Fermented Buffalo Milk with Conjugated Linoleic Acid-Producing Bacteria: Strain Selection and Functional Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains Isolation and Identification
2.2. Screening for CLA Synthesis Potential
2.3. Development of Fermented Buffalo Milk
2.4. Lipid Extraction and Fatty Acid Analysis
2.5. Statistical Analyses
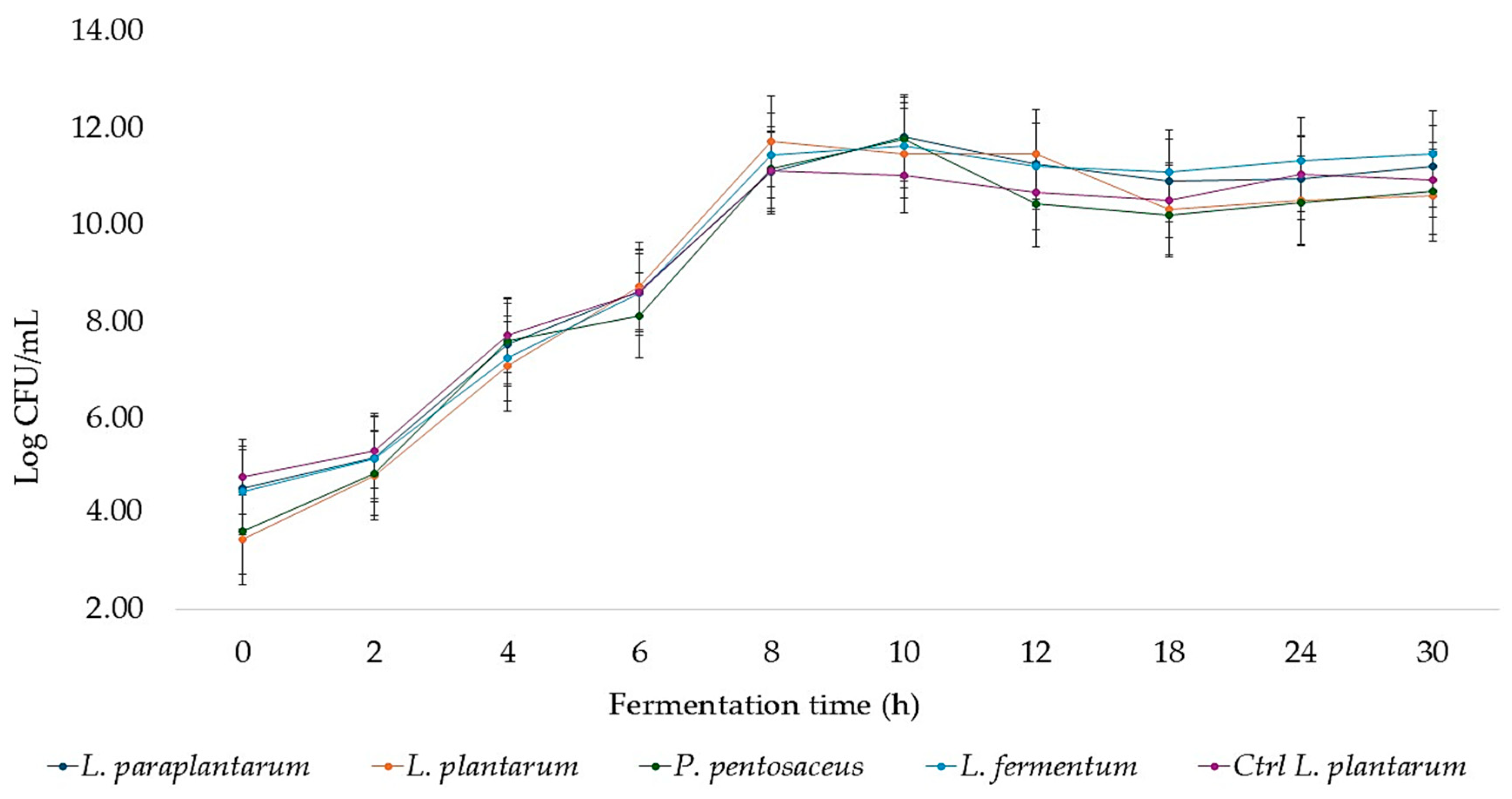
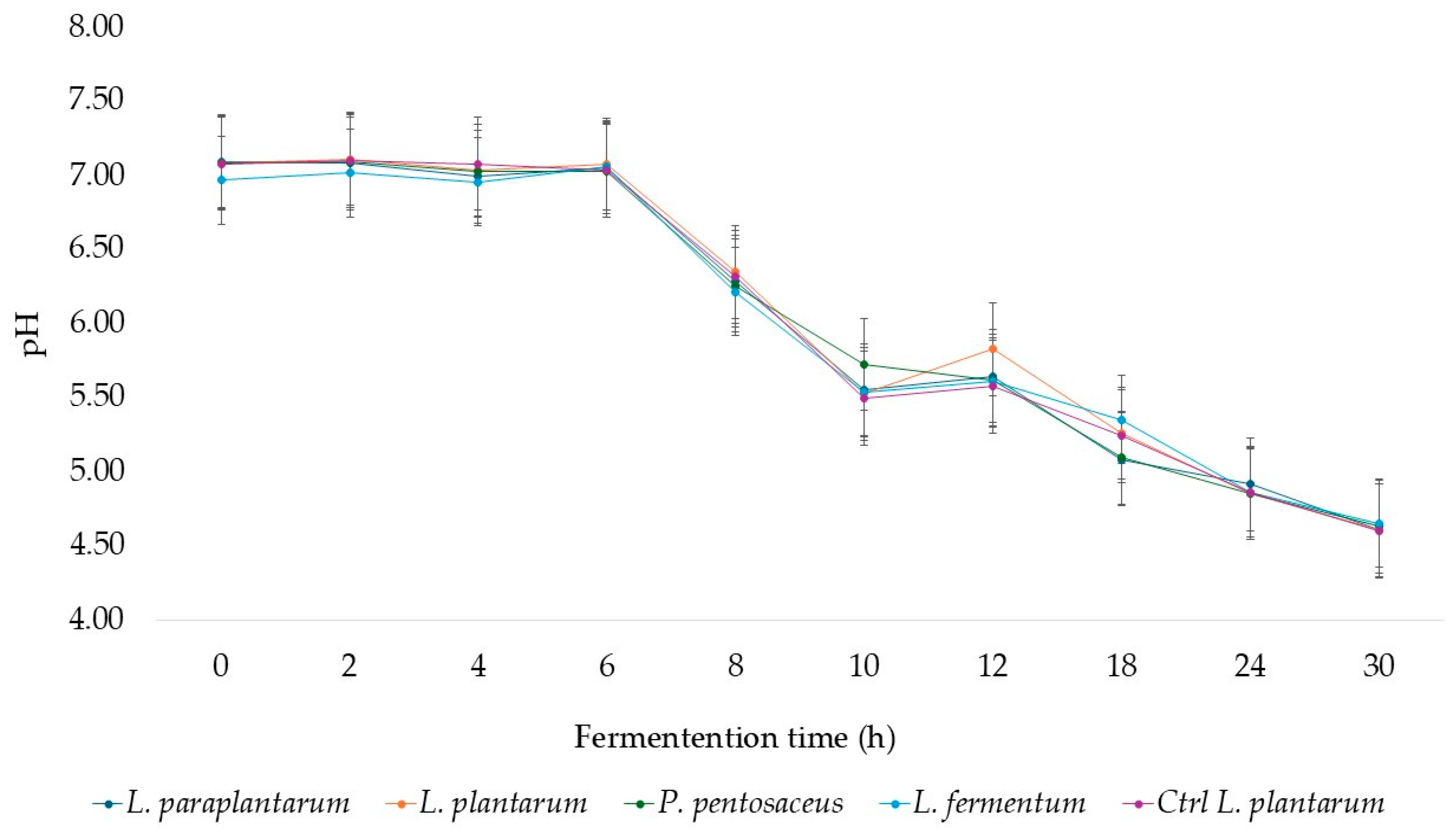
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vieira, C.P.; Rosario, A.I.L.S.; Lelis, C.A.; Rekowsky, B.S.S.; Carvalho, A.P.A.; Rosário, D.K.A.; Elias, T.A.; Costa, M.P.; Foguel, D.; Conte-Junior, C.A. Bioactive Compounds from Kefir and Their Potential Benefits on Health: A Systematic Review and Meta-Analysis. Oxidative Med. Cell. Longev. 2021, 2021, 9081738. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Nouska, C.; Mantzourani, I.; Kourkoutas, Y.; Alexopoulos, A.; Bezirtzoglou, E. Microbiological exploration of different types of Kefir grains. Fermentation 2017, 3, 1. [Google Scholar] [CrossRef]
- da Anunciação, T.A.; Guedes, J.D.S.; Tavares, P.P.L.G.; Borges, F.E.d.M.; Ferreira, D.D.; Costa, J.A.V.; Umsza-Guez, M.A.; Magalhães-Guedes, K.T. Biological Significance of Probiotic Microorganisms from Kefir and Kombucha: A Review. Microorganisms 2024, 12, 1127. [Google Scholar] [CrossRef] [PubMed]
- Miranda, E.S.M.; Santos, J.V.R.; de Miranda, E.P.; de Oliveira, A.T.C.; dos Santos, S.M.L.; Damaceno, M.N. Aplicação de frutas do semiárido brasileiro em produtos alimentícios à base de kefir: Uma revisão sistemática. Res. Soc. Dev. 2021, 10, e349101321079. [Google Scholar] [CrossRef]
- Gao, H.; Yang, B.; Stanton, C.; Ross, R.P.; Zhang, H.; Liu, Z.; Chen, H.; Chen, W. Characteristics of bifidobacterial conjugated fatty acid and hydroxy fatty acid production and its potential application in fermented milk. LWT 2020, 120, 108940. [Google Scholar] [CrossRef]
- Salsinha, A.S.; Pimentel, L.L.; Fontes, A.L.; Gomes, A.M.; Rodríguez-Alcalá, L.M. Microbial Production of Conjugated Linoleic Acid and Conjugated Linolenic Acid Relies on a Multienzymatic System. Microbiol. Mol. Biol. Rev. 2018, 82, e00019-18. [Google Scholar] [CrossRef]
- Vieira, C.P.; Cabral, C.C.; Lima, B.R.d.C.; Paschoalin, V.M.F.; Leandro, K.C.; Conte-Junior, C.A. Lactococcus lactis ssp. cremoris MRS47, a potential probiotic strain isolated from kefir grains, increases cis-9, trans-11-CLA and PUFA contents in fermented milk. J. Funct. Foods 2017, 31, 172–178. [Google Scholar] [CrossRef]
- González, A.; Fullaondo, A.; Rodríguez, J.; Tirnauca, C.; Odriozola, I.; Odriozola, A. Conjugated linoleic acid metabolite impact in colorectal cancer: A potential microbiome-based precision nutrition approach. Nutr. Rev. 2024, 83, e602–e614. [Google Scholar] [CrossRef]
- Vieira, C.P.; da Costa, M.P.; Silva, V.L.d.M.; Delgado, K.F.; Frasão, B.d.S.; Elias, T.A.; Nunes, Y.E.C.d.O.; Gloria, M.B.d.A.; Conte-Junior, C.A. Interactive effect of physicochemical and microbial variables on bioactive amines content during storage of probiotic fermented milk. LWT 2021, 138, 110700. [Google Scholar] [CrossRef]
- Rodríguez-Alcalá, L.M.; Fontecha, J.; de la Hoz, L.; da Silva, V.S.N.; Carvalho, J.E.; Pacheco, M.T.B. CLA-enriched milk powder reverses hypercholesterolemic risk factors in hamsters. Food Res. Int. 2013, 51, 244–249. [Google Scholar] [CrossRef]
- Nasrollahzadeh, A.; Tavani, S.M.; Arjeh, E.; Jafari, S.M. Production of conjugated linoleic acid by lactic acid bacteria; important factors and optimum conditions. Food Chem. X 2023, 20, 100942. [Google Scholar] [CrossRef] [PubMed]
- Zongo, K.; Krishnamoorthy, S.; Moses, J.A.; Yazici, F.; Çon, A.H.; Anandharamakrishnan, C. Total conjugated linoleic acid content of ruminant milk: The world status insights. Food Chem. 2021, 334, 127555. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, H.; Mei, Y.; Yang, B.; Zhao, J.; Stanton, C.; Chen, W. Advances in research on microbial conjugated linoleic acid bioconversion. Prog. Lipid Res. 2024, 93, 101257. [Google Scholar] [CrossRef] [PubMed]
- Basilicata, M.G.; Pepe, G.; Adesso, S.; Ostacolo, C.; Sala, M.; Sommella, E.; Scala, M.C.; Messore, A.; Autore, G.; Marzocco, S.; et al. Antioxidant properties of buffalo-milk dairy products: A β-Lg peptide released after gastrointestinal digestion of buffalo ricotta cheese reduces oxidative stress in intestinal epithelial cells. Int. J. Mol. Sci. 2018, 19, 1955. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Asif, M.; Khan, M.K.; Din, A.; Ullah, R. Triglyceride, fatty acid profile and antioxidant characteristics of low melting point fractions of Buffalo Milk fat. Lipids Health Dis. 2019, 18, 59. [Google Scholar] [CrossRef]
- Verruck, S.; Prudêncio, E.S.; Vieira, C.R.W.; Amante, E.R.; Amboni, R.D.d.M.C. The buffalo Minas Frescal cheese as a protective matrix of Bifidobacterium BB-12 under in vitro simulated gastrointestinal conditions. LWT-Food Sci. Technol. 2015, 63, 1179–1183. [Google Scholar] [CrossRef]
- Da Silva, T.M.S.; Piazentin, A.C.M.; Mendonça, C.M.N.; Converti, A.; Bogsan, C.S.B.; Mora, D.; de Souza Oliveira, R.P. Buffalo milk increases viability and resistance of probiotic bacteria in dairy beverages under in vitro simulated gastrointestinal conditions. J. Dairy Sci. 2020, 103, 7890–7897. [Google Scholar] [CrossRef]
- Aziz, T.; Sarwar, A.; Naveed, M.; Shahzad, M.; Shabbir, M.A.; Dablool, A.S.; Din, J.U.; Khan, A.A.; Naz, S.; Cui, H.; et al. Bio-Molecular analysis of selected food derived Lactiplantibacillus strains for CLA production reveals possibly a complex mechanism. Food Res. Int. 2022, 154, 111031. [Google Scholar] [CrossRef]
- Jang, Y.; Elnar, A.G.; Kang, M.H.; Kim, G.-B. Application of conjugated linoleic acid-producing strain, Bifidobacterium breve JKL2022, in the development of probiotic dairy products. Food Sci. Anim. Resour. 2024; online ahead-of-print. [Google Scholar] [CrossRef]
- Yadav, H.; Jain, S.; Sinha, P. Production of free fatty acids and conjugated linoleic acid in probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei during fermentation and storage. Int. Dairy J. 2007, 17, 1006–1010. [Google Scholar] [CrossRef]
- Van Nieuwenhove, C.; Oliszewski, R.; González, S.; Chaia, A.P. Conjugated linoleic acid conversion by dairy bacteria cultured in MRS broth and buffalo milk. Lett. Appl. Microbiol. 2007, 44, 467–474. [Google Scholar] [CrossRef]
- Van Nieuwenhove, C.P.; Oliszewski, R.; González, S.N.; Chaia, A.B.P. Influence of bacteria used as adjunct culture and sunflower oil addition on conjugated linoleic acid content in buffalo cheese. Food Res. Int. 2007, 40, 559–564. [Google Scholar] [CrossRef]
- de Oliveira, L.G.; Silva, G.O.E.; Barbosa, C.D.; Sant’Anna, F.M.; de Castro, R.D.; Figueiredo, N.C.; Nunes, Á.C.; Lage, A.P.; de Souza, M.R. Lactic acid bacteria isolated from Brazilian Minas artisanal cheeses and their in vitro antagonisms against Mycobacterium bovis BCG. Int. J. Dairy Technol. 2018, 71, 879–886. [Google Scholar] [CrossRef]
- Silva, J.; Castro, R.; Sant’anna, F.; Barquete, R.; Oliveira, L.; Acurcio, L.; Luiz, L.; Sales, G.; Nicoli, J.; Souza, M. In vitro assessment of the probiotic potential of lactobacilli isolated from Minas artisanal cheese produced in the Araxá region, Minas Gerais state, Brazil. Arq. Bras. Med. Veter. Zootec. 2019, 71, 647–657. [Google Scholar] [CrossRef]
- de Sant’Anna, F.M.; Acurcio, L.B.; Alvim, L.B.; de Castro, R.D.; de Oliveira, L.G.; da Silva, A.M.; Nunes, Á.C.; Nicoli, J.R.; Souza, M.R. Assessment of the probiotic potential of lactic acid bacteria isolated from Minas artisanal cheese produced in the Campo das Vertentes region, Brazil. Int. J. Dairy Technol. 2017, 70, 592–601. [Google Scholar] [CrossRef]
- Souza, C.O.; Leite, M.E.Q.; Lasekan, J.; Baggs, G.; Pinho, L.S.; Druzian, J.I.; Ribeiro, T.C.M.; Mattos, Â.P.; Menezes-Filho, J.A.; Costa-Ribeiro, H. Milk protein-based formulas containing different oils affect fatty acids balance in term infants: A randomized blinded crossover clinical trial. Lipids Health Dis. 2017, 16, 78. [Google Scholar] [CrossRef]
- Godinho, F.M.d.S.; Friedrich, M.T.; Modesto, E.C.; Mota, A.d.S.d. Fatty acid profile of buffalo milk produced in southern Brazil. Acta Sci. Anim. Sci. 2024, 46, e63400. [Google Scholar] [CrossRef]
- Terán, V.; Pizarro, P.L.; Zacarías, M.; Vinderola, G.; Medina, R.; Van Nieuwenhove, C. Production of conjugated dienoic and trienoic fatty acids by lactic acid bacteria and bifidobacteria. J. Funct. Foods 2015, 19, 417–425. [Google Scholar] [CrossRef]
- Coakley, M.; Barrett, E.; Murphy, J.; Ross, R.; Devery, R.; Stanton, C. Cheese Manufacture with Milk with Elevated Conjugated Linoleic Acid Levels Caused by Dietary Manipulation. J. Dairy Sci. 2007, 90, 2919–2927. [Google Scholar] [CrossRef]
- Alonso, L.; Cuesta, E.; Gilliland, S. Production of free conjugated linoleic acid by Lactobacillus acidophilus and Lactobacillus casei of human intestinal origin. J. Dairy Sci. 2003, 86, 1941–1946. [Google Scholar] [CrossRef]
- Lv, H.; Ren, D.; Yan, W.; Wang, Y.; Liu, H.; Shen, M. Linoleic acid inhibits Lactobacillus activity by destroying cell membrane and affecting normal metabolism. J. Sci. Food Agric. 2020, 100, 2057–2064. [Google Scholar] [CrossRef]
- Rekowsky, B.S.d.S.; Rosário, A.I.L.d.S.; Da Costa, M.P. Development of a new Brazilian semi-hard (Coalho) Buffalo cheese made with the inclusion of cow milk and functional potential/Desenvolvimento de um novo queijo semiduro brasileiro (Coalho) de búfalo feito com a inclusão de leite de vaca e potencial funcional. Braz. J. Dev. 2021, 7, 96944–96959. [Google Scholar] [CrossRef]
- Rafiq, S.; Huma, N.; Pasha, I.; Sameen, A.; Mukhtar, O.; Khan, M.I. Chemical composition, nitrogen fractions and amino acids profile of milk from different animal species. Asian-Australas. J. Anim. Sci. 2016, 29, 1022–1028. [Google Scholar] [CrossRef]
- Sales, D.C.; Rangel, A.H.D.N.; Urbano, S.A.; Tonhati, H.; Galvão Júnior, J.G.B.; Guilhermino, M.M.; Aguiar, E.M.; Bezerra, M.d.F. Buffalo milk composition, processing factors, whey constituents recovery and yield in manufacturing Mozzarella cheese. Food Sci. Technol. 2018, 38, 328–334. [Google Scholar] [CrossRef]
- Garau, V.; Manis, C.; Scano, P.; Caboni, P. Compositional Characteristics of Mediterranean Buffalo Milk and Whey. Dairy 2021, 2, 469–488. [Google Scholar] [CrossRef]
- Kushwaha, B.P.; Upadhyay, D.; Singh, S.; Maity, S.B.; Singh, K.K.; Misra, A.K. Fatty acid profile of Murrah buffalo milk fat. Buffalo Bull. 2022, 41, 73–79. [Google Scholar] [CrossRef]
- Akgun, A.; Yazici, F.; Gulec, H.A. Effect of reduced fat content on the physicochemical and microbiological properties of buffalo milk yoghurt. LWT 2016, 74, 521–527. [Google Scholar] [CrossRef]
- Dhawi, F.; El-Beltagi, H.S.; Aly, E.; Hamed, A.M. Antioxidant, antibacterial activities and mineral content of buffalo yoghurt fortified with fenugreek and Moringa oleifera seed flours. Foods 2020, 9, 1157. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ayaz, M.; Ajmal, M.; Ellahi, M.Y.; Khalique, A. Antioxidant capacity and fatty acids characterization of heat treated cow and buffalo milk. Lipids Health Dis. 2017, 16, 163. [Google Scholar] [CrossRef]
- Hill, D.; Sugrue, I.; Arendt, E.; Hill, C.; Stanton, C.; Ross, R.P. Recent advances in microbial fermentation for dairy and health. F1000Research 2017, 6, 751. [Google Scholar] [CrossRef]
- Amiri, S.; Rezazadeh-Bari, M.; Alizadeh-Khaledabad, M.; Rezaei-Mokarram, R.; Sowti-Khiabani, M. Fermentation Optimization for Co-production of Postbiotics by Bifidobacterium lactis BB12 in Cheese Whey. Waste Biomass Valorization 2021, 12, 5869–5884. [Google Scholar] [CrossRef]
- Chen, C.; Tong, F.; Sun, R.; Zhang, Y.; Pang, Z.; Liu, X. Screening and Identification of High-Yielding Strains of Conjugated Linoleic Acid and Optimization of Conditions for the Conversion of CLA. Foods 2024, 13, 1830. [Google Scholar] [CrossRef] [PubMed]
- Pereira Da Costa, M.; Conte-Junior, C.A. Chromatographic Methods for the Determination of Carbohydrates and Organic Acids in Foods of Animal Origin. Compr. Rev. Food Sci. Food Saf. 2015, 14, 586–600. [Google Scholar] [CrossRef]
- Medeiros, A.C.; Souza, D.F.; Correia, R.T.P. Effect of incubation temperature, heat treatment and milk source on the yoghurt kinetic acidification. Int. Food Res. J. 2015, 22, 1030–1036. [Google Scholar]
- Baliyan, N.; Maurya, A.K.; Kumar, A.; Agnihotri, V.K.; Kumar, R. Probiotics from the bovine raw milk of Lahaul valley showed cis-9, trans-11 conjugated linoleic acid isomer and antioxidant activity with food formulation ability. LWT 2023, 176, 114553. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, H.N. Effect of Synbiotic Interaction of Fructooligosaccharide and Probiotics on the Acidification Profile, Textural and Rheological Characteristics of Fermented Soy Milk. Food Bioprocess Technol. 2013, 6, 3166–3176. [Google Scholar] [CrossRef]
- Czarnowska-Kujawska, M.; Paszczyk, B. Changes in the folate content and fatty acid profile in fermented milk produced with different starter cultures during storage. Molecules 2021, 26, 6063. [Google Scholar] [CrossRef]
- Paszczyk, B.; Brandt, W.; Łuczyńska, J. Content of conjugated linoleic acid (CLA) and trans isomers of c18:1 and c18:2 acids in fresh and stored fermented milks produced with selected starter cultures. Czech J. Food Sci. 2016, 34, 391–396. [Google Scholar] [CrossRef]
- Tv, R. Current Knowledge on Source and Synthesis of Conjugated Linoleic Acid (CLA): A Review. Adv. Biotechnol. Microbiol. 2017, 7, 555707. [Google Scholar] [CrossRef]
- Andrade, J.C.; Ascenção, K.; Gullón, P.; Henriques, S.M.S.; Pinto, J.M.S.; Rocha-Santos, T.A.P.; Freitas, A.C.; Gomes, A.M. Production of conjugated linoleic acid by food-grade bacteria: A review. Int. J. Dairy Technol. 2012, 65, 467–481. [Google Scholar] [CrossRef]
- Correddu, F.; Serdino, J.; Manca, M.G.; Cosenza, G.; Pauciullo, A.; Ramunno, L.; Macciotta, N.P. Use of multivariate factor analysis to characterize the fatty acid profile of buffalo milk. J. Food Compos. Anal. 2017, 60, 25–31. [Google Scholar] [CrossRef]
- Ren, D.X.; Zou, C.X.; Lin, B.; Chen, Y.L.; Liang, X.W.; Liu, J.X. A comparison of milk protein, amino acid and fatty acid profiles of river buffalo and their F1 and F2 hybrids with swamp buffalo in China. Pak. J. Zool. 2015, 47, 1459–1465. [Google Scholar]
- Teng, F.; Wang, P.; Yang, L.; Ma, Y.; Day, L. Quantification of Fatty Acids in Human, Cow, Buffalo, Goat, Yak, and Camel Milk Using an Improved One-Step GC-FID Method. Food Anal. Methods 2017, 10, 2881–2891. [Google Scholar] [CrossRef]
- Annunziata, G.; Arnone, A.; Ciampaglia, R.; Tenore, G.C.; Novellino, E. Fermentation of foods and beverages as a tool for increasing availability of bioactive compounds. focus on short-chain fatty acids. Foods 2020, 9, 999. [Google Scholar] [CrossRef]
- Gużewska, G.; Monedeiro-Milanowski, M.; Florkiewicz, A.B.; Arendowska, I.; Walczak-Skierska, J.; Białczak, D.; Pomastowski, P.P. Analysis of the Fatty Acid Profile in Cream, Buttermilk Fractions, and Anhydrous Milk Fat: Influence of Physicochemical and Microbiological Parameters on the Fatty Acid Profile. Appl. Sci. 2024, 14, 6117. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Huang, L.; Huang, Z.; Romeih, E.; Yang, P.; Zeng, Q.; Li, L. Effect of Buffalo Breed on the Detailed Milk Composition in Guangxi, China. Foods 2023, 12, 1603. [Google Scholar] [CrossRef] [PubMed]
- Pegolo, S.; Stocco, G.; Mele, M.; Schiavon, S.; Bittante, G.; Cecchinato, A. Factors affecting variations in the detailed fatty acid profile of Mediterranean buffalo milk determined by 2-dimensional gas chromatography. J. Dairy Sci. 2017, 100, 2564–2576. [Google Scholar] [CrossRef]
- Dubey, V.; Ghosh, A.R.; Mandal, B.K. Appraisal of conjugated linoleic acid production by probiotic potential of Pediococcus spp. GS4. Appl. Biochem. Biotechnol. 2012, 168, 1265–1276. [Google Scholar] [CrossRef]
- Dubey, V.; Mishra, A.K.; Ghosh, A.R. Appraisal of the Possible Role of PPARγ Upregulation by CLA of Probiotic Pediococcus pentosaceus GS4 in Colon Cancer Mitigation. PPAR Res. 2023, 2023, 9458308. [Google Scholar] [CrossRef]
- Yadav, R.; Khan, S.H.; Mada, S.B.; Meena, S.; Kapila, R.; Kapila, S. Consumption of Probiotic Lactobacillus fermentum MTCC: 5898-Fermented Milk Attenuates Dyslipidemia, Oxidative Stress, and Inflammation in Male Rats Fed on Cholesterol-Enriched Diet. Probiotics Antimicrob. Proteins 2019, 11, 509–518. [Google Scholar] [CrossRef]
| Species | CLA (mg/mL) | LA Conversion (%) | Food Origin |
|---|---|---|---|
| L. paraplantarum * | 0.99 ± 0.35 a | 65.66 | Jaboticaba juice |
| L. plantarum * | 0.76 ± 0.19 b | 59.56 | Kefir |
| L. plantarum | 0.60 ± 0.05 b | 53.83 | Kefir grains in Jaboticaba juice |
| L. paraplantarum | 0.56 ± 0.07 bcd | 51.87 | Kombucha in Jaboticaba juice |
| L. plantarum | 0.54 ± 0.03 cd | 51.24 | Raw milk |
| L. plantarum | 0.54 ± 0.06 cde | 51.05 | Kombucha in Jaboticaba juice |
| L. plantarum | 0.53 ± 0.10 cde | 50.79 | Kombucha in Jaboticaba juice |
| L. plantarum | 0.52 ± 0.03 cdef | 49.93 | Kefir grains in Jaboticaba juice |
| P. pentosaceus * | 0.52 ± 0.11 cdef | 49.93 | Kefir |
| L. plantarum | 0.50 ± 0.05 cdefg | 49.15 | Kefir grains |
| NI | 0.48 ± 0.07 cdefgh | 48.23 | Raw milk |
| L. plantarum | 0.48 ± 0.11 cdefgh | 47.84 | Kefir |
| L. plantarum | 0.47 ± 0.02 cdefgh | 47.65 | Kefir grains |
| Lim. fermentum * | 0.45 ± 0.02 cdefghi | 46.34 | Raw milk |
| P. pentosaceus | 0.44 ± 0.03 cdefghi | 45.91 | Kefir |
| Leu. mesenteroides | 0.38 ± 0.01 defghij | 42.09 | Cheese counter |
| L. plantarum | 0.37 ± 0.12 defghijk | 41.55 | Kefir |
| Leu. pseudomesenteroides | 0.36 ± 0.08 defghijk | 41.32 | Artesanal cheese |
| Lac. rhamnosus | 0.36 ± 0.12 defghijkl | 41.14 | Artesanal cheese |
| NI | 0.36 ± 0.06 defghijkl | 41.04 | Kefir |
| L. plantarum | 0.35 ± 0.02 defghijkl | 40.38 | Kefir |
| L. plantarum | 0.35 ± 0.02 defghijkl | 40.29 | Kefir |
| P. pentosaceus | 0.35 ± 0.03 defghijkl | 40.15 | Kefir |
| P. pentosaceus | 0.35 ± 0.06 defghijkl | 40.05 | Kefir |
| L. plantarum | 0.34 ± 0.07 defghijkl | 39.86 | Kefir |
| L. plantarum | 0.34 ± 0.02 defghijkl | 39.47 | Kefir |
| Lac. paracasei | 0.33 ± 0.12 defghijkl | 38.97 | Artesanal cheese |
| NI | 0.32 ± 0.02 efghijkl | 38.37 | Kefir |
| L. plantarum | 0.31 ± 0.01 efghijkl | 37.70 | Kefir grains |
| P. pentosaceus | 0.31 ± 0.03 fghijkl | 37.39 | Kefir |
| L. plantarum | 0.30 ± 0.01 fghijkl | 36.48 | Kefir |
| P. pentosaceus | 0.30 ± 0.03 fghijkl | 36.32 | Kefir |
| NI | 0.29 ± 0.01 ghijkl | 36.16 | Kefir |
| Lim. fermentum | 0.29 ± 0.09 ghijkl | 35.88 | Kefir |
| P. pentosaceus | 0.29 ± 0.06 ghijkl | 35.77 | Kefir |
| P. pentosaceus | 0.29 ± 0.09 ghijkl | 35.66 | Ripened artesanal cheese |
| Lactococcus lactis | 0.28 ± 0.00 hijkl | 35.10 | Ripened artesanal cheese |
| P. pentosaceus | 0.28 ± 0.05 hijkl | 34.76 | Kefir |
| L. plantarum | 0.27 ± 0.04 hijkl | 34.53 | Raw milk |
| Lac. paracasei | 0.26 ± 0.00 hijkl | 33.84 | Ripened artesanal cheese |
| Lim. fermentum | 0.26 ± 0.01 hijkl | 33.60 | Kefir |
| P. pentosaceus | 0.26 ± 0.04 hijkl | 33.30 | Kefir |
| L. plantarum | 0.25 ± 0.05 ijkl | 32.15 | Kefir |
| Lac. casei | 0.24 ± 0.01 ijkl | 32.09 | Ripened artesanal cheese |
| Lac. paracasei | 0.24 ± 0.03 ijkl | 31.47 | Ripened artesanal cheese |
| Lactococcus garvieae | 0.22 ± 0.00 ijkl | 30.19 | Artesanal cheese |
| L. plantarum | 0.21 ± 0.03 jkl | 29.33 | Queijo Minas Artesanal |
| Levilactobacillus brevis | 0.21 ± 0.02 jkl | 29.13 | Queijo Artesanal da Serra Geral |
| Lactococcus lactis | 0.21 ± 0.04 jkl | 29.06 | Pingo |
| L. plantarum | 0.21 ± 0.00 jkl | 28.93 | Queijo Artesanal da Serra Geral |
| L. mesenteroides | 0.20 ± 0.08 jkl | 28.31 | Artesanal cheese |
| L. pseudomesenteroides | 0.20 ± 0.00 jkl | 28.31 | Artesanal cheese |
| L. plantarum | 0.19 ± 0.01 jkl | 27.19 | Queijo Minas Artesanal (Maturado) |
| Lac. paracasei | 0.17 ± 0.04 jkl | 24.70 | Queijo Minas Artesanal |
| Lat. curvatus | 0.17 ± 0.04 jkl | 24.62 | Artesanal cheese |
| L. plantarum | 0.16 ± 0.02 jkl | 23.70 | Artesanal cheese |
| L. plantarum | 0.16 ± 0.01 kl | 23.23 | Artesanal cheese |
| L. plantarum * | 0.14 ± 0.03 l | 21.86 | Queijo Minas Artesanal Canastra |
| Fatty Acids Profile | L. paraplantarum | L. plantarum | P. pentosaceus | L. fermentum | Ctrl L. plantarum * | p-Value |
|---|---|---|---|---|---|---|
| Individual fatty acids | ||||||
| C4:0 | 1.56 ± 0.29 b | 1.76 ± 0.18 a | 1.93 ± 0.09 a | 1.04 ± 0.16 c | 1.92 ± 0.11 a | <0.0001 |
| C6:0 | 0.97 ± 0.09 b | 1.05 ± 0.10 a | 1.13 ± 0.06 a | 0.80 ± 0.09 b | 1.13 ± 0.07 a | 0.0006 |
| C8:0 | 0.46 ± 0.04 ab | 0.49 ± 0.04 ab | 0.53 ± 0.03 a | 0.40 ± 0.04 b | 0.52 ± 0.03 a | 0.0024 |
| C10:0 | 0.88 ± 0.08 ab | 0.94 ± 0.08 ab | 1.03 ± 0.06 a | 0.78 ±0.07 b | 0.97 ± 0.07 a | 0.0038 |
| C11:0 | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.02 ± 0.00 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.1611 |
| C12:0 | 1.30 ± 0.12 ab | 1.42 ± 0.12 ab | 1.53 ± 0.08 a | 1.15 ± 0.10 b | 1.44 ± 0.09 a | 0.0036 |
| C13:0 | 0.07 ± 0.01 ab | 0.08 ± 0.01 a | 0.09 ± 0.01 a | 0.06 ± 0.01 b | 0.08 ± 0.01 a | 0.0002 |
| C14:0 | 7.18 ± 0.37 ab | 8.17 ± 1.03 ab | 8.41 ± 0.28 a | 6.62 ± 0.57 b | 8.05 ± 0.06 a | 0.0041 |
| C14:1 n-5 | 0.22 ± 0.07 a | 0.33 ± 0.12 a | 0.27 ± 0.10 a | 0.32 ± 0.02 a | 0.32 ± 0.09 a | 0.1510 |
| C15:0 | 1.39 ± 0.13 ab | 1.46 ± 0.12 ab | 1.58 ± 0.09 a | 1.14 ± 0.11 b | 1.44 ± 0.08 a | 0.0047 |
| C15:1 n-5 | 0.20 ± 0.08 a | 0.32 ± 0.03 a | 0.25 ± 0.03 a | 0.25 ± 0.02 a | 0.28 ± 0.03 a | 0.1010 |
| C16:0 | 24.28 ± 1.69 ab | 27.23 ± 4.21 ab | 27.15 ± 1.51 ab | 22.76 ± 2.11 b | 27.67 ± 1.33 a | 0.0284 |
| C16:1 n-7 | 0.53 ± 0.35 b | 1.21 ± 0.34 ab | 1.05 ± 0.26 ab | 1.10 ± 0.09 ab | 1.17 ± 0.24 a | 0.0480 |
| C17:0 | 1.36 ± 0.33 a | 1.41 ± 0.09 a | 1.60 ± 0.14 a | 1.10 ± 0.11 a | 1.38 ± 0.14 a | 0.0797 |
| C17:1 n-7 | 0.28 ± 0.02 a | 0.32 ± 0.05 a | 0.23 ± 0.16 a | 0.27 ± 0.03 a | 0.32 ± 0.03 a | 0.5829 |
| C18:0 | 15.04 ± 2.16 a | 13.48 ± 0.82 a | 15.11 ± 1.52 a | 10.27 ± 1.07 b | 13.24 ± 1.39 a | 0.0116 |
| C18:1 n-9 c | 20.73 ± 1.32 ab | 24.48 ± 3.04 ab | 24.66 ± 0.55 ab | 20.09 ± 1.91 b | 23.31 ± 0.31 a | 0.0169 |
| C18:2 n-6 t | 0.13 ± 0.09 a | 0.21 ± 0.03 a | 0.23 ± 0.01 a | 0.17 ± 0.03 a | 0.21 ± 0.02 a | 0.1004 |
| C18:2 n-6 c | 1.03 ± 0.14 ab | 1.11 ± 0.08 ab | 1.23 ± 0.08 a | 0.86 ± 0.09 b | 1.09 ± 0.07 a | 0.0106 |
| C18:3 n-3 | 0.49 ± 0.13 ab | 0.47 ± 0.05 ab | 0.60 ± 0.10 a | 0.33 ± 0.03 b | 0.47 ± 0.10 ab | 0.0313 |
| C18:2 c9 t11 CLA | 0.88 ± 0.05 a | 1.09 ± 0.22 a | 1.21 ± 0.02 a | 1.16 ± 0.19 a | 1.03 ± 0.11 a | 0.1170 |
| C20:0 | 0.35 ± 0.04 a | 0.32 ± 0.02 ab | 0.37 ± 0.02 a | 0.25 ± 0.03 b | 0.32 ± 0.02 a | 0.0087 |
| C20:1 n-9 | 0.02 ± 0.03 ab | 0.05 ± 0.03 ab | 0.01 ± 0.01 b | 0.03 ± 0.02 ab | 0.05 ± 0.01 a | 0.0474 |
| C21:0 | 0.04 ± 0.03 a | 0.00 ± 0.00 a | 0.05 ± 0.04 a | 0.00 ± 000 a | 0.00 ± 0.00 a | 0.0747 |
| C20:3 n-6 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.6114 |
| C20:4 n-6 | 0.02 ± 0.04 a | 0.00 ± 0.00 b | 0.04 ± 0.02 a | 0.00 ± 0.00 bb | 0.00 ± 0.00 b | 0.0031 |
| C22:0 | 0.13 ± 0.02 a | 0.11 ± 0.02 ab | 0.14 ± 0.00 a | 0.09 ± 0.02 ab | 0.05 ± 0.04 b | 0.0156 |
| C20:5 n-3 | 0.02 ± 0.02 b | 0.00 ± 0.00 b | 0.05 ± 0.01 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.0002 |
| C23:0 | 0.06 ± 0.04 ab | 0.05 ± 0.04 b | 0.09 ± 0.02 a | 0.06 ± 0.01 ab | 0.00 ± 0.00 b | 0.0020 |
| C24:0 | 0.07 ± 0.04 a | 0.07 ± 0.05 a | 0.09 ± 0.01 a | 0.03 ± 0.03 a | 0.02 ± 0.02 a | 0.1304 |
| C24:1 n-9 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.02 ± 0.01 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.0039 |
| Sums fatty acids | ||||||
| SFA | 55.15 ± 4.93 ab | 58.07 ± 6.38 ab | 60.82 ± 1.64 a | 46.57 ± 4.44 b | 57.23 ± 0.71 a | 0.0086 |
| MUFA | 21.98 ± 1.62 a | 26.71 ± 3.59 a | 26.49 ± 0.93 a | 22.06 ± 2.08 a | 25.45 ± 0.67 a | 0.1577 |
| PUFA | 2.58 ± 0.39 ab | 2.89 ± 0.30 ab | 3.37 ± 0.21 a | 2.52 ± 0.22 b | 2.80 ± 0.08 ab | 0.0408 |
| n-3 | 0.51 ± 0.15 ab | 0.47 ± 0.06 ab | 0.65 ± 0.11 a | 0.33 ± 0.03 b | 0.47 ± 0.10 ab | 0.0205 |
| n-6 | 2.07 ± 0.24 ab | 2.42 ± 0.32 a | 1.51 ± 0.11 b | 2.19 ± 0.21 a | 2.33 ± 0.02 a | 0.0019 |
| Ratios | ||||||
| PUFA:SFA | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.06 ± 0.00 a | 0.05 ± 0.01 a | 0.05 ± 0.00 a | 0.0895 |
| PUFA:MUFA | 0.12 ± 0.01 a | 0.11 ± 0.01 a | 0.13 ± 0.01 a | 0.011 ± 0.01 a | 0.11 ± 0.01 a | 0.3121 |
| MUFA:SFA | 0.40 ± 0.02 a | 0.46 ± 0.02 a | 0.44 ± 0.01 a | 0.47 ± 0.00 a | 0.44 ± 0.02 a | 0.5334 |
| n3:n6 | 0.24 ± 0.62 b | 0.20 ± 0.05 b | 0.43 ± 0.07 a | 0.15 ± 0.07 b | 0.20 ± 0.04 b | 0.0335 |
| DFA | 39.60 ± 4.09 a | 43.08 ± 4.24 a | 44.96 ± 1.52 a | 34.85 ± 4.00 a | 41.49 ± 0.80 a | 0.0381 |
| AI | 2.22 ± 0.11 a | 2.07 ± 0.01 b | 2.09 ± 0.03 b | 2.05 ± 0.03 b | 2.13 ± 0.01 ab | 0.0046 |
| ThI | 3.39 ± 0.23 a | 3.02 ± 0.00 b | 3.09 ± 0.02 b | 2.99 ± 0.03 b | 3.09 ± 0.01 b | 0.0008 |
| hH | 0.74 ± 0.04 a | 0.78 ± 0.02 a | 0.79 ± 0.03 a | 0.77 ± 0.01 a | 0.75 ± 0.02 a | 0.0775 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rekowsky, B.S.d.S.; Fernandez, L.B.R.M.; Alzate, K.G.; Lelis, C.A.; Souza, M.R.d.; Souza, C.O.d.; Silva, B.D.d.; Conte-Júnior, C.A.; Vieira, C.P.; Silva, J.G.d.; et al. Fermented Buffalo Milk with Conjugated Linoleic Acid-Producing Bacteria: Strain Selection and Functional Applications. Dairy 2025, 6, 25. https://doi.org/10.3390/dairy6030025
Rekowsky BSdS, Fernandez LBRM, Alzate KG, Lelis CA, Souza MRd, Souza COd, Silva BDd, Conte-Júnior CA, Vieira CP, Silva JGd, et al. Fermented Buffalo Milk with Conjugated Linoleic Acid-Producing Bacteria: Strain Selection and Functional Applications. Dairy. 2025; 6(3):25. https://doi.org/10.3390/dairy6030025
Chicago/Turabian StyleRekowsky, Bruna Samara dos Santos, Lorena Brandão Rocha Martinez Fernandez, Katherine Gutierrez Alzate, Carini Aparecida Lelis, Marcelo Resende de Souza, Carolina Oliveira de Souza, Bruno Dutra da Silva, Carlos Adam Conte-Júnior, Carla Paulo Vieira, José Givanildo da Silva, and et al. 2025. "Fermented Buffalo Milk with Conjugated Linoleic Acid-Producing Bacteria: Strain Selection and Functional Applications" Dairy 6, no. 3: 25. https://doi.org/10.3390/dairy6030025
APA StyleRekowsky, B. S. d. S., Fernandez, L. B. R. M., Alzate, K. G., Lelis, C. A., Souza, M. R. d., Souza, C. O. d., Silva, B. D. d., Conte-Júnior, C. A., Vieira, C. P., Silva, J. G. d., & Costa, M. P. d. (2025). Fermented Buffalo Milk with Conjugated Linoleic Acid-Producing Bacteria: Strain Selection and Functional Applications. Dairy, 6(3), 25. https://doi.org/10.3390/dairy6030025








