tang, J.; Chang, S.; Qiang, L.; Du, G.; Yue, T.; Yuan, Y. Microencapsulation of Lactobacillus plantarum by spray drying: Protective effects during simulated food processing, gastrointestinal conditions, and in kefir. Int. J. Biol. Macromol. 2022, 194, 539–545. [Google Scholar] [CrossRef]
- Song, S.; Cui, Y.; Ji, X.; Gao, F.; Zhu, H.; Zhu, J.; Liu, X.; Guan, J. Microencapsulation of Lactobacillus plantarum with enzymatic hydrolysate of soybean protein isolate for improved acid resistance and gastrointestinal survival in vitro. Int. J. Food Eng. 2022, 18, 499–511. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, Y.; Xie, Q.; Chen, H.; Zhang, Y.; Hong, Z.; Chen, S.; Zhang, M.e. Microencapsulation of Lactobacillus plantarum with Improved survivability using pufferfish skin gelatin-based wall materials. Mar. Drugs 2024, 22, 124. [Google Scholar] [CrossRef] [PubMed]
- Tee, W.F.; Nazaruddin, R.; Tan, Y.N.; Ayob, M.K. Effects of encapsulation on the viability of potential probiotic Lactobacillus plantarum exposed to high acidity conditions and presence of bile salts. Food Sci Technol Int. 2014, 20, 399–404. [Google Scholar] [CrossRef]
- Bautista Villarreal, M.; Castillo Hernández, S.L.L.; López Uriarte, S.; Barrón González, M.P. Encapsulation of Lactiplantibacillus plantarum and beetroot extract with alginate and effect of capsules on rheological properties and stability of an oil-in-water emulsion model food. Pol. J. Food Nutr. Sci. 2023, 73, 242–252. [Google Scholar] [CrossRef]
- Mahmoud, M.; Abdallah, N.A.; El-Shafei, K.; Tawfik, N.F.; El-Sayed, H.S. Survivability of alginate-microencapsulated Lactobacillus plantarum during storage, simulated food processing and gastrointestinal conditions. Heliyon 2020, 6, e03541. [Google Scholar] [CrossRef]
- Ni, F.; Luo, X.; Zhao, Z.; Yuan, J.; Song, Y.; Liu, C.; Huang, M.; Dong, L.; Xie, H.; Cai, L.; et al. Enhancing viability of Lactobacillus plantarum encapsulated by alginate-gelatin hydrogel beads during gastrointestinal digestion, storage and in the mimic beverage systems. Int. J. Biol. Macromol. 2023, 224, 94–104. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Martin, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Effect of unmodified starch on viability of alginate-encapsulated Lactobacillus fermentum CECT5716. Lebenson. Wiss. Technol. 2013, 53, 480–486. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C.; Nagarajan, M.; Kumar, P.K.; Singh, S.S.; Manvi, D.; Gowda, N.A.N. A comprehensive review on microencapsulation of probiotics: Technology, carriers and current trends. Appl. Food Res. 2023, 3, 100248. [Google Scholar] [CrossRef]
- Hugues-Ayala, A.M.; Sarabia-Sainz, J.A.-i.; González-Rios, H.; Vázquez-Moreno, L.; Ramos-Clamont Montfort, G. Airbrush encapsulation of Lactobacillus rhamnosus GG in dry microbeads of alginate coated with regular buttermilk proteins. Lebenson. Wiss. Technol. 2020, 117, 108639. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Smaoui, S.; Chaari, M.; Varzakas, T.; Can Karaca, A.; Jafari, S.M. Encapsulation of probiotics within double/multiple layer beads/carriers: A concise review. Molecules 2024, 29, 2431. [Google Scholar] [CrossRef]
- Gbassi, G.K.; Vandamme, T.; Ennahar, S.; Marchionni, E. Microencapsulation of Lactobacillus plantarum spp in an alginate matrix coated with whey proteins. Int. J. Food Microbiol. 2009, 129, 103–105. [Google Scholar] [CrossRef]
- Bokkhim, H.; Bansal, N.; Grøndahl, L.; Bhandari, B. Interactions between different forms of bovine lactoferrin and sodium alginate affect the properties of their mixtures. Food Hydrocoll. 2015, 48, 38–46. [Google Scholar] [CrossRef]
- Vega-Bautista, A.; de la Garza, M.; Carrero, J.C.; Campos-Rodríguez, R.; Godínez-Victoria, M.; Drago-Serrano, M.E. The impact of lactoferrin on the growth of intestinal inhabitant bacteria. Int. J. Mol. Sci. 2019, 20, 4707. [Google Scholar] [CrossRef]
- Chen, P.W.; Liu, Z.S.; Kuo, T.C.; Hsieh, M.C.; Li, Z.W. Prebiotic effects of bovine lactoferrin on specific probiotic bacteria. Biometals 2017, 30, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.S.; Chen, P.W. Featured prebiotic agent: The roles and mechanisms of direct and indirect prebiotic activities of lactoferrin and its application in disease control. Nutrients 2023, 15, 2759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, N.; Ashaolu, T.J. Prebiotic and modulatory evidence of lactoferrin on gut health and function. J. Funct. Foods 2023, 108, 105741. [Google Scholar] [CrossRef]
- Kenny, O.; FitzGerald, R.J.; O’Cuinn, G.; Beresford, T.; Jordan, K. Growth phase and growth medium effects on the peptidase activities of Lactobacillus helveticus. Int. Dairy J. 2003, 13, 509–516. [Google Scholar] [CrossRef]
- Kim, W.-S.; Ohashi, M.; Tanaka, T.; Kumura, H.; Kim, G.-Y.; Kwon, I.-K.; Goh, J.-S.; Shimazaki, K. Growth-promoting effects of lactoferrin on L. acidophilus and Bifidobacterium spp. Biometals 2004, 17, 279–283. [Google Scholar] [CrossRef]
- de Sá Almeida, J.S.; de Oliveira Marre, A.T.; Teixeira, F.L.; Boente, R.F.; Domingues, R.; de Paula, G.R.; Lobo, L.A. Lactoferrin and lactoferricin B reduce adhesion and biofilm formation in the intestinal symbionts Bacteroides fragilis and Bacteroides thetaiotaomicron. Anaerobe 2020, 64, 102232. [Google Scholar] [CrossRef]
- O’Halloran, F.; Beecher, C.; Chaurin, V.; Sweeney, T.; Giblin, L. Lactoferrin affects the adherence and invasion of Streptococcus dysgalactiae ssp. dysgalactiae in mammary epithelial cells. J. Dairy Sci. 2016, 99, 4619–4628. [Google Scholar]
- Liu, F.; Zhang, S.; Li, J.; McClements, D.J.; Liu, X. Recent development of lactoferrin-based vehicles for the delivery of bioactive compounds: Complexes, emulsions, and nanoparticles. Trends Food Sci. Technol. 2018, 79, 67–77. [Google Scholar] [CrossRef]
- Akdaşçi, E.; Eker, F.; Duman, H.; Singh, P.; Bechelany, M.; Karav, S. Lactoferrin as a versatile agent in nanoparticle applications: From therapeutics to agriculture. Nanomaterials 2024, 24, 2018. [Google Scholar] [CrossRef] [PubMed]
- Kooshan, Z.; Srinivasan, S.; Janjua, T.I.; Popat, A.; Batra, J. Lactoferrin conjugated radicicol nanoparticles enhanced drug delivery and cytotoxicity in prostate cancer cells. Eur. J. Pharmacol. 2025, 991, 177300. [Google Scholar] [CrossRef]
- Subramaniam, S.; Jeet, V.; Gunter, J.H.; Janjua Khan, T.; Feng, Y.; Clements, J.A.; Srinivasan, S.; Popat, A.; Batra, J. Lactoferrin-encapsulated dichloroacetophenone (DAP) nanoparticles enhance drug delivery and anti-tumor efficacy in prostate cancer. Cancer Lett. 2025, 616, 217522. [Google Scholar] [CrossRef]
- Rajput, H.; Nangare, S.; Khan, Z.; Patil, A.; Bari, S.; Patil, P. Design of lactoferrin functionalized carboxymethyl dextran coated egg albumin nanoconjugate for targeted delivery of capsaicin: Spectroscopic and cytotoxicity studies. Int. J. Biol. Macromol. 2024, 256, 128392. [Google Scholar] [CrossRef]
- Yang, T.; Li, H.; Yu, R.; Yu, X.; Li, Y.; Duan, Z.; Yang, J.; Tao, G.; Huang, A.; Shi, Y. Lactoferrin-alginate-pectin composite hydrogel: Enhancing Lactobacillus plantarum B072 survival, density and biofilm formation. Int. J. Biol. Macromol. 2025, 308, 141983. [Google Scholar] [CrossRef]
- Priya, A.J.; Vijayalakshmi, S.P.; Raichur, A.M. Enhanced survival of probiotic Lactobacillus acidophilus by encapsulation with nanostructured polyelectrolyte layers through layer-by-layer approach. J. Agric. Food Chem. 2011, 59, 11838–11845. [Google Scholar] [CrossRef]
- Liu, B.; Hu, J.; Yao, H.; Zhang, L.; Liu, H. Improved viability of probiotics encapsulated by layer-by-layer assembly using zein nanoparticles and pectin. Food Hydrocoll. 2023, 143, 108899. [Google Scholar] [CrossRef]
- Zhao, R.; Yu, T.; Li, J.; Niu, R.; Liu, D.; Wang, W. Single-cell encapsulation systems for probiotic delivery: Armor probiotics. Adv. Colloid Interface Sci. 2024, 332, 103270. [Google Scholar] [CrossRef]
- Kabary, D.M.; Helmy, M.W.; Elkhodairy, K.A.; Fang, J.-Y.; Elzoghby, A.O. Hyaluronate/lactoferrin layer-by-layer-coated lipid nanocarriers for targeted co-delivery of rapamycin and berberine to lung carcinoma. Colloids Surf. B Biointerfaces 2018, 169, 183–194. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, G.G.; Sarıyıldız, S.; Cholakov, R.; Nalbantsoy, A.; Baler, B.; Aslan, E.; Düzel, A.; Sargın, S.; Göksungur, Y.; Kışla, D. A novel Lactiplantibacillus plantarum strain: Probiotic properties and optimization of the growth conditions by response surface methodology. World J. Microbiol. Biotechnol. 2024, 40, 66. [Google Scholar] [CrossRef] [PubMed]
- Mendoza Madrigal, A.; Duran-Paramo, E.; Valencia del Toro, G.; Chanona-Pérez, J.; Martínez-Ramírez, O.; Arzate-Vázquez, I. Viability kinetics of free and immobilized Bifidobacterium bifidum in presence of food samples under gastrointestinal in vitro conditions. Rev. Mex. Ing. Quím. 2017, 16, 159–168. [Google Scholar] [CrossRef]
- Lo Curto, A.; Pitino, I.; Mandalari, G.; Dainty, J.R.; Faulks, R.M.; John Wickham, M.S. Survival of probiotic lactobacilli in the upper gastrointestinal tract using an in vitro gastric model of digestion. Food Microbiol. 2011, 28, 1359–1366. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Chen, P.W.; Jheng, T.T.; Shyu, C.L.; Mao, F.C. Antimicrobial potential for the combination of bovine lactoferrin or its hydrolysate with lactoferrin-resistant probiotics against foodborne pathogens. J. Dairy Sci. 2013, 96, 1438–1446. [Google Scholar] [CrossRef]
- Oda, H.; Wakabayashi, H.; Yamauchi, K.; Abe, F. Lactoferrin and bifidobacteria. Biometals 2014, 27, 915–922. [Google Scholar] [CrossRef]
- Petschow, B.W.; Talbott, R.D.; Batema, R.P. Ability of lactoferrin to promote the growth of Bifidobacterium spp. in vitro is independent of receptor binding capacity and iron saturation level. J. MedMicrobiol. 1999, 48, 541–549. [Google Scholar] [CrossRef]
- Xu, Y.-G.; Yu, H.; Zhang, L.; Liu, M.; Qiao, X.-Y.; Cui, W.; Jiang, Y.-P.; Wang, L.; Li, Y.-J.; Tang, L.-J. Probiotic properties of genetically engineered Lactobacillus plantarum producing porcine lactoferrin used as feed additive for piglets. Process Biochem. 2016, 51, 719–724. [Google Scholar] [CrossRef]
- Hao, L.; Shan, Q.; Wei, J.; Ma, F.; Sun, P. Lactoferrin: Major Physiological Functions and Applications. Curr. Protein Pept. Sci. 2019, 20, 139–144. [Google Scholar] [CrossRef]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. SpringerPlus 2015, 4, 672. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.A.C.; Abdelsadig, M.S.E.; Conway, B.R.; Merchant, H.A. Using zeta potential to study the ionisation behavior of polymers employed in modified-release dosage forms and estimating their pK(a). Int. J. Pharm X. 2019, 1, 100024. [Google Scholar]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Wen, Y.; Chen, S.; Zhang, X.; Zhang, C.; Liu, X. Unraveling the secrets of probiotic adhesion: An overview of adhesion-associated cell surface components, adhesion mechanisms, and the effects of food composition. Trends Food Sci. Technol. 2025, 159, 104945. [Google Scholar] [CrossRef]
- Van Tassell, M.L.; Miller, M.J. Lactobacillus Adhesion to Mucus. Nutrients 2011, 3, 613–636. [Google Scholar] [CrossRef]
- Ceja-Medina, L.I.; Ortiz-Basurto, R.I.; Medina-Torres, L.; Calderas, F.; Bernad-Bernad, M.J.; González-Laredo, R.F.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; González-Ávila, M.; Andrade-González, I.; et al. Microencapsulation of Lactobacillus plantarum by spray drying with mixtures of mucilage and agave fructans as wall materials. J. Food Process. Eng. 2020, 43, e13436. [Google Scholar] [CrossRef]
- Cao, M.-X.; Qian, Z.-Y.; Liang, Y.-J.; Liu, Q.-Y.; Wang, H.-P.; Meng, Y.; Wang, Y.-S.; Wang, Y. Layer-by-layer coated probiotics with tannic acid-Ca2+ and casein phosphopeptide complexes for caries prevention and enamel remineralization. iScience 2025, 28, 111579. [Google Scholar] [CrossRef] [PubMed]
- Sbehat, M.; Altamimi, M.; Sabbah, M.; Mauriello, G. Layer-by-Layer coating of single-cell Lacticaseibacillus rhamnosus to increase viability under simulated gastrointestinal conditions and use in film formation. Front. Microbiol. 2022, 13, 838416. [Google Scholar] [CrossRef]
- Capela, P.; Hay, T.K.C.; Shah, N.P. Effect of homogenization on bead size and survival of encapsulated probiotic bacteria. Food Res. Int. 2007, 40, 1261–1269. [Google Scholar] [CrossRef]
- Hansen, L.T.; Allan-Wojtas, P.M.; Jin, Y.L.; Paulson, A.T. Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastrointestinal conditions. Food Microbiol. 2002, 19, 35–45. [Google Scholar] [CrossRef]
- Virk, M.S.; Virk, M.A.; Gul, M.; Awais, M.; Liang, Q.; Tufail, T.; Zhong, M.; Sun, Y.; Qayum, A.; El-Salam, E.A.; et al. Layer-by-layer concurrent encapsulation of probiotics and bioactive compounds with supplementation in intermediary layers: An establishing instrument for microbiome recharge, core safety, and targeted delivery. Food Hydrocoll. 2025, 161, 110873. [Google Scholar] [CrossRef]
- Yucel Falco, C.; Sotres, J.; Rascón, A.; Risbo, J.; Cárdenas, M. Design of a potentially prebiotic and responsive encapsulation material for probiotic bacteria based on chitosan and sulfated β-glucan. J. Colloid Interface Sci. 2017, 487, 97–106. [Google Scholar] [CrossRef]
- Ahammed, S.; Liu, F.; Easdani, M.; Saqib, M.N.; Zhong, F. Self-assembly of zein in aqueous acetic acid and ethanol solvents: Effect on mechanical properties of the zein film. Food Packag. Shelf Life 2023, 38, 101120. [Google Scholar] [CrossRef]
- Wei, Y.S.; Feng, K.; Li, S.F.; Hu, T.G.; Linhardt, R.J.; Zong, M.H.; Wu, H. Oral fate and stabilization technologies of lactoferrin: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6341–6358. [Google Scholar] [CrossRef]
- Kato, K.; Tamaki, N.; Saito, Y.; Fujimoto, T.; Sato, A. Amino group PEGylation of bovine lactoferrin by linear polyethylene Glycol-p-nitrophenyl active esters. Biol. Pharm. Bull. 2010, 33, 1253–1255. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, R.; Ke, W.; Liu, Y.; Jiang, C.; Pei, Y. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials 2008, 29, 238–246. [Google Scholar] [CrossRef]
- Agwa, M.M.; Sabra, S. Lactoferrin coated or conjugated nanomaterials as an active targeting approach in nanomedicine. Int. J. Biol. Macromol. 2021, 167, 1527–1543. [Google Scholar] [CrossRef]
- Nojima, Y.; Suzuki, Y.; Iguchi, K.; Shiga, T.; Iwata, A.; Fujimoto, T.; Yoshida, K.; Shimizu, H.; Takeuchi, T.; Sato, A. Development of Poly(ethylene glycol) conjugated Llactoferrin for oral administration. Bioconjug. Chem. 2008, 19, 2253–2259. [Google Scholar] [CrossRef]
- Martoni, C.J.; Srivastava, S.; Damholt, A.; Leyer, G.J. Efficacy and dose response of Lactiplantibacillus plantarum in diarrhea-predominant irritable bowel syndrome. World J. Gastroenterol. 2023, 29, 4451–4465. [Google Scholar] [CrossRef]
- Ramos, C.; Thorsen, L.; Schwan, R.; Jespersen, L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013, 36, 22–29. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, P.T.; Nguyen, T.T.; Nguyen, T.B.; Bui, N.B.; Nguyen, H.T. Efficacy of the incorporation between self-encapsulation and cryoprotectants on improving the freeze-dried survival of probiotic bacteria. J. Appl. Microbiol. 2022, 132, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Luo, L.; Dong, C.; Zheng, X.; Guo, B.; Xia, Y.; Tao, L.; Ai, L. Polysaccharides can improve the survival of Lactiplantibacillus plantarum subjected to freeze-drying. J. Dairy Sci. 2021, 104, 2606–2614. [Google Scholar] [CrossRef] [PubMed]
- Savedboworn, W.; Teawsomboonkit, K.; Surichay, S.; Riansa-Ngawong, W.; Rittisak, S.; Charoen, R.; Phattayakorn, K. Impact of protectants on the storage stability of freeze-dried probiotic Lactobacillus plantarum. Food Sci. Biotechnol. 2019, 28, 795–805. [Google Scholar] [CrossRef]
- Troost, F.J.; Steijns, J.; Saris, W.H.M.; Brummer, R.-J.M. Gastric digestion of bovine lactoferrin in vivo in adults. J. Nutr. 2001, 131, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).