Abstract
This work analyzed the antidiabetic activity of peptides from whey proteins after hydrolysis by Lactobacillus rhamnosus GG and Streptococcus thermophilus SY-102, emphasizing the differences between the proteolytic systems of both bacteria. Peptide fractions from whey proteins may have biological functions, such as antidiabetic functions, which inhibit the DPP-IV enzyme, and lactic acid bacteria could release them. A whey solution of 10% was fermented with selected lactic acid bacteria in monoculture and coculture, analyzing kinetic parameters and the proteolytic profile, using the 2,4,6-trinitrobenzene sulfonic acid technique for free amino groups’ determination and Tris-tricine polyacrylamide gel electrophoresis. An in vitro inhibition assay of the DPP-IV enzyme was used. The kinetic parameters showed a faster duplication rate in the monoculture with L. rhamnosus GG than in the co-culture, which was related to lactic acid production. Co-culture does not have the highest production of free amino groups and peptides. Still, peptide fractions with lower molecular weight (<2 kDa) were found and showed a high DPP-IV inhibitory capacity that was maintained from the middle of the fermentation to the end (55.4%). In comparison, the monoculture of L. rhamnosus GG increased from 0 to 63.3%. This demonstrates that the proteolytic capacity and the proteolytic system of each lactic acid bacteria determine the structure of the released peptides.
1. Introduction
In recent years, inhibition of the enzyme dipeptidyl peptidase IV (DPP-IV) has been pointed out as a new alternative in the treatment of diabetes mellitus II (DM2) [1]. This enzyme is found on the surface of various cells and in the bloodstream [2]. DPP-IV hydrolyzes incretins important in regulating postprandial blood glucose levels [3]. These incretins are glucose-dependent insulinotropic peptides (GIP) and glucagon-like polypeptides (GLP-1). The production of both incretins takes place in the small intestine [4], and subsequently, they act on the pancreatic cells, stimulating insulin production in the β cells. In contrast, α cells inhibited glucagon secretion [5]. However, these incretins lose their bioactive properties when DPP-IV degrades them before they act at the pancreatic cell level. Inhibition of the enzyme DPP-IV turns out to be a target point in regulating glucose concentrations. [6]. In this sense, using bioactive peptides as adjuvants in treating DM2 has long been a subject of interest [7]. The mechanism of action that gives these peptides their antidiabetic effect is centered on inhibiting the DPP-IV enzyme. This inhibition increases the half-life of GIP and GLP-1 with their subsequent activity in pancreatic cells [5]. Recently, obtaining DPP-IV inhibitory peptides from various food matrices has been explored. Thus, whey proteins have been pointed out as a potential source of peptides with considerable antidiabetic activity [8]. It has been reported that the enzymatic hydrolysis of α-lactoalbumin and β-lactoglobulin leads to peptides (LDQWLCEKL, IPAVF, IPAVFK) with high DPP-IV inhibitory activity [7,9]. Similarly, using lactic acid bacteria to hydrolyze milk whey proteins has been considered a profitable alternative in obtaining peptides with antihypertensive, antioxidant, and anticholesterolemic activity, among others [8]. However, the antidiabetic activity of peptides obtained by microbial fermentation has not been investigated extensively. However, recent studies have reported the release of peptides with antidiabetic capacity evaluated through DPP-IV inhibition. Solieri et al. [10] have reported three antidiabetic peptides derived from the fractionation of β-lactoglobulin after a fermentation process by S. thermophilus spp. which had variable molecular weights. The chains were sequences of 7, 9, and 11 amino acids. This same genus of lactic acid bacteria has been used to ferment whey proteins, finding a DPP-IV inhibitory capacity close to IC50 = 50 µmol/L [11]. However, studies on releasing antidiabetic peptides obtained via co-culture fermentation with lactic acid bacteria are practically null.
Therefore, the objective of this study was to determine the inhibitory capacity of the enzyme DPP-IV of peptides generated by the fermentation of milk whey with Streptococcus thermophilus SY-102, Lacticaseibacillus rhamnosus GG, and both bacteria in co-culture.
2. Materials and Methods
2.1. Chemicals and Reagents
Man Rogosa Sharpe broth was supplied by Becto Dickinson Difco (Maryland, MD, USA), bacteriological agar was purchased from Bioxon, BD Lab. (Edo. México, México), and powderer whey was purchased from DairyGold Food Ingredients (UK). β-mercaptoethanol, ethanol, 2,4,6-103 trinitrobenzenesulfonic acid (TNBS), glycine, Gly-Pro-p-nitroanilide and DPP-IV (1 U/mL) were supplied by Sigma-Aldrich Co. (St. Louis, MO, USA). Bradford reagent, acrylamide: bisacrylamide, SDS, ammonium persulfate, TEMED, Comassie Blue G-250, and all electrophoresis reagents were purchased from Bio-Rad (Hercules, CA, USA). Methanol, potassium carbonate, NaOH, acetic acid, KH2PO4/K2HPO4, and HCl were supplied by JT Baker, Thermo Fisher Scientific (CDMX Mexico).
2.2. Culture Preparation
S. thermophilus SY-102 and L. rhamnosus GG were obtained from the Food Biotechnology Laboratory of the Universidad Autónoma Metropolitana campus in Iztapalapa. The microorganisms were previously conditioned in Man Ragosa and Sharpe (MRS) broth and were incubated for 24 h at 42 °C. Subsequently, one milliliter of the inoculated broth was added to 9 mL of powdered whey solution (10% (w/v) Dairy Gold) previously pasteurized at 90 °C for 10 min. It was incubated for 24 h at 42 °C. Then, 1 mL was added to an Erlenmeyer flask with 100 mL of a 10% (w/v) powdered whey solution previously pasteurized (90 °C for 10 min). Each microorganism was conditioned in the same way independently. After incubating for 24 h at 42 °C, the solution was refrigerated. This solution was used as a starter culture, performing a viable count before each inoculation to determine the inoculum concentration in each fermentation.
2.3. Fermentation
Three different whey solutions were prepared to carry out the fermentations. Solutions were prepared with 10% (w/v) powdered whey previously pasteurized (90 °C for 10 min). The first solution was inoculated with Lacticaseibacillus rhamnosus GG, the second with Streptococcus thermophilus SY-102, and the third inoculated with both microorganisms. The initial concentration of bacteria was approximately 1 × 108 CFU for each fermentation. The solutions were incubated at 42 °C, and sampled every 3 h. Titratable acidity and microbial viability were determined. Subsequently, the samples were centrifuged at 24,600× g for 10 min at 4 °C (Eppendorf) to eliminate biomass and high-molecular-weight proteins. The supernatants of the centrifuged samples were stored at −4 °C for later analysis. All samples were analyzed in triplicate.
2.4. Kinetic Parameters of the Microbial Growth
Kinetic parameters of the microbial growth were calculated to evaluate the metabolic differences of lactic acid bacteria. The growth rate (µ) was calculated according to Equation (1). Equations (2) and (3) were used to determine the generation time (g) and the growth constant (K). The initial (N0) and final (Nx) concentration of biomass corresponded to the time interval selected in the logarithmic phase, which was t0 and tx, respectively.
µ = [ln(Nx) − ln(N0)]/(tx − t0)
g = ln(2)/µ
K = 1/g
2.5. Proteolytic Profile Analysis
The characterization of the proteolytic profile was carried out through two different methods. First, free amino groups were determined using the TNBS technique to analyze the concentration of peptides produced. Subsequently, Tris-Tricine polyacrylamide gel electrophoresis (SDS-Tris-Tricine-PAGE) was performed to separate the peptides released during fermentation, and obtain their molecular weight profile.
2.5.1. Free Amino Groups’ Analysis
The free amino groups derived from whey fermentation were determined using the 2,4,6-trinitrobenzene sulfonic acid (TNBS) technique. In amber test tubes, a 125 µL sample volume was mixed with 1 mL of 0.21 M phosphate-buffered solution, pH 8.2. Subsequently, 1 mL of 0.10% TNBS (Sigma Aldrich) was added to 0.21 M phosphate buffer, pH 8.2, followed by vortexing. The tubes were incubated for 1 h at 50 °C in the dark. The reaction was stopped after 60 min, adding 2 mL of 0.1 N hydrochloric acid. Finally, the samples were read in a spectrophotometer at a wavelength of 340 nm (Abs340) against the control. The control was prepared with deionized water, and a glycine concentration curve (0.05 to 0.25 mg/mL; R2 = 0.9963) was used to calculate the concentration of peptide fractions according to Equation (4).
Amino groups concentration (mg/L) = (Abs340 − 0.0139)/0.0046
2.5.2. Tris-Tricine Polyacrylamide Electrophoresis (Tris-Tricine-SDS-PAGE)
To perform polyacrylamide gel electrophoresis, the method proposed by Schägger and Von Jagow [12] was used, considering the modifications proposed by González-Olivares et al. [13]. The protein concentration of the samples was standardized to 150 mg/L via analysis with the Bradford method. Electrophoresis was performed on a separation gel of 16.5% T (concentration of polymer in a 19:1 acrylamide: bisacrylamide ratio and 5% crosslinker (Bio-Rad)). The peptide fractions were filtered with a 0.22 um filter, and 40 µL was placed in an Eppendorf tube with 20 µL of staining buffer (Bio-Rad), 20 µL of deionized water, and 3 µL of β-mercaptoethanol. The vials were incubated at 40 °C for 30 min. A molecular weight standard (Bio-Rad polypeptide standard) was used, which was prepared according to the supplier’s specifications. The standard was incubated at 90 °C for 5 min. The separation gel was polymerized with Tris-HCl buffer at pH = 8.5, calculating the required concentration of acrylamide, 0.5 µL of TEMED (Bio-Rad), and 35 µL of sodium persulfate (Bio-Rad). The run was conducted at 30 V for one hour, rising to 95 V with a run time of 5 to 6 h. At the end of the run, the gels were fixed with a 7.5 acetic acid solution and stained with Coomassie blue G-250 (Bio-Rad) for 2 h. The gels were discolored in a 7% acetic acid solution with 10% ethanol in deionized water. They were placed in an image capture camera (Bio-Rad) and analyzed through the Gel-Doc software integrated into the camera.
2.6. DPP-IV Inhibitory Activity
The inhibitory effect of DPP-IV (D4943-1VL; Sigma-Aldrich, St. Louis, USA) was evaluated spectrophotometrically according to the technique of Nongonierma et al. [14] with some modifications. The substrate (Gly-Pro-p-nitroanilide; Sigma-Aldrich, St. Louis, USA) was dissolved in Tris-HCl buffer (pH 8) at a substrate concentration of 1.6 mM. Subsequently, 100 µL of the sample (AbsM) was added to 100 µL of substrate and incubated at 37 °C for 10 min. Following this incubation, 200 µL of DPP-IV (1 U/mL; Sigma-Aldrich, St. Louis, MO, USA) was added. The reaction was conducted for 60 min at 37 °C, and finished by adding 400 µL of 0.1 M potassium carbonate. The same treatment was followed for preparing the positive control (AbsB), adding 100 µL of Tris-HCl buffer instead of the sample volume. The negative control (AbsC) was designed by adding 100 µL of the sample, 300 µL of Tris-HCl buffer (pH 8), and 400 µL of 0.1 M potassium carbonate. Finally, all the samples and controls were read at 405 nm in a spectrometer (Power Wave XS UV-Biotek; KC Junior software, Kansas, MO, USA). The DPP-IV inhibitory activity was calculated using Equation (5) based on the absorbance of each measurement at 405 nm:
Inhibition of DPP-IV (%) = {[(AbsB − (AbsM-AbsC)]/(AbsB)} × 100
2.7. Statistic Analysis
Results were analyzed using a one-way ANOVA (p = 0.05) and Tukey’s test, using the NCSS statistical software (NCSS 2007, v.0, Kaysville, UT, USA, 2007). All experiments were carried out in triplicate.
3. Results and Discussion
3.1. Microbial Growth and Lactic Acid Production
The results obtained from the fermentation demonstrated that the growth of the microorganisms between the independent cultures and the coculture was different. A more significant change was observed in the monoculture of L. rhamnosus GG, reaching its logarithmic phase close to 12 h of fermentation. On the contrary, in the monoculture of S. thermophilus, a shorter lac phase was observed, reaching its logarithmic phase around six h of fermentation (Table 1). In this sense, other authors reported that lactobacilli species in monocultures tend to present higher growth than streptococcus species [15]. A correlation was observed between growth and the concentration of lactic acid produced. Thus, lactic acid concentrations were higher in the culture of L. rhamnosus GG compared to the culture of S. thermophilus SY-102.

Table 1.
Microbial growth, lactic acid concentration, and kinetic parameters during fermentation with L. rhamnosus GG, S. thermophilus SY-102, and co-culture.
The metabolism rate of lactic acid bacteria is determined by the lactic acid production capacity, especially for homofermentative microorganisms, as is the case of those tested in this study. Thus, as observed in Table 1, the highest production of lactic acid was found in the monoculture with L. rhamnosus GG (5.20 ± 0.0), followed by co-culture (3.24 ± 0.0), and finally, the system of monoculture with S. thermophilus SY-102 (2.61 ± 0.09). These results are closely related to the growth or duplication rate (µ), which followed the same trend as lactic acid production and generation time (g). Likewise, the growth constant was perfectly related to each parameter studied. These kinetic parameters are intended to somehow explain the metabolic behavior of microorganisms according to the environment in which they are found. Castañeda-Ovando et al. [16] related the growth of lactic bacteria in monoculture and co-culture to determine the fundamental differences in the process. Thus, the two main metabolic identification parameters are proteolytic capacity and lactic acid production. In this case, it has been observed that coculture systems are more effective in duplication, generation time, and lactic acid production.
3.2. Free Amino Groups Analysis by TNBS
The concentration of free amino groups after fermentation differed for the three experiments evaluated. In the case of cultures of L. rhamnosus GG and S. thermophilus SY-102 independently, a decrease in the concentration of free amino groups was observed after 12 h of fermentation, which started from 367.28 ± 0.02 to 209.18 ± 0.02 µg/mL for L. rhamnosus GG and from 423.31 ± 0.13 to 167.52 ± 005 µg/mL for S. thermophilus SY-102. Subsequently, around 21 h of fermentation, the concentration of amino groups increased again due to the proteolytic activity of each strain. The concentration of amino groups for L. rhamnosus GG was 293.83 ± 0.05, while for S. thermophilus SY-102, it was 323.87 ± 0.04 at the end of fermentation (Figure 1).
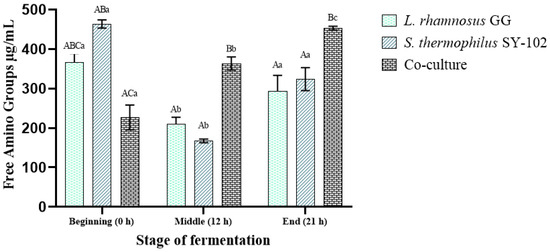
Figure 1.
Production of amino groups released during the fermentation of whey with L. rhamnosus GG, S. thermophilus SY-102, and both microorganisms in co-culture. The results are the average of three determinations ± the standard deviation. (a–c) Lowercase letters indicate a comparison of means between times of the same fermentation system. (A–C) Uppercase letters indicate the comparison of means between fermentation systems with the same fermentation time. Samples with the same letter did not present a significant difference using Tukey‘s test (p < 0.05).
It has been reported that in lactic acid bacteria, proteolytic activity and protein hydrolysis patterns vary considerably between strains [15,16]. These differences could be due to multiple factors, among which the following have been pointed out: (1) changes and mutations in the genes involved in the proteinase expression linked to the cell wall, and (2) the conditions in which the enzymatic activity is carried out [17]. Therefore, the concentration of amino groups and the amino acids released during fermentation will depend on the auxotrophies of each microorganism. In this sense, various studies have indicated that S. thermophilus strains require the incorporation of amino acids such as methionine and glutamine, thus satisfying their auxotrophies while releasing peptides and amino acids to the medium, which is not metabolically necessary [18,19].
Similarly, the activity of the proteolytic system of this microorganism varies in the different stages of fermentation. Thus, it has been observed that during the early stages of fermentation, the proteolytic activity of S. thermophilus species is higher and decreases as the fermentation time elapses. Therefore, it can satisfy its glutamine and methionine requirements at the beginning of fermentation. Similarly, it favors the accumulation in the medium of amino acids such as cysteine and glutamic acid, which are not necessary [15]. On the other hand, L. rhamnosus GG presents auxotrophies that differ from those of S. thermophilus.
An example of this is the use of amino acids such as cysteine. For L. rhamnosus GG, cysteine is an essential amino acid for survival, while for S. thermophilus species, it does not represent a metabolic requirement [15]. In addition, L. rhamnosus GG typically utilizes a wide range of amino acids, including serine, glutamine, proline, and arginine [20]. However, the behavior and proteolytic activity of these strains in monoculture vary when they are in co-culture [21].
In the case of coculture fermentation, the final concentration of released amino groups was higher (453.13 ± 0.06 µg/mL) compared to independent cultures. This trend has been previously reported from milk fermentation using the same strains [21]. Some studies have indicated that in a coculture medium, there is a protocooperative relationship between both microorganisms [22]. This interaction entails a synergistic effect that favors an increase in the concentrations of free amino groups and the obtainment of a greater diversity of peptides [21,22]. Thus, the initial proteolysis is related to the increase in amino groups at the end of fermentation and the type of released peptides. In addition, the biological activity presented in a released peptide is always correlated to the composition of the peptide chain [20]. In this type of system, it has been observed that the initial proteolytic activity is higher in lactobacilli species [22]. The protease associated with its cell wall begins with the hydrolysis of proteins to satisfy its auxotrophies [23]. Subsequently, it excretes amino acids (that are not necessary for it) into the medium used by S. thermophilus species [24].
In the case of S. thermophilus species, the initial proteolytic activity is relatively low when found in coculture. This microorganism will initially use the amino acids excreted by L. rhamnosus GG, so the protease associated with its cell wall will not be as active as that associated with L. rhamnosus. Ultimately, protein hydrolysis will continue via its system of intracellular peptidases and aminopeptidases to cover its specific auxotrophies [20].
Due to these differences, the concentration of free amino groups varies in such a way that the nitrogen needs of each microorganism are initially met. For this reason, there is a decrease, and in the end, an increase due to the hydrolytic activity; eventually, a greater quantity of free amino groups is released, both via excretion and metabolic activity. Thus, in coculture, the production of free amino groups is constant due to the same factors, a constant proteolytic activity, and an accumulation via excretion of peptides from inside the cell. Likewise, the protocooperation between lactic acid bacteria has been discussed, allowing for a constant accumulation of free amino groups.
3.3. Analysis of Profile Peptides by Tris-Tricine-SDS-PAGE
The gels obtained via electrophoresis were analyzed using Gel-Doc software (BioRad, Hercules, CA, USA). This way, the electropherograms corresponding to monocultures and co-cultures were obtained (Figure 2). Peptides between 10 kDa and 2 kDa were obtained for the three fermentation systems. However, due to the specific proteolytic activity of each of the microorganisms, a different profile was presented in each case [25]. Thus, in monoculture fermentations (Figure 2A,B), a more significant accumulation of peptides was observed in the L. rhamnosus GG system towards the end of the fermentation (21 h).

Figure 2.
Separation of peptides by SDS-PAGE from whey fermented by L. rhamnosus GG (A), S. thermophilus SY-102 (B), and coculture (C). Start (0 h), middle (12 h), and end (21 h) of fermentation.
These results coincide with what has been observed in other studies that indicate a higher rate of hydrolysis from lactobacilli cultures compared to streptococcus cultures [18,19]. The production of peptides between 4 and 36 amino acids by L. rhamnosus is carried out from the first fractionation of the proteins by the proteinase linked to its cell wall (PrtR) [23]. Peptides generated by the action of PrtR are incorporated through a system of oligopeptide (Opp), dipeptide, and tripeptide (DtpT, Dpp) transporters [25]. Subsequently, these peptides are broken down into amino acids by a series of intracellular peptidases and aminopeptidases. Once their auxotrophies are satisfied, the unused peptides and amino acids will be excreted into the medium.
Contrary to L. rhamnosus GG, wherein protein hydrolysis is carried out primarily by PrtR, in S. thermophilus species, hydrolysis is carried out mainly by the action of the intracellular peptidase complex (Pep O, Pep X, Pep S, Pep N, Pep C) [19]. For this reason, both the hydrolysis rate and the excretion of short-chain peptides are lower. Compared to lactobacilli species, streptococci have higher amino acid requirements, so initial proteolytic activity should be higher [19]. Therefore, at the beginning of fermentation, the proteinase associated with the cell wall of S. thermophilus spp. (PrtS) has a high activity rate to supply the necessary amino acids from the proteins available in the medium [24]. However, the activity of this proteinase decreases as the fermentation time elapses.
The concentrations of low-molecular-weight peptides obtained from the coculture were higher than those observed in monocultures (Figure 2C). These results are related to the results obtained in the analysis of free amino groups. Coculture systems tend to show a synergy that increases the concentration, size, and composition of the synthesized peptides [22]. This represents an essential factor related to the biological activity that peptides can exert since the bioactivity of specific peptides is related to both the weight and the amino acid composition of the peptide chain [26]. Due to the particularities of the proteolytic system of each strain, it is expected that these have a direct impact on the production of bioactive peptides, which is also closely linked to the structure of the proteolytic system and its mode of action (Figure S1).
In a coculture system, the L. rhamnosus GG PrtR begins protein hydrolysis to satisfy its auxotrophies. The activity of the proteolytic system of L. rhamnosus GG will allow the release of amino acids essential for developing S. thermophilus species in the medium, making evident the protocooperation relationship between both species [27]. In this sense, L. rhamnosus GG will prefer amino acids such as cysteine, serine, or proline, while S. thermophilus species cover their auxotrophies with amino acids such as methionine and glutamine [20,25]. Studies have highlighted that these amino acids are promoters for activating oligopeptide transport systems in all lactic acid bacteria. This is why, more than a system of competition for nutrients, a balance is established by the protocooperation activated in each fermentation [28]. In the end, the peptides and amino acids that are not used are excreted through the cell walls of both microorganisms and released into the medium [29].
Various studies have reported that coculture systems increase the rate of proteolysis, as well as the concentration and diversity of peptides in the medium [18,19,28]. Similarly, it has been observed that peptides obtained from cocultures have more excellent biological activity than those obtained from independent cultures [20]. The peptides obtained from the coculture in this study weighed ≥2 kDa, similar to results reported by other authors who point out a relationship between peptides of smaller size and more significant biological activity [30].
3.4. DPP-IV Inhibitory Activity
DPP-IV inhibition via the action of the peptides generated during the fermentation was evaluated. At the beginning of fermentation, no enzyme inhibitory activity was presented in any of the three systems evaluated (Figure 3), even though whey naturally contains some peptides due to the heat treatment to which it is exposed [31]. However, it could be observed that this activity increases as the fermentation time elapses. This could be directly related to the proteolytic activity of the strains, attributing the inhibitory activity of DPP-IV solely to the peptides generated from microbial metabolism. The results showed a 46.9% inhibition percentage for the L. rhamnosus GG monoculture after 12 h of fermentation. On the contrary, for the monoculture of S. thermophilus SY-102, there was no inhibitory activity of DPP-IV. This same trend was observed towards the end of fermentation (21 h), where the inhibitory activity of DPP-IV only reached 2.35% inhibition.

Figure 3.
DPP-IV inhibitory activity by peptide fractions derived from 10% whey, fermented with L. rhamnosus GG, S. thermophilus SY-102, and coculture of both microorganisms. The results are the average of three determinations ± the standard deviation. (a,b) Lowercase letters indicate a comparison of means between times of the same fermentation system. (A,B) Uppercase letters indicate the comparison of means between fermentation systems with the same fermentation time. Samples with the same letter did not present a significant difference using Tukey’s test (p < 0.05).
This could be related to the results observed in the determination of free amino groups and the electrophoresis analysis, since the proteolytic capacity of S. thermophilus SY-102 was lower than L. rhamnosus GG. It has been reported that lactobacilli species tend to have a higher rate of proteolytic activity, producing higher concentrations of peptides with considerable biological activity [21]. Likewise, it is known that the proteolytic activity of S. thermophilus spp. favors the accumulation of peptides with aromatic amino acids such as phenylalanine, tryptophan, or tyrosine [27]. This has been related to other bioactivities, such as angiotensin-converting enzyme (ACE) inhibition. However, the results observed in this study could indicate that the peptides generated from the culture with S. thermophilus spp. have no DPP-IV inhibitory activity, thus ruling out multiple biological activities.
On the other hand, in the case of monoculture with L. rhamnosus GG, a progressive increase in inhibitory activity was observed. After the start of fermentation, an increase from 46.9% (12 h) to 63.3% (21 h) of DPP-IV inhibition was observed. These results agree with the proteolytic activity of the strain, which was higher. This is consistent with the results observed from the analysis of free amino groups and electrophoresis. Different proteinases linked to the cell wall and their hydrolytic specificities give lactobacilli strains a great capacity to generate peptides of various molecular weights and sequences [18].
Similarly, a high percentage of DPP-IV inhibition was observed in the coculture. In this case, an inhibition percentage of 55.49% was obtained at 12 h of fermentation. Subsequently, at 21 h of fermentation, a slight decrease in activity was observed, reaching a percentage of 53.69%. These results were consistent with what was observed in the electrophoresis analysis, where the accumulation of low-molecular-weight peptides was higher compared to cocultures. The synergistic effect in coculture systems leads to the accumulation a wide variety of peptides [15]. Thus, it has been observed that the biological activity of peptides increases in coculture systems [22] due to the release into the medium of those peptides and amino acids that are unnecessary for microbial development [30].
Additionally, the activity of the specific intracellular peptidase of S. thermophilus spp. favors the excretion of peptides with phenylalanine in their structure [32,33]. On the other hand, the proteolytic system of L. rhamnosus GG will favor the accumulation in the medium of peptides with amino acids such as proline, valine, or isoleucine [18]. In this sense, it has been reported that DPP-IV inhibitors contain in their structure amino acids such as proline, alanine, valine, leucine, and phenylalanine [1,22]. These peptides bind to the active site of DPP-IV competitively. However, some molecular docking studies point to the binding of inhibitors to DPP-IV outside of its active site through non-competitive actions [3]. Likewise, it has been reported that the inhibition mechanism associated with these peptides could be due to preventing the dimeric form of DPP-IV [3,34]. Despite the release of aromatic amino acids by S. thermophilus spp., it is known that the position of these amino acids in the peptide chain is one of the limitations for the peptide to present biological activity [35].
With the results found in this study, the importance of coculture fermentations has been demonstrated for the release of peptide sequences with a biological activity that increases the technological value of the inoculums in lactic fermentations, opening a critical field of study in the processing of functional and nutraceutical food, taking advantage of the differences in proteolytic systems. Fermented foods could never replace medical treatment for a condition such as diabetes. However, finding peptides with antidiabetic characteristics could be the watershed for synthesizing peptide sequences or for the purification of fractions. These could be included as supplements or as functional ingredients in producing functional foods.
4. Conclusions
The use of lactic acid bacteria in the fermentation of whey favors the production of peptides with antidiabetic activity. The combination of L. rhamnosus GG and S. thermophilus SY-102 in a coculture system promotes a more significant proteolytic activity of the strains, increasing the concentration of low-molecular-weight peptide fractions. The inhibitory activity of DPP-IV by the action of the peptides obtained from the monoculture of L. rhamnosus GG and coculture increased during the fermentation time. The results obtained from this study indicate that L. rhamnosus GG in monoculture and a coculture system with S. thermophilus SY-102 generates peptides with high DPP-IV inhibitory activity (>50%). The inhibition could be related to the structure and composition of the peptides generated during fermentation by the action of the proteolytic system of each strain. Obtaining peptides with antidiabetic activity from microbial fermentations represents a viable alternative to assist in treating diabetes mellitus II. The hydrolysis of whey proteins by lactic acid bacteria fermentation is an area of opportunity in the field of human health. Molecular coupling studies will be necessary to elucidate the interaction between the generated peptides and DPP-IV, helping develop new products supplemented with bioactive peptides that will help to treat various pathologies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/dairy4030035/s1, Figure S1: Proteolytic system of S. thermophilus species and L. rhamnosus GG.
Author Contributions
Conceptualization, L.G.G.-O., E.C.-L. and A.E.C.-G.; writing original draft preparation, L.B.O.-R., L.G.G.-O. and E.P.-E.; funding acquisition and experimental support, E.C.-L., A.E.C.-G., J.J.-O. and E.R.-M.; review and editing, L.G.G.-O., E.C.-L. and A.Q.-L.; developing of figures and tables, L.B.O.-R. and L.G.G.-O.; project administration, L.G.G.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
L.G.G.-O., J.J.-O, A.E.C.-G., E.C.-L., E.P.-E. and L.B.O.-R. thank Sistema Nacional de Investigadores (CDMX, Mexico) and CONACyT for the stipend received.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nongonierma, A.B.; FitzGerald, R.J. Susceptibility of milk protein-derived peptides to dipeptidyl peptidase IV (DPP-IV) hydrolysis. Food Chem. 2014, 145, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Flatt, P.R.; Bailey, C.J.; Green, B.D. Dipeptidyl peptidase IV (DPP IV) and related molecules in type 2 diabetes. Front. Biosci. 2008, 13, 3648–3660. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; FitzGerald, R.J. Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J. Food Biochem. 2019, 43, e12451. [Google Scholar] [CrossRef] [PubMed]
- Green, B.D.; Flatt, P.R.; Bailey, C.J. Dipeptidyl peptidase IV (DPP IV) inhibitors: A newly emerging drug class for the treatment of type 2 diabetes. Diabetes Vasc. Dis. Res. 2006, 3, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.P.; SinhaRoy, R.; Pocai, A.; Kelly, T.M.; Scapin, G.; Gao, Y.-D.; Pryor, K.A.D.; Wu, J.K.; Eiermann, G.J.; Xu, S.S. A comparative study of the binding properties, dipeptidyl peptidase-4 (DPP-4) inhibitory activity and glucose-lowering efficacy of the DPP-4 inhibitors alogliptin, linagliptin, saxagliptin, sitagliptin and vildagliptin in mice. Endocrinol. Diabetes Metabol. 2018, 1, e00002. [Google Scholar] [CrossRef]
- Jia, C.; Hussain, N.; Ujiroghene, O.J.; Pang, X.; Zhang, S.; Lu, J.; Liu, L.; Lv, J. Generation and characterization of dipeptidyl peptidase-IV inhibitory peptides from trypsin-hydrolyzed α-lactalbumin-rich whey proteins. Food Chem. 2020, 318, 126333. [Google Scholar] [CrossRef]
- Olvera-Rosales, L.B.; Cruz-Guerrero, A.E.; García-Garibay, J.M.; Gómez-Ruíz, L.C.; Contreras-López, E.; Guzmán-Rodríguez, F.; González-Olivares, L.G. Bioactive peptides of whey: Obtaining, activity, mechanism of action, and further applications. Crit. Rev. Food Sci. Nut. 2022, 1–31. [Google Scholar] [CrossRef]
- Silveira, S.T.; Martínez-Maqueda, D.; Recio, I.; Hernández-Ledesma, B. Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem. 2013, 141, 1072–1077. [Google Scholar] [CrossRef]
- Solieri, L.; Valentini, M.; Cattivelli, A.; Sola, L.; Helal, A.; Martini, S.; Tagliazucchi, D. Fermentation of whey protein concentrate by Streptococcus thermophilus strains releases peptides with biological activities. Process Biochem. 2022, 121, 590–600. [Google Scholar] [CrossRef]
- Helal, A.; Pierri, S.; Tagliazucchi, D.; Solieri, L. Effect of Fermentation with Streptococcus thermophilus Strains on In Vitro Gastro-Intestinal Digestion of Whey Protein Concentrates. Microorganisms 2023, 11, 1742. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H.; Aquila, H.; Von Jagow, G. Coomassie blue-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for direct visualization of polypeptides during electrophoresis. Anal. Biochem. 1988, 173, 201–205. [Google Scholar] [CrossRef] [PubMed]
- González-Olivares, L.G.; Añorve-Morga, J.; Castañeda-Ovando, A.; Contreras-López, E.; Jaimez-Ordaz, J. Peptide separation of commercial fermented milk during refrigerated storage. Food Sci. Technol. 2014, 34, 674–679. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Structure activity relationship modelling of milk protein-derived peptides with dipeptidyl peptidase IV (DPP-IV) inhibitory activity. Peptides 2016, 79, 1–7. [Google Scholar] [CrossRef]
- Liu, E.; Zheng, H.; Shi, T.; Ye, L.; Konno, T.; Oda, M.; Shen, H.; Ji, Z.-S. Relationship between Lactobacillus bulgaricus and Streptococcus thermophilus under whey conditions: Focus on amino acid formation. Int. Dairy J. 2016, 56, 141–150. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pérez-Escalante, E.; Rodríguez-Serrano, G.M.; Martínez-Ramírez, X.; Contreras-López, E.; Jaimez-Ordaz, J.; Añorve-Morga, J.; Nieto-Velázquez, S.; Ramírez-Godínez, J.; González-Olivares, L.G. Selenium accumulation by Lactobacillus isolated from commercial fermented milk: Minimum inhibitory concentration and kinetic growth changes. RMIQ 2022, 21, Bio2824. [Google Scholar] [CrossRef]
- Jensen, M.P.; Ardö, Y. Variation in aminopeptidase and aminotransferase activities of six cheese related Lactobacillus helveticus strains. Int. Dairy J. 2010, 20, 149–155. [Google Scholar] [CrossRef]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of Bioactive Peptides by Lactobacillus Species: From Gene to Application. Front. Microbiol. 2018, 9, 2354. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, G.M.; Garcia-Garibay, J.M.; Cruz-Guerrero, A.E.; Gomez-Ruiz, L.C.; Ayala-Nino, A.; Castaneda-Ovando, A.; Gonzalez-Olivares, L.G. Proteolytic system of Streptococcus thermophilus. J. Microbiol. Biotechnol. 2018, 28, 1581–1588. [Google Scholar] [CrossRef]
- Sun, J.; Chen, H.; Qiao, Y.; Liu, G.; Leng, C.; Zhang, Y.; Lv, X.; Feng, Z. The nutrient requirements of Lactobacillus rhamnosus GG and their application to fermented milk. J. Dairy Sci. 2019, 102, 5971–5978. [Google Scholar] [CrossRef]
- Sebastián-Nicolas, J.L.; Contreras-López, E.; Ramírez-Godínez, J.; Cruz-Guerrero, A.E.; Rodríguez-Serrano, G.M.; Añorve-Morga, J.; Jaimez-Ordaz, J.; Castañeda-Ovando, A.; Pérez-Escalante, E.; Ayala-Niño, A. Milk fermentation by Lacticaseibacillus rhamnosus GG and Streptococcus thermophilus SY-102: Proteolytic profile and ace-inhibitory activity. Fermentation 2021, 7, 215. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, W.; Jin, Y. Peptidomic analysis of milk fermented by Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus. Food Hydrocoll. Health 2021, 1, 100033. [Google Scholar] [CrossRef]
- Courtin, P.; Monnet, V.; Rul, F. Cell-wall proteinases PrtS and PrtB have a different role in Streptococcus thermophilus/Lactobacillus bulgaricus mixed cultures in milk. Microbiology 2002, 148, 3413–3421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, O.K.; Roux, É.; Awussi, A.A.; Miclo, L.; Jardin, J.; Jameh, N.; Dary, A.; Humbert, G.; Perrin, C. Use of a free form of the Streptococcus thermophilus cell envelope protease PrtS as a tool to produce bioactive peptides. Int. Dairy J. 2014, 38, 104–115. [Google Scholar] [CrossRef]
- Griffiths, M.W.; Tellez, A.M. Lactobacillus helveticus: The proteolytic system. Front. Microbiol. 2013, 4, 30. [Google Scholar] [CrossRef]
- Xu, Q.; Hong, H.; Wu, J.; Yan, X. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane: A review. Trends Food Sci. Technol. 2019, 86, 399–411. [Google Scholar] [CrossRef]
- Hu, T.; Cui, Y.; Qu, X. Analysis of the proteolytic system of Streptococcus thermophilus strains CS5, CS9, CS18 and CS20. Int. Dairy J. 2021, 118, 105025. [Google Scholar] [CrossRef]
- Hafeez, Z.; Cakir-Kiefer, C.; Lecomte, X.; Miclo, L.; Dary-Mourot, A. The X-prolyl dipeptidyl-peptidase PepX of Streptococcus thermophilus initially described as intracellular is also responsible for peptidase extracellular activity. J. Dairy Sci. 2019, 102, 113–123. [Google Scholar] [CrossRef]
- Hafeez, Z.; Cakir-Kiefer, C.; Girardet, J.M.; Jardin, J.; Perrin, C.; Dary, A.; Miclo, L. Hydrolysis of milk-derived bioactive peptides by cell-associated extracellular peptidases of Streptococcus thermophilus. Appl. Microbiol. Biotechnol. 2013, 97, 9787–9799. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, Y.; Wang, F.; Yan, J.; Qi, Y.; Ye, M. Protein digestomic analysis reveals the bioactivity of deer antler velvet in simulated gastrointestinal digestion. Food Res. Int. 2017, 96, 182–190. [Google Scholar] [CrossRef]
- Kareb, O.; Aïder, M. Whey and its derivatives for probiotics, prebiotics, synbiotics, and functional foods: A critical review. Probiotics Antimicrob. Prot. 2019, 11, 348–369. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Espla, M.D.; Rul, F. PepS from Streptococcus thermophilus: A new member of the aminopeptidase T family of thermophilic bacteria. Eur. J. Biochem. 1999, 263, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Mandal, S.; Tomar, S.K.; Anand, S. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur. J. Nut. 2015, 54, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Velarde-Salcedo, A.J.; Barrera-Pacheco, A.; Lara-González, S.; Montero-Morán, G.M.; Díaz-Gois, A.; de Mejia, E.G.; de La Rosa, A.P.B. In vitro inhibition of dipeptidyl peptidase IV by peptides derived from the hydrolysis of amaranth (Amaranthus hypochondriacus L.) proteins. Food Chem. 2013, 136, 758–764. [Google Scholar] [CrossRef]
- Martin, M.; Hagemann, D.; Henle, T.; Deussen, A. The angiotensin converting enzyme-inhibitory effects of the peptide isoleucine-tryptophan after oral intake via whey hydrolysate in men. J. Hypertens. 2018, 36, e220. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).