Lactic Microbiota and Metabolites in Raw Cow’s Milk: Implications for Consumer Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Study of the Quality of the Raw Milk Using the Mesophilic Bacterial Count and Thermal Stability Using the 70° and 80° Ethanol Test
2.3. Metataxonomic Analysis of the Samples by Massive Sequencing Using Illumina 16S rRNA
2.3.1. DNA Extraction
2.3.2. Massive Sequencing of the Amplicons of Gene 16S rRNA
2.3.3. Processing of the Sequencing Data and Statistical Analysis
2.4. Metabolomic Analysis of the Samples by UHPLC-Q/TOF MS/MS
2.4.1. Extraction of Metabolites and Untargeted Metabolomic Analysis Based on UHPLC Mass Spectrometry
2.4.2. Data Processing and Statistical Analysis
2.5. Multivariate Correlation Analysis Based on Metabolomics and Microbial Profiles
3. Results
3.1. Study of the Quality and Stability of the Milk in 70° and 80° Ethanol
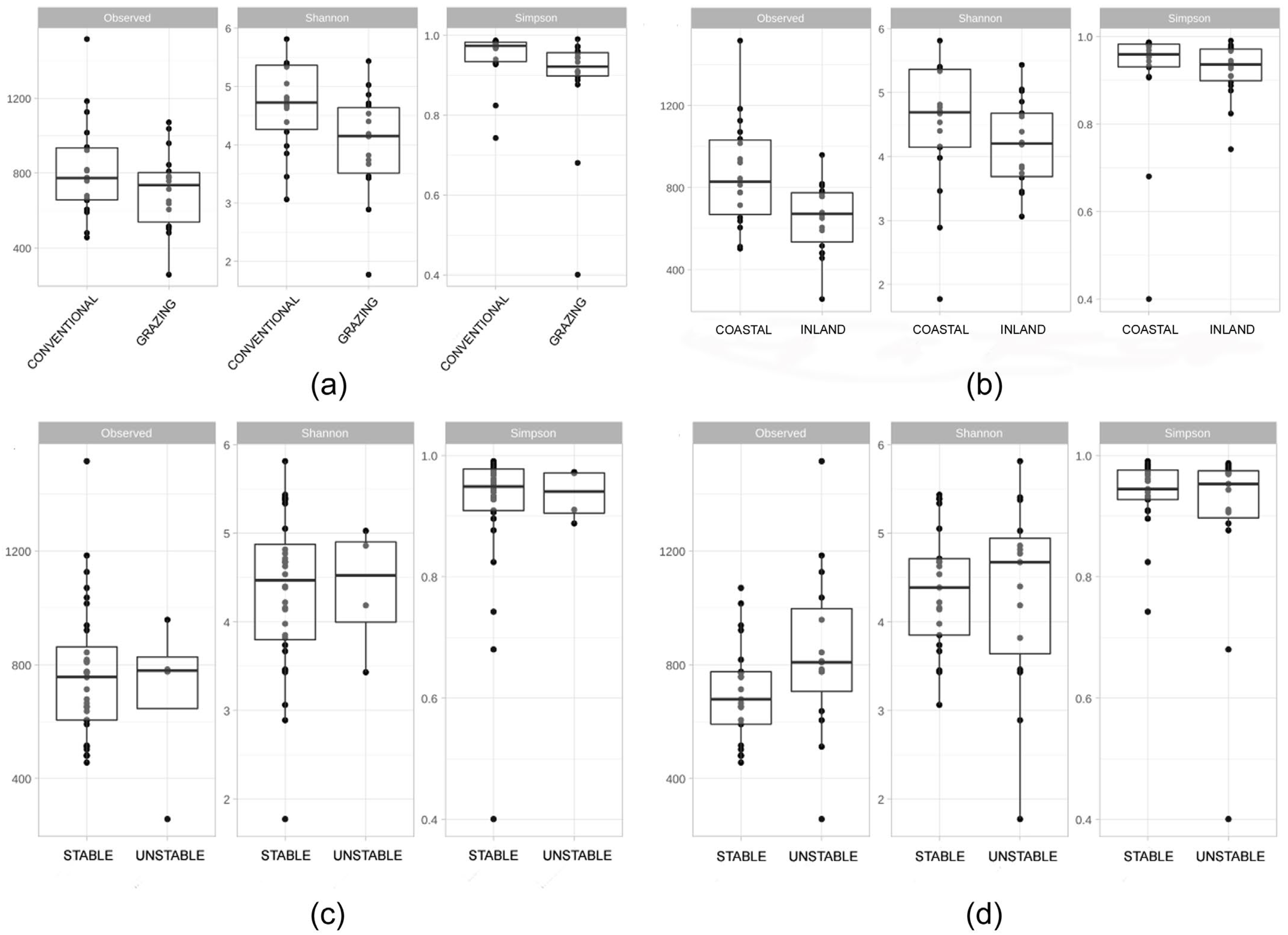
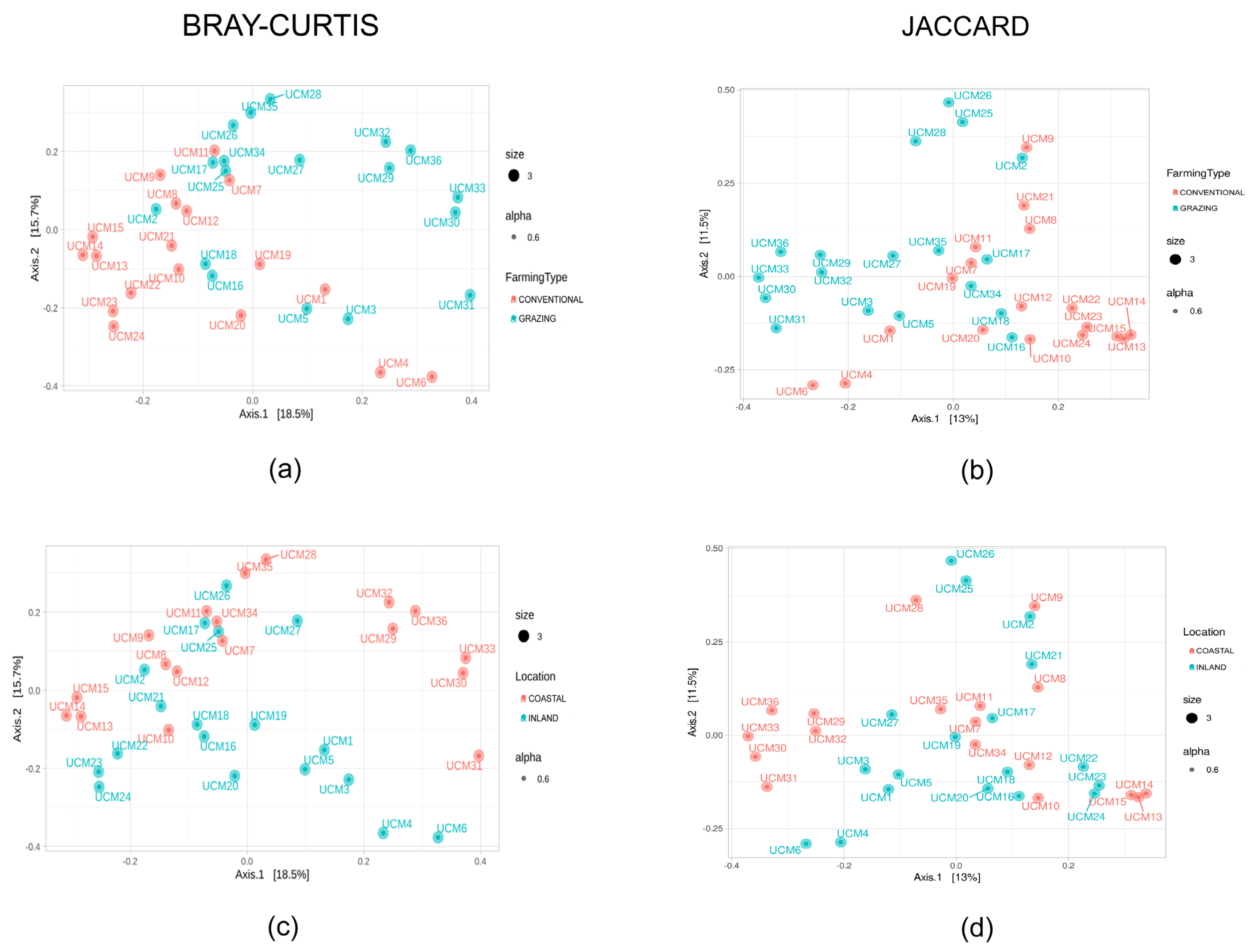
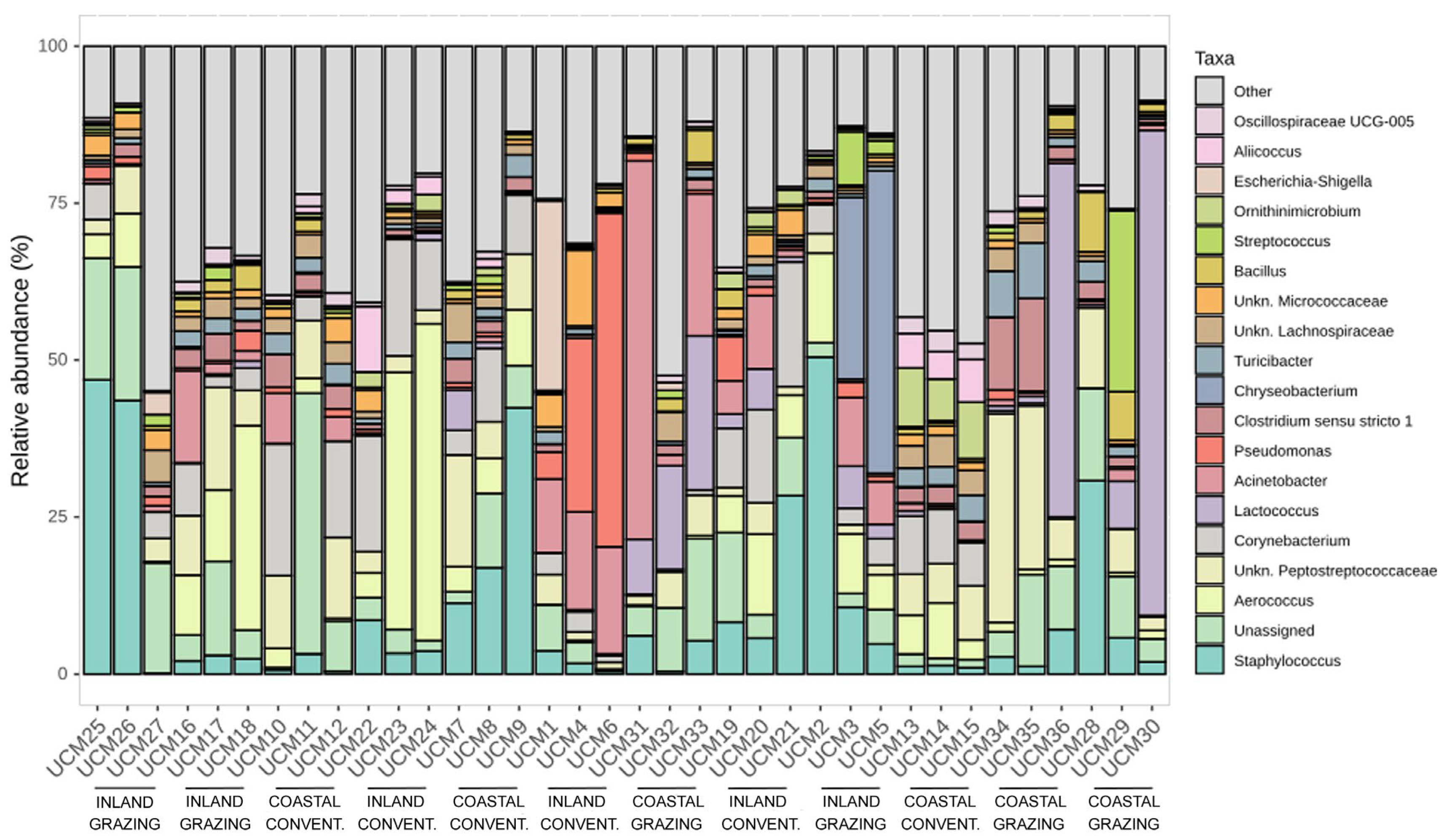
3.2. Metataxonomic Analysis
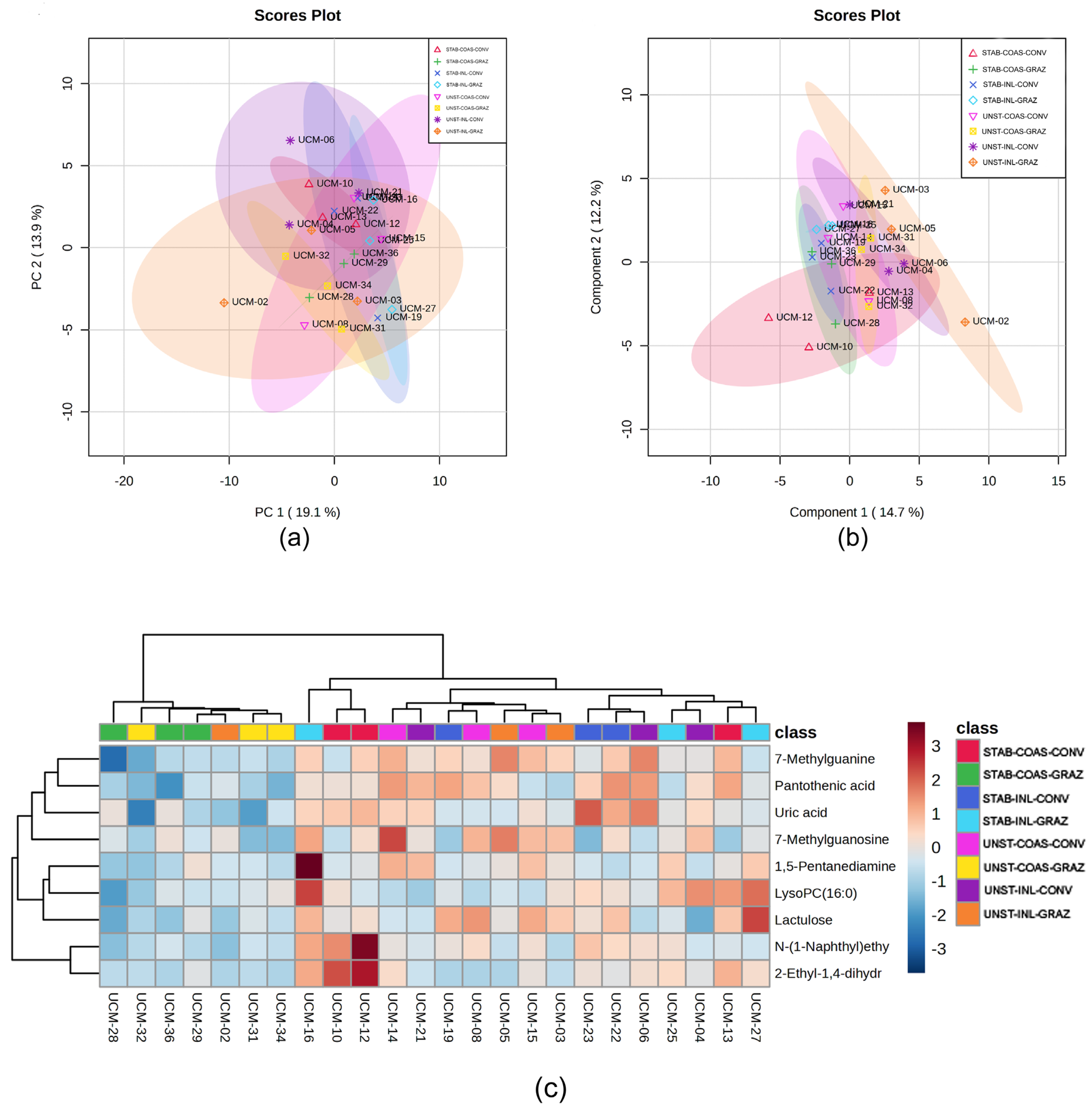
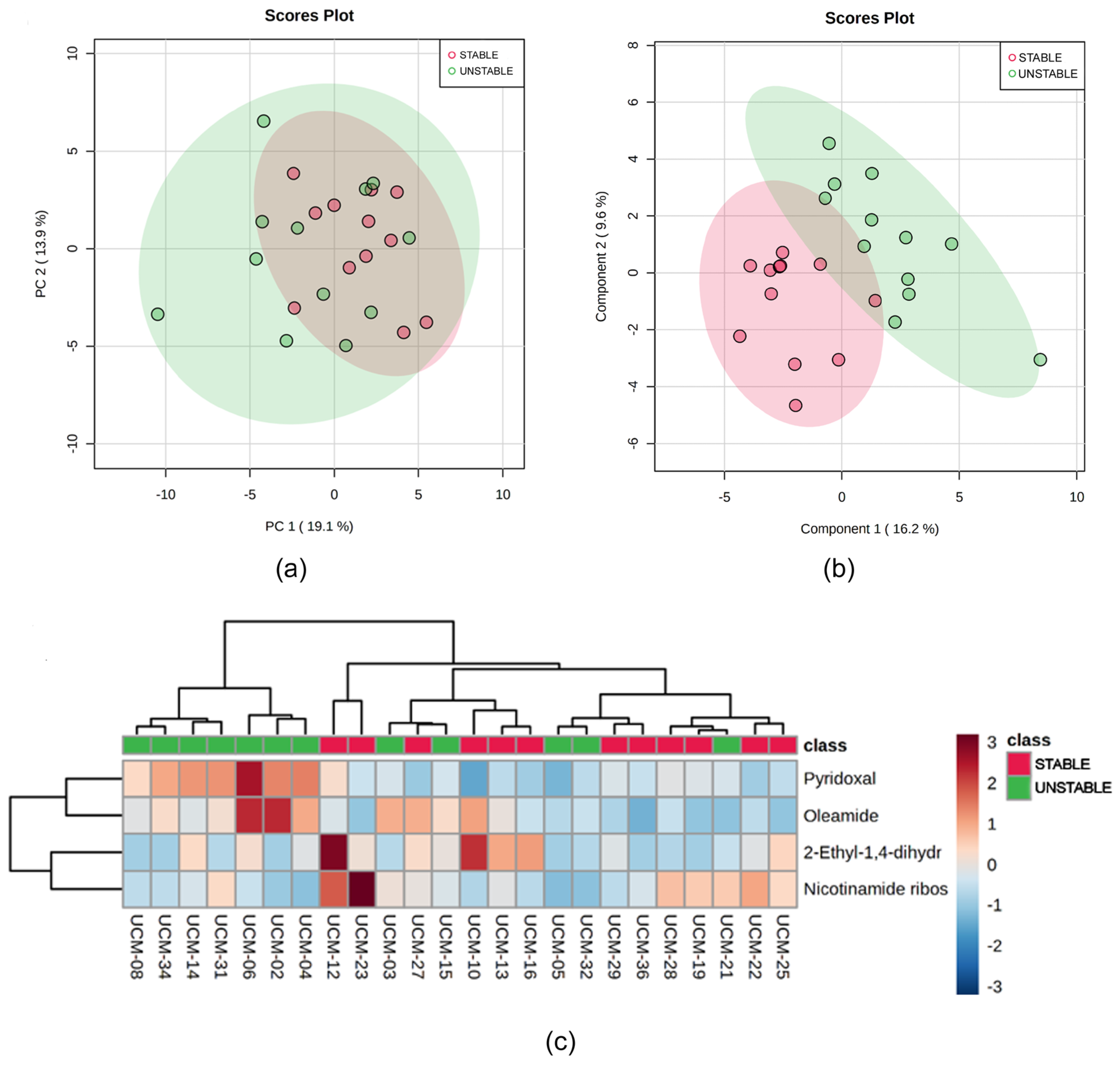
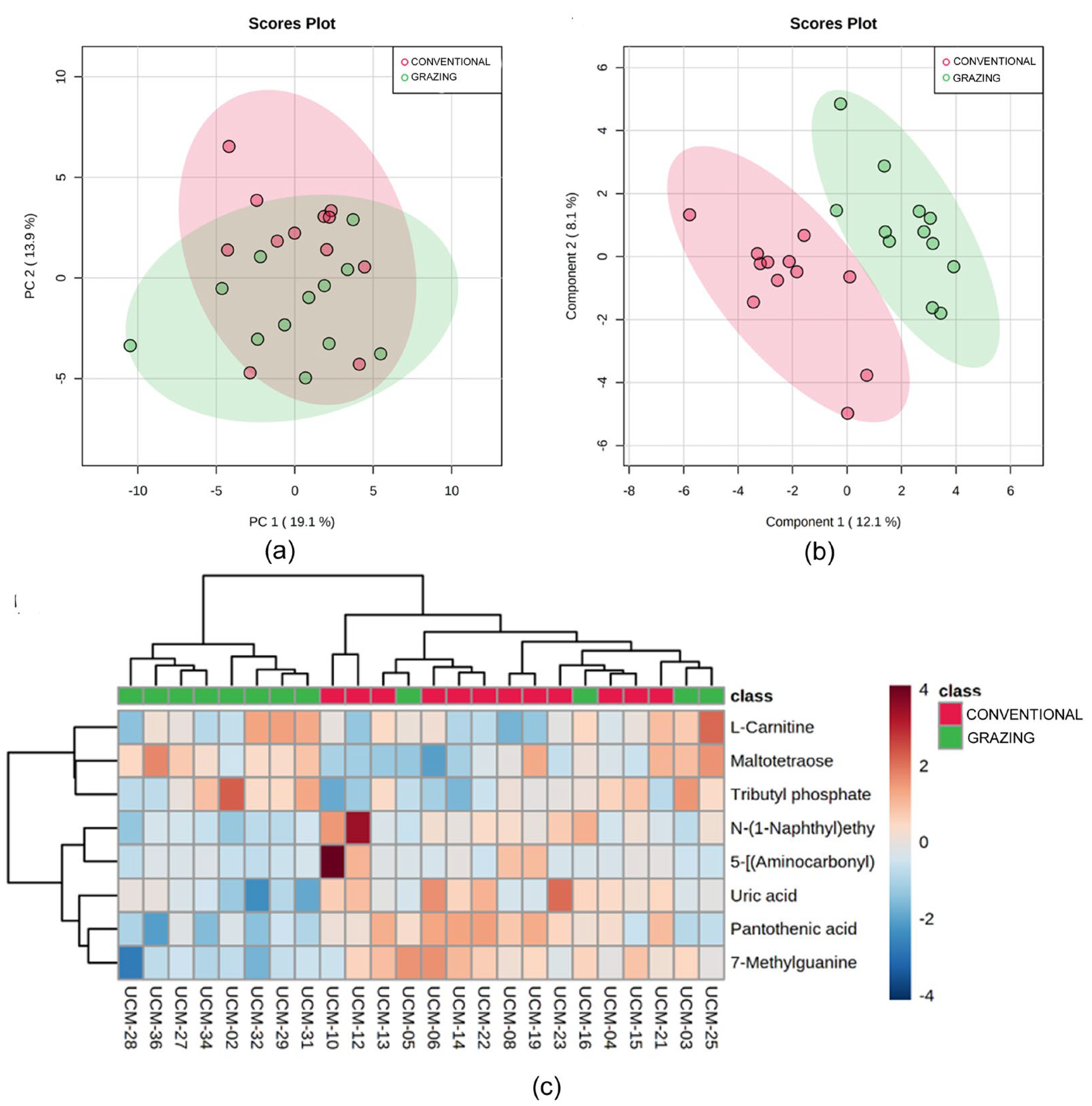
3.3. Metabolomic Analysis
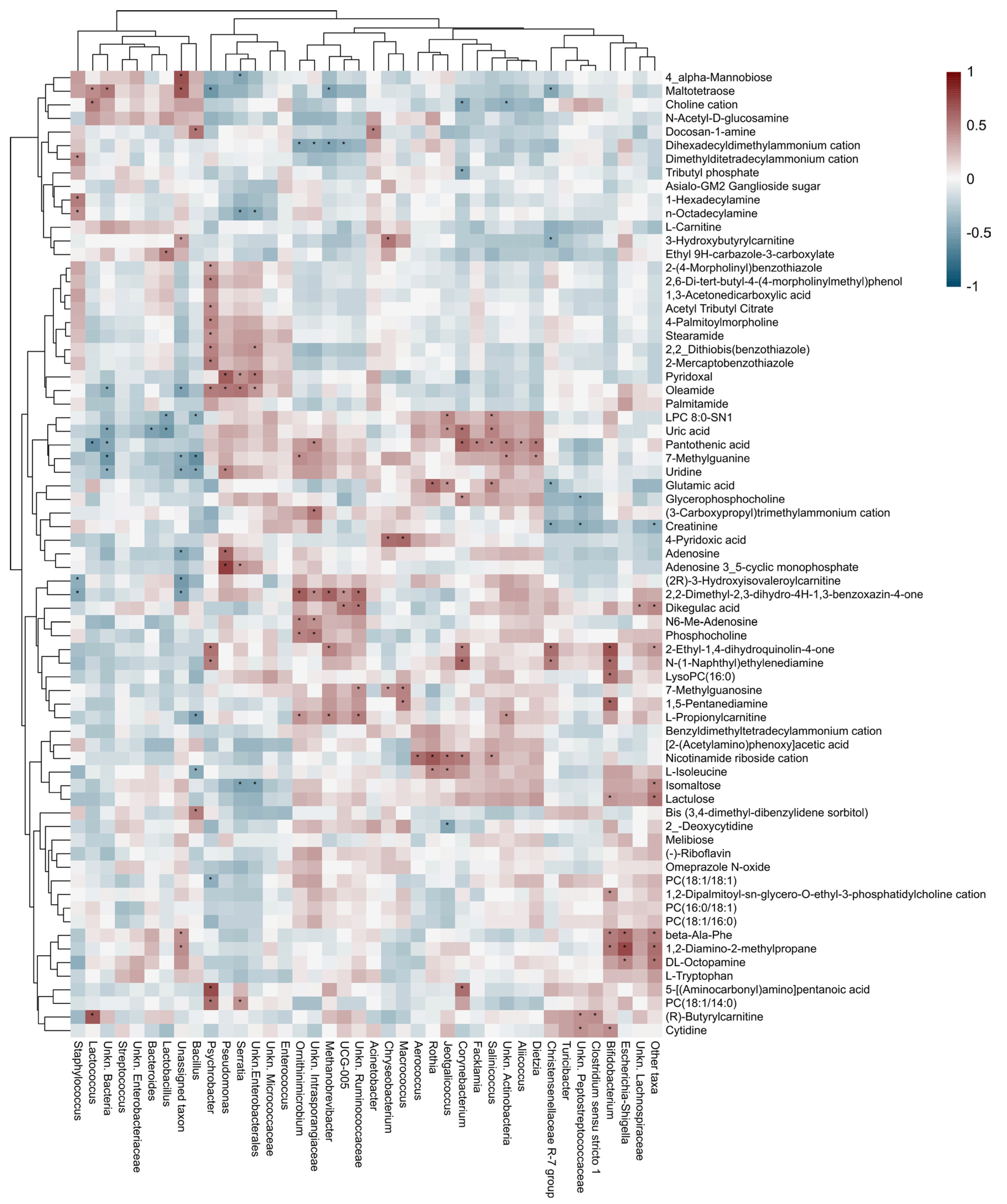
3.4. Correlation Between the Metabolomics and Metataxonomic Profiles Observed
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haug, A.; Hostmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Xu, Y.; Yang, J.; Du, L.; Li, K.; Zhou, Y. Milk consumption and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses in humans. Nutr. Metab. 2021, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Mansson, H.L. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef]
- Addis, M.F.; Tanca, A.; Uzzau, S.; Oikonomou, G.; Bicalho, R.C.; Moroni, P. The bovine milk microbiota: Insights and perspectives from -omics studies. Mol. Biosyst. 2016, 12, 2359–2372. [Google Scholar] [CrossRef] [PubMed]
- Taponen, S.; McGuinness, D.; Hiitiö, H.; Simojoki, H.; Zadoks, R.; Pyörälä, S. Bovine milk microbiome: A more complex issue than expected. Vet. Res. 2019, 50, 44. [Google Scholar] [CrossRef]
- Derakhshani, H.; Fehr, K.B.; Sepehri, S.; Francoz, D.; De Buck, J.; Barkema, H.W.; Plaizier, J.C.; Khafipour, E. Invited review: Microbiota of the bovine udder: Contributing factors and potential implications for udder health and mastitis susceptibility. J. Dairy Sci. 2018, 101, 10605–10625. [Google Scholar] [CrossRef]
- Kuehn, J.S.; Gorden, P.J.; Munro, D.; Rong, R.; Dong, Q.; Plummer, P.J.; Wang, C.; Phillips, G.J. Bacterial community profiling of milk samples as a means to understand culture-negative bovine clinical mastitis. PLoS ONE 2013, 8, e61959. [Google Scholar] [CrossRef]
- Oikonomou, G.; Bicalho, M.L.; Meira, E.; Rossi, R.E.; Foditsch, C.; Machado, V.S.; Teixeira, A.G.; Santisteban, C.; Schukken, Y.H.; Bicalho, R.C. Microbiota of cow’s milk; distinguishing healthy, sub-clinically and clinically diseased quarters. PLoS ONE 2014, 9, e85904. [Google Scholar] [CrossRef]
- Oikonomou, G.; Machado, V.S.; Santisteban, C.; Schukken, Y.H.; Bicalho, R.C. Microbial diversity of bovine mastitic milk as described by pyrosequencing of metagenomic 16s rDNA. PLoS ONE 2012, 7, e47671. [Google Scholar] [CrossRef]
- Doyle, C.J.; Gleeson, D.; O’Toole, P.W.; Cotter, P.D. Impacts of Seasonal Housing and Teat Preparation on Raw Milk Microbiota: A High-Throughput Sequencing Study. Appl. Environ. Microbiol. 2017, 83, e02694-16. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef] [PubMed]
- Callon, C.; Duthoit, F.; Delbès, C.; Ferrand, M.; Le Frileux, Y.; De Crémoux, R.; Montel, M.C. Stability of microbial communities in goat milk during a lactation year: Molecular approaches. Syst. Appl. Microbiol. 2007, 30, 547–560. [Google Scholar] [CrossRef]
- Vithanage, N.R.; Yeager, T.R.; Jadhav, S.R.; Palombo, E.A.; Datta, N. Comparison of identification systems for psychrotrophic bacteria isolated from raw bovine milk. Int. J. Food Microbiol. 2014, 189, 26–38. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. Molecular approaches to analysing the microbial composition of raw milk and raw milk cheese. Int. J. Food Microbiol. 2011, 150, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Kebede, B.; Chen, G.; McComb, K.; Frew, R. Effects of the vat pasteurization process and refrigerated storage on the bovine milk metabolome. J. Dairy Sci. 2020, 103, 2077–2088. [Google Scholar] [CrossRef]
- Suh, J.H. Critical review: Metabolomics in dairy science—Evaluation of milk and milk product quality. Food Res. Int. 2022, 154, 110984. [Google Scholar] [CrossRef] [PubMed]
- Cavero-Redondo, I.; Alvarez-Bueno, C.; Sotos-Prieto, M.; Gil, A.; Martinez-Vizcaino, V.; Ruiz, J.R. Milk and Dairy Product Consumption and Risk of Mortality: An Overview of Systematic Reviews and Meta-Analyses. Adv. Nutr. 2019, 10, S97–S104. [Google Scholar] [CrossRef]
- Givens, D.I. Milk in the diet: Good or bad for vascular disease? Proc. Nutr. Soc. 2012, 71, 98–104. [Google Scholar] [CrossRef]
- Tieri, M.; Ghelfi, F.; Vitale, M.; Vetrani, C.; Marventano, S.; Lafranconi, A.; Godos, J.; Titta, L.; Gambera, A.; Alonzo, E.; et al. Whole grain consumption and human health: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2020, 71, 668–677. [Google Scholar] [CrossRef]
- Deeth, H.C.; Khusniati, T.; Datta, N.; Wallace, R.B. Spoilage patterns of skim and whole milks. J. Dairy. Res. 2002, 69, 227–241. [Google Scholar] [CrossRef]
- UNE-EN ISO 4833-2:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Colony Count at 30 Degrees C by the Surface Plating Technique. ISO: Geneva, Switzerland, 2014.
- Roggenbuck, M.; Bærholm Schnell, I.; Blom, N.; Bælum, J.; Bertelsen, M.F.; Sicheritz-Pontén, T.; Sørensen, S.J.; Gilbert, M.T.P.; Graves, G.R.; Hansen, L.H. The microbiome of New World vultures. Nat. Commun. 2014, 5, 5498. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome. Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 2018, 57, 289–300. [Google Scholar] [CrossRef]
- Machado, S.G.; Baglinière, F.; Marchand, S.; Van Coillie, E.; Vanetti, M.C.; De Block, J.; Heyndrickx, M. The Biodiversity of the Microbiota Producing Heat-Resistant Enzymes Responsible for Spoilage in Processed Bovine Milk and Dairy Products. Front. Microbiol. 2017, 8, 302. [Google Scholar] [CrossRef]
- Ledenbach, L.H.; Marshall, R.T. Microbiological Spoilage of Dairy Products. In Compendium of the Microbiological Spoilage of Foods and Beverages; Sperber, W.H., Doyle, M.P., Eds.; Springer: New York, NY, USA, 2009; pp. 41–67. [Google Scholar]
- Singh, H. Heat stability of milk. Int. J. Dairy Technol. 2004, 57, 111–119. [Google Scholar] [CrossRef]
- Lejeune, J.T.; Rajala-Schultz, P.J. Food safety: Unpasteurized milk: A continued public health threat. Clin. Infect. Dis. 2009, 48, 93–100. [Google Scholar] [CrossRef]
- Sundekilde, U.K.; Gustavsson, F.; Poulsen, N.A.; Glantz, M.; Paulsson, M.; Larsen, L.B.; Bertram, H.C. Association between the bovine milk metabolome and rennet-induced coagulation properties of milk. J. Dairy. Sci. 2014, 97, 6076–6084. [Google Scholar] [CrossRef]
- Sundekilde, U.K.; Frederiksen, P.D.; Clausen, M.R.; Larsen, L.B.; Bertram, H.C. Relationship between the metabolite profile and technological properties of bovine milk from two dairy breeds elucidated by NMR-based metabolomics. J. Agric. Food Chem. 2011, 59, 7360–7367. [Google Scholar] [CrossRef] [PubMed]
- Harzia, H.; Kilk, K.; Joudu, I.; Henno, M.; Kart, O.; Soomets, U. Comparison of the metabolic profiles of noncoagulating and coagulating bovine milk. J. Dairy. Sci. 2012, 95, 533–540. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Vázquez-Fresno, R.; Serra-Cayuela, A.; Dong, E.; Mandal, R.; Hennessy, D.; McAuliffe, S.; Dillon, P.; Wishart, D.S.; Stanton, C.; et al. Pasture Feeding Changes the Bovine Rumen and Milk Metabolome. Metabolites 2018, 8, 27. [Google Scholar] [CrossRef]
- Rocchetti, G.; Gallo, A.; Nocetti, M.; Lucini, L.; Masoero, F. Milk metabolomics based on ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to discriminate different cows feeding regimens. Food Res. Int. 2020, 134, 109279. [Google Scholar] [CrossRef] [PubMed]
- Bellassi, P.; Rocchetti, G.; Nocetti, M.; Lucini, L.; Masoero, F.; Morelli, L. A Combined Metabolomic and Metagenomic Approach to Discriminate Raw Milk for the Production of Hard Cheese. Foods 2021, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Bonizzi, I.; Buffoni, J.N.; Feligini, M.; Enne, G. Investigating the relationship between raw milk bacterial composition, as described by intergenic transcribed spacer-PCR fingerprinting, and pasture altitude. J. Appl. Microbiol. 2009, 107, 1319–1329. [Google Scholar] [CrossRef]
- Antunes, I.C.; Bexiga, R.; Pinto, C.; Roseiro, L.C.; Quaresma, M.A.G. Cow’s Milk in Human Nutrition and the Emergence of Plant-Based Milk Alternatives. Foods 2022, 12, 99. [Google Scholar] [CrossRef]
- Thorning, T.K.; Raben, A.; Tholstrup, T.; Soedamah-Muthu, S.S.; Givens, I.; Astrup, A. Milk and dairy products: Good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr. Res. 2016, 60, 32527. [Google Scholar] [CrossRef]
- Alemayehu, D.; Hannon, J.A.; McAuliffe, O.; Ross, R.P. Characterization of plant-derived lactococci on the basis of their volatile compounds profile when grown in milk. Int. J. Food Microbiol. 2014, 172, 57–61. [Google Scholar] [CrossRef]
- Alothman, M.; Hogan, S.A.; Hennessy, D.; Dillon, P.; Kilcawley, K.N.; O’Donovan, M.; Tobin, J.; Fenelon, M.A.; O’Callaghan, T.F. The “Grass-Fed” Milk Story: Understanding the Impact of Pasture Feeding on the Composition and Quality of Bovine Milk. Foods 2019, 8, 350. [Google Scholar] [CrossRef]
- Coorevits, A.; De Jonghe, V.; Vandroemme, J.; Reekmans, R.; Heyrman, J.; Messens, W.; De Vos, P.; Heyndrickx, M. Comparative analysis of the diversity of aerobic spore-forming bacteria in raw milk from organic and conventional dairy farms. Syst. Appl. Microbiol. 2008, 31, 126–140. [Google Scholar] [CrossRef]
- Hagi, T.; Kobayashi, M.; Nomura, M. Molecular-based analysis of changes in indigenous milk microflora during the grazing period. Biosci. Biotechnol. Biochem. 2010, 74, 484–487. [Google Scholar] [CrossRef]
- Vacheyrou, M.; Normand, A.C.; Guyot, P.; Cassagne, C.; Piarroux, R.; Bouton, Y. Cultivable microbial communities in raw cow milk and potential transfers from stables of sixteen French farms. Int. J. Food Microbiol. 2011, 146, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Rigauts, C.; Aizawa, J.; Taylor, S.L.; Rogers, G.B.; Govaerts, M.; Cos, P.; Ostyn, L.; Sims, S.; Vandeplassche, E.; Sze, M.; et al. R othia mucilaginosa is an anti-inflammatory bacterium in the respiratory tract of patients with chronic lung disease. Eur. Respir. J. 2022, 59, 2101293. [Google Scholar] [CrossRef]
- Uranga, C.C.; Arroyo, P., Jr.; Duggan, B.M.; Gerwick, W.H.; Edlund, A. Commensal Oral Rothia mucilaginosa Produces Enterobactin, a Metal-Chelating Siderophore. mSystems 2020, 5, e00161-20. [Google Scholar] [CrossRef]
- Petrov, V.A.; Fernández-Peralbo, M.A.; Derks, R.; Knyazeva, E.M.; Merzlikin, N.V.; Sazonov, A.E.; Mayboroda, O.A.; Saltykova, I.V. Biliary Microbiota and Bile Acid Composition in Cholelithiasis. Biomed. Res. Int. 2020, 2020, 1242364. [Google Scholar] [CrossRef] [PubMed]
- Fariq, A.; Yasmin, A.; Jamil, M. Production, characterization and antimicrobial activities of bio-pigments by Aquisalibacillus elongatus MB592, Salinicoccus sesuvii MB597, and Halomonas aquamarina MB598 isolated from Khewra Salt Range, Pakistan. Extremophiles 2019, 23, 435–449. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, S.M. Developing an emulsion model system containing canthaxanthin biosynthesized by Dietzia natronolimnaea HS-1. Int. J. Biol. Macromol. 2012, 51, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.L.d.P.A.; Vigoder, H.C.; Nascimento, J.n.d.S. Kocuria spp. in Foods: Biotechnological Uses and Risks for Food Safety. Appl. Food Biotechnol. 2021, 8, 79–88. [Google Scholar] [CrossRef]
- Friman, M.J.; Eklund, M.H.; Pitkala, A.H.; Rajala-Schultz, P.J.; Rantala, M.H.J. Description of two Serratia marcescens associated mastitis outbreaks in Finnish dairy farms and a review of literature. Acta Vet. Scand. 2019, 61, 54. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Liu, H.; Zhao, S.; Wang, J.; Zheng, N. Characterization of Pseudomonas spp. and Associated Proteolytic Properties in Raw Milk Stored at Low Temperatures. Front. Microbiol. 2017, 8, 2158. [Google Scholar] [CrossRef] [PubMed]
- Amat, S.; Holman, D.B.; Schmidt, K.; Menezes, A.C.B.; Baumgaertner, F.; Winders, T.; Kirsch, J.D.; Liu, T.; Schwinghamer, T.D.; Sedivec, K.K.; et al. The Nasopharyngeal, Ruminal, and Vaginal Microbiota and the Core Taxa Shared across These Microbiomes in Virgin Yearling Heifers Exposed to Divergent In Utero Nutrition during Their First Trimester of Gestation and in Pregnant Beef Heifers in Response to Mineral Supplementation. Microorganisms 2021, 9, 2011. [Google Scholar] [CrossRef]
- Tavella, T.; Rampelli, S.; Guidarelli, G.; Bazzocchi, A.; Gasperini, C.; Pujos-Guillot, E.; Comte, B.; Barone, M.; Biagi, E.; Candela, M.; et al. Elevated gut microbiome abundance of Christensenellaceae, Porphyromonadaceae and Rikenellaceae is associated with reduced visceral adipose tissue and healthier metabolic profile in Italian elderly. Gut Microbes 2021, 13, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Camara, A.; Konate, S.; Tidjani Alou, M.; Kodio, A.; Togo, A.H.; Cortaredona, S.; Henrissat, B.; Thera, M.A.; Doumbo, O.K.; Raoult, D.; et al. Clinical evidence of the role of Methanobrevibacter smithii in severe acute malnutrition. Sci. Rep. 2021, 11, 5426. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, F.F.; Farias, D.P. Psychobiotics: An emerging alternative to ensure mental health amid the COVID-19 outbreak? Trends Food Sci. Technol. 2020, 103, 386–387. [Google Scholar] [CrossRef]
- Sharma, M.; Wasan, A.; Sharma, R.K. Recent developments in probiotics: An emphasis on Bifidobacterium. Food Biosci. 2021, 41, 100993. [Google Scholar] [CrossRef]
- Berti Zanella, P.; Donner Alves, F.; Guerini de Souza, C. Effects of beta-alanine supplementation on performance and muscle fatigue in athletes and non-athletes of different sports: A systematic review. J. Sports Med. Phys. Fit. 2017, 57, 1132–1141. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Salvadore, G.; DiazGranados, N.; Ibrahim, L.; Latov, D.; Wheeler-Castillo, C.; Baumann, J.; Henter, I.D.; Zarate, C.A., Jr. New therapeutic targets for mood disorders. Sci. World J. 2010, 10, 713–726. [Google Scholar] [CrossRef]
- Kot, T.V.; Pettit-Young, N.A. Lactulose in the management of constipation: A current review. Ann. Pharmacother. 1992, 26, 1277–1282. [Google Scholar] [CrossRef]
- Karakan, T.; Tuohy, K.M.; Janssen-van Solingen, G. Low-Dose Lactulose as a Prebiotic for Improved Gut Health and Enhanced Mineral Absorption. Front. Nutr. 2021, 8, 672925. [Google Scholar] [CrossRef]
- Garattini, S. Glutamic acid, twenty years later. J. Nutr. 2000, 130, 901s–909s. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Zeisel, S.H. Nutritional importance of choline for brain development. J. Am. Coll. Nutr. 2004, 23, 621s–626s. [Google Scholar] [CrossRef]
- Bissett, D.L.; Oblong, J.E.; Berge, C.A. Niacinamide: A B Vitamin that Improves Aging Facial Skin Appearance. Dermatol. Surg. 2005, 31, 860–865. [Google Scholar] [CrossRef]
- Yiasemides, E.; Sivapirabu, G.; Halliday, G.M.; Park, J.; Damian, D.L. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis 2009, 30, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Maccubbin, D.; Tipping, D.; Kuznetsova, O.; Hanlon, W.A.; Bostom, A.G. Hypophosphatemic Effect of Niacin in Patients without Renal Failure: A Randomized Trial. Clin. J. Am. Soc. Nephrol. 2010, 5, 582–589. [Google Scholar] [CrossRef]
- Bingley, P.J.; Mahon, J.L.; Gale, E.A.M.; European Nicotinamide Diabetes Intervention Trial Group. Insulin Resistance and Progression to Type 1 Diabetes in the European Nicotinamide Diabetes Intervention Trial (ENDIT). Diabetes Care 2008, 31, 146–150. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Bouladoux, N.; Claesen, J.; Chen, Y.E.; Byrd, A.L.; Constantinides, M.G.; Merrill, E.D.; Tamoutounour, S.; Fischbach, M.A.; Belkaid, Y. Contextual control of skin immunity and inflammation by Corynebacterium. J. Exp. Med. 2018, 215, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Rumberger, J.A.; Napolitano, J.; Azumano, I.; Kamiya, T.; Evans, M. Pantethine, a derivative of vitamin B5 used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low– to moderate–cardiovascular risk North American subjects: A triple-blinded placebo and diet-controlled investigation. Nutr. Res. 2011, 31, 608–615. [Google Scholar] [CrossRef]
- Stover, P.J.; Field, M.S. Vitamin B-6. Adv. Nutr. 2015, 6, 132–133. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Souza, P.R.; Peghini, B.C.; da Silva, J.S.; Cardoso, C.R. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [PubMed]
- Mueller, G.P.; Driscoll, W.J. Biosynthesis of oleamide. Vitam. Horm. 2009, 81, 55–78. [Google Scholar] [CrossRef] [PubMed]
- Morello, E.; Pérez-Berezo, T.; Boisseau, C.; Baranek, T.; Guillon, A.; Bréa, D.; Lanotte, P.; Carpena, X.; Pietrancosta, N.; Hervé, V.; et al. Pseudomonas aeruginosa Lipoxygenase LoxA Contributes to Lung Infection by Altering the Host Immune Lipid Signaling. Front. Microbiol. 2019, 10, 1826. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Leeds Jennifer, A.; Meredith Timothy, C. Pseudomonas aeruginosa Directly Shunts β-Oxidation Degradation Intermediates into De Novo Fatty Acid Biosynthesis. J. Bacteriol. 2012, 194, 5185–5196. [Google Scholar] [CrossRef]
- Jaskulska, A.; Gach-Janczak, K.; Drogosz-Stachowicz, J.; Janecki, T.; Janecka, A.E. Synthesis and Anticancer Properties of New 3-Methylidene-1-sulfonyl-2,3-dihydroquinolin-4(1H)-ones. Molecules 2022, 27, 3597. [Google Scholar] [CrossRef]
- Nilsen, A.; Miley, G.P.; Forquer, I.P.; Mather, M.W.; Katneni, K.; Li, Y.; Pou, S.; Pershing, A.M.; Stickles, A.M.; Ryan, E.; et al. Discovery, Synthesis, and Optimization of Antimalarial 4(1H)-Quinolone-3-Diarylethers. J. Med. Chem. 2014, 57, 3818–3834. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Nilov, D.; Maluchenko, N.; Kurgina, T.; Pushkarev, S.; Lys, A.; Kutuzov, M.; Gerasimova, N.; Feofanov, A.; Švedas, V.; Lavrik, O.; et al. Molecular Mechanisms of PARP-1 Inhibitor 7-Methylguanine. Int. J. Mol. Sci. 2020, 21, 2159. [Google Scholar] [CrossRef]
- Cowling, V.H. Regulation of mRNA cap methylation. Biochem. J. 2009, 425, 295–302. [Google Scholar] [CrossRef]
- Tan, M.A.; Sharma, N.; An, S.S.A. Phyto-Carbazole Alkaloids from the Rutaceae Family as Potential Protective Agents against Neurodegenerative Diseases. Antioxidants 2022, 11, 493. [Google Scholar] [CrossRef]
- Slattery, C.; Cotter, P.D.; W. O’Toole, P. Analysis of Health Benefits Conferred by Lactobacillus Species from Kefir. Nutrients 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed]

| Feeding Regimen | Geographical Location of the Herd | Number of Samples | ID of the Samples |
|---|---|---|---|
| Grazing | Inland | 9 | UCM2; UCM3; UCM5; UCM16; UCM17; UCM18; UCM25; UCM26; UCM27 |
| Coastal | 9 | UCM28; UCM29; UCM30; UCM31; UCM32; UCM33; UCM34; UCM35; UCM36 | |
| Conventional | Inland | 9 | UCM1; UCM4;UCM6; UCM19; UCM20; UCM21; UCM22; UCM23; UCM24 |
| Coastal | 9 | UCM7; UCM8; UCM9; UCM10; UCM11; UCM12; UCM13; UCM14; UCM15 |
| Feeding Regimen | Geographic Location of the Herd | Number of Samples | ID of the Samples | |
|---|---|---|---|---|
| Stable for the 80° Ethanol Test | Unstable for the 80° Ethanol Test | |||
| Grazing | Inland | 6 | UCM16; UCM25; UCM27 | UCM2; UCM3; UCM5 |
| Coastal | 6 | UCM28; UCM29; UCM36 | UCM31; UCM32; UCM34 | |
| Conventional | Inland | 6 | UCM19; UCM22; UCM23 | UCM4;UCM6; UCM21 |
| Coastal | 6 | UCM10; UCM12; UCM13 | UCM8; UCM14; UCM15 | |
| Retention Time (min) | Metabolite Name | Molecular Formula | m/z | Adduct Type |
|---|---|---|---|---|
| 0.55 | Glutamic acid | C5H9NO4 | 148.0597 | [M+H]+ |
| 0.59 | Maltotetraose | C24H42O21 | 667.22 | [M+H]+ |
| 0.594 | Choline cation | C5H14NO | 104.1073_60.0808 | [Cat]+_[M+H-CH4N2]+ |
| 0.594 | Creatinine | C4H7N3O | 114.0663 | [M+H]+ |
| 0.596 | L-Carnitine | C7H15NO3 | 162.1109 | [M+H]+ |
| 0.608 | Glycerophosphocholine | C8H20NO6P | 296.0656_280.0914_258.1110 | [M+K]+_[M+Na]+_[M+H]+ |
| 0.616 | N-Acetyl-D-glucosamine | C8H15NO6 | 222.0974_204.0867 | [M+H]+_[M+H-H2O]+ |
| 0.616 | Asialo-GM2 Ganglioside sugar | C20H35NO16 | 546.1978 | [M+H]+ |
| 0.62 | Phosphocholine | C5H14NO4P | 184.0727 | [M+H]+ |
| 0.644 | 4alpha-Mannobiose/beta-lactose | C12H22O11 | 707.223 | [2M+Na]+ |
| 0.649 | Melibiose | C12H22O11 | 325.1132_360.1507 | [M+H-H2O]+_[M+NH4]+ |
| 0.722 | 1,2-Diamino-2-methylpropane | C4H12N2 | 72.081 | [M+H-NH3]+ |
| 0.819 | Cytidine | C9H13N3O5 | 487.1792_244.0934 | [2M+H]+_[M+H]+ |
| 0.819 | 1,5-Pentanediamine | C5H14N2 | 86.0959 | [M+H-NH3]+ |
| 0.962 | 2′-Deoxycytidine | C9H13N3O4 | 112.0507 | [M+H-C5H8O3]+ |
| 0.973 | 2,2-Dimethyl-2,3-dihydro-4H-1,3-benzoxazin-4-one | C10H11NO2 | 178.0864 | [M+H]+ |
| 0.978 | Nicotinamide riboside cation | C11H15N2O5 | 123.0555 | [Cat-C5H8O4]+ |
| 1.024 | Uric acid | C5H4N4O3 | 169.0355 | [M+H]+ |
| 1.116 | Isomaltose/beta-lactose | C12H22O11 | 365.1053 | [M+Na]+ |
| 1.155 | 3-Hydroxybutyrylcarnitine | C11H21NO5 | 248.1492 | [M+H]+ |
| 1.17 | 7-Methylguanine | C6H7N5O | 166.0722 | [M+H]+ |
| 1.222 | N6-Me-Adenosine | C11H15N5O4 | 282.1196 | [M+H]+ |
| 1.289 | Lactulose | C12H22O11 | 365.1055 | [M+Na]+ |
| 1.289 | L-Isoleucine | C6H13NO2 | 132.1016 | [M+H]+ |
| 1.306 | DL-Octopamine | C8H11NO2 | 136.0753 | [M+H-H2O]+ |
| 1.399 | Uridine | C9H12N2O6 | 245.0771 | [M+H]+ |
| 1.702 | L-Propionylcarnitine | C10H19NO4 | 218.1393 | [M+H]+ |
| 1.821 | Adenosine | C10H13N5O4 | 268.1042 | [M+H]+ |
| 1.874 | 4-Pyridoxic acid | C8H9NO4 | 184.0608 | [M+H]+ |
| 1.91 | (2R)-3-Hydroxyisovaleroylcarnitine | C12H23NO5 | 262.1651 | [M+H]+ |
| 1.91 | Pyridoxal | C8H9NO3 | 150.0548 | [M+H-H2O]+ |
| 1.917 | Adenosine 3′,5′-cyclic monophosphate | C10H12N5O6P | 330.06 | [M+H]+ |
| 2.087 | beta-Ala-Phe | C12H16N2O3 | 166.086 | [M+H-C3H5NO]+ |
| 2.125 | 5-[(Aminocarbonyl)amino]pentanoic acid/d-Alanyl-D-Alanine | C6H12N2O3 | 100.076 | [M+H-CH3NO2]+ |
| 2.191 | 7-Methylguanosine | C11H15N5O5 | 298.1151 | [M+H]+ |
| 2.369 | Ethyl 9H-carbazole-3-carboxylate | C15H13NO2 | 240.1025 | [M+H]+ |
| 2.421 | Pantothenic acid | C9H17NO5 | 242.1004_220.1191 | [M+Na]+_[M+H]+ |
| 2.426 | (R)-Butyrylcarnitine | C11H21NO4 | 232.1555 | [M+H]+ |
| 2.531 | 2-Ethyl-1,4-dihydroquinolin-4-one | C11H11NO | 174.0913 | [M+H]+ |
| 2.8 | L-Tryptophan | C11H12N2O2 | 205.0982_188.0701 | [M+H]+_[M+H-NH3]+ |
| 4.031 | N-(1-Naphthyl)ethylenediamine | C12H14N2 | 187.1234 | [M+H]+ |
| 4.489 | (-)-Riboflavin | C17H20N4O6 | 377.1458 | [M+H]+ |
| 7.354 | 2-Mercaptobenzothiazole | C7H5NS2 | 167.9937 | [M+H]+ |
| 7.379 | 2,2′-Dithiobis(benzothiazole) | C14H8N2S4 | 332.9646 | [M+H]+ |
| 7.798 | LPC (8:0) | C16H34NO7P | 384.2142 | [M+H]+ |
| 7.95 | 2-(4-Morpholinyl)benzothiazole | C11H12N2OS | 221.0747 | [M+H]+ |
| 8.373 | 2,6-Di-tert-butyl-4-(4-morpholinylmethyl)phenol | C19H31NO2 | 306.2433 | [M+H]+ |
| 9.341 | Bis (3,4-dimethyl-dibenzylidene sorbitol) | C24H30O6 | 432.2391_119.0852_135.0802_415.2123_453.1677 | [M+NH4]+_[M+H-C15H20O6]+_[M+H-C15H20O5]+_[M+H]+_[M+K]+ |
| 9.429 | LPC (16:0) | C24H50NO7P | 496.3404 | [M+H]+ |
| 9.59 | 1-Hexadecylamine | C16H35N | 242.2846 | [M+H]+ |
| 9.765 | Tributyl phosphate | C12H27O4P | 267.1721 | [M+H]+ |
| 9.87 | Docosan-1-amine | C22H47N | 326.3792 | [M+H]+ |
| 9.962 | n-Octadecylamine | C18H39N | 270.316 | [M+H]+ |
| 10.04 | 1,3-Acetonedicarboxylic acid | C5H6O5 | 129.0183 | [M+H-H2O]+ |
| 10.352 | Acetyl Tributyl Citrate | C20H34O8 | 425.216 | [M+Na]+ |
| 10.531 | PC(18:1_14:0) | C40H78NO8P | 732.5511 | [M+H]+ |
| 10.536 | PC(16:0_18:1) | C42H82NO8P | 760.585 | [M+H]+ |
| 10.611 | PC(18:1_18:1) | C44H84NO8P | 786.6008 | [M+H]+ |
| 10.627 | 1,2-Dipalmitoyl-sn-glycero-O-ethyl-3-phosphatidylcholine cation | C42H85NO8P | 734.5695 | [Cat-C2H4]+ |
| 10.629 | PC(18:1_16:0) | C42H82NO8P | 760.5867 | [M+H]+ |
| 10.822 | Palmitamide | C16H33NO | 256.2644 | [M+H]+ |
| 10.927 | Oleamide | C18H35NO | 282.2797 | [M+H]+ |
| 11.394 | 4-Palmitoylmorpholine | C20H39NO2 | 326.3062 | [M+H]+ |
| 11.528 | Stearamide | C18H37NO | 284.2953 | [M+H]+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, M.C.; Soria, E.; Llama-Palacios, A.; Almirón, F.; Valdés, A.; Cifuentes, A.; Hernández, M.; Ciudad, M.J.; Collado, L. Lactic Microbiota and Metabolites in Raw Cow’s Milk: Implications for Consumer Health. Dairy 2025, 6, 24. https://doi.org/10.3390/dairy6030024
Sánchez MC, Soria E, Llama-Palacios A, Almirón F, Valdés A, Cifuentes A, Hernández M, Ciudad MJ, Collado L. Lactic Microbiota and Metabolites in Raw Cow’s Milk: Implications for Consumer Health. Dairy. 2025; 6(3):24. https://doi.org/10.3390/dairy6030024
Chicago/Turabian StyleSánchez, María Carmen, Eduardo Soria, Arancha Llama-Palacios, Franco Almirón, Alberto Valdés, Alejandro Cifuentes, Marta Hernández, María José Ciudad, and Luis Collado. 2025. "Lactic Microbiota and Metabolites in Raw Cow’s Milk: Implications for Consumer Health" Dairy 6, no. 3: 24. https://doi.org/10.3390/dairy6030024
APA StyleSánchez, M. C., Soria, E., Llama-Palacios, A., Almirón, F., Valdés, A., Cifuentes, A., Hernández, M., Ciudad, M. J., & Collado, L. (2025). Lactic Microbiota and Metabolites in Raw Cow’s Milk: Implications for Consumer Health. Dairy, 6(3), 24. https://doi.org/10.3390/dairy6030024








