Benzimidazole Derivatives: A Review of Advances in Synthesis, Biological Potential, Computational Modelling, and Specialized Material Functions
Abstract
1. Introduction
2. Benzimidazole
3. Methods for Preparing Benzimidazole Derivatives
3.1. Synthesis of Benzimidazole from 2-Haloanilines
3.2. Synthesis of Benzimidazole from o-Phenylenediamine
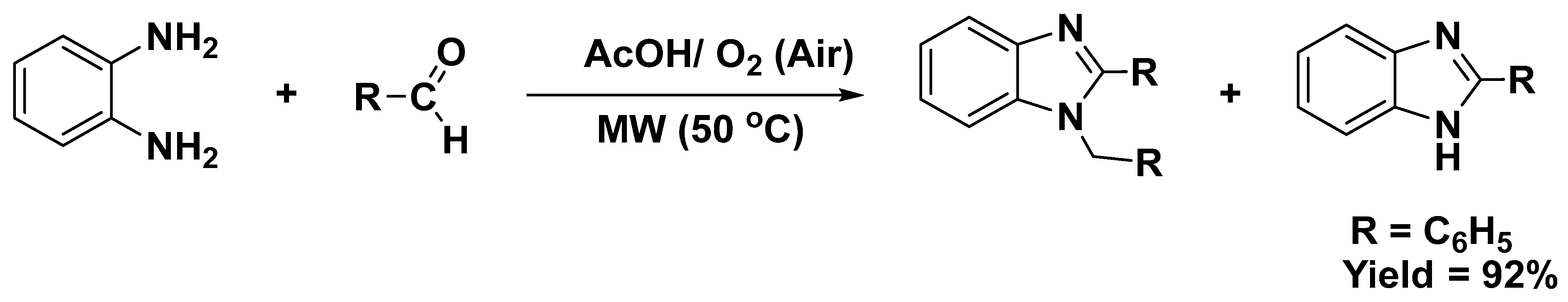
3.2.1. Reaction with Aldehydes
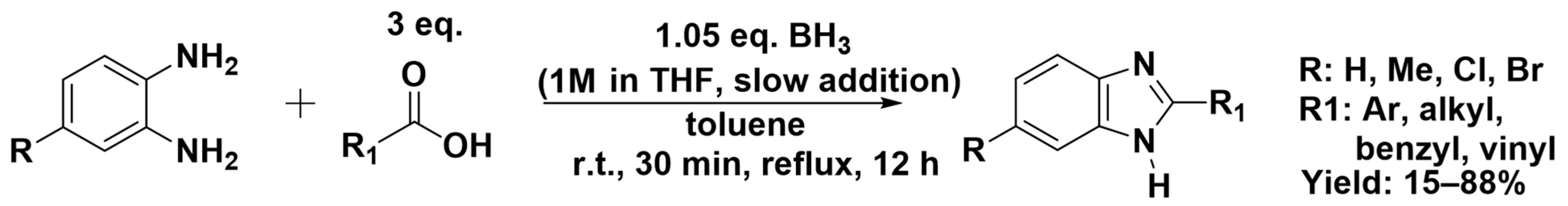
3.2.2. Reaction with Carboxylic Acids
3.3. Synthesis of Benzimidazole from Aromatic and Heteroaromatic 2-Nitroamines
3.4. Synthesis of Benzimidazole from 2-Aminobenzylamines
3.5. Synthesis of Benzimidazole Using Copper (II) Oxide Catalysts
3.6. Green Synthesis
- Microwave technology, which is used to accelerate chemical reactions and save time and energy;
- Photochemical reactions, which are catalysed by light and allow for improved productivity in safer environments;
- Reactions in aqueous medium, which is an environmentally friendly solvent, instead of toxic materials;
- Ultrasound methods, which are used to accelerate organic reactions and make them more efficient;
- Catalytic reactions, which rely on chemical catalysts to increase the reaction rate and reduce the need for additional materials.
4. Biological Activity of Benzimidazole Derivatives
4.1. Anticancer Activity
4.2. Anti-Inflammatory Activity
4.3. Antioxidant Activity
4.4. Antimicrobial Activity
4.5. Other Biological Applications
5. Density Functional Theory and Molecular Docking
5.1. DFT for the Study of Benzimidazole Derivatives
5.2. Molecular Docking Techniques
Applications of Molecular Docking in Benzimidazole Derivatives
6. Molecular Dynamics and Its Applications in Theoretical Chemistry
Applications of Molecular Dynamics in the Study of Benzimidazole Derivatives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Mulla, A. A review: Biological importance of heterocyclic compounds. Der Pharma Chem. 2017, 9, 141–147. [Google Scholar]
- Kabir, E.; Uzzaman, M. A review on biological and medicinal impact of heterocyclic compounds. Results Chem. 2022, 4, 100606. [Google Scholar] [CrossRef]
- Alheety, N.F.; Mssdf, A.K.; Besbes, R.; Alheety, M.A. Influence of Ring Size on Hydrogen Storage Performance of cis-Aziridine Derivatives: A Thermodynamic Investigation. Macromol. Symp. 2025, e70208. [Google Scholar] [CrossRef]
- Alheety, N.F.; Al-Hadithy, S.; Alheety, M.A.; Besbes, R. Color Fastness of Newly Synthesized Thiadiazole Derivatives According to ISO 105 on Cotton Fiber. Macromol. Symp. 2025, e70210. [Google Scholar] [CrossRef]
- Gomtsyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Alheety, N.F.; Awad, S.A.; Abdulkareem, R.; Alheety, M.A.; Jebur, I.K. Synthesis and Characterization of Benzimidazole-Based Scaffolds (ICSEM 2025). Macromol. Symp. 2025, e70153. [Google Scholar] [CrossRef]
- Adnan, L.A.; Alheety, N.F.; Majeed, A.H.; Alheety, M.A.; Akbaş, H. Novel organic-inorganic nanohybrids (MnO2 and Ag nanoparticles functionalized 5-methoxy-2-mercaptobenzimidazole): One step synthesis and characterization. Mater. Today Proc. 2021, 42, 2700–2705. [Google Scholar] [CrossRef]
- Alheety, N.F.; Raouafi, N.; Ibrahim, S.S.; Al-Kubaisi, A.A.; Alheety, M.A.; Besbes, R. Synthesis, characterization, DFT and molecular docking of novel organic-inorganic nanohybrids synthesized from silver nanoparticles with benzothiazole derivatives. J. Indian Chem. Soc. 2025, 102, 101761. [Google Scholar] [CrossRef]
- Farhan, M.A.; Nief, O.A.; Ali, W.B. New photostabilizers for poly (vinyl chloride) derived from heterocyclic compounds. J. Med. Pharm. Chem. Res. 2022, 4, 525–543. [Google Scholar]
- Ibrahim, W.A.; Farhan, M.A.; Abdulateef, M.H. Synthesis and evaluation of biological activity of some newsalicylic acid derivatives. Biochem. Cell. Arch. 2020, 20, 3727–3732. [Google Scholar]
- Abd El-Salam, N.M.; Mostafa, M.S.; Ahmed, G.A.; Alothman, O.Y. Synthesis and Antimicrobial Activities of Some New Heterocyclic Compounds Based on 6-Chloropyridazine-3(2H)-thione. J. Chem. 2013, 2013, 890617. [Google Scholar] [CrossRef]
- Azab, M.E.; Youssef, M.M.; El-Bordany, E.A. Synthesis and Antibacterial Evaluation of Novel Heterocyclic Compounds Containing a Sulfonamido Moiety. Molecules 2013, 18, 832–844. [Google Scholar] [CrossRef]
- Alheety, N.F.; Mohammed, L.A.; Majeed, A.H.; Aydin, A.; Ahmed, K.D.; Alheety, M.A.; Guma, M.A.; Dohare, S. Antiproliferative and antimicrobial studies of novel organic-inorganic nanohybrids of ethyl 2-((5-methoxy-1H-benzo[d]imidazol-2-yl)thio)acetate (EMBIA) with TiO2 and ZnO. J. Mol. Struct. 2023, 1274, 134489. [Google Scholar] [CrossRef]
- Cao, X.; Sun, Z.; Cao, Y.; Wang, R.; Cai, T.; Chu, W.; Hu, W.; Yang, Y. Design, Synthesis, and Structure–Activity Relationship Studies of Novel Fused Heterocycles-Linked Triazoles with Good Activity and Water Solubility. J. Med. Chem. 2014, 57, 3687–3706. [Google Scholar] [CrossRef]
- Salem, M.S.; Sakr, S.I.; El-Senousy, W.M.; Madkour, H.M.F. Synthesis, Antibacterial, and Antiviral Evaluation of New Heterocycles Containing the Pyridine Moiety. Arch. Der Pharm. 2013, 346, 766–773. [Google Scholar] [CrossRef]
- El-Sawy, E.R.; Ebaid, M.S.; Abo-Salem, H.M.; Al-Sehemi, A.G.; Mandour, A.H. Synthesis, anti-inflammatory, analgesic and anticonvulsant activities of some new 4,6-dimethoxy-5-(heterocycles)benzofuran starting from naturally occurring visnagin. Arab. J. Chem. 2014, 7, 914–923. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, K.; Tan, N.-Y.; Qiu, R.-H.; Liu, W.; Luo, N.-L.; Tong, L.; Au, C.-T.; Luo, Z.-Q.; Yin, S.-F. Synthesis, characterization and anti-proliferative activity of heterocyclic hypervalent organoantimony compounds. Eur. J. Med. Chem. 2014, 79, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, Y.N.; Barakat, A.; Al-Majid, A.M.; Alshahrani, S.; Yousuf, S.; Choudhary, M.I. Synthesis, reactions and biological activity of some new bis-heterocyclic ring compounds containing sulphur atom. Chem. Cent. J. 2013, 7, 112. [Google Scholar] [CrossRef]
- Hossain, M.; Nanda, A.K. A review on heterocyclic: Synthesis and their application in medicinal chemistry of imidazole moiety. Science 2018, 6, 83–94. [Google Scholar] [CrossRef]
- Guendouz, A.; Ettahiri, W.; Adardour, M.; Lazrak, J.; El Assiri, E.H.; Taleb, A.; Hammouti, B.; Rais, Z.; Baouid, A.; Taleb, M. New benzimidazole derivatives as efficient organic inhibitors of mild steel corrosion in hydrochloric acid medium: Electrochemical, SEM/EDX, MC, and DFT studies. J. Mol. Struct. 2025, 1321, 139901. [Google Scholar] [CrossRef]
- Vasava, M.S.; Bhoi, M.N.; Rathwa, S.K.; Jethava, D.J.; Acharya, P.T.; Patel, D.B.; Patel, H.D. Benzimidazole: A milestone in the field of medicinal chemistry. Mini Rev. Med. Chem. 2020, 20, 532–565. [Google Scholar] [CrossRef]
- El Rashedy, A.A.; Aboul-Enein, H.Y. Benzimidazole derivatives as potential anticancer agents. Mini Rev. Med. Chem. 2013, 13, 399–407. [Google Scholar]
- Keri, R.S.; Hiremathad, A.; Budagumpi, S.; Nagaraja, B.M. Comprehensive review in current developments of benzimidazole-based medicinal chemistry. Chem. Biol. Drug Des. 2015, 86, 19–65. [Google Scholar]
- Marinescu, M.; Tudorache, D.G.; Marton, G.I.; Zalaru, C.-M.; Popa, M.; Chifiriuc, M.-C.; Stavarache, C.-E.; Constantinescu, C. Density functional theory molecular modeling, chemical synthesis, and antimicrobial behaviour of selected benzimidazole derivatives. J. Mol. Struct. 2017, 1130, 463–471. [Google Scholar] [CrossRef]
- Martin, E. o-Phenylenediamine. Org. Synth. 2003, 19, 70. [Google Scholar]
- Ke, F.; Zhang, P.; Xu, Y.; Lin, X.; Lin, J.; Lin, C.; Xu, J. Microwave-Assisted Nickel-Catalyzed Synthesis of Benzimidazoles: Ammonia as a Cheap and Nontoxic Nitrogen Source. Synlett 2018, 29, 2722–2726. [Google Scholar] [CrossRef]
- Bahrami, K.; Khodaei, M.M.; Kavianinia, I. A Simple and Efficient One-Pot Synthesis of 2-Substituted Benzimidazoles. Synthesis 2007, 2007, 547–550. [Google Scholar] [CrossRef]
- Cui, W.; Kargbo, R.B.; Sajjadi-Hashemi, Z.; Ahmed, F.; Gauuan, J.F. Efficient one-pot synthesis of 2-substituted benzimidazoles from triacyloxyborane intermediates. Synlett 2012, 2012, 247–250. [Google Scholar] [CrossRef]
- Hanan, E.J.; Chan, B.K.; Estrada, A.A.; Shore, D.G.; Lyssikatos, J.P. Mild and General One-Pot Reduction and Cyclization of Aromatic and Heteroaromatic 2-Nitroamines to Bicyclic 2H-Imidazoles. Synlett 2010, 2010, 2759–2764. [Google Scholar] [CrossRef]
- Hati, S.; Kumar Dutta, P.; Dutta, S.; Munshi, P.; Sen, S. Accessing Benzimidazoles via a Ring Distortion Strategy: An Oxone Mediated Tandem Reaction of 2-Aminobenzylamines. Org. Lett. 2016, 18, 3090–3093. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Ramana, T.; Purkait, N.; Ali, M.A.; Paul, R.; Punniyamurthy, T. Ligand-free copper-catalyzed synthesis of substituted benzimidazoles, 2-aminobenzimidazoles, 2-aminobenzothiazoles, and benzoxazoles. J. Org. Chem. 2009, 74, 8719–8725. [Google Scholar] [CrossRef]
- Azarifar, D.; Pirhayati, M.; Maleki, B.; Sanginabadi, M.; Yami, N.R. Acetic acid-promoted condensation of o-phenylenediamine with aldehydes into 2-aryl-1-(arylmethyl)-1H-benzimidazoles under microwave irradiation. J. Serbian Chem. Soc. 2010, 75, 1181–1189. [Google Scholar] [CrossRef]
- Alheety, N.F.; Mssdf, A.K.; Alheety, A.J.; Aldahham, B.J.; Raouafi, N.; Alheety, M.A.; Besbes, R. Benzimidazole Macromolecules from 2-Mercaptobenzimidazole: Synthesis, Characterization, and Molecular Docking Studies. Macromol. Symp. 2025, 414, 2400233. [Google Scholar]
- Clayton, C.; Abbott, L., Jr. Inhibitory effects of certain benzimidazole derivatives on the production of azo dye liver tumors. Cancer Res. 1958, 18, 94–97. [Google Scholar]
- Alheety, N.F.; Aldahham, B.J.; Raouafi, N.; Mohammed, A.M.; Alheety, M.A.; Besbes, R. Molecular Docking Studies of Novel Benzimidazole Macromolecules. Macromol. Symp. 2025, 414, 2400232. [Google Scholar] [CrossRef]
- Maekawa, K.; Ohtani, J. Synthesis and Biological Activities of Benzimidazole Derivatives from Peptides. Agric. Biol. Chem. 1977, 41, 811–818. [Google Scholar]
- Lazer, E.S.; Matteo, M.R.; Possanza, G.J. Benzimidazole derivatives with atypical antiinflammatory activity. J. Med. Chem. 1987, 30, 726–729. [Google Scholar] [CrossRef]
- Meegalla, S.K.; Stevens, G.J.; McQueen, C.A.; Chen, A.Y.; Yu, C.; Liu, L.F.; Barrows, L.R.; LaVoie, E.J. Synthesis and pharmacological evaluation of isoindolo [1, 2-b] quinazolinone and isoindolo [2, 1-a] benzimidazole derivatives related to the antitumor agent batracylin. J. Med. Chem. 1994, 37, 3434–3439. [Google Scholar] [PubMed]
- Pham, E.C.; Le, T.V.T.; Truong, T.N. Design, synthesis, bio-evaluation, and in silico studies of some N-substituted 6-(chloro/nitro)-1 H-benzimidazole derivatives as antimicrobial and anticancer agents. RSC Adv. 2022, 12, 21621–21646. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, H.A.; Alam, M.M.; Elhenawy, A.A.; Malebari, A.M.; Nazreen, S. Synthesis, antiproliferative, docking and DFT studies of benzimidazole derivatives as EGFR inhibitors. J. Mol. Struct. 2022, 1253, 132265. [Google Scholar] [CrossRef]
- Shaharyar, M.; Mazumder, A. Benzimidazoles: A biologically active compounds. Arab. J. Chem. 2017, 10, S157–S173. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Xiong, S.; Xiong, R.; Liu, J.; Zou, L.; Lei, X.; Cao, X.; Xie, Z.; Chen, Y.; et al. Design, synthesis and biological evaluation of chrysin benzimidazole derivatives as potential anticancer agents. Nat. Prod. Res. 2018, 32, 2900–2909. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Wang, J.; Li, C.-M.; Ahn, S.; Barrett, C.M.; Dalton, J.T.; Li, W.; Miller, D.D. Design, synthesis, and biological evaluation of stable colchicine binding site tubulin inhibitors as potential anticancer agents. J. Med. Chem. 2014, 57, 7355–7366. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Qin, Y.-J.; Yang, N.; Zhang, Y.-L.; Liu, C.-H.; Zhu, H.-L. Synthesis, biological evaluation, and molecular docking studies of novel 1-benzene acyl-2-(1-methylindol-3-yl)-benzimidazole derivatives as potential tubulin polymerization inhibitors. Eur. J. Med. Chem. 2015, 99, 125–137. [Google Scholar]
- Kamal, A.; Reddy, T.S.; Polepalli, S.; Shalini, N.; Reddy, V.G.; Rao, A.S.; Jain, N.; Shankaraiah, N. Synthesis and biological evaluation of podophyllotoxin congeners as tubulin polymerization inhibitors. Bioorg. Med. Chem. 2014, 22, 5466–5475. [Google Scholar] [CrossRef]
- Pathare, B.; Bansode, T. Biological active benzimidazole derivatives. Results Chem. 2021, 3, 100200. [Google Scholar] [CrossRef]
- Sharma, R.; Bali, A.; Chaudhari, B.B. Synthesis of methanesulphonamido-benzimidazole derivatives as gastro-sparing antiinflammatory agents with antioxidant effect. Bioorg. Med. Chem. Lett. 2017, 27, 3007–3013. [Google Scholar]
- Saha, P.; Brishty, S.R.; Rahman, S.A. Pharmacological screening of substituted benzimidazole derivatives. Dhaka Univ. J. Pharm. Sci. 2021, 20, 95–102. [Google Scholar] [CrossRef]
- Kálai, T.; Balog, M.; Szabó, A.; Gulyás, G.; Jekő, J.; Sümegi, B.; Hideg, K. New Poly(ADP-ribose) Polymerase-1 Inhibitors with Antioxidant Activity Based on 4-Carboxamidobenzimidazole-2-ylpyrroline and -tetrahydropyridine Nitroxides and Their Precursors. J. Med. Chem. 2009, 52, 1619–1629. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Brishty, S.R.; Saha, P.; Al Mahmud, Z.; Rahman, S.A. Synthesis and Evaluation of Analgesic and Antioxidant Activities of Substituted Benzimidazole Derivatives: Synthesis and Evaluation of Analgesic and Antioxidant Activities. Dhaka Univ. J. Pharm. Sci. 2020, 19, 37–46. [Google Scholar] [CrossRef]
- Taha, M.; Mosaddik, A.; Rahim, F.; Ali, S.; Ibrahim, M.; Almandil, N.B. Synthesis, antiglycation and antioxidant potentials of benzimidazole derivatives. J. King Saud Univ.-Sci. 2020, 32, 191–194. [Google Scholar] [CrossRef]
- Archie, S.R.; Das, B.K.; Hossain, M.S.; Kumar, U.; Rouf, A.S. Synthesis and antioxidant activity of 2-substituted-5-nitro benzimidazole derivatives. Int. J. Pharm. Pharm. Sci. 2017, 9, 308–310. [Google Scholar] [CrossRef]
- Abd, S.; Soliman, F. Synthesis, some reactions, cytotoxic evaluation and antioxidant study of novel benzimidazole derivatives. Der Pharma Chem. 2015, 7, 71–84. [Google Scholar]
- Wanda, C.R. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. Antimicrobial agents. J. Anal. Pharm. Res. 2017, 4, 73–77. [Google Scholar] [CrossRef]
- Alheety, N.F.; Majeed, A.H.; Alheety, M.A. Silver Nanoparticles Anchored 5-methoxy benzimidazol thiomethanol (MBITM): Modulate, Characterization and Comparative Studies on MBITM and Ag-MBITM Antibacterial Activities. J. Phys. Conf. Ser. 2019, 1294, 052026. [Google Scholar] [CrossRef]
- El-Gohary, N.S.; Shaaban, M.I. Synthesis and biological evaluation of a new series of benzimidazole derivatives as antimicrobial, antiquorum-sensing and antitumor agents. Eur. J. Med. Chem. 2017, 131, 255–262. [Google Scholar] [CrossRef]
- Ajani, O.O.; Aderohunmu, D.V.; Olorunshola, S.J.; Ikpo, C.O.; Olanrewaju, I.O. Facile synthesis, characterization and antimicrobial activity of 2-alkanamino benzimidazole derivatives. Orient. J. Chem. 2016, 32, 109. [Google Scholar] [CrossRef]
- Jasim, K.H.; Ersan, R.H.; Sadeeq, R.; Salim, S.; Mahmood, S.; Fadhil, Z. Fluorinated benzimidazole derivatives: In vitro antimicrobial activity. Bioorg. Med. Chem. Rep. 2023, 6, 1–8. [Google Scholar] [CrossRef]
- Xiang, S.; Li, Y.; Khan, S.N.; Zhang, W.; Yuan, G.; Cui, J. Exploiting the Anticancer, Antimicrobial and Antiviral Potential of Naphthoquinone Derivatives: Recent Advances and Future Prospects. Pharmaceuticals 2025, 18, 350. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xia, M.-B.; Bertsetseg, D.; Wang, Y.-H.; Bao, X.-L.; Zhu, W.-B.; Tao, X.; Chen, P.-R.; Tang, H.-S.; Yan, Y.-J.; et al. Design, synthesis and biological evaluation of novel fluoro-substituted benzimidazole derivatives with anti-hypertension activities. Bioorg. Chem. 2020, 101, 104042. [Google Scholar] [CrossRef]
- Khan, M.T.; Razi, M.T.; Jan, S.U.; Mukhtiar, M.; Gul, R.; Hussain, A.; Rabbani, I. Synthesis, characterization and antihypertensive activity of 2-phenyl substituted benzimidazoles. Pak. J. Pharm. Sci 2018, 31, 1067–1074. [Google Scholar] [PubMed]
- Wu, Z.; Bao, X.-L.; Zhu, W.-B.; Wang, Y.-H.; Phuong Anh, N.T.; Wu, X.-F.; Yan, Y.-J.; Chen, Z.-L. Design, Synthesis, and Biological Evaluation of 6-Benzoxazole Benzimidazole Derivatives with Antihypertension Activities. ACS Med. Chem. Lett. 2019, 10, 40–43. [Google Scholar] [CrossRef]
- Kumar, J.R.; Jawahar L., J.; Pathak, D. Synthesis of benzimidazole derivatives: As anti-hypertensive agents. J. Chem. 2006, 3, 278–285. [Google Scholar] [CrossRef]
- Zhu, W.; Da, Y.; Wu, D.; Zheng, H.; Zhu, L.; Wang, L.; Yan, Y.; Chen, Z. Design, synthesis and biological evaluation of new 5-nitro benzimidazole derivatives as AT1 antagonists with anti-hypertension activities. Bioorg. Med. Chem. 2014, 22, 2294–2302. [Google Scholar] [CrossRef]
- Huo, X.; Hou, D.; Wang, H.; He, B.; Fang, J.; Meng, Y.; Liu, L.; Wei, Z.; Wang, Z.; Liu, F.-W. Design, synthesis, in vitro and in vivo anti-respiratory syncytial virus (RSV) activity of novel oxizine fused benzimidazole derivatives. Eur. J. Med. Chem. 2021, 224, 113684. [Google Scholar] [CrossRef]
- Chen, M.; Su, S.; Zhou, Q.; Tang, X.; Liu, T.; Peng, F.; He, M.; Luo, H.; Xue, W. Antibacterial and antiviral activities and action mechanism of flavonoid derivatives with a benzimidazole moiety. J. Saudi Chem. Soc. 2021, 25, 101194. [Google Scholar] [CrossRef]
- Ibba, R.; Carta, A.; Madeddu, S.; Caria, P.; Serreli, G.; Piras, S.; Sestito, S.; Loddo, R.; Sanna, G. Inhibition of Enterovirus A71 by a Novel 2-Phenyl-Benzimidazole Derivative. Viruses 2021, 13, 58. [Google Scholar] [CrossRef]
- Hue, B.T.B.; Nguyen, P.H.; De, T.Q.; Van Hieu, M.; Jo, E.; Van Tuan, N.; Thoa, T.T.; Anh, L.D.; Son, N.H.; La Duc Thanh, D. Benzimidazole derivatives as novel zika virus inhibitors. ChemMedChem 2020, 15, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Meher, C.P.; Meher, V.K.; Pradhan, D.; Meher, A.K.; Sahu, S.C.; Sahu, S. Screening of a Benzimidazole Derivative for Anthelmintic Activity with” Rule of 5” Approach. J. Pharm. Res. Int. 2021, 33, 1982–1988. [Google Scholar] [CrossRef]
- Anichina, K.; Argirova, M.; Tzoneva, R.; Uzunova, V.; Mavrova, A.; Vuchev, D.; Popova-Daskalova, G.; Fratev, F.; Guncheva, M.; Yancheva, D. 1H-benzimidazole-2-yl hydrazones as tubulin-targeting agents: Synthesis, structural characterization, anthelmintic activity and antiproliferative activity against MCF-7 breast carcinoma cells and molecular docking studies. Chem. -Biol. Interact. 2021, 345, 109540. [Google Scholar] [CrossRef]
- Alheety, N.F.; Al-Kubaisi, A.A.; Benassi, E.; Raouafi, N.; Maamer, C.B.; Alheety, M.A.; Besbes, R. Benzimidazole Derivatives as Potential Inhibitors of Monkeypox A41 Protein: DFT Calculations, Molecular Docking, ADME, and Toxicity Assessments. ChemistrySelect 2025, 10, e01475. [Google Scholar] [CrossRef]
- Meher, C.P.; Sahu, A.K.; Dash, S.K.; Sahoo, R.K.; Sahu, S.C.; Gupta, D.K. Designing and Synthesis of some Novel 2-Substituted Benzimidazole Derivatives and their Evaluation for Antimicrobial and Anthelmintic Activity. Asian J. Res. Chem. 2019, 12, 58–62. [Google Scholar] [CrossRef]
- Kaliyaperumal, S.; Pattanayak, P. Synthesis, Characterization and In Vitro Evaluation for Antimicrobial and Anthelmintic Activity of Novel Benzimidazole Substituted 1, 3, 4-Thiadiazole Schiff’s Bases. FABAD J. Pharm. Sci. 2021, 46, 261–270. [Google Scholar]
- Campos-Almazan, M.I.; Flores-Ramos, M.; Hernández-Campos, A.; Castillo, R.; Sierra-Campos, E.; Torgeson, K.; Peti, W.; Valdez-Solana, M.; Oria-Hernández, J.; Mendez, S.T. Design, synthesis, kinetic, molecular dynamics, and hypoglycemic effect characterization of new and potential selective benzimidazole derivatives as Protein Tyrosine Phosphatase 1B inhibitors. Bioorg. Med. Chem. 2021, 48, 116418. [Google Scholar] [CrossRef] [PubMed]
- Dik, B.; Coşkun, D.; Bahçivan, E.; Üney, K. Potential antidiabetic activity of benzimidazole derivative albendazole and lansoprazoledrugs in different doses in experimental type 2 diabetic rats. Turk. J. Med. Sci. 2021, 51, 1579–1586. [Google Scholar] [CrossRef]
- Deswal, L.; Verma, V.; Kumar, D.; Kaushik, C.P.; Kumar, A.; Deswal, Y.; Punia, S. Synthesis and antidiabetic evaluation of benzimidazole-tethered 1, 2, 3-triazoles. Arch. Der Pharm. 2020, 353, 2000090. [Google Scholar]
- Babkov, D.A.; Zhukowskaya, O.N.; Borisov, A.V.; Babkova, V.A.; Sokolova, E.V.; Brigadirova, A.A.; Litvinov, R.A.; Kolodina, A.A.; Morkovnik, A.S.; Sochnev, V.S. Towards multi-target antidiabetic agents: Discovery of biphenyl-benzimidazole conjugates as AMPK activators. Bioorg. Med. Chem. Lett. 2019, 29, 2443–2447. [Google Scholar] [CrossRef]
- Kwak, H.J.; Pyun, Y.M.; Kim, J.Y.; Pagire, H.S.; Kim, K.Y.; Kim, K.R.; Dal Rhee, S.; Jung, W.H.; Song, J.S.; Bae, M.A. Synthesis and biological evaluation of aminobenzimidazole derivatives with a phenylcyclohexyl acetic acid group as anti-obesity and anti-diabetic agents. Bioorg. Med. Chem. Lett. 2013, 23, 4713–4718. [Google Scholar] [CrossRef] [PubMed]
- Sakr, H.M.; Ayyad, R.R.; Mahmoud, K.; Mansour, A.M.; Ahmed, A.G. Design, synthesis of analgesics and anticancer of some new derivatives of benzimidazole. Int. J. Org. Chem. 2021, 11, 144–169. [Google Scholar] [CrossRef]
- Raka, S.C.; Rahman, A.; Hussain, F.; Rahman, S.A. Synthesis, characterization and in vitro, in vivo, in silico biological evaluations of substituted benzimidazole derivatives. Saudi J. Biol. Sci. 2022, 29, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, D.V.; Spasov, A.A.; Vassiliev, P.M.; Skripka, M.O.; Miroshnikov, M.V.; Kochetkov, A.N.; Eliseeva, N.V.; Lifanova, Y.V.; Kuzmenko, T.A.; Divaeva, L.N.; et al. Synthesis and Pharmacological Evaluation of Novel 2,3,4,5-tetrahydro[1,3]diazepino[1,2-a]benzimidazole Derivatives as Promising Anxiolytic and Analgesic Agents. Molecules 2021, 26, 6049. [Google Scholar] [CrossRef]
- Saha, P.; Brishty, S.R.; Rahman, S.A. Synthesis and evaluation of disubstituted benzimidazole derivatives as potential analgesic and antidiarrheal agents. Indian J. Pharm. Sci 2020, 82, 222–229. [Google Scholar] [CrossRef]
- Aydin, S.; Beis, R.; Can, Ö. Analgesic and antispasmodic activities of 2-(2-nitro-phenyl)-1H-benzimidazole 5-carboxylic acid: Evidence for the importance of the 2-(o-substituted phenyl) group. Die Pharm.-Int. J. Pharm. Sci. 2003, 58, 405–408. [Google Scholar]
- Navarrete-Vázquez, G.; Moreno-Diaz, H.; Aguirre-Crespo, F.; León-Rivera, I.; Villalobos-Molina, R.; Munoz-Muniz, O.; Estrada-Soto, S. Design, microwave-assisted synthesis, and spasmolytic activity of 2-(alkyloxyaryl)-1H-benzimidazole derivatives as constrained stilbene bioisosteres. Bioorg. Med. Chem. Lett. 2006, 16, 4169–4173. [Google Scholar] [CrossRef] [PubMed]
- Tantray, M.A.; Khan, I.; Hamid, H.; Alam, M.S.; Dhulap, A.; Kalam, A. Synthesis of benzimidazole-linked-1, 3, 4-oxadiazole carboxamides as GSK-3β inhibitors with in vivo antidepressant activity. Bioorg. Chem. 2018, 77, 393–401. [Google Scholar] [CrossRef]
- Theivendren, P.; Subramanian, A.; Murugan, I.; Joshi, S.D.; More, U.A. Graph theoretical analysis, insilico modeling, design, and synthesis of compounds containing benzimidazole skeleton as antidepressant agents. Chem. Biol. Drug Des. 2017, 89, 714–722. [Google Scholar] [CrossRef]
- Turan, N.; Özkay, Ü.D.; Can, N.Ö.; Can, Ö.D. Investigating the antidepressant-like effects of some benzimidazole-piperidine derivatives by in-vivo experimental methods. Lett. Drug Des. Discov. 2019, 16, 341–346. [Google Scholar] [CrossRef]
- Mathew, B.; Suresh, J.; Anbazhagan, S. Development of novel (1-H) benzimidazole bearing pyrimidine-trione based MAO-A inhibitors: Synthesis, docking studies and antidepressant activity. J. Saudi Chem. Soc. 2016, 20, S132–S139. [Google Scholar] [CrossRef]
- Tantray, M.A.; Khan, I.; Hamid, H.; Alam, M.S.; Dhulap, A.; Kalam, A. Synthesis of benzimidazole-based 1, 3, 4-oxadiazole-1, 2, 3-triazole conjugates as glycogen synthase kinase-3β inhibitors with antidepressant activity in in vivo models. RSC Adv. 2016, 6, 43345–43355. [Google Scholar] [CrossRef]
- Siddiqui, N.; Alam, M.S.; Sahu, M.; Yar, M.S.; Alam, O.; Siddiqui, M.J.A. Antidepressant, analgesic activity and SAR studies of substituted benzimidazoles. Asian J. Pharm. Res. 2016, 6, 170–174. [Google Scholar] [CrossRef]
- Latif, A.; Bibi, S.; Ali, S.; Ammara, A.; Ahmad, M.; Khan, A.; Al-Harrasi, A.; Ullah, F.; Ali, M. New multitarget directed benzimidazole-2-thiol-based heterocycles as prospective anti-radical and anti-Alzheimer’s agents. Drug Dev. Res. 2021, 82, 207–216. [Google Scholar] [CrossRef]
- Adalat, B.; Rahim, F.; Taha, M.; Alshamrani, F.J.; Anouar, E.H.; Uddin, N.; Shah, S.A.A.; Ali, Z.; Zakaria, Z.A. Synthesis of benzimidazole–based analogs as anti Alzheimer’s disease compounds and their molecular docking studies. Molecules 2020, 25, 4828. [Google Scholar] [CrossRef]
- Chaves, S.; Hiremathad, A.; Tomás, D.; Keri, R.S.; Piemontese, L.; Santos, M.A. Exploring the chelating capacity of 2-hydroxyphenyl-benzimidazole based hybrids with multi-target ability as anti-Alzheimer’s agents. New J. Chem. 2018, 42, 16503–16515. [Google Scholar] [CrossRef]
- Noor, A.; Qazi, N.G.; Nadeem, H.; Khan, A.-u.; Paracha, R.Z.; Ali, F.; Saeed, A. Synthesis, characterization, anti-ulcer action and molecular docking evaluation of novel benzimidazole-pyrazole hybrids. Chem. Cent. J. 2017, 11, 85. [Google Scholar] [CrossRef]
- Katsura, Y.; Inoue, Y.; Nishino, S.; Tomoi, M.; Itoh, H.; Takasugi, H. Studies on antiulcer drugs. III. Synthesis and antiulcer activities of imidazo [1, 2-a] pyridinylethyl-benzoxazoles and related compounds. A novel class of histamine H2-receptor antagonists. Chem. Pharm. Bull. 1992, 40, 1424–1438. [Google Scholar] [CrossRef]
- Spasov, A.; Kovalev, G.; Bakumov, P.; Reshetov, M.; Anisimova, V.; Avdiunina, N. The effect on gastric secretion and the anti-ulcer action of imidazo [1, 2-A] benzimidazole derivatives. Farmakol. I Toksikol. 1990, 53, 30–33. [Google Scholar]
- Yamada, S.-I.; Goto, T.; Shimanuki, E.; Narita, S.-I. Syntheses and Antiulcer Activities of Novel 2-[(6, 7, 8, 9-Tetrahydro-5H-cyclohepta [b] pyridin-9-yl) sulfinyl]-1H-benzimidazole Analogues. Chem. Pharm. Bull. 1994, 42, 718–720. [Google Scholar] [CrossRef] [PubMed]
- Maste, M.; Jeyarani, P.; Kalekar, M.; Bhat, A. Synthesis and Evaluation of Benzimidazole Derivatives for Anti-tubercular and Antimicrobial Activities. Asian J. Res. Chem. 2011, 4, 1055–1058. [Google Scholar]
- Yar, M.S.; Abdullah, M.M.; Majeed, J. In vitro anti-tubercular screening of newly synthesized benzimidazole derivatives. World Acad. Sci. Engr. Tech 2009, 55, 593–599. [Google Scholar]
- Shingalapur, R.V.; Hosamani, K.M.; Keri, R.S. Synthesis and evaluation of in vitro anti-microbial and anti-tubercular activity of 2-styryl benzimidazoles. Eur. J. Med. Chem. 2009, 44, 4244–4248. [Google Scholar] [CrossRef]
- Sabale, P.; Bhagwat, D.; Sabale, V. Synthesis and anti-tubercular activity of substituted phenylpyrazole having benzimidazole ring. Res. J. Pharm. Technol. 2018, 11, 3599–3608. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Khuntia, A.; Yellasubbaiah, N.; Ayyanna, C.; Sudha, B.N.; Harika, M.S. Design, synthesis of novel azo derivatives of benzimidazole as potent antibacterial and anti tubercular agents. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 646–651. [Google Scholar] [CrossRef]
- Kore, P.S.; Lokpaure, S.G.; Jawarkar, S.V.; Mohite, S.K.; Magdum, C.S. Synthesis and anti-tubercular activity of some n-substituted and 2-substituted benzimidazole derivatives. Asian J. Res. Chem. 2014, 7, 137–140. [Google Scholar]
- Siddiki, A.A.; Bairwa, V.K.; Telvekar, V.N. Synthesis and biological evaluation of novel N’(4-aryloxybenzylidene)-1H-benzimidazole-2 carbohydrazide derivatives as anti-tubercular agents. Comb. Chem. High Throughput Screen. 2014, 17, 630–638. [Google Scholar] [CrossRef]
- Rida, S.M.; El-Hawash, S.A.M.; Fahmy, H.T.Y.; Hazzaa, A.A.; El-Meligy, M.M.M. Synthesis of novel benzofuran and related benzimidazole derivatives for evaluation ofin vitro anti-HIV-1, anticancer and antimicrobial activities. Arch. Pharmacal Res. 2006, 29, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Demirayak, Ş.; Mohsen, U.A.; Karaburun, A.Ç. Synthesis and anticancer and anti-HIV testing of some pyrazino [1, 2-a] benzimidazole derivatives. Eur. J. Med. Chem. 2002, 37, 255–260. [Google Scholar]
- Pan, T.; He, X.; Chen, B.; Chen, H.; Geng, G.; Luo, H.; Zhang, H.; Bai, C. Development of benzimidazole derivatives to inhibit HIV-1 replication through protecting APOBEC3G protein. Eur. J. Med. Chem. 2015, 95, 500–513. [Google Scholar] [CrossRef]
- Yadav, G.; Ganguly, S.; Murugesan, S.; Dev, A. Synthesis, anti-HIV, antimicrobial evaluation and structure activity relationship studies of some novel benzimidazole derivatives. Anti-Infect. Agents 2015, 13, 65–77. [Google Scholar] [CrossRef]
- Demirayak, Ş.; Mohsen, U.A. Anticancer and anti-HIV activities of some pyrido/pyrazino-benzimidazole derivatives. Acta Pharm. Sci. 1998, 40. [Google Scholar]
- Miller, J.F.; Turner, E.M.; Gudmundsson, K.S.; Jenkinson, S.; Spaltenstein, A.; Thomson, M.; Wheelan, P. Novel N-substituted benzimidazole CXCR4 antagonists as potential anti-HIV agents. Bioorg. Med. Chem. Lett. 2010, 20, 2125–2128. [Google Scholar] [CrossRef]
- Vásquez, D.; Lagos, C.F.; Mella-Raipán, J.; González, L.; Ebensperger, R.; Alvarez-Figueroa, M.J.; Sáez, E.; Pessoa-Mahana, H.; Araya-Secchp, R.; González-Wong, A. 1-benzoyl-2-(2-nitrophenyl)-1H-benzimidazole derivatives: A novel approach to the development of new HIV-1 reverse transcriptase inhibitors. J. Chil. Chem. Soc. 2007, 52, 1281–1287. [Google Scholar] [CrossRef]
- Alheety, N.F.; Al-Kubaisi, A.A.; Alhadidi, H.A.; Alheety, M.A.; Jebur, I.K. Molecular Docking of Benzimidazole Derivatives as Potential Serotonin Transporter (SERT) Inhibitors in Depression Disease (ICSEM 2025). Macromol. Symp. 2025, e70148. [Google Scholar] [CrossRef]
- Alheety, N.F.; Al-Kubaisi, A.A.; Mukhlif, M.Y.; Alheety, M.A.; Besbes, R. Structure-Based Design and ADME Screening of Benzimidazole Derivatives as Potential Therapeutics for Human Metapneumovirus (HMPV) (ICSEM 2025). Macromol. Symp. 2025, e70159. [Google Scholar] [CrossRef]
- Kumar, B.; Rao, P. Synthesis and structural studies on transition metal complexes derived from 1-(2-thienyl)-1-ethanole-1H-benzimidazole. Asian J. Chem. 2006, 18, 3060–3064. [Google Scholar]
- Devivar, R.V.; Kawashima, E.; Revankar, G.R.; Breitenbach, J.M.; Kreske, E.D.; Drach, J.C.; Townsend, L.B. Benzimidazole Ribonucleosides: Design, Synthesis, and Antiviral Activity of Certain 2-(Alkylthio)-and 2-(Benzylthio)-5, 6-dichloro-1-(. beta.-D-ribofuranosyl) benzimidazoles. J. Med. Chem. 1994, 37, 2942–2949. [Google Scholar] [CrossRef]
- Guo, X.-Z.; Shi, L.; Wang, R.; Liu, X.-X.; Li, B.-G.; Lu, X.-X. Synthesis and biological activities of novel nonpeptide angiotensin II receptor antagonists based on benzimidazole derivatives bearing a heterocyclic ring. Bioorg. Med. Chem. 2008, 16, 10301–10310. [Google Scholar] [CrossRef]
- Khanum, G.; Ali, A.; Shabbir, S.; Fatima, A.; Alsaiari, N.; Fatima, Y.; Ahmad, M.; Siddiqui, N.; Javed, S.; Gupta, M. Vibrational Spectroscopy, Quantum Computational and Molecular Docking Studies on 2-[(1H-benzimidazol-1-yl)-methyl] benzoic acid. Crystals 2022, 12, 337. [Google Scholar] [CrossRef]
- Zouaghi, M.O.; Bensalah, D.; Hassen, S.; Arfaoui, Y.; Mansour, L.; Özdemir, N.; Bülbül, H.; Gurbuz, N.; Özdemir, I.; Hamdi, N. Benzimidazole derivatives as a new scaffold of anticancer agents: Synthesis, optical properties, crystal structure and DFT calculations. Heliyon 2024, 10, e32905. [Google Scholar] [CrossRef] [PubMed]
- Alheety, N.F.; Yassin, H.A.; Karakosh, A.J.; Aldahham, B.J.M.; Ali, S.H.; Ahmed, K.D.; Dylan, B.A.; Nuaman, H.A.; Alheety, M.A. Synthesis and In Silico Study of Silver Nanoparticles Composite with Novel Schiff Base. Macromol. Symp. 2025, 414, 2400234. [Google Scholar] [CrossRef]
- Al-Kubaisi, A.A.; Awad, S.A.; Monawer, E.M.; Abbood, R.S.; Mukhlif, M.Y.; Omar, T.A.; Abbas, J.A.; Khalaf, E.M.; Alheety, N.F. Biogenic Silver Nanowires Embedded in Benzoxazolylthiomethanol Matrix (Ag NPs-BOTM): An Antibacterial Activity, DFT investigation and Molecular Docking Analysis of a New Organic–Inorganic Nanohybrid. J. Mol. Struct. 2025, 1353, 144764. [Google Scholar] [CrossRef]
- Tahlan, S.; Kumar, S.; Ramasamy, K.; Lim, S.M.; Shah, S.A.A.; Mani, V.; Narasimhan, B. In-silico molecular design of heterocyclic benzimidazole scaffolds as prospective anticancer agents. BMC Chem. 2019, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Almalki, A.S.; Nazreen, S.; Elbehairi, S.E.I.; Asad, M.; Shati, A.A.; Alfaifi, M.Y.; Alhadhrami, A.; Elhenawy, A.A.; Alorabi, A.Q.; Asiri, A.M. Design, synthesis, anticancer activity and molecular docking studies of new benzimidazole derivatives bearing 1, 3, 4-oxadiazole moieties as potential thymidylate synthase inhibitors. New J. Chem. 2022, 46, 14967–14978. [Google Scholar] [CrossRef]
- Alheety, N.F.; Al-Kubaisi, A.A.; Yassin, H.A.; Alheety, M.A. Novel Benzoxazole Derivatives as Potential Acetylcholinesterase (AChE) Inhibitors in Alzheimer’s Disease: Molecular Docking and Pharmacokinetics Analysis (ICSEM 2025). Macromol. Symp. 2025, e70156. [Google Scholar] [CrossRef]
- Thapa, S.; Biradar, M.S.; Nargund, S.L.; Ahmad, I.; Agrawal, M.; Patel, H.; Lamsal, A. Synthesis, molecular docking, molecular dynamic simulation studies, and antitubercular activity evaluation of substituted benzimidazole derivatives. Adv. Pharmacol. Pharm. Sci. 2024, 2024, 9986613. [Google Scholar] [CrossRef]
- Srivastava, R.; Gupta, S.K.; Naaz, F.; Gupta, P.S.S.; Yadav, M.; Singh, V.K.; Singh, A.; Rana, M.K.; Gupta, S.K.; Schols, D. Alkylated benzimidazoles: Design, synthesis, docking, DFT analysis, ADMET property, molecular dynamics and activity against HIV and YFV. Comput. Biol. Chem. 2020, 89, 107400. [Google Scholar] [CrossRef] [PubMed]
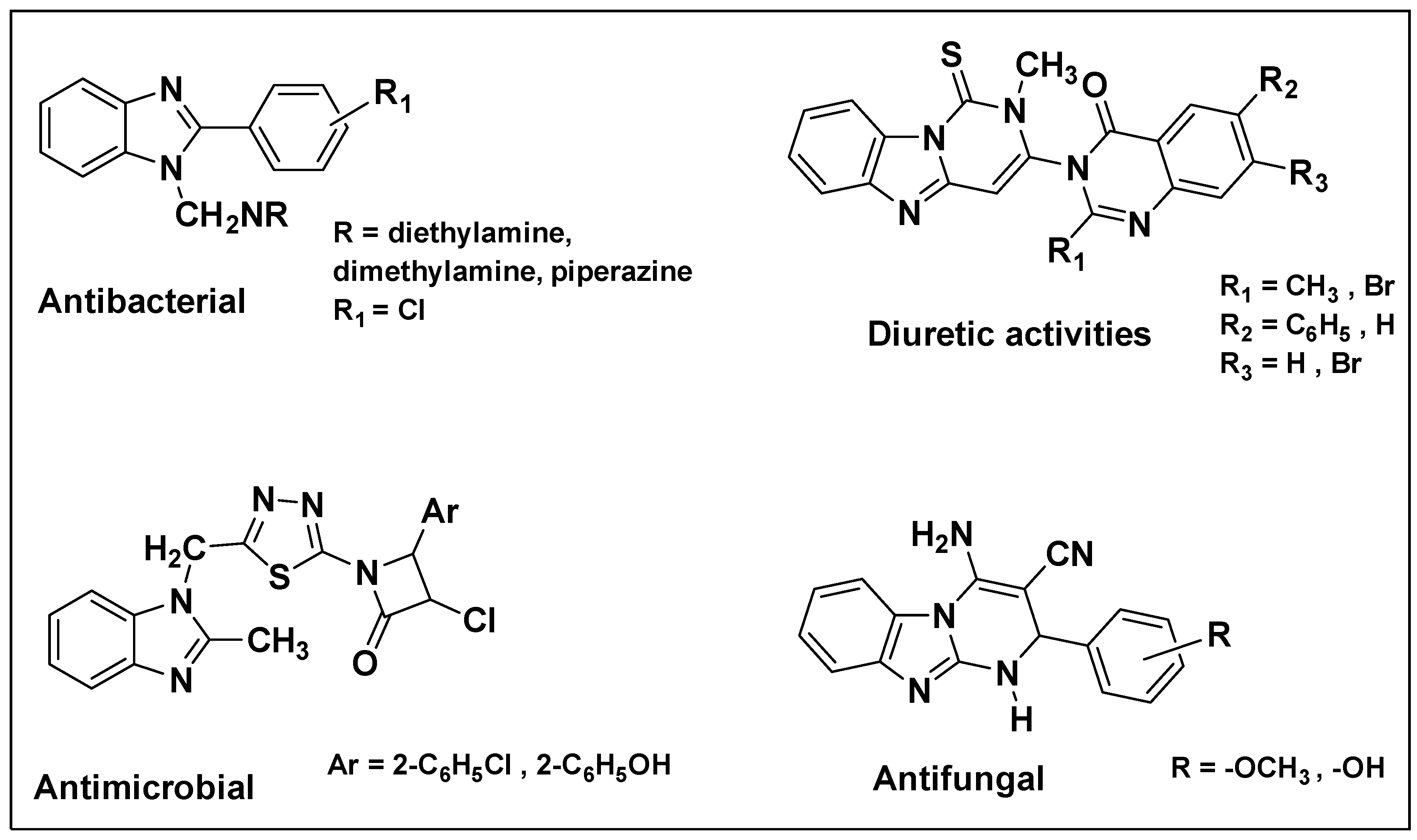
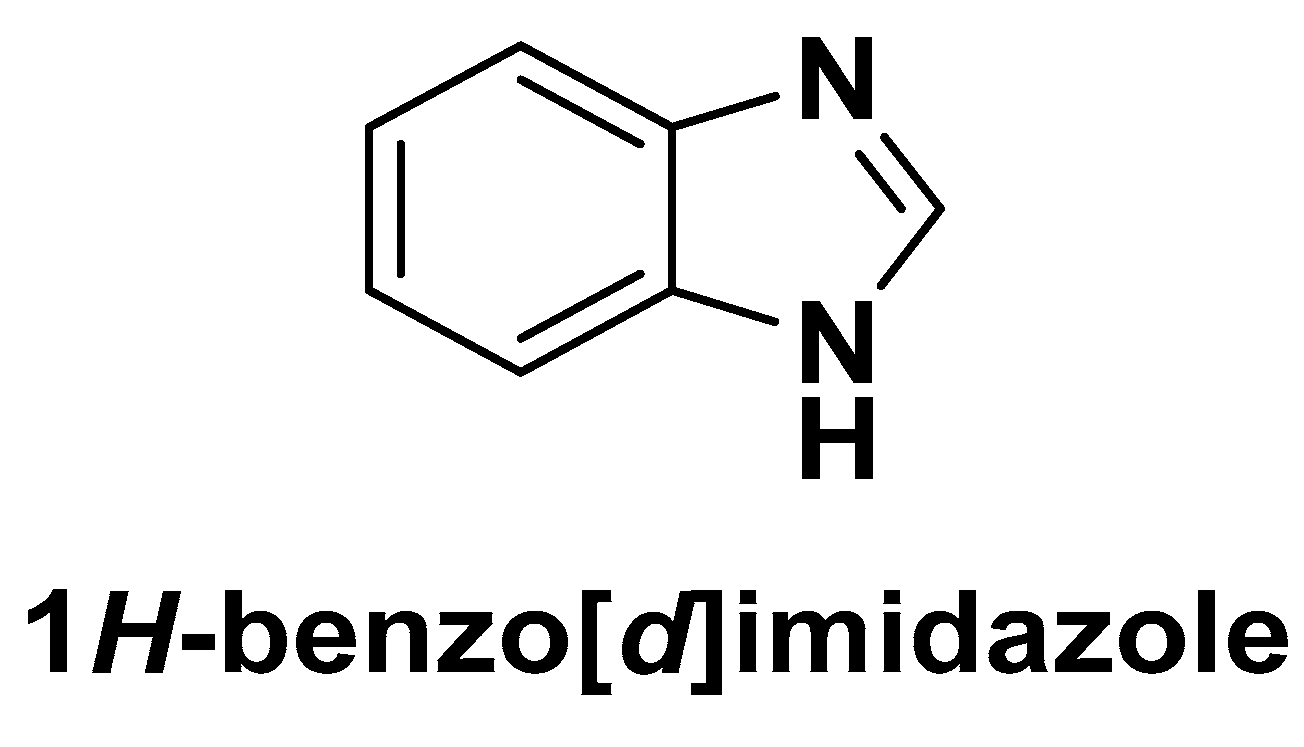
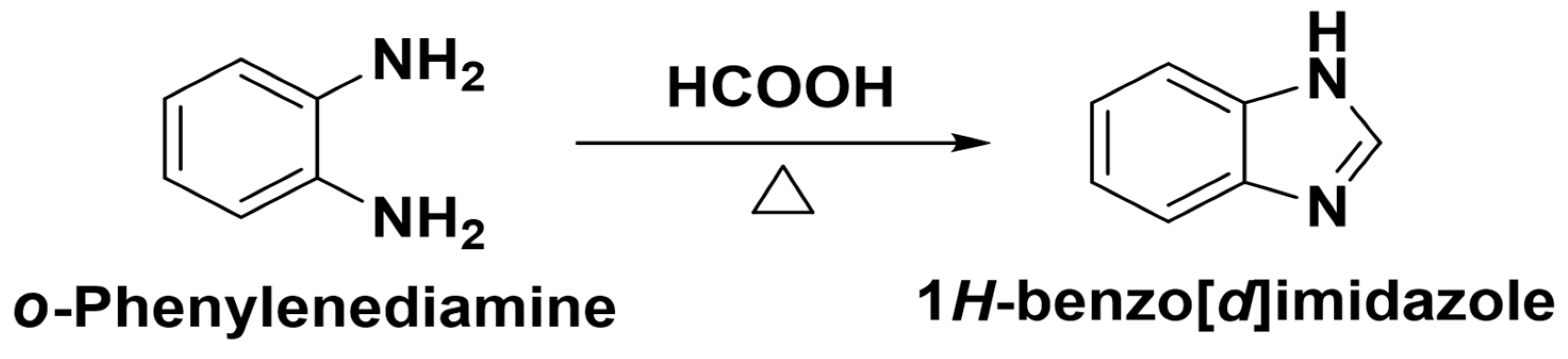
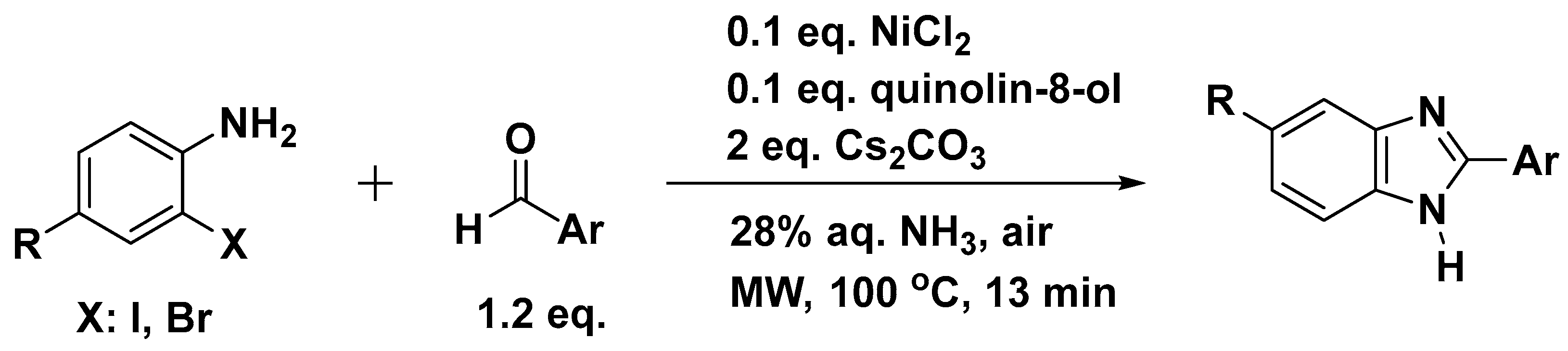
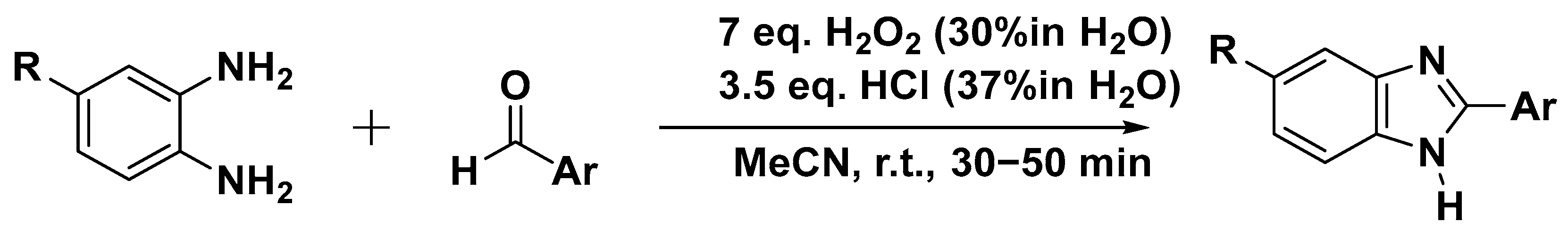
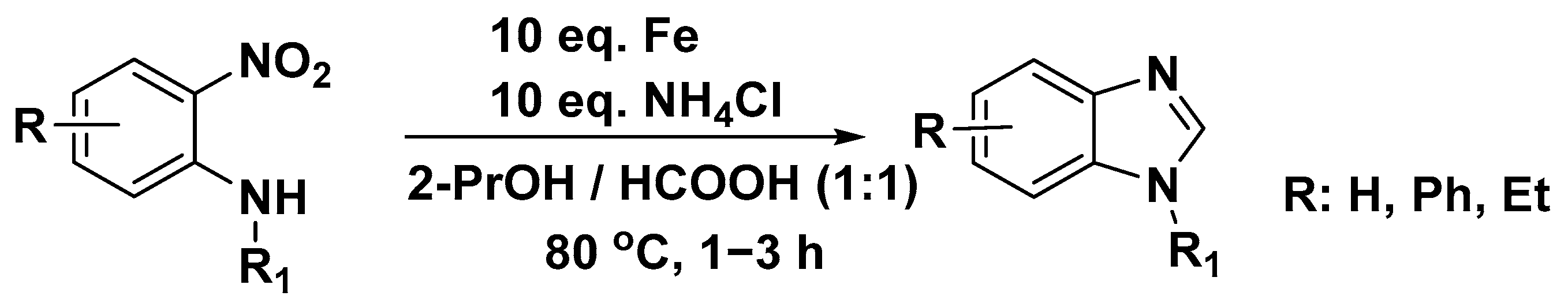
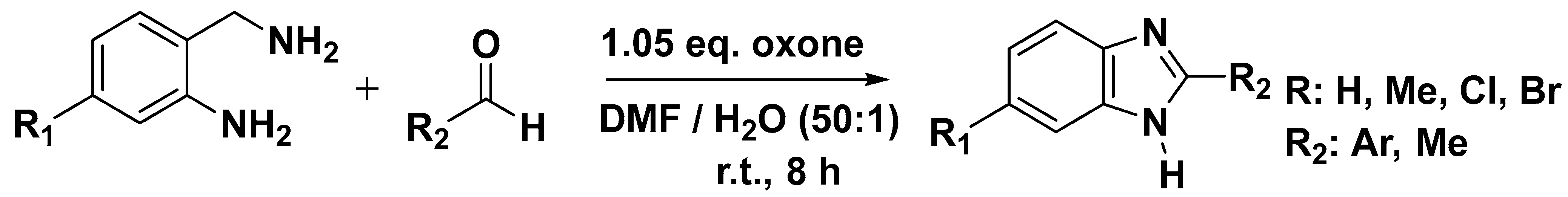
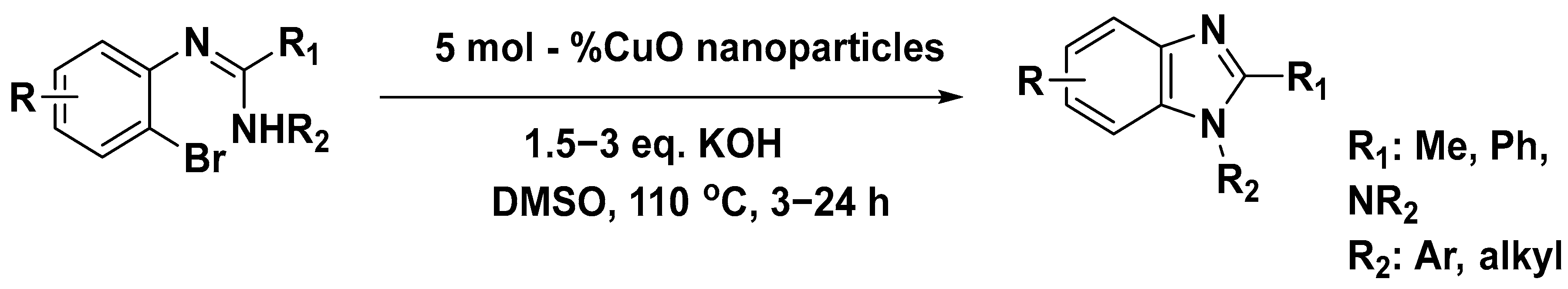


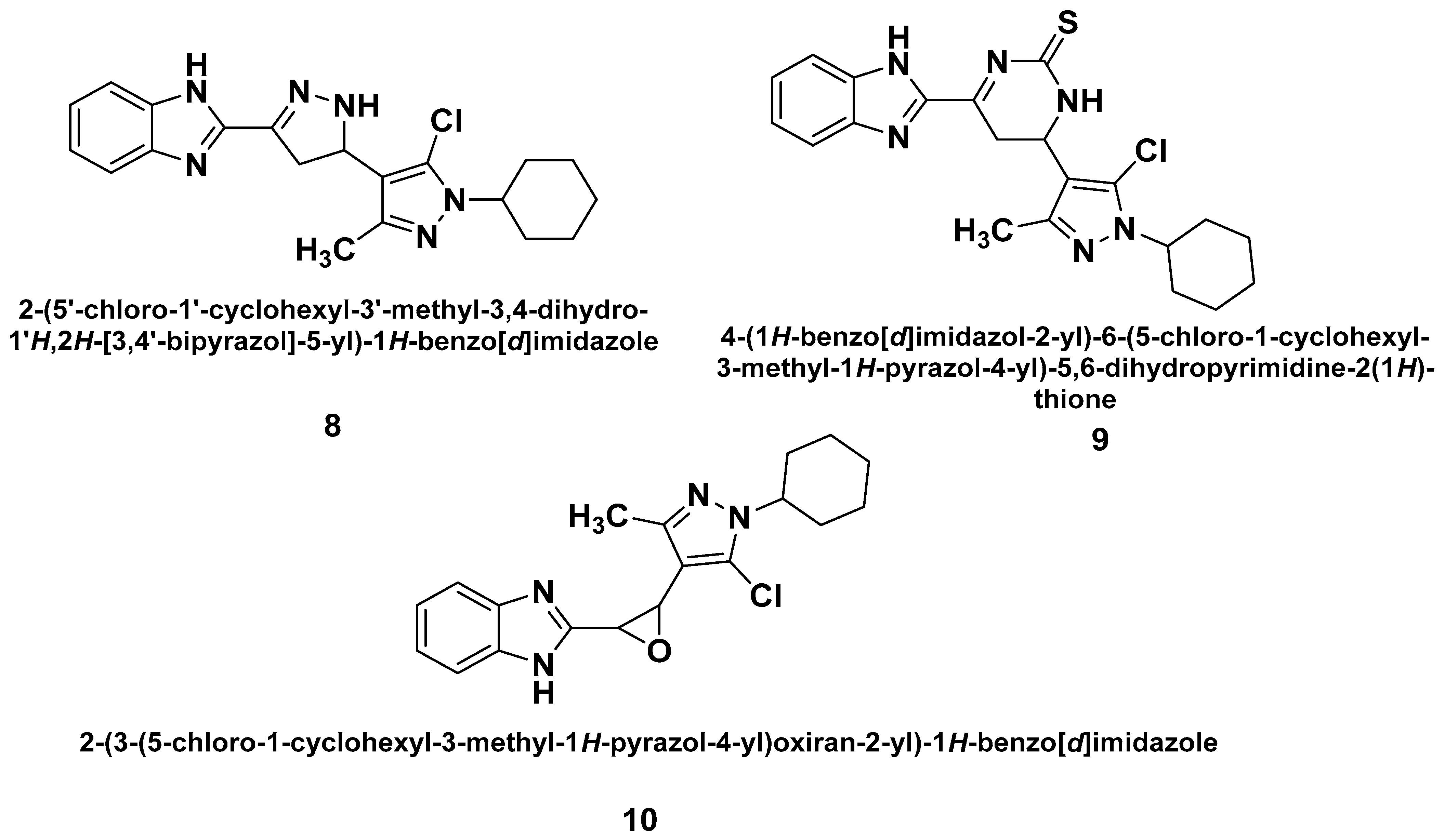
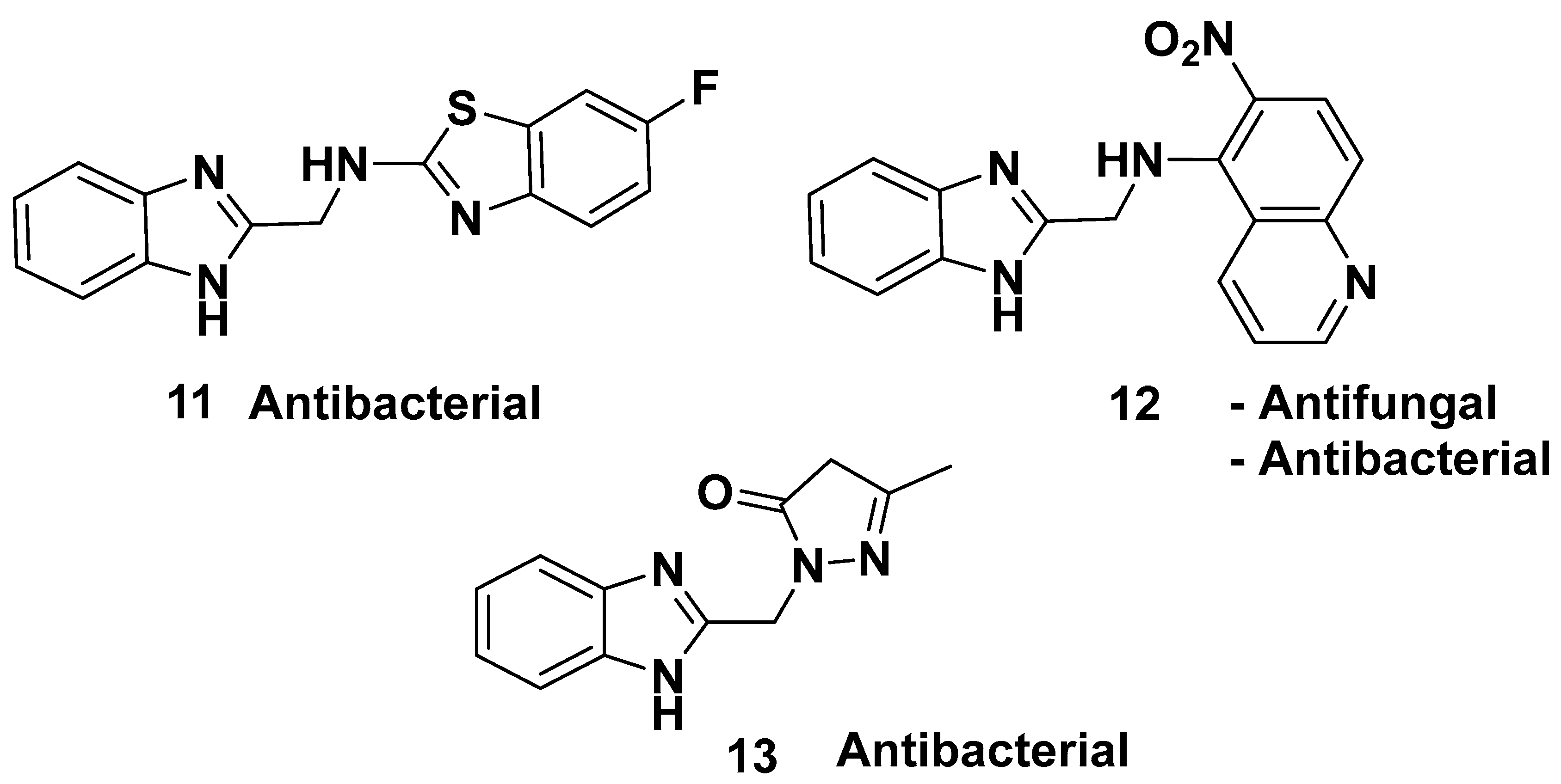

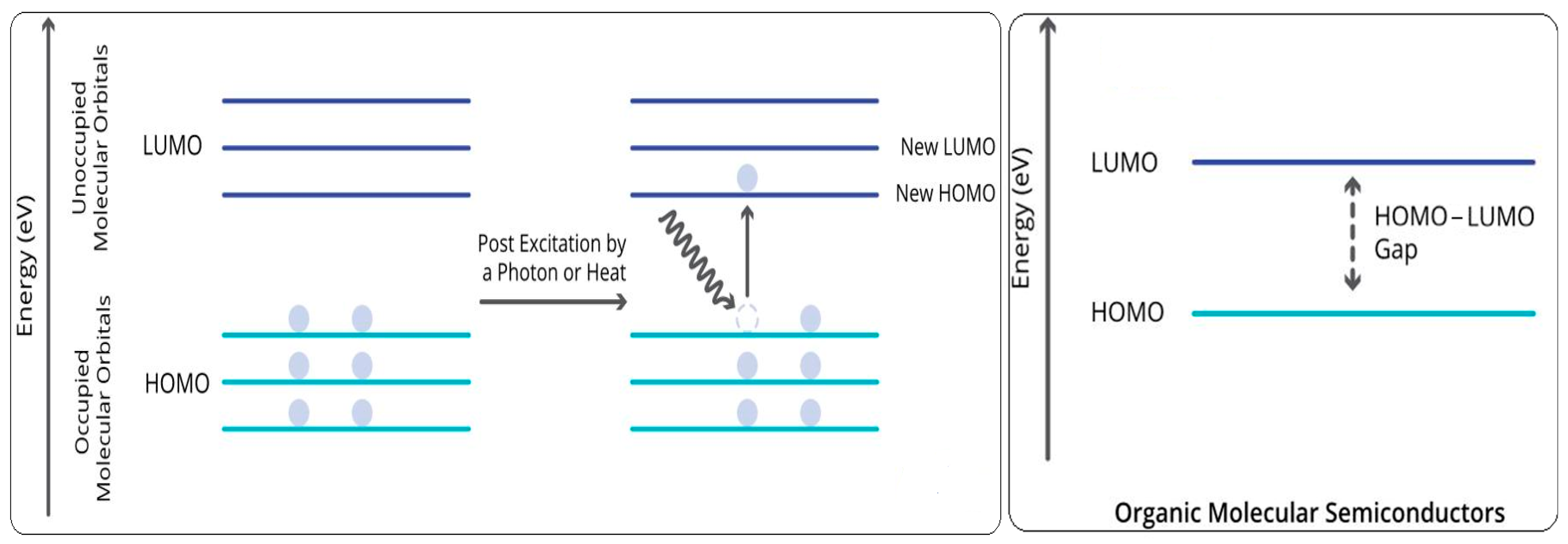
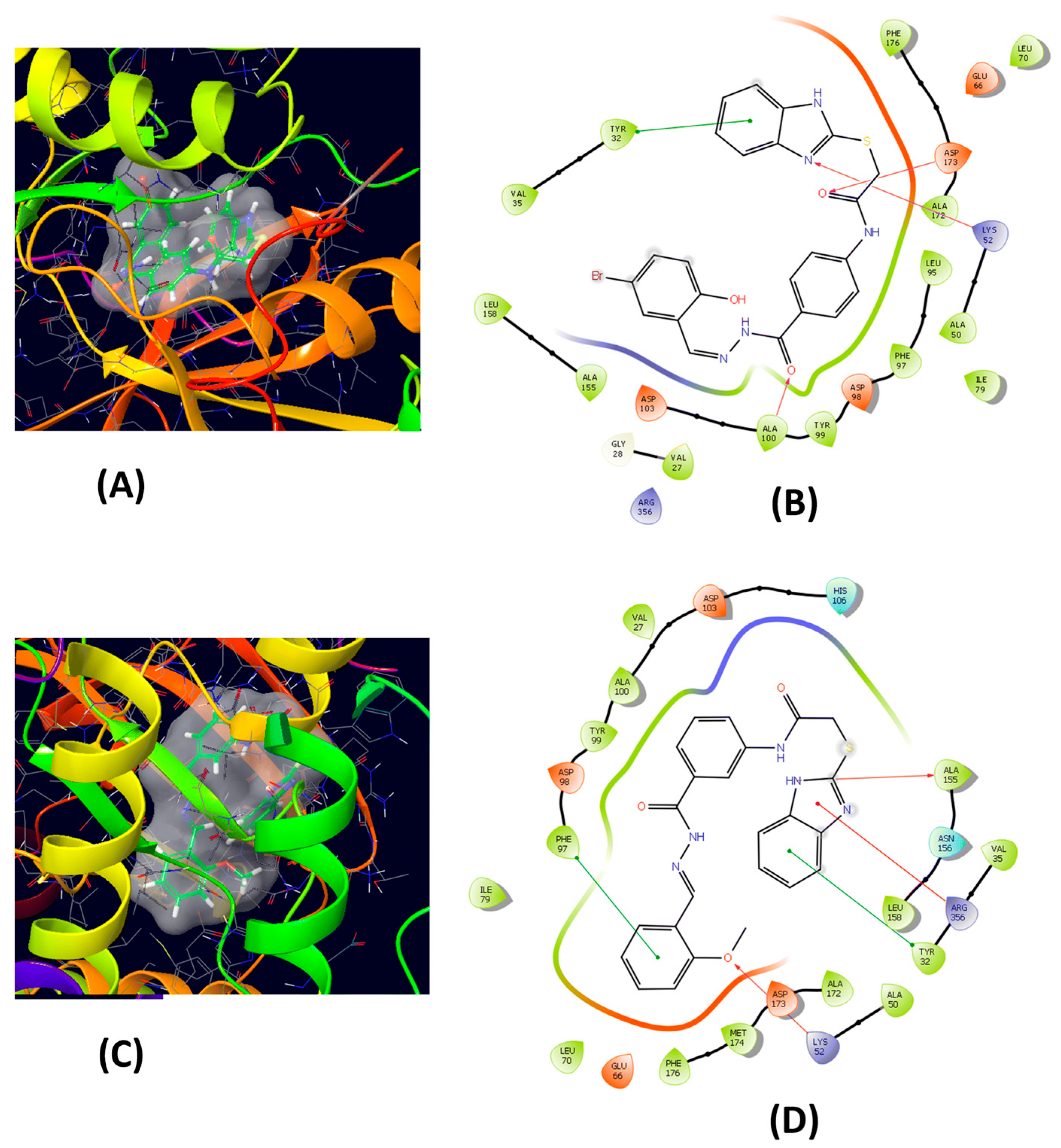
| Method | Starting Materials | Conditions | Yield (%) | Reference |
|---|---|---|---|---|
| Condensation | o-Phenylenediamine + aldehydes | H2O2/HCl, solvent-free, RT | 82–95 | [27] |
| Nitro-reduction | 2-Nitroanilines | Fe/NH4Cl, formic acid | 70–90 | [29] |
| Microwave synthesis | O-Phynylenedianine (OPD) + aldehydes | MW, AcOH | 88–96 | [32] |
| Compound | Target | Activity | Cell Lines | Reference |
|---|---|---|---|---|
| Benzimidazole-fluoroalkyl | Tubulin | IC50 = 30 Nm | A549, HepG2 | [43] |
| 1-Benzoyl-2-(indol-3-yl) | Tubulin | GI50 = 2.4–5.1 µM | A549, HepG2, MCF-7 | [44] |
| Benzimidazole-pyrazole | Tubulin (colchicine site) | Potent inhibitor | HeLa | [45] |
| Compound | Activity | MIC (µg/mL) | Organism | Reference |
|---|---|---|---|---|
| 11 | Antibacterial | 0.524 | S. aureus | [58] |
| 13 | Antibacterial | 0.489 | B. cereus | [58] |
| 17 | Antibacterial | 42 mm (zone) | K. pneumoniae | [58] |
| Compound | Chemical Structure | Biological Activity | Mechanism of Action | Reference |
|---|---|---|---|---|
| 26 |  | Antiviral | Monkeypox A41 protein inhibitor | [73] |
| 27 |  | Antidepressant | Selective Serotonin Reuptake Inhibitor (SSRI) | [114] |
| 28 | 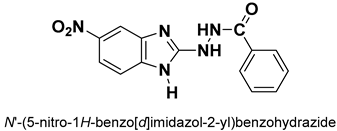 | Antiviral | Inhibitor of HMPV F protein-mediated membrane fusion | [115] |
| 29 | 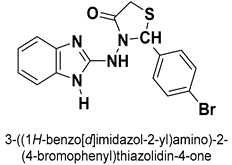 | Antimicrobial | Dual-target bacterial cell division and DNA replication inhibitor | [33] |
| 30 |  | Anti-asthmatic | Selective β2-adrenergic receptor agonist | [116] |
| 31 |  | Antiviral | Inhibits viral DNA polymerase | [117] |
| 32 | 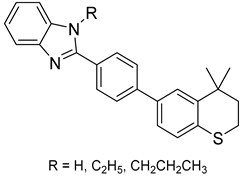 | Antidiabetic | Enhances insulin sensitivity | [116] |
| 33 |  | Hypotensive | Angiotensin II receptor antagonist | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Alheety, N.F.; Awad, S.A.; Alheety, M.A.; Darwesh, M.Y.; Abbas, J.A.; Besbes, R. Benzimidazole Derivatives: A Review of Advances in Synthesis, Biological Potential, Computational Modelling, and Specialized Material Functions. Chemistry 2026, 8, 1. https://doi.org/10.3390/chemistry8010001
Alheety NF, Awad SA, Alheety MA, Darwesh MY, Abbas JA, Besbes R. Benzimidazole Derivatives: A Review of Advances in Synthesis, Biological Potential, Computational Modelling, and Specialized Material Functions. Chemistry. 2026; 8(1):1. https://doi.org/10.3390/chemistry8010001
Chicago/Turabian StyleAlheety, Nuaman F., Sameer A. Awad, Mustafa A. Alheety, Mohanned Y. Darwesh, Jalal A. Abbas, and Rafaâ Besbes. 2026. "Benzimidazole Derivatives: A Review of Advances in Synthesis, Biological Potential, Computational Modelling, and Specialized Material Functions" Chemistry 8, no. 1: 1. https://doi.org/10.3390/chemistry8010001
APA StyleAlheety, N. F., Awad, S. A., Alheety, M. A., Darwesh, M. Y., Abbas, J. A., & Besbes, R. (2026). Benzimidazole Derivatives: A Review of Advances in Synthesis, Biological Potential, Computational Modelling, and Specialized Material Functions. Chemistry, 8(1), 1. https://doi.org/10.3390/chemistry8010001









