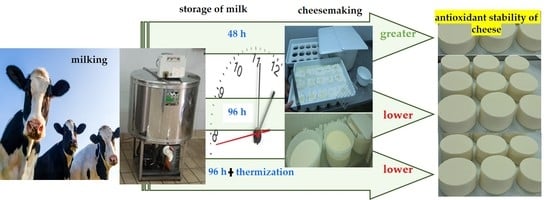
Effect of Storage and Heat Treatment of Milk Destined for Cheese Production on Its Oxidative Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Milk
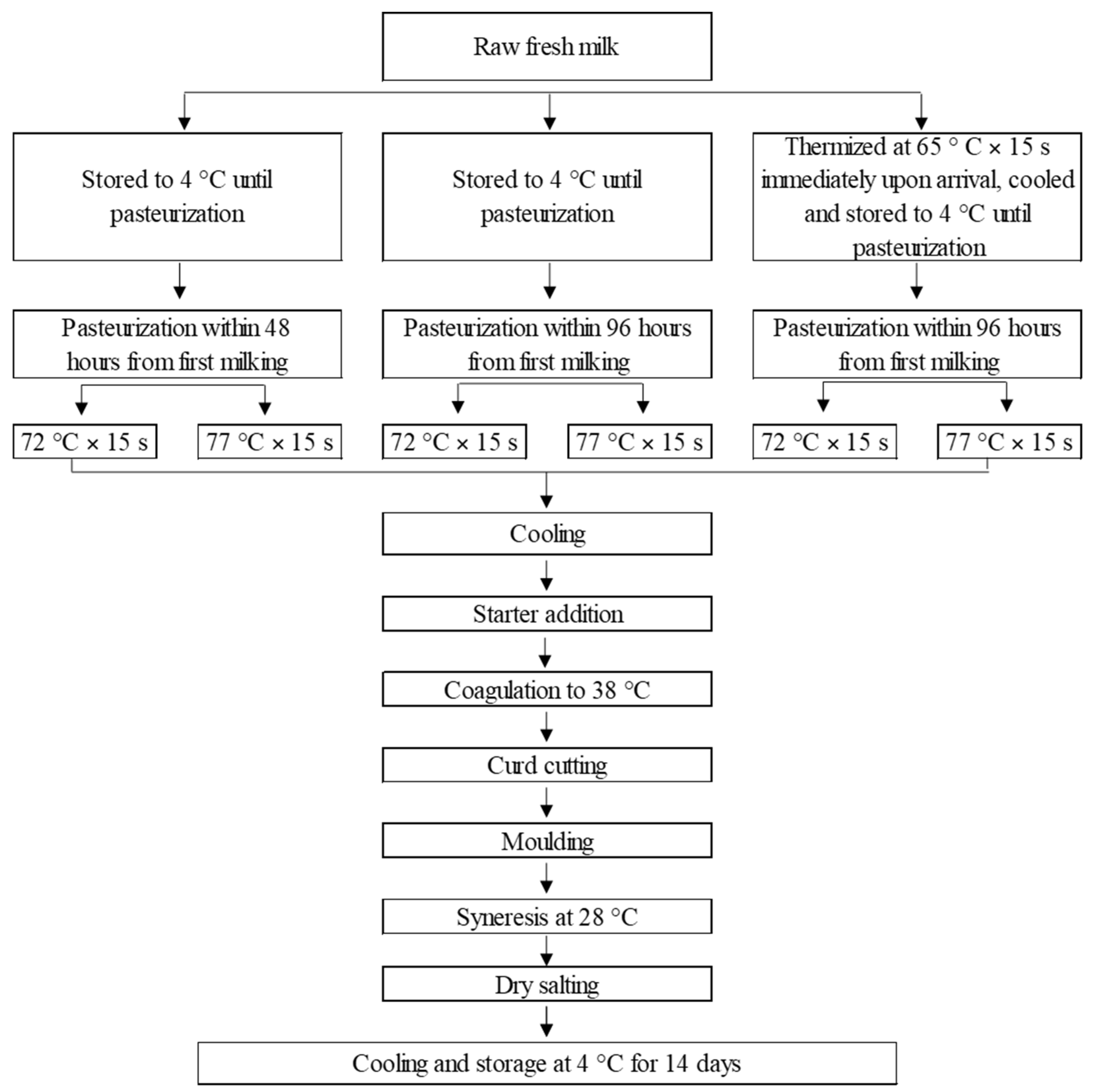
2.2. Milk Treatments
- -
- raw fresh milk: pasteurization within 48 h of the first milking (F);
- -
- raw stored milk: pasteurization within 96 h of the first milking (S);
- -
- thermized and stored milk: thermization process (65 °C for 15 s) immediately upon arrival at the dairy and cooling, pasteurization within 96 h of the first milking (T).
2.3. Cheese Production
2.4. Milk Analysis
2.5. Cheese Analysis
2.6. Lactoperoxidase Activity Method
2.7. FAST Method
- -
- fluorescence of tryptophan (Trp-F) at 290 nm excitation and 340 nm emission,
- -
- fluorescence of advanced Maillard’s products (AMP-F) at 350 nm excitation and 440 nm emission and expressed in arbitrary units (a.u.).
2.8. Antioxidant Activity—DPPH Method
2.9. Statistical Analysis
3. Results
3.1. Chemical Characteristics of Raw Milk
3.2. Characteristics of Pasteurized Milk
3.2.1. Lactoperoxidase Activity
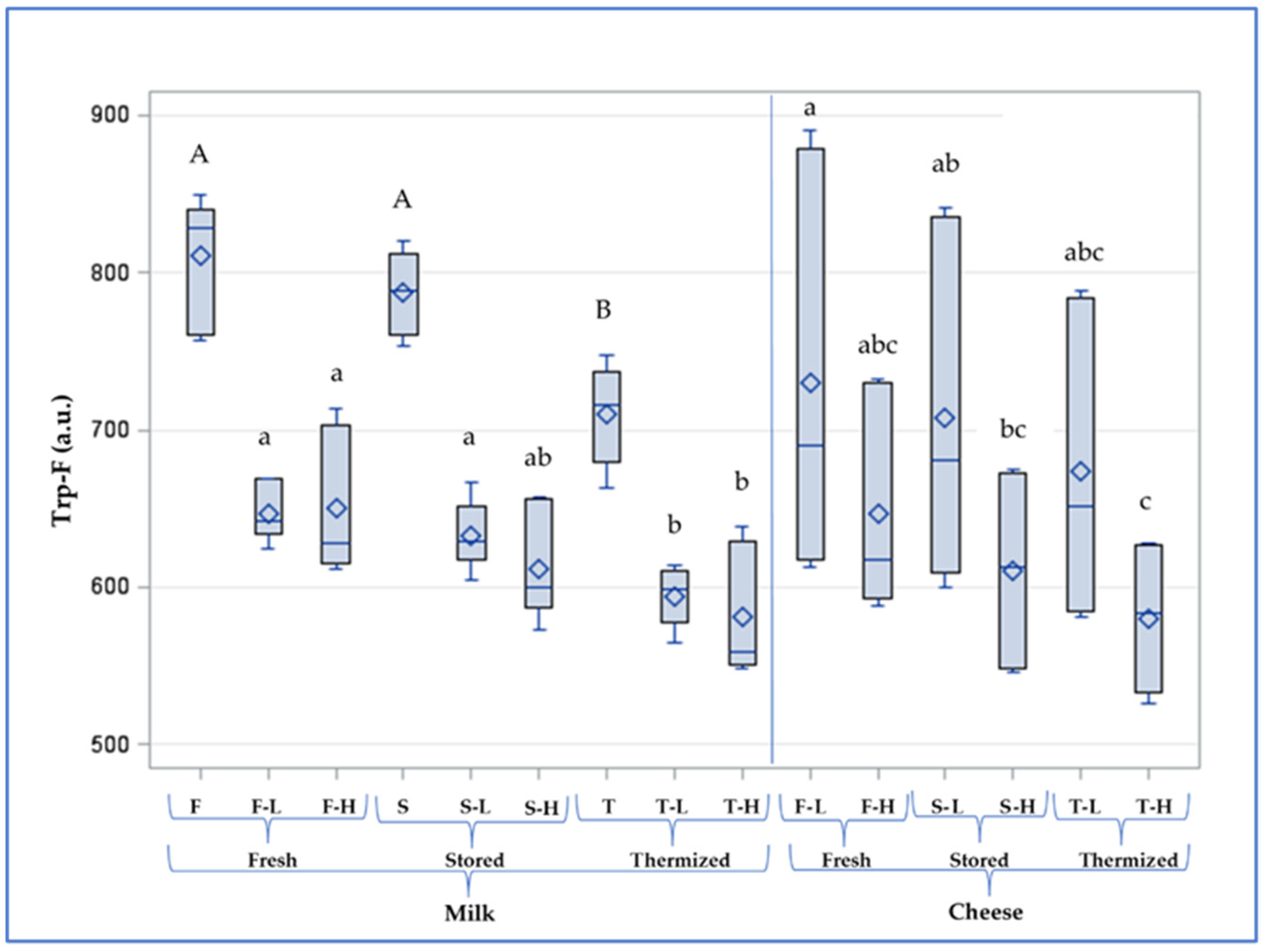
3.2.2. Protein Denaturation and the FAST Index
3.2.3. Antioxidant Activity
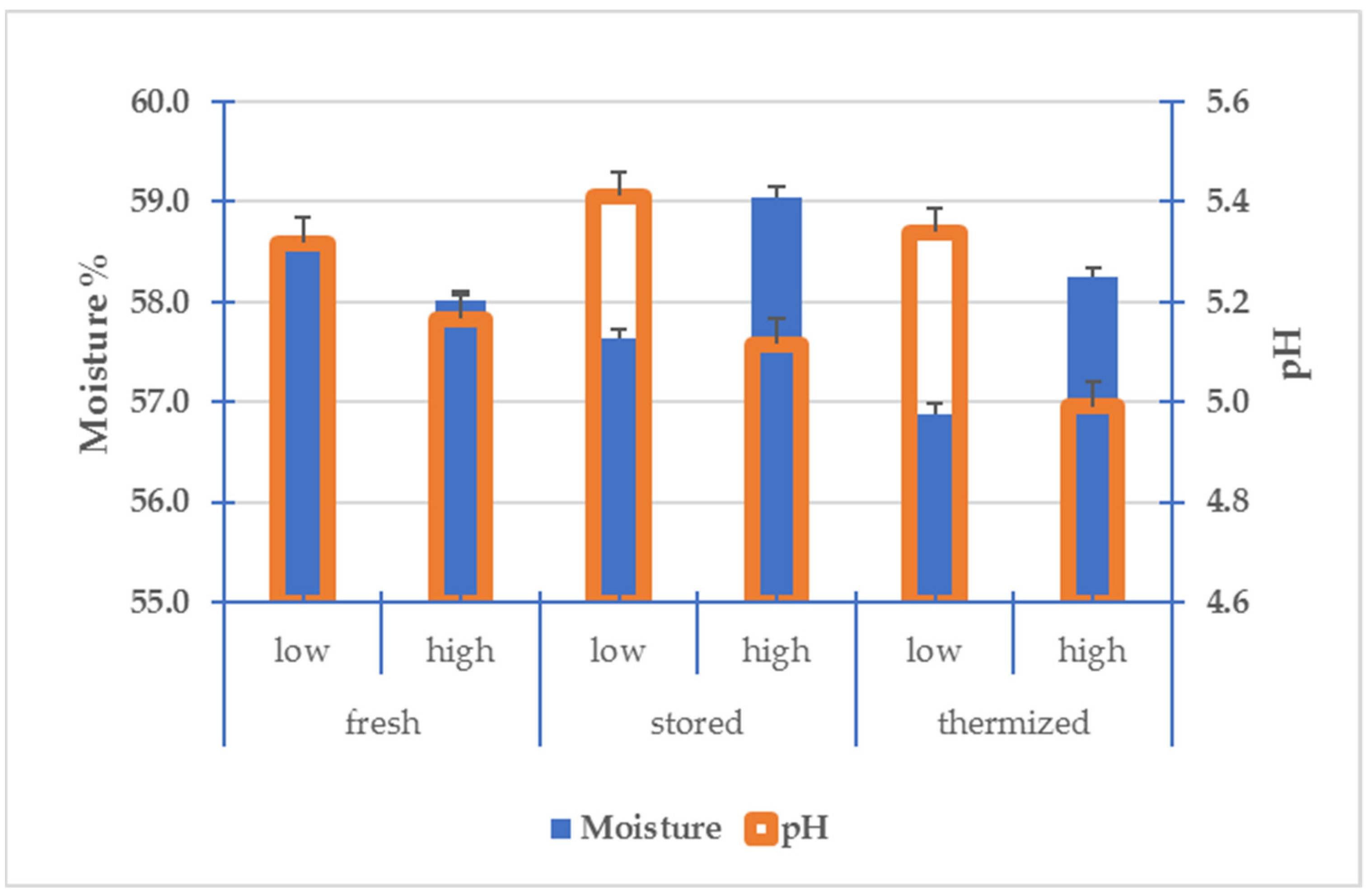
3.3. Chemical and Physical Characteristics of Cheese
3.4. Characteristics of Cheese in Comparison with Milk
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Council of the European Union. Council Regulation (EC) No 2597/1997 of 18 December 1997. Laying down additional rules on the common organization of the market in milk and milk products for drinking milk. Off. J. Eur. Union 1997, 351, 13–15. [Google Scholar]
- Council of the European Union. Commission Regulation (EC) No 1107/96 of 12 June 1996 on the registration of geographical indications and designations of origin under the procedure laid down in Article 17 of Council Regulation (EEC) No 2081/92. Off. J. Eur. Union 1996, 148, 1–10. [Google Scholar]
- Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union 2011, 304, 18–41. [Google Scholar]
- CLAL-Italia: Produzione Latte ed Utilizzazione. 2019. Available online: https://www.clal.it/?section=bilancio_approv2&year=2019 (accessed on 31 May 2021).
- Sahilli, Y.Ç. Determination of antioxidant activities in milks obtained from simmental breed of cattle. J. BAUN Inst. Sci. Technol. 2018, 20, 565–571. [Google Scholar] [CrossRef][Green Version]
- Lindmark-Månsson, H.; Åkesson, B. Antioxidative factors in milk. Br. J. Nutr. 2000, 84. [Google Scholar] [CrossRef]
- Khan, I.T.; Bule, M.; Ullah, R.; Nadeem, M.; Asif, S.; Niaz, K. The antioxidant components of milk and their role in processing, ripening, and storage: Functional food. Vet. World 2019, 12, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F.; Kelly, A.L. Chemistry and Biochemistry of Milk Constituents. In Food Biochemistry and Food Processing; Hui, Y.H., Ed.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 425–452. ISBN 0813803780. [Google Scholar]
- Grażyna, C.; Hanna, C.; Adam, A.; Magdalena, B.M. Natural antioxidants in milk and dairy products. Int. J. Dairy Technol. 2017, 70, 165–178. [Google Scholar] [CrossRef]
- Nielsen, J.H.; Østdal, H.; Andersen, H.J. The Influence of Ascorbic Acid and Uric Acid on the Oxidative Stability of Raw and Pasteurized Milk. ACS Symp. Ser. 2002, 807, 126–137. [Google Scholar] [CrossRef]
- Agabriel, C.; Cornu, A.; Journal, C.; Sibra, C.; Grolier, P.; Martin, B. Tanker milk variability according to farm feeding practices: Vitamins A and E, carotenoids, color, and terpenoids. J. Dairy Sci. 2007, 90, 4884–4896. [Google Scholar] [CrossRef] [PubMed]
- Alothman, M.; Hogan, S.A.; Hennessy, D.; Dillon, P.; Kilcawley, K.N.; O’Donovan, M.; Tobin, J.; Fenelon, M.A.; O’Callaghan, T.F. The “grass-fed” milk story: Understanding the impact of pasture feeding on the composition and quality of bovine milk. Foods 2019, 8, 350. [Google Scholar] [CrossRef]
- De la Torre-Santos, S.; Royo, L.J.; Martínez-Fernández, A.; Chocarro, C.; Vicente, F. The mode of grass supply to dairy cows impacts on fatty acid and antioxidant profile of milk. Foods 2020, 9, 1256. [Google Scholar] [CrossRef] [PubMed]
- Havemose, M.S.; Weisbjerg, M.R.; Bredie, W.L.P.; Nielsen, J.H. Influence of feeding different types of roughage on the oxidative stability of milk. Int. Dairy J. 2004, 14, 563–570. [Google Scholar] [CrossRef]
- Smet, K.; Raes, K.; De Block, J.; Herman, L.; Dewettinck, K.; Coudijzer, K. A change in antioxidative capacity as a measure of onset to oxidation in pasteurized milk. Int. Dairy J. 2008, 18, 520–530. [Google Scholar] [CrossRef]
- Calligaris, S.; Manzocco, L.; Anese, M.; Nicoli, M.C. Effect of heat-treatment on the antioxidant and pro-oxidant activity of milk. Int. Dairy J. 2004, 14, 421–427. [Google Scholar] [CrossRef]
- Kastrup Dalsgaard, T.; Sørensen, J.; Bakman, M.; Vognsen, L.; Nebel, C.; Albrechtsen, R.; Nielsen, J.H. Light-induced protein and lipid oxidation in cheese: Dependence on fat content and packaging conditions. Dairy Sci. Technol. 2010, 90, 565–577. [Google Scholar] [CrossRef]
- Zulueta, A.; Maurizi, A.; Frígola, A.; Esteve, M.J.; Coli, R.; Burini, G. Antioxidant capacity of cow milk, whey and deproteinized milk. Int. Dairy J. 2009, 19, 380–385. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO 9622); International Dairy Federation (IDF 141). Milk and Liquid Milk Products—Guidelines for the Application of Mid-Infrared Spectrometry; ISO: Geneva, Switzerland; IDF: Brussels, Belgium, 2013. [Google Scholar]
- International Dairy Federation (IDF). Cheese and Processed Cheese Products. Determination of Dry Matter; FIL-IDF: Brussels, Belgium, 1986. [Google Scholar]
- Association of Official Analytical Chemists International (AOAC). Official Methods of Analysis, 17th ed.; AOAC Intl.: Gaithersburg, MD, USA, 2000. [Google Scholar]
- International Organization for Standardization (ISO 21543); International Dairy Federation (IDF 201). Milk Products- Guidelines for the Application of Near Infrared Spectrometry; ISO: Geneva, Switzerland; IDF: Brussels, Belgium, 2006. [Google Scholar]
- Rinaldi, S.; Palocci, G.; Giovanni, S.D.; Iacurto, M.; Tripaldi, C. Chemical Characteristics and Oxidative Stability of Buffalo Mozzarella Cheese Produced with Fresh and Frozen Curd. Molecules 2021, 26, 1405. [Google Scholar] [CrossRef] [PubMed]
- Marìn, E.; Sànchez, L.; Pérez, M.D.; Puyol, P.; Calvo, M. Effect of Heat Treatment on Bovine Lactoperoxidase Activity in Skim Milk: Kinetic and Thermodynamic Analysis. J. Food Sci. 2003, 68, 89–93. [Google Scholar] [CrossRef]
- Birlouez-Aragon, I.; Nicolas, M.; Metais, A.; Marchond, N.; Grenier, J.; Calvo, D. A rapid fluorimetric method to estimate the heat treatment of liquid milk. Int. Dairy J. 1998, 8, 771–777. [Google Scholar] [CrossRef]
- Tessier, F.J.; Gadonna-Widehem, P.; Laguerre, J.C. The fluorimetric FAST method, a simple tool for the optimization of microwave pasteurization of milk. Mol. Nutr. Food Res. 2006, 50, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Unal, G. Antioxidant activity of commercial dairy products. Agro Food Ind. Hi Tech. 2012, 23, 39–42. [Google Scholar]
- Statistical Analysis System Institute (SAS). User’s Guide: Statistics, Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2011. [Google Scholar]
- ANAFIJ: Associazione Nazionale Allevatori della Razza Frisona e Jersey Italiana. Medie Produzioni Latte/Grasso/Proteine Vacche Razza Frisona Controllate. Dati Nazionali 2011–2020. Available online: http://www.anafi.it/it/pubblicazioni-statistiche/medie-produzioni-nazionali-link (accessed on 31 May 2021).
- Seifu, E.; Buys, E.M.; Donkin, E.F. Significance of the lactoperoxidase system in the dairy industry and its potential applications: A review. Trends Food Sci. Technol. 2005, 16, 137–154. [Google Scholar] [CrossRef]
- Fonteh, F.A.; Grandison, A.S.; Lewis, M.J. Variations of lactoperoxidase activity and thiocyanate content in cows’ and goats’ milk throughout lactation. J. Dairy Res. 2002, 69, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Ozer, B. Natural Anti-Microbial Systems|Lactoperoxidase and Lactoferrin. In Encyclopedia of Food Microbiology; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 930–935. [Google Scholar]
- Reiter, B. Lactoperoxidase system of bovine milk. In The Lactoperoxidase System: Chemistry and Biological Significance; Pruitt, K.M., Tenovuo, J.O., Eds.; Marcel Dekker: New York, NY, USA, 1985; pp. 123–141. [Google Scholar]
- Barrett, N.E.; Grandison, A.S.; Lewis, M.J. Contribution of the lactoperoxidase system to the keeping quality of pasteurized milk. J. Dairy Res. 1999, 66, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Leclère, J.; Birlouez-Aragon, I.; Meli, M. Fortification of milk with iron-ascorbate promotes lysine glycation and tryptophan oxidation. Food Chem. 2002, 76, 491–499. [Google Scholar] [CrossRef]
- Birlouez-Aragon, I.; Sabat, P.; Gouti, N. A new method for discriminating milk heat treatment. Int. Dairy J. 2002, 12, 59–67. [Google Scholar] [CrossRef]
- Birlouez-Aragon, I.; Leclere, J.; Quedraogo, C.L.; Birlouez, E.; Grongnet, J.-F. The FAST method, a rapid approach of the nutritional quality of heat-treated foods. Nahrung/Food 2001, 45, 201–205. [Google Scholar]
- Chen, J.; Lindmark-Månsson, H.; Gorton, L.; Åkesson, B. Antioxidant capacity of bovine milk as assayed by spectrophotometric and amperometric methods. Int. Dairy J. 2003, 13, 927–935. [Google Scholar] [CrossRef]
- Cloetens, L.; Panee, J.; Åkesson, B. The antioxidant capacity of milk—The application of different methods in vitro and in vivo. Cell. Mol. Biol. 2013, 59, 43–57. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 1–13. [Google Scholar] [CrossRef]
- Lewis, M.J. Thermal processing. In Food Processing Handbook; Brennan, J.G., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 33–70. ISBN 3-527-30719-2. [Google Scholar]
- Rukke, E.O.; Sørhaug, T.; Stepaniak, L. Heat treatment of milk: Thermization of Milk. Encycl. Dairy Sci. Second Ed. 2011, 693–698. [Google Scholar] [CrossRef]
- Guinee, T.P.; Fenelon, M.A.; Mulholland, E.O.; O’Kennedy, B.T.; O’Brien, N.; Reville, W.J. The influence of milk pasteurization temperature and pH at curd milling on the composition, texture and maturation of reduced fat cheddar cheese. Int. J. Dairy Technol. 1998, 51, 1–10. [Google Scholar] [CrossRef]
- Rynne, N.M.; Beresford, T.P.; Kelly, A.L.; Guinee, T.P. Effect of milk pasteurization temperature and in situ whey protein denaturation on the composition, texture and heat-induced functionality of half-fat Cheddar cheese. Int. Dairy J. 2004, 14, 989–1001. [Google Scholar] [CrossRef]
- Lucey, J.A.; Fox, P.F. Importance of Calcium and Phosphate in Cheese Manufacture: A Review. J. Dairy Sci. 1993, 76, 1714–1724. [Google Scholar] [CrossRef]
- Pereira, R.N.; Teixeira, J.A.; Vicente, A.A. Exploring the denaturation of whey proteins upon application of moderate electric fields: A kinetic and thermodynamic study. J. Agric. Food Chem. 2011, 59, 11589–11597. [Google Scholar] [CrossRef]
- Lucas, A.; Rock, E.; Chamba, J.-F.; Verdier-Metz, I.; Brachet, P.; Coulon, J.-B. Respective effects of milk composition and the cheese-making process on cheese compositional variability in components of nutritional interest. Lait 2006, 86, 21–41. [Google Scholar] [CrossRef]
- Erdman, J.W.J.; Poor, C.L.; Dietz, J.M. Factors affecting the bioavailability of vitamin A, carotenoids, and vitamin E. Food Technol. 1988, 42, 214–221. [Google Scholar]
- De Ritter, E. Stability characteristics of vitamins in processed foods. Food Technol. 1976, 30, 48–54. [Google Scholar]
- Tripaldi, C.; Rinaldi, S.; Palocci, G.; Di Giovanni, S.; Campagna, M.C.; Di Russo, C.; Zottola, T. Chemical and microbiological characteristics of homogenised ricotta cheese produced from buffalo whey. Ital. J. Food Sci. 2020, 32, 292–309. [Google Scholar] [CrossRef]
- Fedele, E.; Bergamo, P. Protein and lipid oxidative stresses during cheese manufacture. J. Food Sci. 2001, 66, 932–935. [Google Scholar] [CrossRef]
- Reitznerová, A.; Uleková, M.; Nagy, J.; Marcinčák, S.; Semjon, B.; Čertík, M.; Klempová, T. Lipid peroxidation process in meat and meat products: A comparison study of malondialdehyde determination between modified 2-thiobarbituric acid spectrophotometric method and reverse-phase high-performance liquid chromatography. Molecules 2017, 22, 1988. [Google Scholar] [CrossRef] [PubMed]
- Wiking, L.; Nielsen, J.H. The influence of oxidation on proteolysis in raw milk. J. Dairy Res. 2004, 71, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ayaz, M.; Ajmal, M.; Ellahi, M.Y.; Khalique, A. Antioxidant capacity and fatty acids characterization of heat treated cow and buffalo milk. Lipids Health Dis. 2017, 16, 1–10. [Google Scholar] [CrossRef]
- Miranda, M.; Muriach, M.; Almansa, I.; Jareño, E.; Bosch-Morell, F.; Romero, F.J.; Silvestre, D. Oxidative status of human milk and its variations during cold storage. BioFactors 2004, 20, 129–137. [Google Scholar] [CrossRef]
- Bertino, E.; Giribaldi, M.; Baro, C.; Giancotti, V.; Pazzi, M.; Peila, C.; Tonetto, P.; Arslanoglu, S.; Moro, G.E.; Cavallarin, L.; et al. Effect of prolonged refrigeration on the lipid profile, lipase activity, and oxidative status of human milk. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.; Peila, C.; Cresi, F.; Maggiora, E.; Sottemano, S.; Gazzolo, D.; Arslanoglu, S.; Coscia, A. Donor human milk: Effects of storage and heat treatment on oxidative stress markers. Front. Pediatr. 2018, 6, 1–5. [Google Scholar] [CrossRef]
- Van Aardt, M.; Duncan, S.E.; Marcy, J.E.; Long, T.E.; O’Keefe, S.F.; Nielsen-Sims, S.R. Aroma analysis of light-exposed milk stored with and without natural and synthetic antioxidants. J. Dairy Sci. 2005, 88, 881–890. [Google Scholar] [CrossRef]
- Macdonald, L.E.; Brett, J.; Kelton, D.; Majowicz, S.E.; Snedeker, K.; Sargeant, J.M. A systematic review and meta-analysis of the effects of pasteurization on milk vitamins, and evidence for raw milk consumption and other health-related outcomes. J. Food Prot. 2011, 74, 1814–1832. [Google Scholar] [CrossRef]
- Panfili, G.; Manzi, P.; Pizzoferrato, L. Influence of thermal and other manufacturing stresses on retinol isomerization in milk and dairy products. J. Dairy Res. 1998, 65, 253–260. [Google Scholar] [CrossRef]
- Pihlanto, A. Antioxidative peptides derived from milk proteins. Int. Dairy J. 2006, 16, 1306–1314. [Google Scholar] [CrossRef]

| Parameter | Average ± sd | Minimum | Maximum |
|---|---|---|---|
| Fat (%) | 3.91 ± 0.08 | 3.78 | 4.01 |
| Protein (%) | 3.49 ± 0.04 | 3.43 | 3.54 |
| Casein (%) | 2.74 ± 0.03 | 2.69 | 2.77 |
| Lactose (%) | 4.80 ± 0.03 | 4.73 | 4.86 |
| pH | 6.68 ± 0.02 | 6.65 | 6.71 |
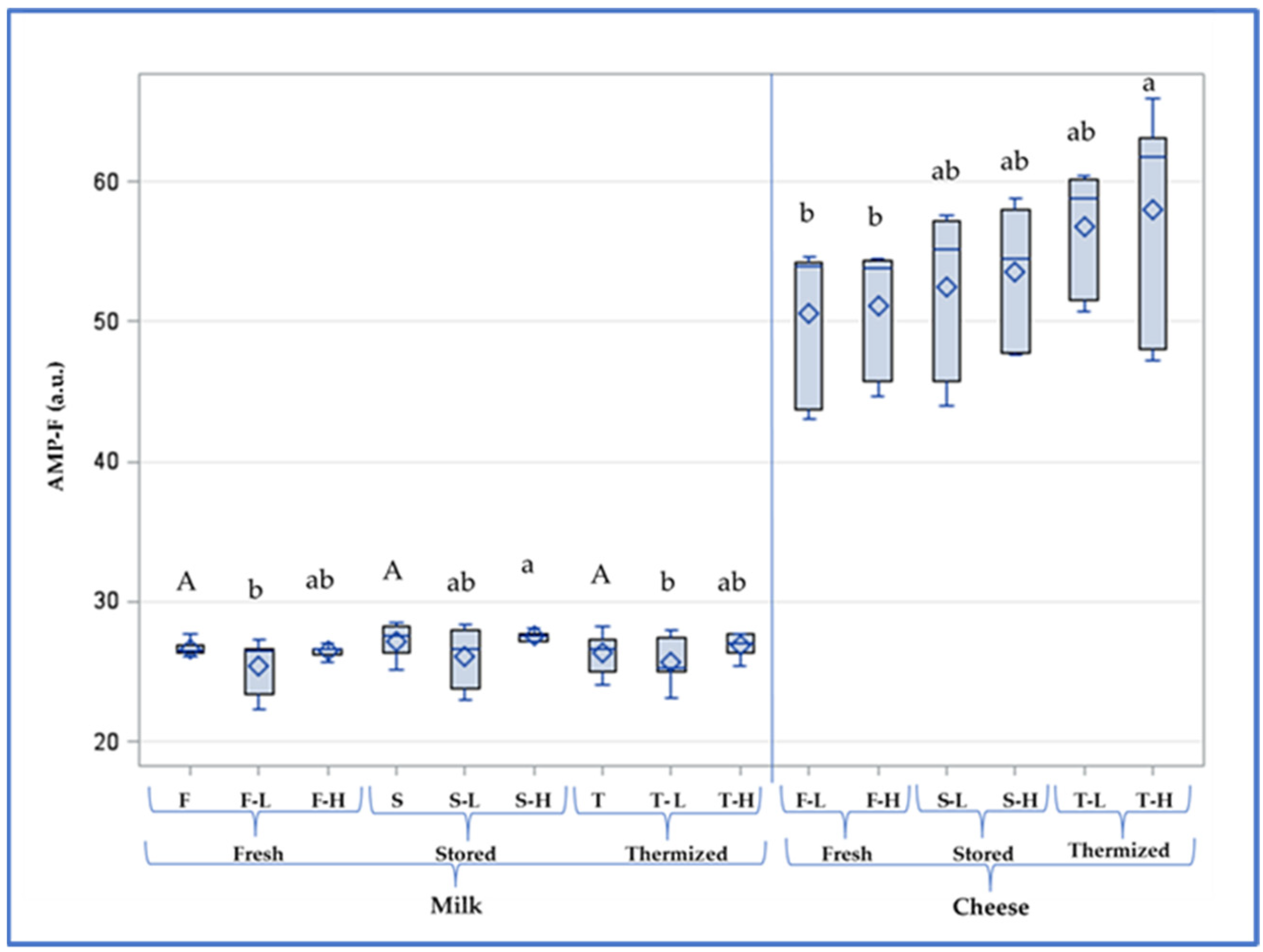
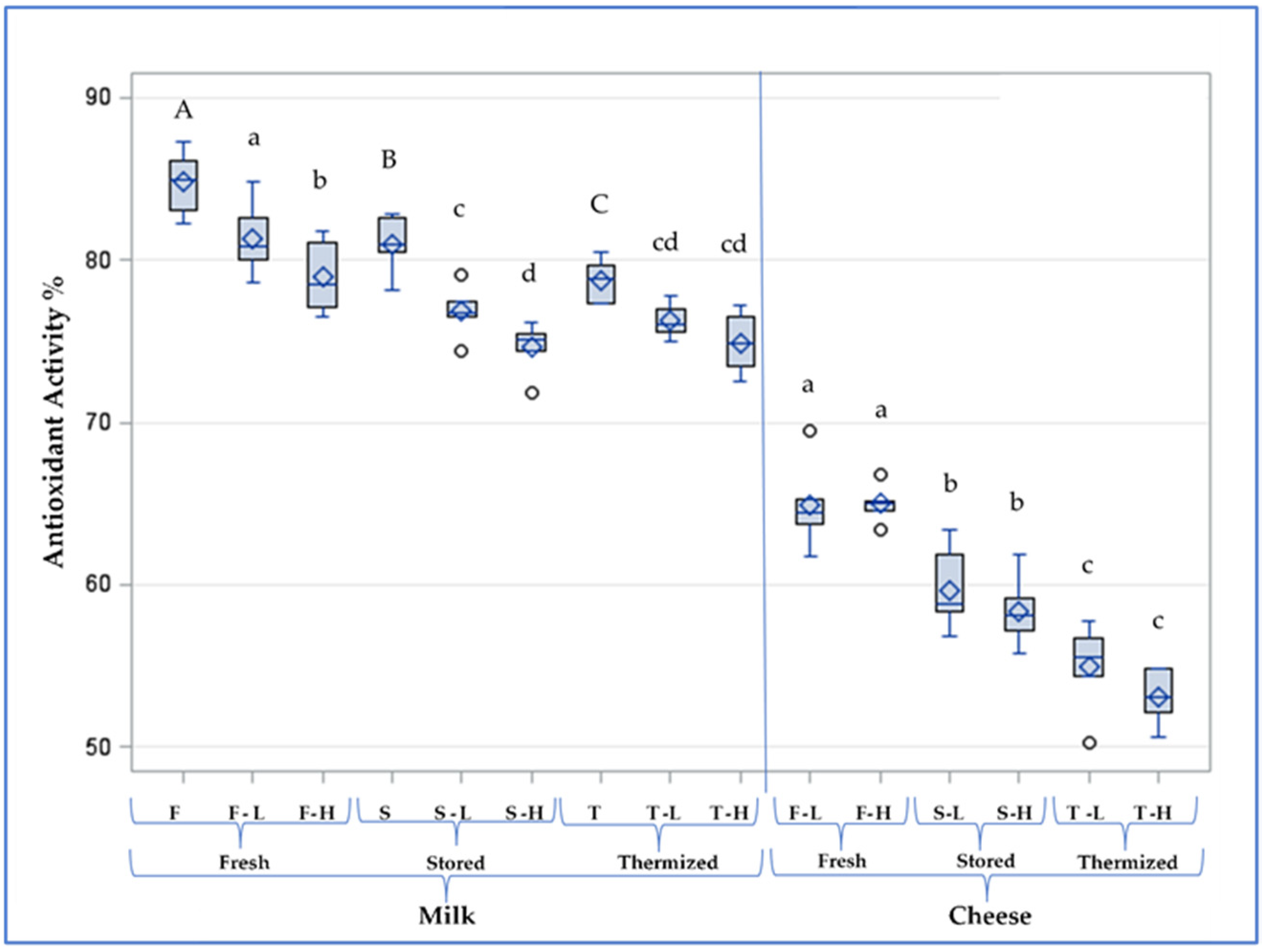
| Milk | Lactoperoxidase U mL−1 | Trp-F a.u. | AMP-F a.u. | FAST Index | Antioxidant Activity % | |
|---|---|---|---|---|---|---|
| Fresh | F-L | 2.53 a | 647 a | 25.4 b | 3.94 c | 81.3 a |
| F-H | 0.64 b | 650 a | 26.5 ab | 4.09 bc | 78.9 b | |
| Stored | S-L | 2.37 a | 633 a | 26.1 ab | 4.13 bc | 76.8 c |
| S-H | 0.68 b | 612 ab | 27.5 a | 4.38 ab | 74.7 d | |
| Thermized | T-L | 2.10 a | 594 b | 25.7 b | 4.33 abc | 76.2 cd |
| T-H | 0.56 b | 581 b | 26.9 ab | 4.65 a | 74.9 cd |
| pH | Moisture % | Ashes % | Protein % | Fat % | ||
|---|---|---|---|---|---|---|
| Fresh | F-L | 5.32 ab | 58.5 | 2.79 | 19.3 a | 22.2 a |
| F-H | 5.17 abc | 58.0 | 2.79 | 18.3 bc | 20.9 ab | |
| Stored | S-L | 5.41 a | 57.6 | 2.78 | 19.0 ab | 21.2 ab |
| S-H | 5.12 abc | 59.1 | 2.75 | 18.0 c | 20.4 ab | |
| Thermized | T-L | 5.34 ab | 56.9 | 2.69 | 19.6 a | 22.0 a |
| T-H | 4.99 c | 58.2 | 2.70 | 18.3 bc | 19.4 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripaldi, C.; Rinaldi, S.; Palocci, G.; Di Giovanni, S.; Claps, S.; Buttazzoni, L. Effect of Storage and Heat Treatment of Milk Destined for Cheese Production on Its Oxidative Characteristics. Dairy 2021, 2, 585-601. https://doi.org/10.3390/dairy2040046
Tripaldi C, Rinaldi S, Palocci G, Di Giovanni S, Claps S, Buttazzoni L. Effect of Storage and Heat Treatment of Milk Destined for Cheese Production on Its Oxidative Characteristics. Dairy. 2021; 2(4):585-601. https://doi.org/10.3390/dairy2040046
Chicago/Turabian StyleTripaldi, Carmela, Simona Rinaldi, Giuliano Palocci, Sabrina Di Giovanni, Salvatore Claps, and Luca Buttazzoni. 2021. "Effect of Storage and Heat Treatment of Milk Destined for Cheese Production on Its Oxidative Characteristics" Dairy 2, no. 4: 585-601. https://doi.org/10.3390/dairy2040046
APA StyleTripaldi, C., Rinaldi, S., Palocci, G., Di Giovanni, S., Claps, S., & Buttazzoni, L. (2021). Effect of Storage and Heat Treatment of Milk Destined for Cheese Production on Its Oxidative Characteristics. Dairy, 2(4), 585-601. https://doi.org/10.3390/dairy2040046