Terpene-Functionalized 3,5-Bis(benzylidene)-4-piperidones: Synthesis, Cytotoxicity Properties, In Silico and In Vitro Studies
Abstract
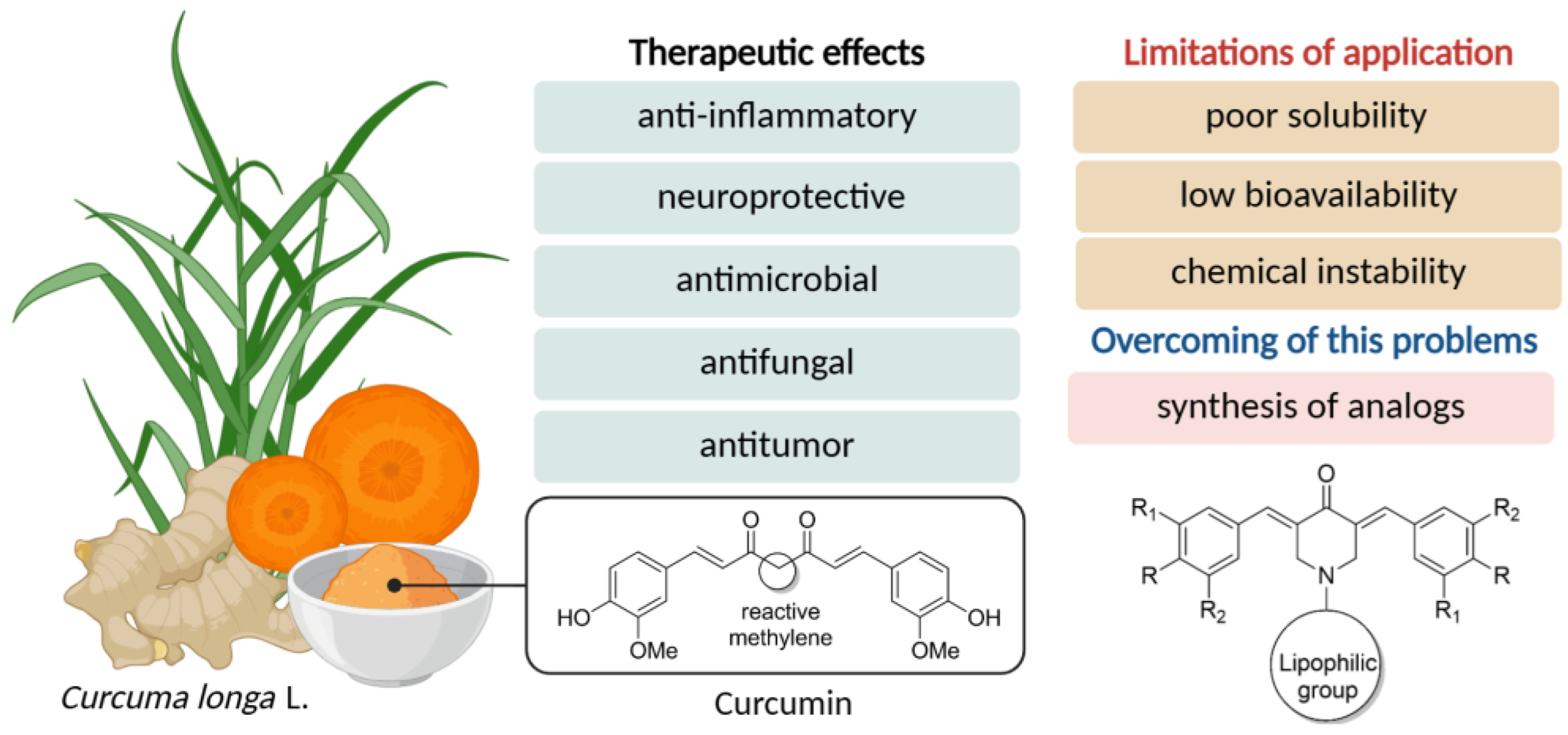
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
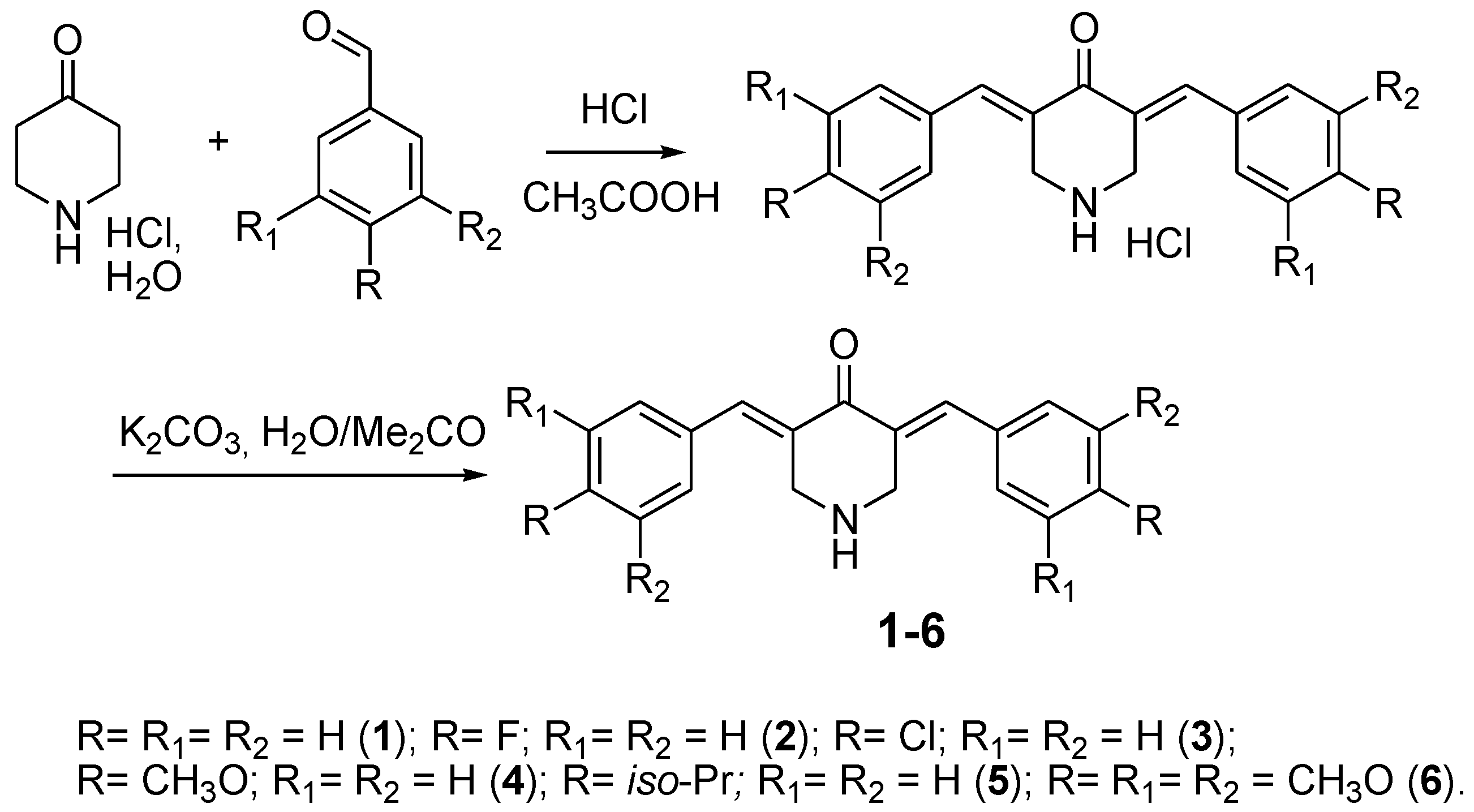
2.2. General Procedure of 3,5-Bis(benzylidene)-4-piperidones (1–6) Synthesis
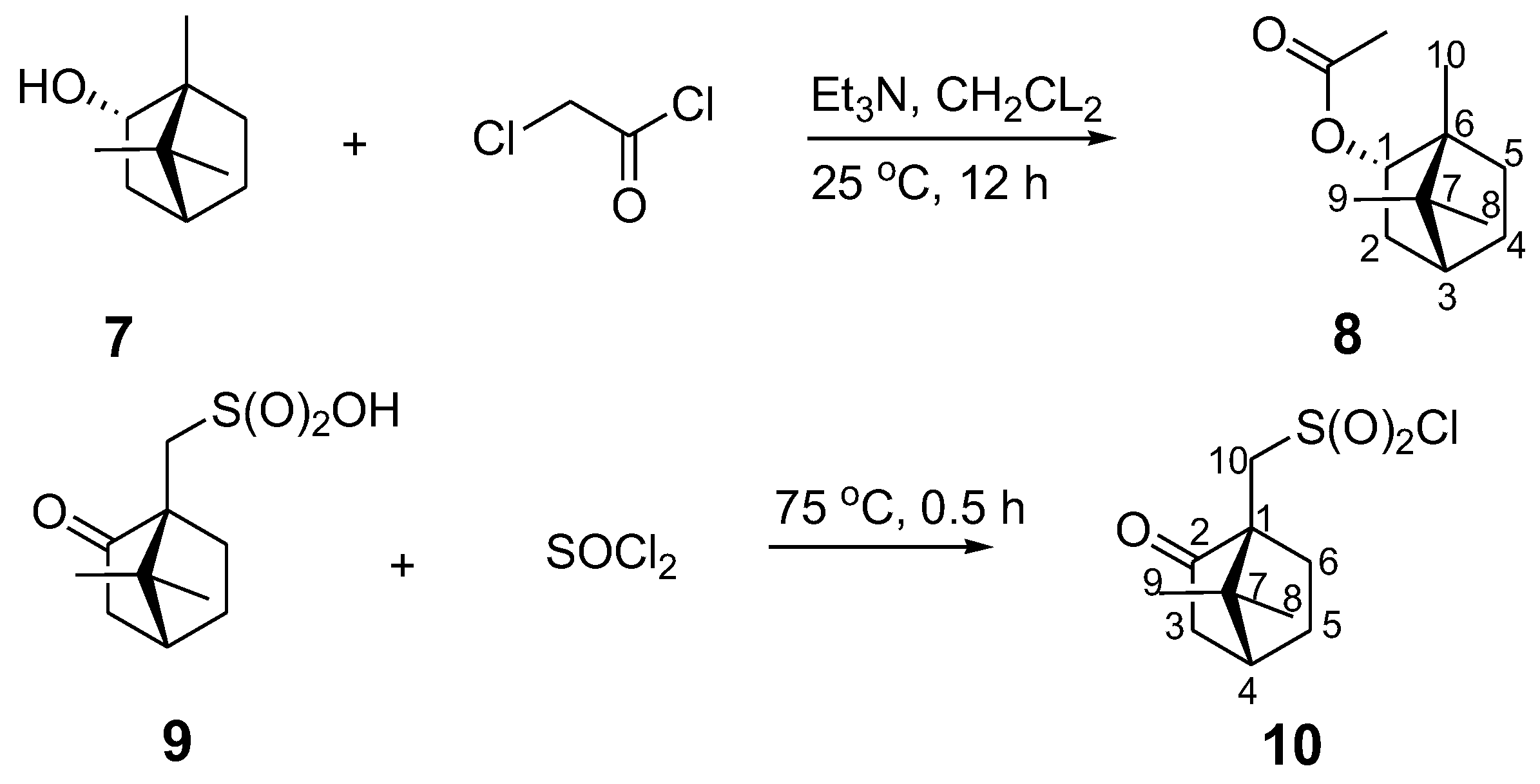
2.3. Synthesis of (1S,2R,4S)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-yl 3-Chloroacetate (8) and (1S)-(+)-Camphor-10-sulfonyl chloride (10)
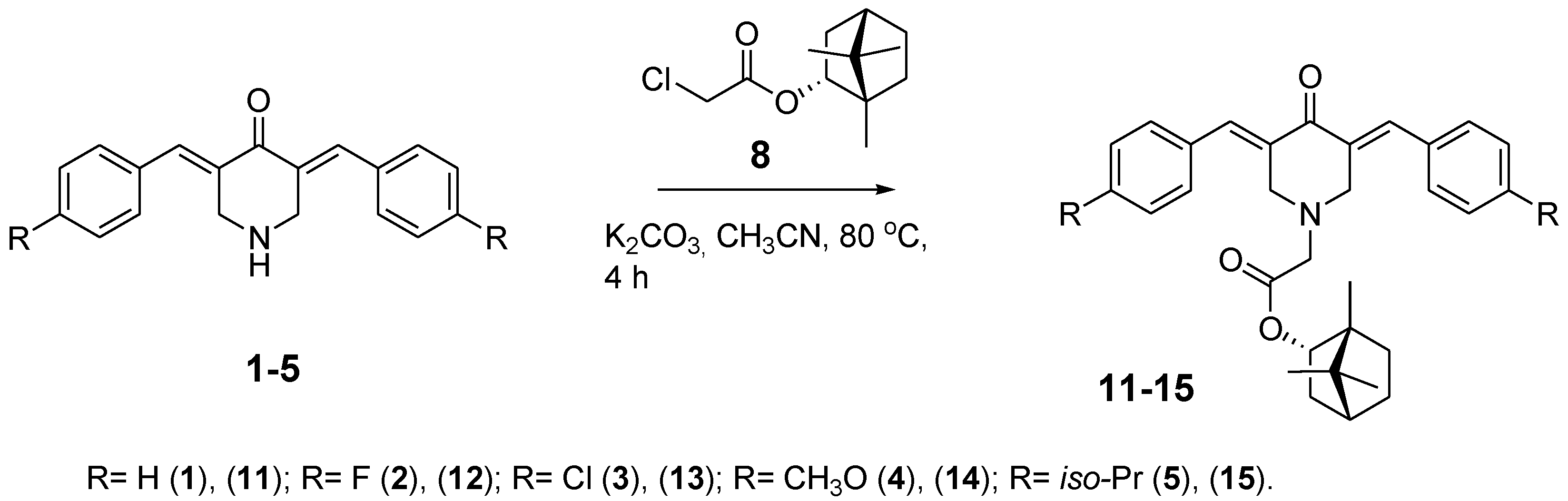
2.4. General Procedure of (1S,2R,4S)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-yl (E,E)-3,5-Dibenzylidene-4-oxo-1-piperidineacetate Synthesis (11–15)
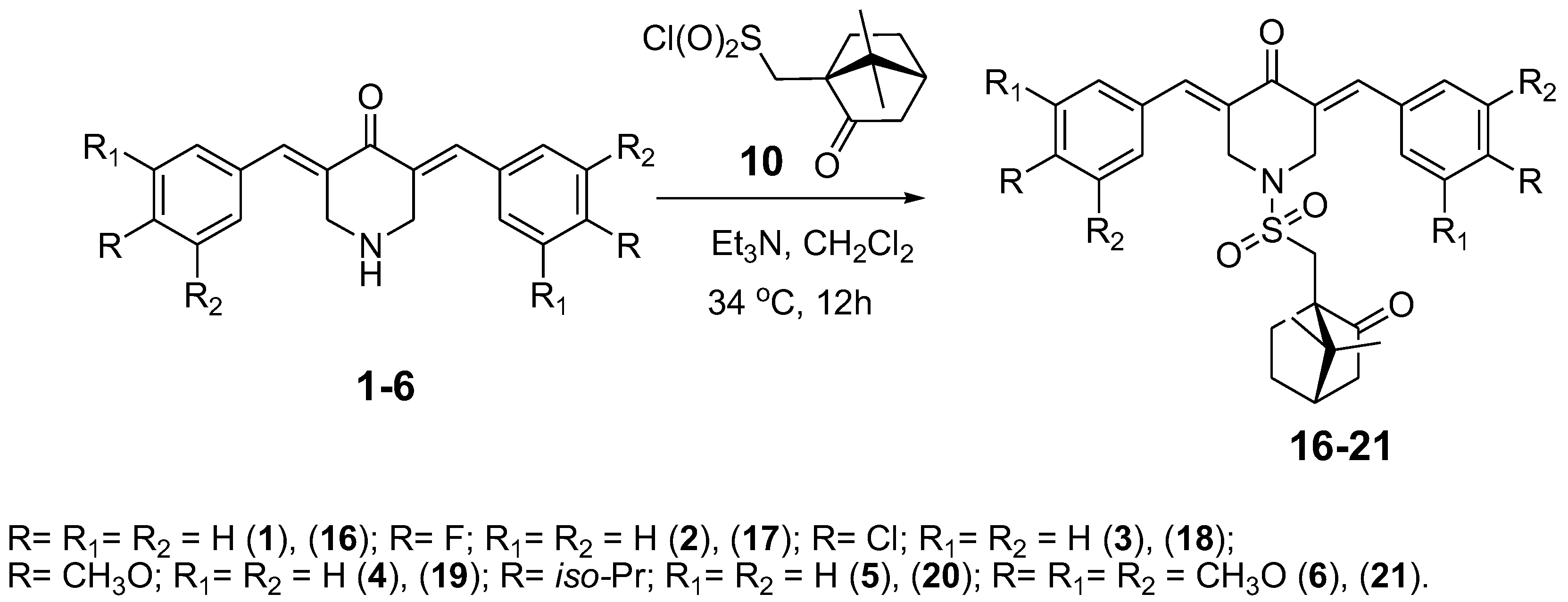
2.5. General Procedure of 3,5-Bis(benzylidene)-1-[(7,7-dimethyl-2-oxobicyclo[2.2.1]heptan-1-yl)methylsulfonyl]-4-piperidones Synthesis (16–21)
2.6. Cell Culture
2.7. Cell Viability Assay (MTT)
2.8. In Silico Toxicological and ADME Profiles
3. Results
3.1. Chemistry
3.2. Cytotoxic Profile of 3,5-bis(benzylidene)-piperidones and Their Analogs (11–21)
3.3. In Silico Toxicological Cytotoxic Profile
3.4. In Silico ADME Profile of Synthesized Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADME | Absorption, distribution, metabolism, excretion |
| CLC-Pred | Cell-line cytotoxicity predictor 2.0 |
| Bcl-2 | Apoptosis regulator Bcl-2 |
| Bax | Apoptosis regulator Bax |
| IL-6 | Interleukin 6 |
| TNF-α | Tumor necrosis factor alpha |
| NMR | Nuclear magnetic resonance spectroscopy |
| IC50 | The half maximal inhibitory concentration |
| MW | Molecular weight |
| nHA | H-bond acceptors |
| nHD | H-bond donors |
| nRot | Rotatable bonds |
| TPSA | Total polar surface area |
| Pgp | P-glycoprotein |
| HIA | Human intestinal absorption |
| TLC | Thin-layer chromatography |
| UV | Ultraviolet |
| HPLC | High performance liquid chromatography |
| DMEM | Dulbecco’s modified Eagle medium |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide |
| DMSO | Dimethyl sulfoxide |
| Cell Lines | |
| HCT116 | Colon cancer |
| A431 | Epidermoid carcinoma |
| HepG2 | Liver cancer |
| QGY-7703 | Hepatocellular carcinoma |
| SMMC-7721 | Hepatocarcinoma |
| A549 | Lung carcinoma |
| MCF-7 | Breast cancer |
| HeLa | Cervical cancer |
| SW-480 | Colon adenocarcinoma |
| WI-38 | Fibroblasts |
| NIH3T3 | Fibroblasts |
| KM12 | Colon adenocarcinoma |
| HCC2998 | Colon adenocarcinoma |
| SR | Adult immunoblastic lymphoma |
| K562 | Erythroleukemia |
| HCC1937 | Breast Carcinoma |
| HeLa | Cervical adenocarcinoma |
| NCI-H522 | Non-small cell lung carcinoma |
| NCI-H1650 | Bronchoalveolar carcinoma |
| SK-MEL-1 | Metastatic melanoma |
| CCRF-CEM | Childhood T acute lymphoblastic leukemia |
| PC-3 | Prostate carcinoma |
| 8505C | Thyroid gland undifferentiated (anaplastic) carcinoma |
| M19-MEL | Melanoma |
| A2780cisR | Cisplatin-resistant ovarian carcinoma |
| Caco-2 | Colorectal adenocarcinoma |
| MDCK | Kidney |
References
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Neuroprotective Effects of Curcumin in Neurodegenerative Diseases. Foods 2024, 13, 1774. [Google Scholar] [CrossRef]
- Rai, M.; Feitosa, C.M.; Ingle, A.P.; Golinska, P. Harnessing bioactive nanocurcumin and curcumin nanocomposites to combat microbial pathogens: A comprehensive review. Crit. Rev. Biotechnol. 2025, 45, 1306–1328. [Google Scholar] [CrossRef]
- Amini, S.M.; M, I.G.; Farahyar, S.; Khodavaisy, S.; Roudbary, M.; Pirhajati Mahabadi, V.; Mahmoudi, S. Antifungal activity of green-synthesized curcumin-coated silver nanoparticles alone and in combination with fluconazole and itraconazole against Candida and Aspergillus species. Curr. Med. Mycol. 2023, 9, 38–44. [Google Scholar]
- Narayanan, V.S.; Muddaiah, S.; Shashidara, R.; Sudheendra, U.S.; Deepthi, N.C.; Samaranayake, L. Variable antifungal activity of curcumin against planktonic and biofilm phase of different candida species. Indian J. Dent. Res. 2020, 31, 145–148. [Google Scholar] [CrossRef]
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin. Cancer Biol. 2022, 80, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.V.; Mishra, S. Nano-Curcumin for Cancer Therapy: A Strategic Approach to Improve Bioavailability and Modulate Tumor Resistance. Chem. Biodivers. 2025, e02012. [Google Scholar] [CrossRef]
- Aleksandrova, Y.; Neganova, M. Antioxidant Senotherapy by Natural Compounds: A Beneficial Partner in Cancer Treatment. Antioxidants 2025, 14, 199. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Mohammed, D.M.; Alkafaas, S.S.; Ghosh, S.; Negm, S.H.; Salem, H.M.; Fahmy, M.A.; Mosa, W.F.A.; Ibrahim, E.H.; et al. Curcumin, an active component of turmeric: Biological activities, nutritional aspects, immunological, bioavailability, and human health benefits—A comprehensive review. Front. Immunol. 2025, 16, 1603018. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Li, Y. Curcumin: A potential anti-photoaging agent. Front. Pharmacol. 2025, 16, 1559032. [Google Scholar] [CrossRef] [PubMed]
- Gronich, N. Central Nervous System Medications: Pharmacokinetic and Pharmacodynamic Considerations for Older Adults. Drugs Aging 2024, 41, 507–519. [Google Scholar] [CrossRef]
- Blommaert, A.G.S.; Weng, J.H.; Dorville, A.; McCort, I.; Ducos, B.; Durieux, C.; Roques, B.P. Cholecystokinin peptidomimetics as selective CCK-B antagonists: Design, synthesis, and in vitro and in vivo biochemical properties. J. Med. Chem. 1993, 36, 2868–2877. [Google Scholar] [CrossRef]
- Bellier, B.; McCort-Tranchepain, I.; Ducos, B.; Danascimento, S.; Meudal, H.; Noble, F.; Garbay, C.; Roques, B.P. Synthesis and biological properties of new constrained CCK-B antagonists: Discrimination of two affinity states of the CCK-B receptor on transfected CHO cells. J. Med. Chem. 1997, 40, 3947–3956. [Google Scholar] [CrossRef]
- Wanka, L.; Iqbal, K.; Schreiner, P.R. The lipophilic bullet hits the targets: Medicinal chemistry of adamantane derivatives. Chem. Rev. 2013, 113, 3516–3604. [Google Scholar] [CrossRef]
- Li, W.; Yan, W.; Liu, Y.; Hou, G.; Li, C. Treatment of rheumatoid arthritis with curcumin analog 3,5-bis(arylidene)-4-piperidone. Future Med. Chem. 2023, 15, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Neganova, M.E.; Aleksandrova, Y.R.; Sharova, E.V.; Smirnova, E.V.; Artyushin, O.I.; Nikolaeva, N.S.; Semakov, A.V.; Schagina, I.A.; Akylbekov, N.; Kurmanbayev, R.; et al. Conjugates of 3,5-Bis(arylidene)-4-piperidone and Sesquiterpene Lactones Have an Antitumor Effect via Resetting the Metabolic Phenotype of Cancer Cells. Molecules 2024, 29, 2765. [Google Scholar] [CrossRef]
- Neganova, M.E.; Aleksandrova, Y.R.; Nikolaeva, N.S.; Brel, V.K. Synthesis and biological testing of 3,5-bis(arylidene)-4-piperidone conjugates with 2,5-dihydro-5H-1,2-oxaphospholenes. Bioorg. Med. Chem. Lett. 2022, 74, 128940. [Google Scholar] [CrossRef]
- Artyushin, O.I.; Sharova, E.V.; Smirnova, E.V.; Semakov, A.V.; Aleksandrova, Y.R.; Neganova, M.E.; Brel, V.K. Synthesis of conjugates of sesquiterpene lactones with 3,5-bis(arylidene)piperidin-4-ones as potential nf-κb modulators using phase transfer catalysis conditions. Russ. Chem. Bull. 2024, 73, 3389–3398. [Google Scholar] [CrossRef]
- Jha, A.; Duffield, K.M.; Ness, M.R.; Ravoori, S.; Andrews, G.; Bhullar, K.S.; Rupasinghe, H.P.; Balzarini, J. Curcumin-inspired cytotoxic 3,5-bis(arylmethylene)-1-(N-(ortho-substituted aryl)maleamoyl)-4-piperidones: A novel group of topoisomerase II alpha inhibitors. Bioorg. Med. Chem. 2015, 23, 6404–6417. [Google Scholar] [CrossRef] [PubMed]
- Seliem, I.A.; Panda, S.S.; Girgis, A.S.; Tran, Q.L.; Said, M.F.; Bekheit, M.S.; Abdelnaser, A.; Nasr, S.; Fayad, W.; Soliman, A.A.F.; et al. Development of Isatin-Based Schiff Bases Targeting VEGFR-2 Inhibition: Synthesis, Characterization, Antiproliferative Properties, and QSAR Studies. ChemMedChem 2022, 17, e202200164. [Google Scholar] [CrossRef] [PubMed]
- Makarov, M.V.; Rybalkina, E.Y.; Brel, V.K. 3, 5-Bis (Arylidene)-4-Piperidones Modified by Bisphosphonate Groups as Novel Anticancer Agents. Phosphorus Sulfur. Silicon Relat. Elem. 2015, 190, 741–746. [Google Scholar] [CrossRef]
- Makarov, M.V.; Rybalkina, E.Y.; Khrustalev, V.N.; Röschenthaler, G.V. Modification of 3, 5-bis (arylidene)-4-piperidone pharmacophore by phosphonate group using 1, 2, 3-triazole cycle as a linker for the synthesis of new cytostatics. Med. Chem. Res. 2015, 24, 1753–1762. [Google Scholar] [CrossRef]
- Makarov, M.V.; Rybalkina, E.Y.; Anikina, L.V.; Pukhov, S.A.; Klochkov, S.G.; Mischenko, D.V.; Neganova, M.E.; Khrustalev, V.N.; Klemenkova, Z.S.; Brel, V.K. 1,5-Diaryl-3-oxo-1,4-pentadienes based on (4-oxopiperidin-1-yl)(aryl)methyl phosphonate scaffold: Synthesis andantitumor properties. Med. Chem. Res. 2016, 26, 140–152. [Google Scholar] [CrossRef]
- Neganova, M.E.; Smirnova, E.V.; Sharova, E.V.; Artyushin, O.I.; Alexandrova, Y.R.; Yandulova, E.Y.; Nikolaeva, N.S.; Brel, V.K. Design of Conjugates Based on Sesquiterpene Lactones with Polyalkoxybenzenes by “Click” Chemistry to Create Potential Anticancer Agents. Molecules 2022, 27, 8411. [Google Scholar] [CrossRef]
- Sokolova, A.S.; Yarovaya, O.I.; Artyushin, O.I.; Sharova, E.V.; Baev, D.S.; Mordvinova, E.D.; Shcherbakov, D.N.; Shnaider, T.A.; Nikitina, T.V.; Esaulkova, I.L.; et al. Design, synthesis and antiviral evaluation of novel conjugates of the 1,7,7-trimethylbicyclo[2.2.1]heptane scaffold and saturated N-heterocycles via 1,2,3-triazole linker. Arch. Pharm. 2024, 357, e2300549. [Google Scholar] [CrossRef]
- Katiyar, P.; Kalpana; Srivastava, A.; Singh, C.M. Investigation of Benzimidazole Derivatives in Molecular Targets for Breast Cancer Treatment: A Comprehensive Review. Crit. Rev. Oncog. 2025, 30, 43–58. [Google Scholar] [CrossRef]
- Dimmock, J.; Padmanilayam, M.; Puthucode, R.; Nazarali, A.; Motaganahalli, N.; Zello, G.; Quail, J.W.; Oloo, E.O.; Kraatz, H.B.; Prisciak, J.S.; et al. A Conformational and Structure—Activity Relationship Study of Cytotoxic 3,5-Bis(arylidene)-4-piperidones and Related N-Acryloyl Analogues. J. Med. Chem. 2001, 44, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.S.; Yarovaya, O.I.; Shtro, A.A.; Borisova, M.S.; Morozova, E.A.; Tolstikova, T.G.; Zarubaev, V.V.; Salakhutdinov, N.F. Synthesis and biological activity of heterocyclic borneol derivatives. Chem. Heterocycl. Compd. 2017, 53, 371–377. [Google Scholar] [CrossRef]
- Huynh, U.; McDonald, S.L.; Lim, D.; Uddin, M.N.; Wengryniuk, S.E.; Dey, S.; Coltart, D.M. Formation, Alkylation, and Hydrolysis of Chiral Nonracemic N-Amino Cyclic Carbamate Hydrazones: An Approach to the Enantioselective A-Alkylation of Ketones. J. Org. Chem. 2018, 83, 12951. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blazquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef]
- Lagunin, A.A.; Rudik, A.V.; Pogodin, P.V.; Savosina, P.I.; Tarasova, O.A.; Dmitriev, A.V.; Ivanov, S.M.; Biziukova, N.Y.; Druzhilovskiy, D.S.; Filimonov, D.A.; et al. CLC-Pred 2.0: A Freely Available Web Application for In Silico Prediction of Human Cell Line Cytotoxicity and Molecular Mechanisms of Action for Druglike Compounds. Int. J. Mol. Sci. 2023, 24, 1689. [Google Scholar] [CrossRef]
- Fu, L.; Shi, S.; Yi, J.; Wang, N.; He, Y.; Wu, Z.; Peng, J.; Deng, Y.; Wang, W.; Wu, C.; et al. ADMETlab 3.0: An updated comprehensive online ADMET prediction platform enhanced with broader coverage, improved performance, API functionality and decision support. Nucleic Acids Res. 2024, 52, W422–W431. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Shrestha, A.; Park, S.; Bist, G.; Kunwar, S.; Kadayat, T.M.; Jang, H.; Seo, M.; Sheen, N.; Kim, S.; et al. Identification of new halogen-containing 2,4-diphenyl indenopyridin-5-one derivative as a boosting agent for the anticancer responses of clinically available topoisomerase inhibitors. Eur. J. Med. Chem. 2022, 227, 113916. [Google Scholar] [CrossRef]
- Li, J.J.; Ma, J.; Xin, Y.B.; Quan, Z.S.; Tian, Y.S. Synthesis and pharmacological evaluation of 2,3-diphenyl acrylonitriles-bearing halogen as selective anticancer agents. Chem. Biol. Drug Des. 2018, 92, 1419–1428. [Google Scholar] [CrossRef]
- Upadhyay, R.; Tandel, P.; Patel, A.B. Halogen-based quinazolin-4(3H)-one derivatives as MCF-7 breast cancer inhibitors: Current developments and structure-activity relationship. Arch Pharm 2025, 358, e2400740. [Google Scholar] [CrossRef]
- Neganova, M.E.; Klochkov, S.G.; Aleksandrova, Y.R.; Osipov, V.N.; Avdeev, D.V.; Pukhov, S.A.; Gromyko, A.V.; Aliev, G. New Spirocyclic Hydroxamic Acids as Effective Antiproliferative Agents. Anticancer. Agents Med. Chem. 2021, 21, 597–610. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Radchenko, O.S.; Shubina, L.K.; Balaneva, N.N.; Bode, A.M.; Stonik, V.A.; Dong, Z. Evaluation of cancer-preventive activity and structure-activity relationships of 3-demethylubiquinone Q2, isolated from the ascidian Aplidium glabrum, and its synthetic analogs. Pharm. Res. 2006, 23, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Traversi, G.; Staid, D.S.; Fiore, M.; Percario, Z.; Trisciuoglio, D.; Antonioletti, R.; Morea, V.; Degrassi, F.; Cozzi, R. A novel resveratrol derivative induces mitotic arrest, centrosome fragmentation and cancer cell death by inhibiting gamma-tubulin. Cell Div. 2019, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Forero-Doria, O.; Guzman, L.; Jimenez-Aspee, F.; Echeverria, J.; Wehinger, S.; Valenzuela, C.; Araya-Maturana, R.; Martínez-Cifuentes, M. An In Vitro and In Silico Study of Antioxidant Properties of Curcuminoid N-alkylpyridinium Salts: Initial Assessment of Their Antitumoral Properties. Antioxidants 2022, 11, 1104. [Google Scholar] [CrossRef]
- Sazdova, I.; Keremidarska-Markova, M.; Dimitrova, D.; Mitrokhin, V.; Kamkin, A.; Hadzi-Petrushev, N.; Bogdanov, J.; Schubert, R.; Gagov, H.; Avtanski, D.; et al. Anticarcinogenic Potency of EF24: An Overview of Its Pharmacokinetics, Efficacy, Mechanism of Action, and Nanoformulation for Drug Delivery. Cancers 2023, 15, 5478. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, H.L.; Ling, M.; Zhang, S.; Ma, F.X.; Zhang, H.C.; Lv, X.A. The Curcumin Analog EF24 Inhibits Proliferation and Invasion of Triple-Negative Breast Cancer Cells by Targeting the Long Noncoding RNA HCG11/Sp1 Axis. Mol. Cell Biol. 2022, 42, e0016321. [Google Scholar] [CrossRef]
- Ferro, Y.; Maurotti, S.; Tarsitano, M.G.; Lodari, O.; Pujia, R.; Mazza, E.; Lascala, L.; Russo, R.; Pujia, A.; Montalcini, T. Therapeutic Fasting in Reducing Chemotherapy Side Effects in Cancer Patients: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2666. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Wang, X.; Xie, S. Side-effects of hyperthermic intraperitoneal chemotherapy in patients with gastrointestinal cancers. PeerJ 2023, 11, e15277. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, P.; Lisanti, C.; Garutti, M.; Buriolla, S.; Alberti, M.; Mazzeo, R.; Puglisi, F. Chemotherapy in patients with early breast cancer: Clinical overview and management of long-term side effects. Expert. Opin. Drug Saf. 2022, 21, 1341–1355. [Google Scholar] [CrossRef] [PubMed]
- Kumboonma, P.; Senawong, T.; Saenglee, S.; Senawong, G.; Somsakeesit, L.; Yenjai, C.; Phaosiri, C. New Histone Deacetylase Inhibitors and Anticancer Agents From Curcuma Longa. Med. Chem. Res. 2019, 28, 1773–1782. [Google Scholar] [CrossRef]
- Namwan, N.; Senawong, G.; Phaosiri, C.; Kumboonma, P.; Somsakeesit, L.O.; Samankul, A.; Leerat, C.; Senawong, T. HDAC Inhibitory and Anti-Cancer Activities of Curcumin and Curcumin Derivative CU17 against Human Lung Cancer A549 Cells. Molecules 2022, 27, 4014. [Google Scholar] [CrossRef]
- Permsuwan, U.; Thongprasert, S.; Sirichanchuen, B. Cost-Utility Analysis of First-Line Pemetrexed Plus Cisplatin in Non-Small Cell Lung Cancer in Thailand. Value Health Reg. Issues 2020, 21, 9–16. [Google Scholar] [CrossRef]
- Katayama, S.; Kobayashi, Y.; Takamoto, A.; Edamura, K.; Sadahira, T.; Iwata, T.; Nishimura, S.; Sako, T.; Wada, K.; Araki, M.; et al. Impact of paclitaxel, cisplatin, and gemcitabine as first-line chemotherapy in cisplatin-fit and -unfit patients with advanced/metastatic urothelial carcinoma. Urol. Oncol. 2021, 39, 731.e25–731.e32. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, T.; Fei, Y.; Zhang, S.; Yang, Y.; Chen, Z.; Zhu, R.; Deng, S.; Zhang, T.; Wu, D.; et al. RTA-408 overcomes cisplatin-resistant lung cancer by inhibiting WWP1-mediated NCOA4 ubiquitination to induce ferritinophagy and ferroptosis. Free Radic. Biol. Med. 2025, 238, 595–610. [Google Scholar] [CrossRef]
- Panse, N.; Gerk, P.M. The Caco-2 Model: Modifications and enhancements to improve efficiency and predictive performance. Int. J. Pharm. 2022, 624, 122004. [Google Scholar] [CrossRef]
- Alam, M.S.; Anwar, M.J.; Maity, M.K.; Azam, F.; Jaremko, M.; Emwas, A.H. The Dynamic Role of Curcumin in Mitigating Human Illnesses: Recent Advances in Therapeutic Applications. Pharmaceuticals 2024, 17, 1674. [Google Scholar] [CrossRef] [PubMed]
- Barsony, O.; Szaloki, G.; Turk, D.; Tarapcsak, S.; Gutay-Toth, Z.; Bacso, Z.; Holb, I.J.; Székvölgyi, L.; Szabó, G.; Csanády, L.; et al. A single active catalytic site is sufficient to promote transport in P-glycoprotein. Sci. Rep. 2016, 6, 24810. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Duan, Z.; Wang, X. An Update on Circumventing Multidrug Resistance in Cancer by Targeting P-Glycoprotein. Curr. Cancer Drug Targets 2018, 18, 677–696. [Google Scholar] [CrossRef]
- Kondo, S.; Miyake, M. Simultaneous Prediction Method for Intestinal Absorption and Metabolism Using the Mini-Ussing Chamber System. Pharmaceutics 2023, 15, 2732. [Google Scholar] [CrossRef]
- Mardaneh, P.; Lavian, S.; Bagherniya, M.; Roufogalis, B.D.; Sahebkar, A. Synthetic Curcumin Analogs in the Treatment of Cancer: A Literature Review. Curr. Med. Chem. 2025, 32, 3366–3388. [Google Scholar] [CrossRef]
- MaruYama, T.; Miyazaki, H.; Komori, T.; Osana, S.; Shibata, H.; Owada, Y.; Kobayashi, S. Curcumin analog GO-Y030 inhibits tumor metastasis and glycolysis. J. Biochem. 2023, 174, 511–518. [Google Scholar] [CrossRef] [PubMed]
| Compound/ Cell Line | IC50 of Cytotoxic Effect, µM | ||||
|---|---|---|---|---|---|
| A549 1 | MCF-7 2 | HeLa 3 | SW-480 4 | WI-38 5 | |
| 1 | 50.28 ± 1.74 | 28.04 ± 0.53 | 31.12 ± 1.62 | 96.47 ± 1.03 | 80.33 ± 0.11 |
| 2 | 58.19 ± 1.83 | 13.81 ± 0.19 | 25.10 ± 0.01 | 89.25 ± 0.48 | 68.15 ± 0.03 |
| 3 | 53.24 ± 0.69 | 14.15 ± 0.23 | 23.08 ± 0.06 | 97.50 ± 1.49 | 69.76 ± 0.24 |
| 4 | 62.74 ± 1.50 | 28.01 ± 0.82 | 51.00 ± 2.19 | 99.93 ± 3.56 | 90.16 ± 0.17 |
| 5 | >100 | >100 | >100 | >100 | >100 |
| 6 | 76.14 ± 1.03 | 35.85 ± 0.33 | 60.01 ± 2.64 | 82.89 ± 3.60 | >100 |
| 11 | 59.10 ± 0.18 | 31.12 ± 3.34 | 40.43 ± 0.98 | >100 | >100 |
| 12 | 73.24 ± 1.00 | 15.56 ± 0.06 | 25.74 ± 0.45 | >100 | 75.19 ± 0.93 |
| 13 | 54.11 ± 2.28 | 19.24 ± 0.39 | 41.12 ± 1.23 | >100 | 68.36 ± 2.56 |
| 14 | 68.09 ± 1.35 | 49.85 ± 1.56 | 56.18 ± 2.94 | >100 | >100 |
| 15 | >100 | >100 | >100 | >100 | >100 |
| 16 | 48.54 ± 0.62 | 30.87 ± 0.21 | 42.15 ± 1.30 | 57.34 ± 2.96 | 68.74 ± 0.81 |
| 17 | 20.63 ± 2.20 | 9.21 ± 0.74 | 22.60 ± 0.50 | 36.89 ± 0.28 | 49.38 ±0.42 |
| 18 | 17.79 ± 1.17 | 9.27 ± 0.40 | 25.14 ± 0.97 | 52.09 ± 1.44 | 44.03 ± 0.44 |
| 19 | 33.17 ± 0.66 | 19.74 ± 0.39 | 30.10 ± 0.58 | 42.24 ± 1.57 | 55.07 ± 1.58 |
| 20 | 99.01 ± 4.23 | 80.19 ± 0.07 | 87.38 ± 2.94 | 96.57 ± 3.80 | >100 |
| 21 | 21.18 ± 0.36 | 9.94 ± 0.02 | 27.95 ± 2.12 | 50.46 ± 0.29 | 65.21 ± 3.19 |
| Curcumin | 76.21 ± 1.73 | 44.02 ± 2.29 | 65.72 ± 0.70 | >100 | 81.07 ± 2.98 |
| Compound/Cell Line | IC50, µM |
|---|---|
| MCF-7 | |
| 16 | 25.64 ± 1.91 |
| 17 | 3.25 ± 0.76 |
| 18 | 3.16 ± 0.71 |
| 19 | 7.31 ± 0.43 |
| 20 | 23.47 ± 1.33 |
| 21 | 0.67 ± 0.01 |
| Pa 1 | Pi 2 | Cell Line | Description |
|---|---|---|---|
| 0.530 | 0.017 | KM12 | Colon adenocarcinoma |
| 0.419 | 0.036 | HCC2998 | Colon adenocarcinoma |
| 0.412 | 0.030 | SR | Adult immunoblastic lymphoma |
| 0.333 | 0.076 | K562 | Erythroleukemia |
| 0.314 | 0.226 | HCC1937 | Breast Carcinoma |
| 0.310 | 0.113 | HeLa | Cervical adenocarcinoma |
| 0.297 | 0.075 | NCI-H522 | Non-small cell lung carcinoma |
| 0.295 | 0.094 | NCI-H1650 | Bronchoalveolar carcinoma |
| 0.285 | 0.275 | SK-MEL-1 | Metastatic melanoma |
| 0.272 | 0.077 | CCRF-CEM | Childhood T acute lymphoblastic leukemia |
| 0.264 | 0.138 | PC-3 | Prostate carcinoma |
| 0.261 | 0.162 | 8505C | Thyroid gland undifferentiated (anaplastic) carcinoma |
| 0.256 | 0.133 | M19-MEL | Melanoma |
| Compound | Physicochemical Properties | |||||||
|---|---|---|---|---|---|---|---|---|
| MW 1 | Volume 2 | nHA 3 | nHD 4 | nRot 5 | TPSA 6 | logS 7 | LogP 8 | |
| 16 | 489.20 | 505.66 | 5 | 0 | 5 | 71.52 | −5.10 | 3.91 |
| 17 | 525.18 | 517.80 | 5 | 0 | 5 | 71.52 | −5.49 | 4.27 |
| 18 | 557.12 | 536.08 | 5 | 0 | 5 | 71.52 | −5.61 | 4.74 |
| 19 | 549.22 | 557.83 | 7 | 0 | 7 | 89.98 | −5.26 | 4.02 |
| 20 | 573.29 | 609.44 | 5 | 0 | 7 | 71.52 | −5.51 | 5.66 |
| 21 | 669.26 | 662.18 | 11 | 0 | 11 | 126.90 | −5.27 | 3.25 |
| Curcumin | 368.13 | 381.04 | 6 | 2 | 8 | 93.06 | −3.62 | 2.15 |
| Compound | Properties of Drug-Likeness | ||||||
|---|---|---|---|---|---|---|---|
| Permeability | Pgp Inhibitor | Pgp Substrate | HIA 1 | F20% 2 | F30% 3 | ||
| Caco-2 | MDCK | ||||||
| 16 | −4.86 | 1.6−5 | yes | - | excellent | excellent | good |
| 17 | −4.73 | 1.6−5 | yes | - | excellent | excellent | excellent |
| 18 | −4.75 | 1.6−5 | yes | - | excellent | excellent | excellent |
| 19 | −4.72 | 1.5−5 | yes | - | excellent | good | bad |
| 20 | −4.86 | 1.4−5 | yes | - | excellent | good | bad |
| 21 | −4.76 | 1.4−5 | yes | - | excellent | excellent | excellent |
| Curcumin | −5.47 | 1.3−5 | - | - | excellent | bad | bad |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandrova, Y.; Neganova, M.; Tapalova, A.; Sokolova, A.; Rodionov, A.; Shagina, I.; Appazov, N.; Brel, V. Terpene-Functionalized 3,5-Bis(benzylidene)-4-piperidones: Synthesis, Cytotoxicity Properties, In Silico and In Vitro Studies. Chemistry 2025, 7, 167. https://doi.org/10.3390/chemistry7050167
Aleksandrova Y, Neganova M, Tapalova A, Sokolova A, Rodionov A, Shagina I, Appazov N, Brel V. Terpene-Functionalized 3,5-Bis(benzylidene)-4-piperidones: Synthesis, Cytotoxicity Properties, In Silico and In Vitro Studies. Chemistry. 2025; 7(5):167. https://doi.org/10.3390/chemistry7050167
Chicago/Turabian StyleAleksandrova, Yulia, Margarita Neganova, Anipa Tapalova, Anastasiya Sokolova, Alexey Rodionov, Inna Shagina, Nurbol Appazov, and Valery Brel. 2025. "Terpene-Functionalized 3,5-Bis(benzylidene)-4-piperidones: Synthesis, Cytotoxicity Properties, In Silico and In Vitro Studies" Chemistry 7, no. 5: 167. https://doi.org/10.3390/chemistry7050167
APA StyleAleksandrova, Y., Neganova, M., Tapalova, A., Sokolova, A., Rodionov, A., Shagina, I., Appazov, N., & Brel, V. (2025). Terpene-Functionalized 3,5-Bis(benzylidene)-4-piperidones: Synthesis, Cytotoxicity Properties, In Silico and In Vitro Studies. Chemistry, 7(5), 167. https://doi.org/10.3390/chemistry7050167









