1. Introduction
Milk and dairy products hold significant nutritional value in the human diet, supplying essential energy, protein, vitamins, and minerals [
1,
2,
3,
4]. Various factors influence milk composition, including diet, breed, parity, environmental conditions, feeding and management practices, season, and lactation state [
5,
6]. Among these factors, lipids constitute the most variable fraction of milk and are highly responsive to dietary modifications in terms of composition and concentration [
1,
3]. The chain length and degree of unsaturation of fatty acids (FAs) are critical determinants of milk fat quality concerning human health. These factors are closely linked to lipid digestion in bovine species. Lipids in the diet undergo lipolysis and biohydrogenation processes mediated by rumen bacteria, leading to the saturation of most consumed unsaturated FAs (UFAs). The progression of this process is influenced by the characteristics (type and quantity) of lipids and the type of diet [
1,
4,
7]. Moreover, it should be noted that some natural fatty materials, such as fat from meat and ruminant milk, contain trans FAs, including vaccenic acid (TVA) and conjugated linoleic acid (CLA). Conjugated linoleic acid comprises a family of positional and geometric isomers, all of which are conjugated dienes of linoleic acid (18:2). These FAs have been reported to offer beneficial health effects, including hypocholesterolemic, anticarcinogenic, antiatherogenic properties, modulation of the immune response, and improvement of bone mineralization [
8,
9,
10]. It has been reported that the consumed amount of CLA needed to produce observable health benefits ranges from 0.8 to 3.0 g/d [
11]. TVA, an intermediate in CLA formation, also possesses beneficial health properties, including the reduction of cardiovascular disease risk and potential inhibition of tumor growth [
12]. Additionally, oleic acid and TVA found in milk have also been shown to have beneficial effects on human health, similar to CLA. Oleic acid reduces total cholesterol and low-density lipoprotein (LDL) [
13], while TVA is desaturated to CLA in the human body at a rate of 19% [
14]. Furthermore, odd- and branched-chain FAs (OBCFAs) are unique components of ruminant fat, representing the primary contribution of BFAs to human nutrition. The quantification of milk BFAs has garnered significant interest in recent years. Due to their origin and the correlations observed between diet and ruminal microbial population, OBCFAs are considered potential biological indicators of ruminal function [
15]. The potential inhibitory effects on tumor cells [
16] and the reduced risk of cardiovascular disease, associated with the consumption of these FAs further underscore their beneficial effects on human health, including a lower risk of developing type 2 diabetes [
17]. Specifically, 15:0 and 17:0 iso and anteiso have been found to enhance the fluidity of cell membranes [
18]. The concentration and composition of milk fat can be readily modified through dietary adjustments [
19]. For example, increasing the proportion of forage in the diet compared to concentrate results in higher concentrations of OBCFAs in the milk. Similarly, a diet rich in grass silage could elevate the total content of OBCFAs in milk. The profile of OBCFAs in cow’s milk is primarily influenced by the FAs in the diet and FA metabolism in the rumen [
20]. Therefore, understanding the origin of OBCFAs in milk and manipulating the diet of dairy cows to produce milk enriched with odd- and branched-chain FAs can be important both scientifically and industrially.
Prior studies have investigated milk FAs and their correlation with human health using certain indices. The atherogenic index (AI) is indicative of the risk impact on cardiovascular diseases. A higher AI suggests a greater risk of such diseases [
21]. Additionally, the ratio between hypocholesterolemic (18:1, 18:2, and 18:3) and hypercholesterolemic FAs (12:0; 14:0, and 16:0), denoted as H/H, is associated with AI [
22]. Another index associated with human health is the n-6/n-3 ratio [
23]. Excessive levels of n-6, commonly found in Western diets, can hinder human enzymatic systems, contributing to the development of certain diseases. Conversely, higher levels of n-3 can have the opposite effects [
23,
24]. Furthermore, the technological potential of milk for butter production was assessed using the spreadability index (SI) [
25].
Moreover, pasture-based systems are regarded as more environmentally friendly, animal welfare-conscious, and sustainable, compared to confinement systems [
26]. Additionally, milk and dairy products from these systems offer potential nutritional benefits and market opportunities due to their improved composition, compared to those derived from total mixed ration (TMR) systems [
27,
28]. Pasture-based systems are commonly employed in regions with mild climates like South America and Oceania, owing to their low production costs, which are favored by climate conditions and forage accessibility [
29]. However, even in these systems, the use of reserves and concentrates is necessary to ensure the fulfillment of energy requirements and nutrient quality [
29,
30]. In Uruguay, pasture utilization is crucial for reducing production costs and maintaining satisfactory production levels [
29]. Furthermore, it has been widely reported that including pasture improves the FA profile (FAP) in milk and dairy products [
31,
32]. For instance, milk and dairy products from systems with a high proportion of pasture exhibit higher levels of UFAs and CLA, and lower proportions of saturated fatty acids (SFAs), compared to systems with low levels or no access to pasture [
25,
33,
34,
35,
36,
37,
38]. Therefore, the ability to modify the composition of milk fat through pasture utilization and strategic supplementation can serve as a tool to differentiate dairy products, resulting in milk and dairy products with a healthier FAP for human consumption. It is important to consider that this management approach has its limitations. For instance, the addition of UFAs to the diet increases CLA production, but excessive amounts can result in a lower percentage of milk fat by reducing the production of new FAs in the udder [
7,
39]. Moreover, seasonality, particularly in pasture-based systems, affects the FAP in milk and dairy products. Climate factors influencing the thermal comfort of cows, primarily heat stress in summer, impact the lipid catabolism of the animals [
40]. Additionally, seasonality impacts the quality of pastures [
41], thus significantly influencing the quality of milk fat. In this regard, higher levels of monounsaturated FAs (MUFAs), polyunsaturated FAs (PUFAs), and CLA, along with a lower n-6/n-3 ratio and palmitic acid content, have been reported in milk and cheeses in spring compared to fall [
42,
43,
44]. This may result from lower pasture availability and increased utilization of preserved forages in temperate countries during fall, leading to decreased FA quality in the cows’ diet [
29,
43].
In the past, the consumption of dairy fat (such as milk cream, butter, or cheese) raised concerns among consumers due to its high levels of SFAs, which had been linked to elevated cholesterol levels, arteriosclerosis, and cardiovascular diseases [
21,
45]. However, recent reviews and meta-analyses have concluded that milk consumption has at least a neutral effect on various health outcomes, and cow’s milk consumption may even be beneficial for osteoporosis, cardiovascular disease, stroke, type II diabetes, and certain cancers [
46,
47,
48]. Regarding organoleptic characteristics, it has been reported that modifications in the FAP result in changes in the texture, sensory, and nutritional quality of butter [
49]. In this regard, butter from pasture-based systems has shown better nutritional and rheological quality, compared to butter from confinement systems using TMR [
50]. Additionally, higher levels of PUFAs, including CLA, and a higher yellow index have been reported in milk from pasture-based systems compared to TMR systems [
51].
This study aimed to evaluate the FAP (mainly OBCFAs) in milk obtained from farms located in the northwest region of Uruguay, using feeding strategies with varying pasture content (classified as high and low pasture in the diet) during two seasons (spring and fall). The physical and chemical properties of butter were investigated to assess the impact of varying FAs profiles on the technological and nutritional properties of high-fat dairy products.
2. Materials and Methods
2.1. Experimental Design, Localization, and Sample Analysis
Six dairy farms located in the northwest region of Uruguay (Salto, Paysandú and Río Negro provinces) were selected according to the pasture intake, milk production, and somatic cell count from the previous year. Two treatments were created according to pasture intake: High Pasture (HP) (>65% pasture of total dry matter intake: DMI) and Low Pasture (LP) (<35% pasture of total DMI).
The study was conducted during two seasons: spring 2021 and fall 2022. Milk samples were collected from bulk tanks (if there were multiple tanks, a proportional mixture was prepared based on the volumes of each tank), considering that the milk originated from two or four milkings. Sampling was conducted fortnightly, completing a total of five periods for each season. The samples were extracted in 15 mL Falcon tubes, frozen for transfer, and subsequently analyzed in the laboratory of Food Technology at the School of Chemistry, Universidad de la República. Concurrently, feed samples (provided to dairy cows), including pasture and supplements (concentrate and reserves), were collected. In each dairy farm, the following records were kept: herd management, milk production, feeding routine (type of supplement, quantity, and composition), type of pasture (allocation, availability, and species), milk production, and number of milking cows.
During the spring, the HP treatment had an average of 74% pasture and 26% supplement, with a forage/concentrate ratio of 79:21, while the LP treatment had 10% pasture and 90% supplement, with a forage/concentrate ratio of 68:32. In the fall, the HP had an average of 45% pasture and 55% supplement, with a forage/concentrate ratio of 76:24, while the LP had 3% pasture and 97% supplement, with a forage/concentrate ratio of 61:39.
Pastures consisted of a variety of grass species (Festuca arundinacea, Avena sativa) and legumes (Medicago sativa, Lotus corniculatus, Trifolium pretense, Trifolium repens), as well as natural grassland. The ingredients used in commercial dairies varied over time, depending on available conserved forage and the market availability of grains and by-products for concentrate. The most commonly used conserved forages were whole-plant maize or sorghum silage, as well as grass hay, silage, or haylage (such as oat, lucerne, and moha). Concentrate mixes could include ground corn grain, rice bran, soybean expeller, soybean meal, barley rootlets, urea, yeast, and minerals.
Daily pasture DMI (kg DM/cow) was estimated using the energy balance method according to National Research Council (NRC) [
52] guidelines, as the amount of pasture required to provide the remaining energy needed to meet the cow’s net energy (NE) requirement, not supplied by supplements (reserves and concentrates). Cow NE requirements were estimated as the sum of maintenance and milk production requirements [
53]. The average data for neutral detergent fiber (NDF), acid detergent fiber (ADF), and ether extract (EE) of each group (pasture and supplement) are presented in
Table 1.
Table 2 shows the FAP of pasture and supplement in each treatment (HP and LP) (averaged over the experimental period).
Butter Production
Butter was produced from two contrasting dairy farms based on diet and management: grazing + supplement (GRZ) and confinement cows with total mixed ration (TMR) (C: confined). At the time of milk extraction for butter production, a sample of feed (pasture and supplement) was obtained from each producer in each season. For the C dairy farm in spring and fall, the total mixed ration consisted of ground corn grain and soybean expeller for concentrate, haylage (
Medicago sativa) as conserved forage, urea, and minerals. For the GRZ dairy farm in spring and fall, the supplement comprised corn grain and barley rootlet. Pastures consisted of
Avena sativa and
Medicago sativa in the fall, and
Trifolium pretense and
Lolium multiflorum. The FAP in the diet of cows from each farm (GRZ and C) is shown in
Table 3.
For both producers (contrasting conditions: GRZ and C), in each season (spring and fall), two butter productions were carried out on consecutive days in triplicate. For each butter production, 60 L of bulk milk (MilkB) was obtained and immediately transported under refrigerated conditions (4 °C) to the dairy pilot plant of the Universidad Tecnológica del Uruguay (UTEC) (La Paz, Colonia, Uruguay) for processing. Two batches of fresh cream (5 L each; 40–42% fat) were separated at 45 °C from pre-pasteurized milk. The cream was then stored overnight at 10 °C for maturation. Subsequently, both cream samples were churned in a rotary churn (Edibon España) at 26 rpm and 13 °C. After grain formation, the butter was washed with 1.7 L of water at 4 °C, and finally, it was kneaded until it formed into butter. Portions of butter were clarified for further analysis using the British Standards Method 769 (BSI 1961 54) [
54].
2.2. Sample Analysis
2.2.1. Fatty Acid Profile in Milk and Butter
Milk and butter samples (3 g each) were extracted using the Rose-Gottlieb technique [
55]. Analyses were conducted in triplicate, and FAs methyl esters were prepared following the IUPAC 2.301 protocol, [
56] and analyzed by gas chromatography, according to the AOCS Ce 1c-89 and AOCS Ce 1f-96 protocols [
57]. The gas chromatograph was a Shimadzu (Kyoto, Japan) model 2014 equipped with a Supelco (Bellefonte, PA, USA) SP 2560 (100 m × 0.25 mm × 0.2 mm) capillary column and a flame ionization detector (FID). The injection volume of the samples was 1 μL. The temperature program used was as follows: an initial temperature of 90 °C for 2 min, then an increase to 175 °C at a rate of 20 °C/min, maintained for 35 min, followed by an increase to 240 °C at a rate of 15 °C/min, maintained for 25 min. Peak identification was achieved through the analysis of authentic standards. Standards and reagents used for the analysis were supplied by Sigma-Aldrich (Burlington, MA, USA). The milk fat compositions were expressed in grams of each individual FA per 100 g of total fat. The atherogenicity index (AI) was calculated as (12:0 + 4 × 14:0 + 16:0)/(MUFAs + PUFAs) [
21], and the hypocholesterolemic/hypercholesterolemic FA (H/H) ratio was calculated as (18:1 cis + 18:2 cis + 18:3 cis)/(12:0 + 14:0 + 16:0) [
22]. The spreadability index (SI) was calculated as the ratio of 16:0 to 18:1, as proposed by O’Callaghan et al. [
25].
2.2.2. Fatty Acid Profile in Pasture and Supplement
Fat was extracted from the different samples (1 g each) using the Hara and Radin [
58] technique, with a mixture of hexane and isopropanol (3:2). Initially, approximately 1 g of milled samples was weighed into a tube, and 20 mL of the solvent mixture was added. Extraction was conducted at room temperature (20 °C) with magnetic stirring for 90 min. Subsequently, centrifugation was performed (Hermle, model Z 200 A, Gosheim, Germany) at 3000 rpm for 15 min. Following centrifugation, 5 mL of the solvent mixture was added for complete lipid extraction. Finally, the total solvent mixture was removed using a nitrogen flow at 40 °C until the weight of the lipids remained constant. Analyses were conducted in triplicate, and the FAP was determined as previously described for milk and butter.
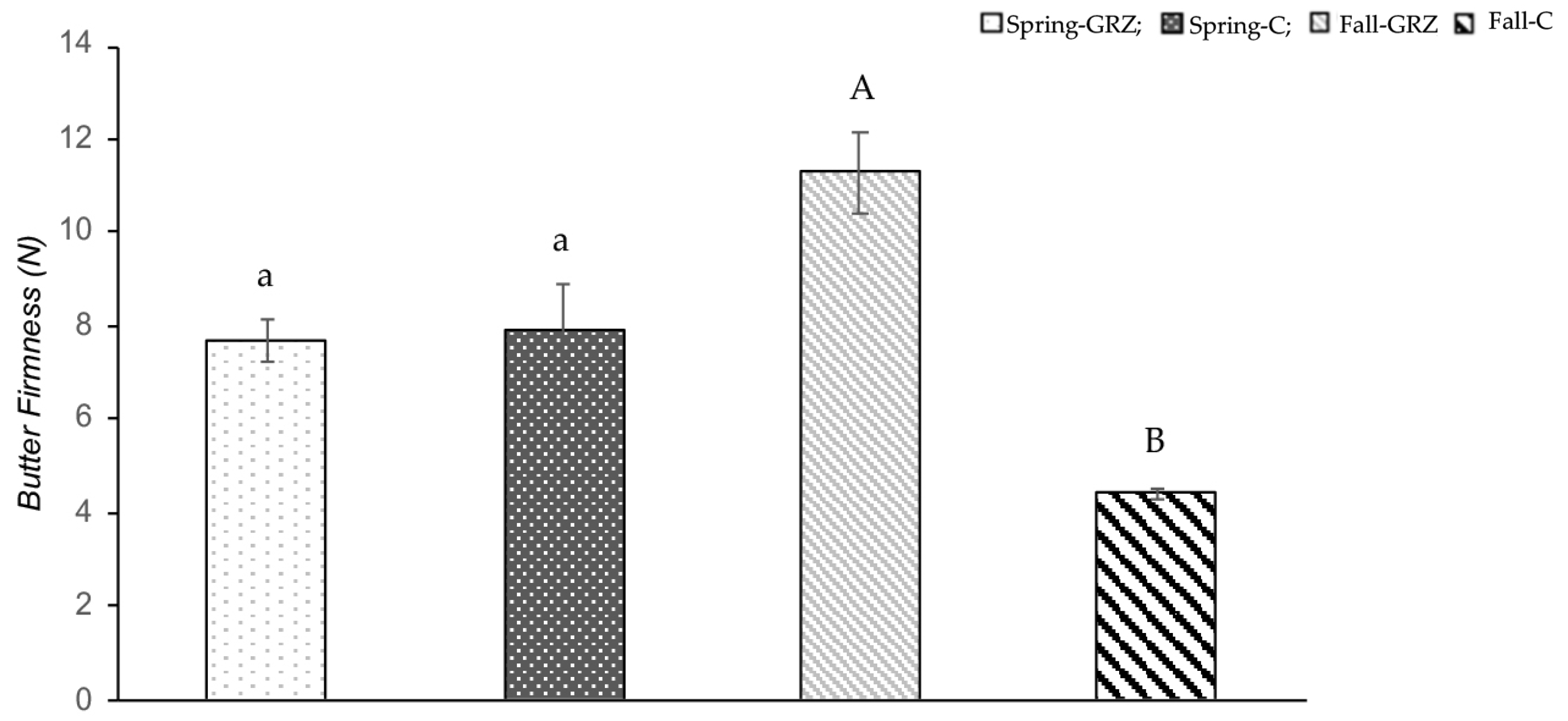
2.2.3. Butter Firmness
The firmness of butter was measured at 10 °C using a Texture Analyzer (Brookfield CT3 50k, MA, USA) following the procedure previously described [
59], with modifications. The procedure consisted of testing the cutting force at a depth of 16 mm applied to a given sample, which was stored at 10 °C for 12 h, at a speed of 2 mm/s using a TA53 cutting wire probe. The force of cutting between 8 and 16 mm was reported as the firmness of the sample. Each sample was measured six times.
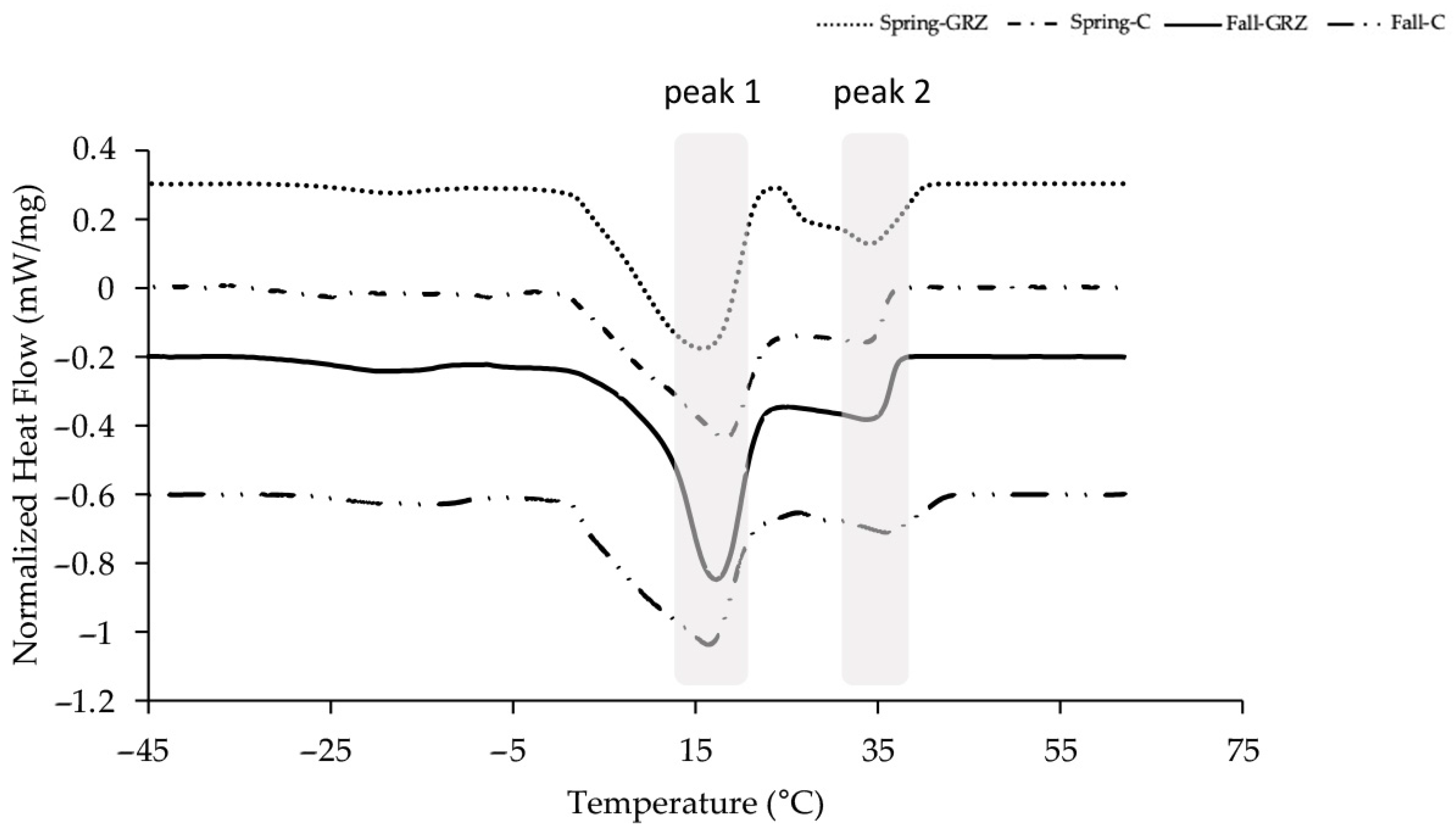
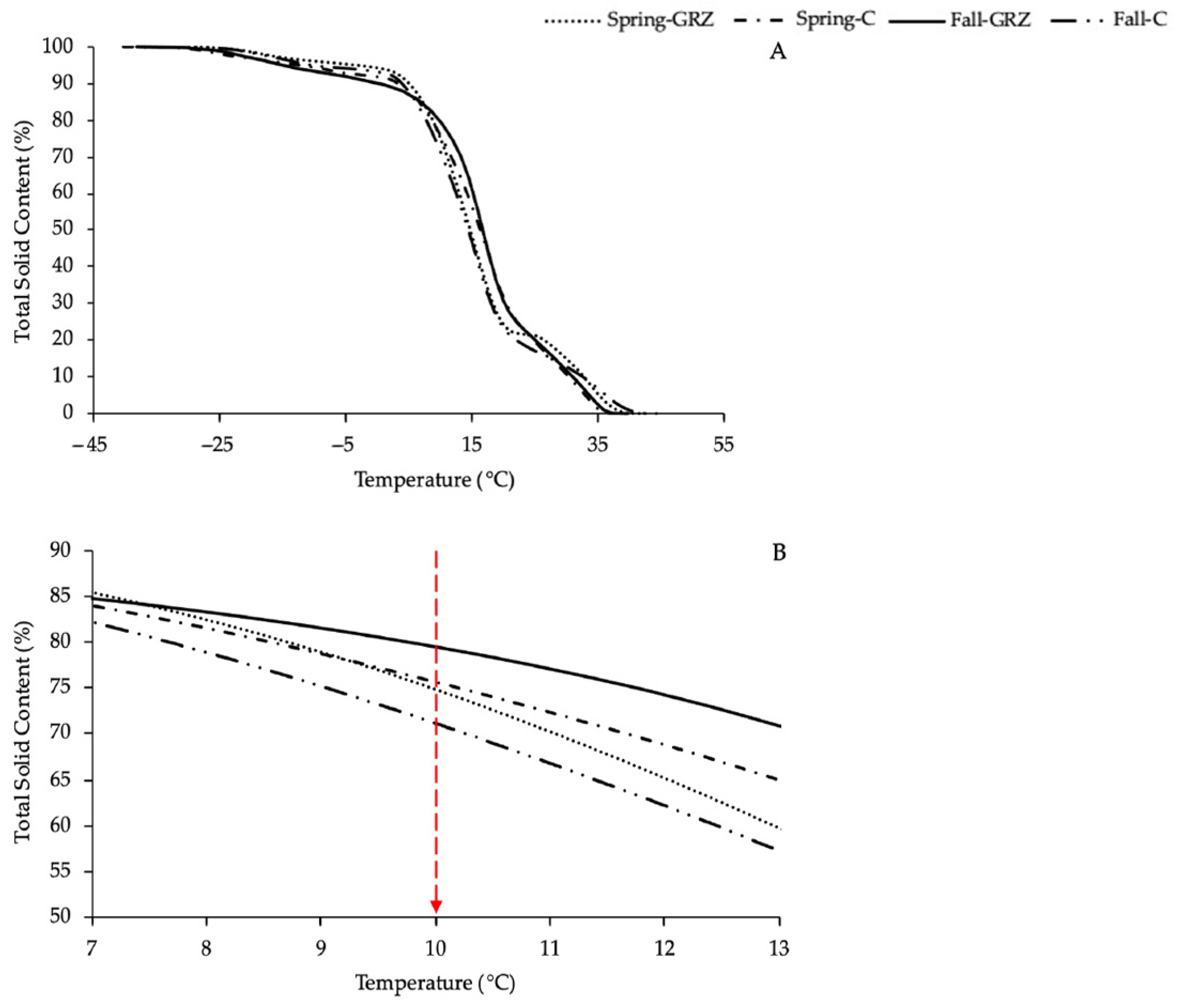
2.2.4. Differential Scanning Calorimeter (DSC)
A Shimadzu differential scanning calorimeter (DSC-60A plus, Shimadzu Co., Kyoto, Japan) was used to examine the melting and crystallization properties of milk fat from butter. Oxygen (99.999% purity) was used as the purge gas. The DSC was calibrated using high-purity indium (m.p. 156.6 °C, ∆Hf = 28.45 J g−1), according to standard DSC procedures. Clarified butter samples of about 10 mg were placed in open aluminum pans and inserted into the heating chamber of the DSC cell, with an empty aluminum pan as the reference. Prior to analysis, the samples were heated at 50 °C for 5 min to melt all crystals and nuclei. Subsequently, they were tempered in a freezer at −20 °C for 48 h. The final crystallization was carried out in the DSC by cooling from 2 °C to −50 °C and left at −50 °C for 30 min. After that time, the melting was studied by heating the sample at 5 °C/min from −50 °C to 60 °C. At least duplicate determinations were carried out. Characteristic data were obtained using the Shimadzu DSC-TA 60A plus Version 2.21 software. The thermograms were integrated using the TA60 Version 2.21 software. From this integration, the fraction of liquid formed for each temperature was determined, and the curve of solids percentage vs. temperature was constructed.
2.2.5. Color
Clarified butter was measured at 50 °C using a Minolta Chroma-Meter CR-400 colorimeter. The parameters L*, a*, and b* were recorded in triplicate (Chiyodaku, Tokyo, Japan).
2.3. Statistical Analysis
For each season, data on fat content, FAP, and nutritional quality indices (AI, H/H, n-6/n-3) in milk were analyzed using repeated measures ANOVA with PROC GLIMMIX of SAS 9.04 (SAS Institute Inc., Cary, NC, USA). The statistical models included treatment (HP and LP), period (1 to 5), and the interaction between treatment and period. Farms within each treatment were considered as random effects. The Kenward–Roger approximation was utilized to calculate the denominator degrees of freedom for the fixed-effects tests of the model. Least squares (LS) means were generated using the LSMEANS/DIFF option, and post hoc comparisons were conducted with the Tukey test.
For butter variables (FAP, SI, firmness, and color), analyses were performed using one-way ANOVA, followed by post hoc Tukey tests, to compare the two contrasting farms (GRZ and C) in each season. Results were considered significant at p ≤ 0.05. Data are presented as mean ± SEM (standard error of the mean). The temperature and fusion enthalpy variables obtained from the DSC were analyzed using descriptive statistics. The mean ± SD data for both variables are shown. All variables were performed using SAS software 9.04. (SAS Institute Inc., Cary, NC, USA).