Combined Effects of Human Chorionic Gonadotropin and Intravaginal Progesterone Device Treatment in the Early Luteal Phase After Artificial Insemination on Conception Rate in Lactating Dairy Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
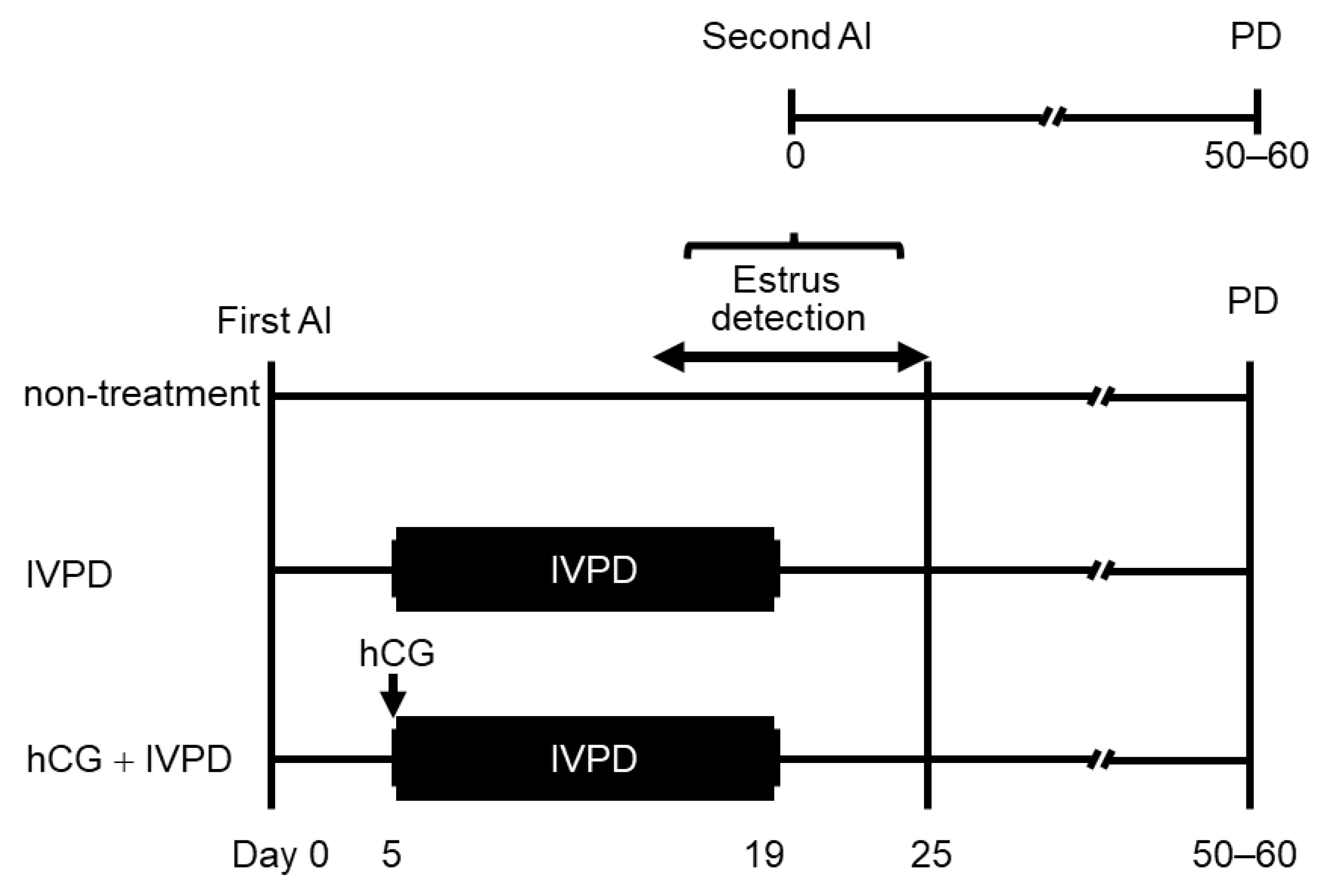
2.2. Experimental Design
2.3. Statistical Analysis
3. Results
3.1. Effects of Treatment, Farm, AI Number, and Interaction of Treatment and AI Number on Conception Rate of First AI
3.2. Effects of Treatment, Farm, AI Number, and Interaction of Treatment and AI Number on Re-Estrus Detection Rate up to 25 Days After First AI
3.3. Day to Re-Estrus from First AI in Non-Treatment, IVPD, and hCG + IVPD Groups in Non-Pregnant Cows
3.4. Effects of Treatment, Farm, AI Number, and Interaction of Treatment and AI Number on Conception Rate of Second AI
3.5. Effects of Treatment, Farm, AI Number, and Interaction of Treatment and AI Number on Cumulative Pregnancy Rate
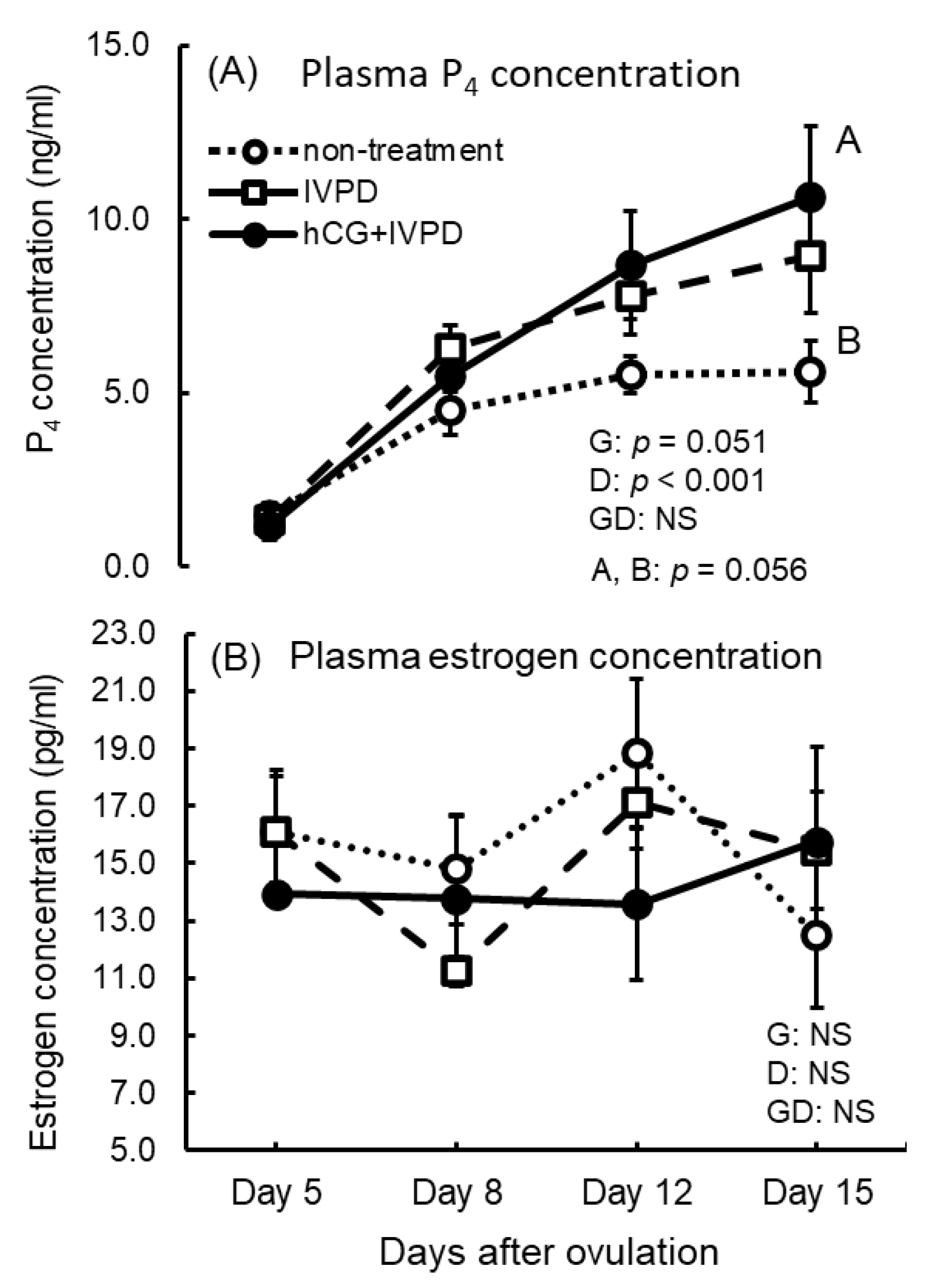
3.6. Plasma P4 and E Concentrations in Non-Treatment, IVPD, and hCG + IVPD Treatment Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial insemination |
| CIDR | Controlled internal drug release |
| CL | Corpus luteum |
| DF | Dominant follicle |
| DM | Dry matter |
| E | Estrogen |
| EIA | Enzyme immunoassay |
| hCG | Human chorionic gonadotropin |
| IVPD | Intravaginal progesterone device |
| P4 | Progesterone |
| PGF2α | Prostaglandin F2α |
References
- Bartlett, P.C.; Kirk, J.H.; Mather, E.C. Repeated insemination in Michigan Holstein Friesian cattle: Incidence, descriptive epidemiology and estimated economic impact. Theriogenology 1986, 26, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Heersche, G., Jr.; Nebel, R.L. Measuring efficiency and accuracy of detection of estrus. J. Dairy Sci. 1994, 77, 2754–2761. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.E.; Thatcher, W.W.; Pool, L.; Overton, M.W. Effect of human chorionic gonadotropin on luteal function and reproductive performance of high-producing lactating Holstein dairy cows. J. Anim Sci. 2001, 79, 2881–2894. [Google Scholar] [CrossRef]
- Hanlon, D.W.; Jarratt, G.M.; Davidson, P.J.; Millar, A.J.; Douglas, V.L. The effect of hCG administration five days after insemination on the first service conception rate of anestrous dairy cows. Theriogenology 2005, 63, 1938–1945. [Google Scholar] [CrossRef]
- Nascimento, A.B.; Bender, R.W.; Souza, A.H.; Ayres, H.; Araujo, R.R.; Guenther, J.N.; Sartori, R.; Wiltbank, M.C. Effect of treatment with human chorionic gonadotropin on day 5 after timed artificial insemination on fertility of lactating dairy cows. J. Dairy Sci. 2013, 96, 2873–2882. [Google Scholar] [CrossRef]
- Larson, S.F.; Butler, W.R.; Currie, W.B. Pregnancy rates in lactating dairy cattle following supplementation of progesterone after artificial insemination. Anim. Reprod. Sci. 2007, 102, 172–179. [Google Scholar] [CrossRef]
- Friedman, E.; Roth, Z.; Voet, H.; Lavon, Y.; Wolfenson, D. Progesterone supplementation postinsemination improves fertility of cooled dairy cows during the summer. J. Dairy Sci. 2012, 95, 3092–3099. [Google Scholar] [CrossRef]
- Garcia-Ispierto, I.; López-Gatius, F. Progesterone supplementation in the early luteal phase after artificial insemination improves conception rates in high-producing dairy cows. Theriogenology 2017, 90, 20–24. [Google Scholar] [CrossRef]
- Izumi, T.; Miura, R.; Sobu, N.; Hirase, A.; Yoneyama, O.; Miyake, Y.-I.; Haneda, S.; Matsui, M. Effects of human chorionic gonadotropin and intravaginal progesterone device treatment after artificial inseminations on the reproductive performance of normal and repeat breeder lactating dairy cows. J. Reprod. Dev. 2020, 66, 523–528. [Google Scholar] [CrossRef]
- Starbuck, G.R.; Mann, G.E. Differential effects of exogenous progesterone administration at different stages of the luteal phase on endogenous oestradiol concentration in cows. Reprod. Domest. Anim. 2010, 45, 283–286. [Google Scholar] [CrossRef]
- Stock, A.E.; Fortune, J.E. Ovarian follicular dominance: Relationship between prolonged growth of the ovulatory follicle and endocrine parameters. Endocrinology 1993, 132, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.S.; Portaluppi, M.A.; Tenhouse, D.E.; Lloyd, A.; Eborn, D.R.; Kacuba, S.; DeJarnette, J.M. Interventions after artificial insemination: Conception rates, pregnancy survival, and ovarian responses to gonadotropin-releasing hormone, human chorionic gonadotropin, and progesterone. J. Dairy Sci. 2007, 90, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.O.; Statz, L.R.; Domingues, R.R.; Andrade, J.P.N.; Wiltbank, M.C.; Martins, J.P.N. Accessory corpus luteum induced by human chorionic gonadotropin on day 7 or days 7 and 13 of the estrous cycle affected follicular and luteal dynamics and luteolysis in lactating Holstein cows. J. Dairy Sci. 2022, 105, 2631–2650. [Google Scholar] [CrossRef]
- Diaz, T.; Schmitt, E.J.; de la Sota, R.L.; Thatcher, M.J.; Thatcher, W.W. Human chorionic gonadotropin-induced alterations in ovarian follicular dynamics during the estrous cycle of heifers. J. Anim. Sci. 1998, 76, 1929–1936. [Google Scholar] [CrossRef]
- Garcia-Ispierto, I.; López-Helguera, I.; Serrano-Pérez, B.; Paso, V.; Tuono, T.; Ramon, A.; Mur-Vovales, R.; Tutusaus, J.; López-Gatius, F. Progesterone supplementation during the time of pregnancy recognition after artificial insemination improves conception rates in high-producing dairy cows. Theriogenology 2016, 85, 1343–1347. [Google Scholar] [CrossRef]
- Miyamoto, A.; Okuda, K.; Schweigert, F.J.; Schams, D. Effects of basic fibroblast growth factor, transforming growth factor-beta and nerve growth factor on the secretory function of the bovine corpus luteum in vitro. J. Endocrinol. 1992, 135, 103–114. [Google Scholar] [CrossRef]
- Takenouchi, N.; Oshima, K.; Shimada, K.; Takahashi, M. The development of a sensitive enzyme immunoassay for the determination of estrone and estradiol-17 beta in bovine blood plasma based on the same homologous combination with antiserum and steroid-enzyme conjugate. J. Vet. Med. Sci. 2004, 66, 1315–1321. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Mann, G.E.; Fray, M.D.; Lamming, G.E. Effects of time of progesterone supplementation on embryo development and interferon-t production in the cow. Vet. J. 2005, 171, 500–503. [Google Scholar] [CrossRef]
- Funeshima, N.; Noguchi, T.; Onizawa, Y.; Yaginuma, H.; Miyamura, M.; Tsuchiya, H.; Iwata, H.; Kuwayama, T.; Hamano, S.; Shirasuna, K. The transfer of parthenogenetic embryos following artificial insemination in cows can enhance pregnancy recognition via the secretion of interferon tau. J. Reprod. Dev. 2019, 65, 443–450. [Google Scholar] [CrossRef]
- Claypool, C.K.; Spencer, J.A.; Zoca, S.M.; Shafii, B.; Price, W.J.; Ahmadzadeh, A.; Rimbey, N.R.; Dalton, J.C. Short communication: Reproduction outcomes in dairy heifers following a 14-d progesterone insert presynchronization protocol. J. Dairy Sci. 2019, 102, 11730–11735. [Google Scholar] [CrossRef] [PubMed]
- Binelli, M.; Thatcher, W.W.; Mattos, R.; Baruselli, P.S. Antiluteolytic strategies to improve fertility in cattle. Theriogenology. 2001, 56, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E.; Bazer, F.W. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front. Biosci. 2002, 7, 879–898. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.R.; Ginther, O.J.; Ferreira, J.C.; Palhão, M.M.; Beg, M.A.; Wiltbank, M.C. Role of follicular estradiol-17beta in timing of luteolysis in heifers. Biol. Reprod. 2009, 81, 426–437. [Google Scholar] [CrossRef]
- Silvia, W.J.; Lewis, G.S.; McCracken, J.A.; Thatcher, W.W.; Wilson, L., Jr. Hormonal regulation of uterine secretion of prostaglandin F2 alpha during luteolysis in ruminants. Biol. Reprod. 1991, 45, 655–663. [Google Scholar] [CrossRef]
- Lopez, H.; Caraviello, D.Z.; Satter, L.D.; Fricke, P.M.; Wiltbank, M.C. Relationship between level of milk production and multiple ovulations in lactating dairy cows. J. Dairy Sci. 2005, 88, 2783–2793. [Google Scholar] [CrossRef]
- Madureira, A.M.L.; Burnett, T.A.; Borchardt, S.; Heuwieser, W.; Baes, C.F.; Vasconcelos, J.L.M.; Cerri, R.L.A. Plasma concentrations of progesterone in the preceding estrous cycle are associated with the intensity of estrus and fertility of Holstein cows. PLoS ONE 2021, 16, e0248453. [Google Scholar] [CrossRef]
| Factor | Class | Conception Rate (%) | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|---|
| Treatment | Non-treatment † | 31.5 (57/181) | Reference | ||
| IVPD ‡ | 48.6 (36/74) | 1.33 | 0.68–2.61 | 0.411 | |
| hCG + IVPD § | 56.3 (49/87) | 2.24 | 1.25–4.01 | 0.006 | |
| Farm | A | 41.9 (103/246) | Reference | ||
| B | 40.6 (39/96) | 0.77 | 0.47–1.29 | 0.322 | |
| AI number | ≤3 | 40.3 (108/268) | Reference | ||
| >3 | 45.9 (34/74) | 0.43 | 0.17–1.13 | 0.086 | |
| Treatment × AI number | Non-treatment × ≤3 | 34.2 (51/149) | Reference | ||
| Non-treatment × >3 | 18.8 (6/32) | 0.44 | 0.17–1.15 | 0.093 | |
| IVPD × ≤3 | 40.4 (19/47) | 1.30 | 0.67–2.56 | 0.440 | |
| IVPD × >3 | 63.0 (17/27) | 3.27 | 1.39–7.65 | 0.006 | |
| hCG + IVPD × ≤3 | 52.8 (38/72) | 2.15 | 1.21–3.81 | 0.008 | |
| hCG + IVPD × >3 | 73.3 (11/15) | 5.28 | 1.60–17.40 | 0.006 |
| Factor | Class | Re-Estrus Detection Rate (%) | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|---|
| Treatment | Non-treatment † | 34.7 (43/124) | Reference | ||
| IVPD ‡ | 78.9 (30/38) | 5.06 | 2.00–12.80 | 0.0006 | |
| hCG + IVPD § | 60.5 (23/38) | 2.69 | 1.19–6.08 | 0.017 | |
| Farm | A | 43.4 (62/143) | Reference | ||
| B | 59.6 (34/57) | 1.71 | 0.87–3.39 | 0.121 | |
| AI number | ≤3 | 45.0 (72/160) | Reference | ||
| >3 | 60.0 (24/40) | 1.60 | 0.66–3.91 | 0.303 |
| Factor | Class | Conception Rate (%) | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|---|
| Treatment | Non-treatment ‡ | 39.5 (17/43) | Reference | ||
| IVPD § | 33.3 (10/30) | 0.35 | 0.04–3.02 | 0.339 | |
| hCG + IVPD ¶ | 56.5 (13/23) | 0.72 | 0.04–13.20 | 0.822 | |
| Farm | A | 41.9 (26/62) | Reference | ||
| B | 41.2 (14/34) | 0.97 | 0.40–2.35 | 0.950 | |
| AI number | ≤3 | 40.3 (29/72) | Reference | ||
| >3 | 45.8 (11/24) | 2.28 | 0.56–9.25 | 0.248 |
| Factor | Class | Cumulative Pregnancy Rate (%) | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|---|
| Treatment | Non-treatment † | 40.9 (74/181) | Reference | ||
| IVPD ‡ | 62.1 (46/74) | 1.75 | 0.90–3.39 | 0.098 | |
| hCG + IVPD § | 71.3 (62/87) | 3.63 | 1.67–5.53 | <0.001 | |
| Farm | A | 52.4 (129/246) | Reference | ||
| B | 55.2 (53/96) | 0.91 | 0.55–1.51 | 0.711 | |
| AI number | ≤3 | 51.1 (137/268) | Reference | ||
| >3 | 60.8 (45/74) | 0.84 | 0.38–1.84 | 0.656 | |
| Treatment × AI number | Non-treatment × ≤3 | 41.6 (62/149) | Reference | ||
| Non-treatment × >3 | 37.5 (12/32) | 0.84 | 0.38–1.85 | 0.668 | |
| IVPD × ≤3 | 55.3 (26/47) | 1.74 | 0.90–3.36 | 0.101 | |
| IVPD × >3 | 74.0 (20/27) | 4.01 | 1.60–10.10 | 0.003 | |
| hCG + IVPD × ≤3 | 68.1 (49/72) | 2.99 | 1.65–5.41 | <0.001 | |
| hCG + IVPD × >3 | 86.7 (13/15) | 9.12 | 1.99–41.9 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, R.; Izumi, T.; Wada, Y.; Hagita, Y.; Iga, K.; Sobu, N.; Hirase, A.; Yoneyama, O.; Miyake, Y.-i.; Tajima, T.; et al. Combined Effects of Human Chorionic Gonadotropin and Intravaginal Progesterone Device Treatment in the Early Luteal Phase After Artificial Insemination on Conception Rate in Lactating Dairy Cows. Dairy 2025, 6, 26. https://doi.org/10.3390/dairy6030026
Miura R, Izumi T, Wada Y, Hagita Y, Iga K, Sobu N, Hirase A, Yoneyama O, Miyake Y-i, Tajima T, et al. Combined Effects of Human Chorionic Gonadotropin and Intravaginal Progesterone Device Treatment in the Early Luteal Phase After Artificial Insemination on Conception Rate in Lactating Dairy Cows. Dairy. 2025; 6(3):26. https://doi.org/10.3390/dairy6030026
Chicago/Turabian StyleMiura, Ryotaro, Taiki Izumi, Yuriko Wada, Yujiro Hagita, Kosuke Iga, Natsumi Sobu, Akiya Hirase, Osamu Yoneyama, Yo-ichi Miyake, Tsuyoshi Tajima, and et al. 2025. "Combined Effects of Human Chorionic Gonadotropin and Intravaginal Progesterone Device Treatment in the Early Luteal Phase After Artificial Insemination on Conception Rate in Lactating Dairy Cows" Dairy 6, no. 3: 26. https://doi.org/10.3390/dairy6030026
APA StyleMiura, R., Izumi, T., Wada, Y., Hagita, Y., Iga, K., Sobu, N., Hirase, A., Yoneyama, O., Miyake, Y.-i., Tajima, T., Ajito, T., Haneda, S., & Matsui, M. (2025). Combined Effects of Human Chorionic Gonadotropin and Intravaginal Progesterone Device Treatment in the Early Luteal Phase After Artificial Insemination on Conception Rate in Lactating Dairy Cows. Dairy, 6(3), 26. https://doi.org/10.3390/dairy6030026





