Abstract
To address the greenhouse effect and environmental pollution stemming from fossil fuels, the development of new energy sources is widely regarded as a critical pathway toward achieving carbon neutrality. Microalgae, as a feedstock for third-generation biofuels, have emerged as a research hotspot for producing biojet fuel due to their high photosynthetic efficiency, non-competition with food crops, and potential for carbon reduction. This paper provides a systematic review of technological advancements in the catalytic hydrogenation of microalgal oil for biojet fuel production. It specifically focuses on the reaction mechanisms and catalyst design involved in the hydrogenation–deoxygenation and cracking/isomerization processes within the Oil-to-Jet (OTJ) pathway. Furthermore, the paper compares the performance differences among various catalyst support materials and between precious and non-precious metal catalysts. Finally, it outlines the current landscape of policy support and progress in industrialization projects globally.
1. Introduction
Recent years have witnessed a rapid acceleration in societal advancement. This progress, however, has been heavily reliant on the rampant exploitation of fossil fuels, which, despite driving economic expansion, has simultaneously imposed severe challenges on the climate and environment. Carbon dioxide (CO2) and other greenhouse gases generated during the industrialization process have become the primary drivers of global temperature rise [1]. To actively address environmental pollution and other complex issues, China formally proposed the carbon neutrality goal in 2020, aiming to ensure that annual CO2 emissions are offset by emission reductions, thereby achieving the vision of net-zero CO2 emissions. China has also committed to reaching peak CO2 emissions by 2030 and striving to achieve carbon neutrality by 2060 [2].
In the “Opinions on Fully, Accurately, and Comprehensively Implementing the New Development Philosophy and Doing a Good Job in Carbon Peaking and Carbon Neutrality Work” document issued in September 2021 [3], it was emphasized that efforts should be made to expand the utilization pathways of non-mineral resources and actively promote the replacement of renewable energy nationwide. Under the carbon neutrality context, finding alternatives to traditional fuels holds significant strategic importance [4]. Among numerous alternative energy sources, biomass is a material resource comparable to coal, oil, and natural gas, capable of forming large-scale industrial clusters. Therefore, the production of liquid bio-oil from renewable biomass has become a research hotspot across industries [5]. Additionally, given its numerous advantages, including renewability, high yield, low pollution, and zero CO2 emissions, biomass energy is considered one of the most ideal alternatives to fossil fuels [6,7], particularly in the field of aviation jet fuel production.
Aviation jet fuel, as the fuel for aircraft, primarily consists of hydrocarbons ranging from C8 to C16, specifically including n-alkanes, isoalkanes, cycloalkanes, and aromatic hydrocarbons [8,9]. The composition of jet fuel is primarily alkanes, which can be further classified into straight-chain alkanes, isoalkanes, and cycloalkanes. Due to their high hydrogen-to-carbon ratio, alkanes release a high calorific value during combustion. Additionally, since they do not contain other heteroatoms, their combustion process only produces CO2 and H2O, significantly reducing environmental pollution. Additionally, a certain amount of isomeric alkanes and cycloalkanes can effectively lower the pour point of aviation fuel, mitigating the constraints imposed by environmental factors on aviation transportation [10]. Internationally, mainstream aviation fuel production technologies rely on traditional petrochemical industries, which are technically mature and relatively cost-effective. Given the constraints imposed by raw material availability, there is a pressing need to develop sustainable alternatives to traditional aviation fuels. This paper centers on the research progress in catalytic hydrogenation technology for processing microalgae-based oils into aviation fuel. Representative of third-generation biofuels, microalgae oil presents considerable development potential, with future pathways and product portfolios trending toward diversification [3].
2. Microalgae-Based Biofuel
In the field of biofuels, microalgae are widely regarded as third-generation biofuels. Microalgae have extremely high photosynthetic efficiency, grow rapidly, and have high oil content, while also effectively capturing CO2 emissions from factories. Life cycle assessments indicate that compared to traditional jet fuels, the production process of algal biojet fuels reduces greenhouse gas emissions by 76% [11]. These characteristics have made algal biomass a research hotspot in the energy industry [12] (Figure 1).
Figure 1.
Microalgae-based biofuels contribute to carbon neutrality.
First-generation biofuels, represented by corn and soybeans, are produced through transesterification reactions that convert triglycerides into biodiesel components such as methyl esters or ethyl esters of fatty acids [13]. In contrast, microalgae oil has the significant advantage of not competing with food crops for land, thereby safeguarding food reserves and maintaining global food security. Second-generation biofuels are represented by non-food oils such as waste cooking oil, palm oil, and jatropha oil, which are processed into biofuels through hydrogenation and isomerization. This method is similar to that used for microalgae oil, and it is currently the primary source of biofuels. However, the limited availability of raw materials significantly constrains the development of second-generation biofuels [14,15]. As a third-generation biofuel, microalgae can be cultivated in saline or wastewater, capturing CO2 from the atmosphere and producing lipids without requiring large spaces. Their short growth cycles enhance land use efficiency and eliminate concerns about weather and environmental impacts on feedstocks, making them pivotal for scaling up biofuel production. Table 1 compares microalgae with first- and second-generation biofuels [16,17,18,19].

Table 1.
Comparison of third-generation biodiesel feedstocks [20].
There are thousands of different microalgae species, each possessing distinct characteristics [21]. Therefore, selecting the appropriate species is an essential step in this process. Before cultivating microalgae for aviation fuel production, lipid productivity and lipid quality are two factors that require consideration. Research has demonstrated that lipid productivity serves as the optimal indicator for determining whether a specific microalgae species is suitable for biofuel production [22]. Chlorella, Dunaliella, Microcystis, and Dunaliella are three of the most well-known among thousands of different microalgae species due to their ability to produce lipids [23]. The cultivation process of microalgae is relatively straightforward compared to terrestrial plants, and microalgae production does not necessitate changes in land use [24]. After cultivation, microalgal cells are harvested using methods such as centrifugation, filtration, flocculation, or other physical separation techniques. The choice of harvesting method depends on factors including cell size, density, and the desired biomass purity. The harvested biomass is then subjected to drying processes, such as spray drying or freeze drying, to reduce moisture content and facilitate downstream processing. Subsequently, cell walls are disrupted through mechanical crushing, enzymatic treatment, or ultrasonication to release intracellular oils. Oil extraction employs techniques such as solvent extraction (e.g., hexane extraction), supercritical fluid extraction using CO2, or mechanical pressing to obtain crude oil. The efficiency of oil extraction significantly impacts the overall yield and quality of biofuel [25].
Extracted microalgal oils are subsequently subjected to esterification or transesterification for conversion into products like fatty acid methyl esters (FAME) and triglycerides (TAG). This catalytic process, which uses alcohols like methanol with an acid or base catalyst, converts the lipids into biodiesel. A more energy- and time-efficient alternative is to bypass the drying step altogether by performing oil extraction and catalytic conversion directly on wet algal biomass [26]. Figure 2 below shows a simplified version of the entire process diagram.
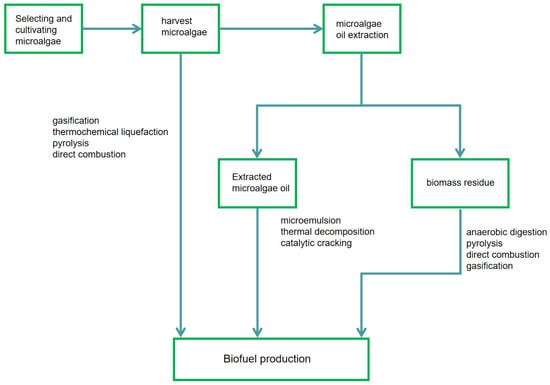
Figure 2.
Steps in algae energy production [20].
In recent years, with the advancement of global energy transition and carbon neutrality goals, microalgae biofuels have emerged as a core strategic focus for many countries due to their unique sustainability advantages. The United States, the European Union, China, and others have incorporated microalgae technology into their national energy innovation plans, accelerating research and development and industrialization through policy support and financial investment. For example, the EU’s Renewable Energy Directive II (RED II) [27] explicitly requires that the proportion of sustainable biofuels in aviation fuel be increased to over 32%, while setting strict standards for greenhouse gas emissions reductions across the entire lifecycle (requiring reductions of over 60%). Microalgae-based aviation fuel, with its low carbon footprint (carbon reduction potential of 70–90%) and non-food feedstock characteristics, has become one of the key pathways to meet RED II requirements. Meanwhile, the American Society for Testing and Materials revised the D7566 standard in 2021 [28], formally incorporating microalgae-based synthetic paraffin kerosene (HEFA-SPK) [29] into the aviation fuel certification system, thereby removing technical barriers to its commercial application. The relevant performance standards and specifications are as follows in Table 2.

Table 2.
Fuel performance specifications [28].
Driven by both policy and standards, the microalgae industry is entering a golden era of technological breakthroughs and large-scale production. The “2050 China Energy Science and Technology Development Strategy Plan” explicitly states that microalgae-based carbon sequestration technology and its energy utilization have been established as an important potential development direction for China’s future energy system [31]. In the future, with further advancements in synthetic biology, photobioreactor optimization, and low-cost harvesting technologies, the production efficiency and economic viability of microalgae aviation fuel will significantly improve, making it one of the core solutions for the aviation industry to achieve its decarbonization goals. This process will not only reshape the global energy landscape, but also accelerate the evolution of the “blue bioeconomy” [32] toward efficient, circular, and low-carbon directions.
3. Biojet Fuel Production Process
Currently, the mainstream production processes for green aviation fuels primarily encompass the following four technical routes: Oil-To-Jet (OTJ) technology, whose core processes include hydrogenation deoxygenation, hydrogenation cracking, and isomerization reactions of natural oils; Alcohol-To-Jet (ATJ) technology, based on dehydration and oligomerization reactions of biomass fermentation products; Sugar-to-Jet (STJ) technology, which involves condensation reactions of biomass hydrolysis products followed by hydrogenation refining; and Gas-to-Jet (GTJ) technology, which uses biomass gasification products through Fischer-Tropsch synthesis and hydrogenation upgrading processes.
This paper will systematically elaborate on the reaction pathways and process characteristics of the aforementioned four technical approaches, with a focus on analyzing the catalytic hydrogenation process—currently the most mature and widely applicable for industrial implementation.
3.1. Gas-to-Jet
Gas-to-Jet (GTJ) refers to the thermal decomposition of bio-oils and other biomass feedstocks at high temperatures into small molecules such as carbon monoxide and hydrogen. Under specific reaction conditions, carbon monoxide and hydrogen undergo polymerization reactions in the presence of a catalyst to form straight-chain alkane hydrocarbons (Figure 3). This catalytic conversion process, which uses synthesis gas (CO + H2), as a feedstock to produce liquid fuels, is known as the Fischer-Tropsch (FT) synthesis reaction [33]. The biomass aviation fuel produced via Fischer-Tropsch synthesis is sulfur-free and contains only trace amounts of aromatic compounds. The selection of catalysts should consider their adsorption capacity for synthesis gas; catalysts containing metals such as Co and Fe typically exhibit higher activity in Fischer-Tropsch synthesis. Currently, Fischer-Tropsch synthesis technology is primarily applied in the industrial production of traditional biofuels (including diesel, gasoline, and kerosene) [33]. The Fischer-Tropsch synthesis process for producing bio-aviation kerosene offers significant technical advantages, particularly in terms of its lower requirements for feedstock purity and composition, and the technology has achieved a high level of industrial maturity. However, its drawbacks are also evident, with high costs being the most significant. Additionally, the products obtained through Fischer-Tropsch synthesis are relatively dispersed, necessitating the removal of short-chain hydrocarbons via component distillation to meet the raw material requirements for subsequent hydrogenation, isomerization, and cracking processes [34].
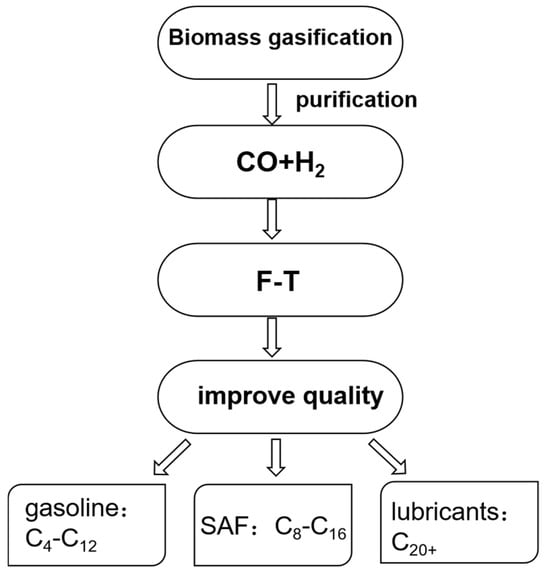
Figure 3.
Production of biojet fuel using Fischer-Tropsch synthesis technology.
3.2. Alcohol-Based Oil
Alcohol-to-jet fuel technology involves the conversion of biomass-derived alcohols such as methanol and ethanol into biojet fuel through catalytic reactions. The process begins by converting initial feedstocks (e.g., lignocellulosic biomass) into alcohols, which are then dehydrated to produce olefins. These olefins undergo polymerization to form long-chain olefins, which are further processed through hydrogenation to yield the target jet fuel product [34,35,36] (Figure 4). Bio-alcohol (ATJ) production of biojet fuel offers significant advantages in terms of feedstock sustainability and long-term emissions reduction potential. However, its primary drawback lies in the currently high production costs and technical complexity, particularly the multi-step catalytic conversion process required to convert alcohols into aviation fuel.
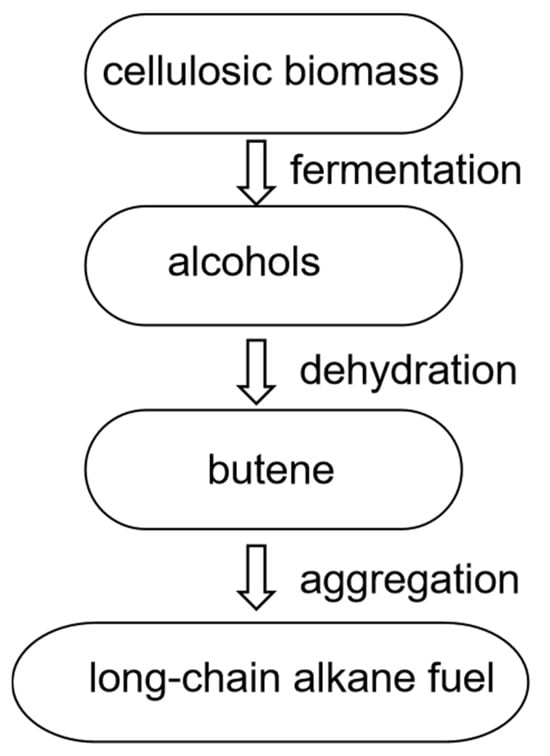
Figure 4.
Production of biojet fuel using the alcohol-to-jet (ATJ) technology route.
3.3. Sugar-Based Oil
Sugar-to-Jet (STJ) involves a preparation process that begins with biomass pretreatment and hydrolysis, primarily utilizing sugar-containing biomass or cellulose-based waste materials; followed by hydrolysis, where polysaccharides are hydrolyzed into monosaccharides through acid/enzyme catalysis, then undergo condensation reactions. Subsequently, the monosaccharides are converted into oxygen-containing intermediates via acid catalysis or biocatalysis, which are further condensed to form ketone or aldehyde compounds with carbon chain lengths of C8-C16. Finally, hydrogenation upgrading, deoxygenation, and isomerization are performed, similar to the oil-to-jet process. The reaction pathway is shorter than the traditional ATJ pathway, offering significant emission reduction potential. However, this technology is still in its infancy, currently only at the small-scale trial or demonstration stage globally, with no large-scale industrial applications to date.
3.4. Oil-Based Oil
Oil-to-Jet (OTJ) technology, as the most mature process route in the current field of biojet fuel production, primarily encompasses two technical pathways: the two-step method and the one-step method. At present, industrial-scale production of biojet fuel is exclusively carried out using the two-step process. In the two-step process, the first stage involves catalytic hydrogenation to saturate the unsaturated bonds in the molecular structure of triglycerides, producing saturated triglycerides, which are then subjected to dehydrogenation to obtain long-chain n-alkanes [37]. In the second stage, the long-chain n-alkanes undergo hydrogen cracking-isomerization reactions to ultimately produce branched-chain isomerized alkanes [38]. Experimental results show that the target products not only exhibit excellent low-temperature flow properties but also significantly improved viscosity characteristics. Although the two-step method is widely applied, it faces challenges in industrial practice, including a lengthy process flow, high equipment costs, significant hydrogen consumption, and difficulties in industrial-scale operation. Additionally, each step requires separation of products from byproducts, all of which contribute to increased production costs. Given the limitations of the two-step process, our research team conducted a literature review and found that the academic community currently tends to favor a one-step process route, which involves simultaneously catalyzing hydrogenation deoxygenation and alkene cracking reactions to directly convert bio-oils into aviation kerosene components. The key points of this technology are twofold: first, the selection of the deoxygenation pathway, and second, the selection of the catalyst in the reaction [39,40].
During catalytic hydrogenation, the first inevitable step is to remove oxygen elements from the oil. This reaction system primarily involves three conversion pathways: decarboxylation (DCO2), decarbonylation (DCO), and hydrogenation-deoxygenation (HDO) [41], as shown in Figure 5. Since hydrogenation deoxygenation, hydrogenation desulfurization, and hydrogenation denitrogenation reactions share similar catalytic mechanisms, this process not only effectively removes oxygen from the raw material but also simultaneously removes impurities such as nitrogen, phosphorus, and sulfur. Additionally, it promotes the hydrogenation saturation of unsaturated double bonds, ultimately producing long-chain alkanes within the C16-C18 range [10].
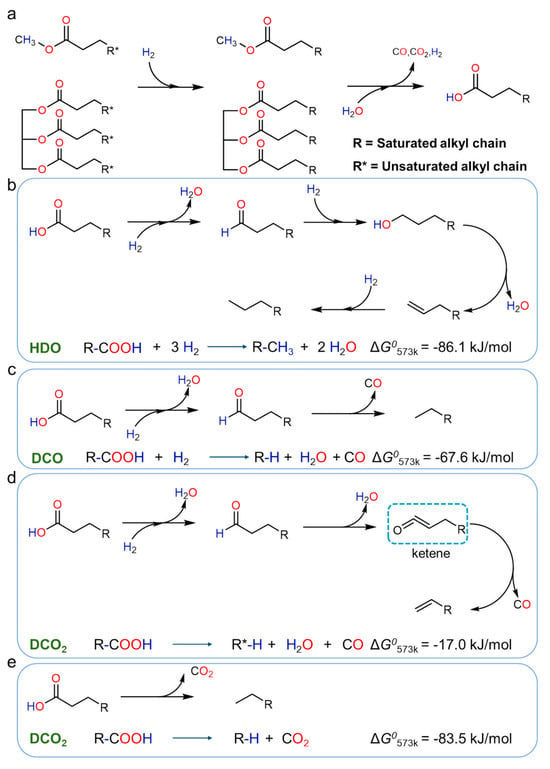
Figure 5.
Microalgal oil HDO treatment reaction pathways: (a) Hydrogenation of microalgal oil to saturated fatty acids. (b) HDO pathway of saturated fatty acids. (c) DCO pathway of saturated fatty acids. (d) Direct DCO2 pathway of saturated fatty acids. (e) Alternative DCO2 pathway via ketone intermediates [25]. Reproduced from ref. [25]. with permission from Chemical Engineering Science, copyright 2025.
The HDO process demonstrates optimal performance in terms of liquid product yield (with the number of carbon atoms in the alkane chain remaining constant). Although the initial hydrogen consumption of the DCO and DCO2 processes is lower than that of the HDO process, when considering the methanation side reactions of CO and CO2 (CO methanation consumes 3 mol H2; CO2 methanation consumes 4 mol H2), the total hydrogen consumption of the DCO and DCO2 processes increases to 5 mol, significantly higher than the 4 mol of the HDO process. Experimental data indicate that the HDO process outperforms the DCO and DCO2 processes in both hydrogen utilization efficiency and liquid product yield [42].
The OTJ technology route can significantly reduce the oxygen content of oxygen-containing compounds such as phenols, esters, and aldehydes through hydrogenation reforming, decarboxylation, decarbonylation, and direct deoxygenation, demonstrating notable effects in upgrading bio-oil quality. Through this route, animal and plant oils, as well as waste oils such as grease trap oil, can be completely converted into biojet fuel. Compared to these routes, OTJ not only has relatively high economic viability but also balances environmental sustainability. Additionally, it can utilize existing petrochemical hydrogenation facilities, making it the most commercially mature technology route with broad application prospects [43,44]. A detailed comparison of several routes is presented in Table 3 below.

Table 3.
Comparative analysis table of main technological pathways for sustainable aviation fuels.
4. Hydrogenation Catalyst
Central to the OTJ technology route are high-performance hydrodeoxygenation catalysts, necessitating high activity, strong selectivity, and robust stability. Prominent catalyst compositions incorporate noble metals (Pt, Pd), transition metals/oxides (Co, Mo), amorphous alloys, and transition metal carbides, nitrides, or phosphides. These catalysts are structured around active metal sites dispersed on a support, and their synergistic interaction governs overall performance. While the metal sites drive hydrodeoxygenation, the acidic sites on the support activate C-C and C-H bonds through a carbocation mechanism. The strategic selection of the support thus allows for tuning the cracking/isomerization balance to achieve efficient oil upgrading [45]. This section will focus on these two aspects.
4.1. Commonly Used Carriers
The carrier serves as the framework in the catalyst, primarily functioning to disperse metal components and prevent particle migration and aggregation, thereby significantly enhancing the catalyst’s thermal stability [46]. Additionally, carrier materials with different physicochemical properties have a significant impact on the catalyst’s catalytic activity. A carrier with appropriate acidity is essential for the hydrogenation deoxygenation of compounds with high oxygen content [5]. Mesoporous zeolites with large pore sizes help reactants and intermediates overcome diffusion and adsorption limitations at metal active sites [47]. The following sections will introduce and compare three types of carriers: oxide carriers, carbon-based carriers, and molecular sieve carriers.
4.1.1. Oxide Carrier
There are various types of oxide carriers, primarily including Al2O3, TiO2, and SiO2, with their acid strength ordered as follows: Al2O3 > TiO2 > SiO2. In hydrogenation deoxygenation reactions, different carriers exhibit distinct properties: for instance, Al2O3 carriers possess strong acidity, which can enhance catalytic activity through dehydration reactions, but may lead to catalyst coking; TiO2 is more resistant to high-temperature hydrothermal environments (especially in liquid-phase reactions) than Al2O3, reducing carrier structural collapse and sintering of active components. Additionally, TiO2 carriers have moderate acidic sites, but their raw material costs are relatively high. SiO2 carriers facilitate the high dispersion of active components; however, their surfaces interact with oxygen-containing compounds via weak hydrogen bonds, resulting in limited deoxygenation efficiency [42].
4.1.2. Carbon Material Carrier
Carbon materials, as common carrier materials, are widely used in various heterogeneous catalytic reactions and are also commonly employed in the hydrogenation and deoxygenation of bio-oil. Before being used as catalyst carriers, biochar materials typically require simple activation treatment. Through various physical and chemical methods, the structural properties and surface functionality of biochar are modified to increase its surface area and enhance surface acidity. Common methods for modifying carbon materials include nitrogen doping, sulfonation, and in situ carbonization synthesis. Activated carbon (AC) materials, due to their unique structural characteristics—such as high porosity, large specific surface area, excellent adsorption capacity, and cost advantages—show significant potential in HDO reactions [48].
4.1.3. Molecular Sieve Carrier
Molecular sieves are widely used as carriers for HDO reactions due to their controllable surface acidity, high specific surface area, low cost, and regular pore structure. The pore size of molecular sieves can be adjusted using physical or chemical methods.
Molecular sieves are primarily classified into three types: microporous molecular sieves (pore size < 2 nm), which selectively adsorb straight-chain alkanes; mesoporous molecular sieves (pore size 2–50 nm), suitable for adsorbing branched-chain alkanes; and macroporous molecular sieves (pore size > 50 nm), capable of accommodating highly branched macromolecules. During the reaction process, the pore size limitations of molecular sieves often affect the reaction process. For example, the pore size limitations of microporous molecular sieves, due to the molecular size effect, make it difficult for larger molecules to diffuse into the microporous channels, resulting in insufficient contact with the catalyst active sites. Converting microporous molecular sieves into mesoporous molecular sieves using an alkaline treatment method is an effective strategy to address this issue. During the alkaline treatment process, sodium hydroxide solution is typically used as the treatment reagent [49]. After pore expansion treatment, the density of acidic sites in the molecular sieve significantly increases, thereby significantly enhancing the synergistic catalytic effect between the metal components and the molecular sieve carrier [42].
(1) Microporous molecular sieves:
Microporous molecular sieves are molecular sieve carriers with pore sizes less than 2 nm, with representative examples including ZSM-22 and ZSM-5 [15], both of which are high Si/Al ratio molecular sieve carriers. ZSM-5 molecular sieves feature a unique pore structure and excellent thermal stability [50], finding widespread application in petroleum cracking and alcohol-to-oil processes.
(2) Mesoporous molecular sieves:
Mesoporous molecular sieves have been developed for the conversion of large molecules due to their larger pore sizes. Common mesoporous molecular sieves include MCM-41, SBA-15, and Y-type molecular sieves. Among these, MCM-41 has a three-dimensional mesoporous structure with weak interaction with active components. SBA-15 has a two-dimensional hexagonal symmetric structure with superior thermal stability compared to MCM-41 (thermal gravimetric analysis shows an increased decomposition temperature of 80 °C) [15]. Compared to microporous molecular sieves, mesoporous structures are more conducive to the catalytic conversion of macromolecular reactants. For example, Y zeolite, with its unique octahedral structure, is considered an efficient catalyst for cracking reactions and is currently widely used in heavy oil processing processes such as fluid catalytic cracking (FCC). However, traditional microporous Y zeolites are limited by their narrow pore structures, leading to significant mass transfer resistance and reduced catalytic efficiency. To address this issue, mesoporous Y zeolites, with their enlarged pore sizes and excellent diffusion properties, demonstrate significant advantages in the catalytic conversion of macromolecular reactants. Studies have confirmed their high catalytic efficiency in related reactions [49].
Since there is limited literature on macroporous molecular sieves, indicating their limited application scope, further discussion is omitted in this paper. In the field of bio-oil catalysis, the Boateng research team [51] systematically screened three molecular sieve catalysts—H-Y, H-ZSM-5, and H-Beta—based on their skeletal structure characteristics and acidic site distribution properties for use in the thermal cracking of biomass to produce oil. Experimental data indicate that H-ZSM-5 molecular sieves with higher acid strength exhibit superior pyrolysis reaction catalytic activity. Through three parallel experiments examining the influence of the Si/Al ratio of H-ZSM-5 molecular sieves, it was found that when Si/Al = 23, the catalyst more effectively promotes the selective production of alkane products. In addition, the use of sulfonation modification technology to regulate the acidity of molecular sieve catalysts can also significantly improve the catalytic efficiency of converting algal biomass into hydrocarbon fuels [52].
4.2. Active Metal Center
Based on the active metal components, one-step hydrogenation–deoxygenation catalysts for bio-oil can be divided into two major categories: precious metal systems and non-precious metal systems. The following sections will discuss these two categories separately.
4.2.1. Precious Metal Catalyst
Precious metals include Ag, Au, and platinum group metals (Re, Rh, Ru, Ir, Os, Pd, and Pt), totaling nine metallic elements. When precious metals are used as catalyst centers, they must be combined with a carrier. Common supports include single metal oxides (TiO2, SiO2), composite oxides of two or more metals, molecular sieves (ZSM-5, H-Y, etc.), and carbon materials of various structures [53]. Additionally, incorporating precious metals can improve the stability of acidic molecular sieves and enhance the reusability of catalysts. In precious metal catalysts, besides single-metal-loaded catalysts, there are also dual-precious-metal-loaded catalysts. Research indicates that in dual-component precious metal catalyst systems, there is a significant synergistic effect between the two metal components, and this interaction effectively promotes the hydrogenation–deoxygenation (HDO) reaction of phenolic compounds [53].
Precious metal catalysts typically exhibit high hydrogenation activity [53]. Numerous studies have shown that precious metal catalysts supported on acidic carriers not only exhibit high activity but also excellent stability, making them an effective alternative to traditional CoMoS/Al2O3 catalysts [54]. A comparison of traditional CoMo catalysts with other precious metal catalysts is shown in Table 4 and Table 5.

Table 4.
Traditional CoMo catalysts.

Table 5.
Precious metal catalysts.
Biller et al. [55] employed CoMo and NiMo catalysts for the hydrogenation of bio-crude oil derived from microalgal hydrothermal liquefaction (HTL). Experiments demonstrated that at 405 °C, both catalysts significantly reduced oxygen content (by approximately 85%) and nitrogen content (to 2.4% for NiMo and 2.7% for CoMo) in the bio-crude oil while effectively desulfurizing it. The hydrogenated oil primarily consisted of alkanes with improved boiling range distribution, enabling separation of high proportions of gasoline and diesel fractions. Notably, the hydrotreated oil from CoMo at 405 °C contained hydrocarbons ranging from C9 to C26, predominantly C15 and C16. This composition aligns with the hydrocarbon distribution characteristic of HEFA aviation fuel, indicating its potential as an aviation fuel alternative.
Pongsiriyakul et al. [60]. discovered in their research on catalytic upgrading of microalgal hydrothermal liquefaction (HTL) bio-crude oil that the Ni-Cu-Re/γ-Al2O3 ternary catalyst exhibited outstanding comprehensive performance. Operating at 350 °C and 75 bar hydrogen pressure for 4 h, this catalyst achieved a 58.5 wt% upgraded bio-oil yield with 64.6% energy recovery, resulting in an overall energy recovery rate of 46.4%. Its carbon efficiency reached 74.3%, significantly outperforming both single-metal and bimetallic catalysts. The upgraded bio-oil exhibits substantially enhanced hydrocarbon content with a broad carbon number distribution, making it particularly suitable for aviation biofuel production. C12–C18 alkanes constitute over 65% of the composition, including 30.94% C15 and 13.48% C17, meeting aviation fuel carbon number requirements. The catalyst demonstrated outstanding performance in deoxygenation and desulfurization while effectively suppressing excessive cracking and coking, exhibiting excellent potential for industrial application.
Although precious metal catalysts exhibit high activity toward deoxygenation products, their high cost limits their large-scale commercial application. Therefore, identifying an alternative metal with high catalytic activity and low cost remains crucial.
4.2.2. Non-Precious Metal Catalyst
The main representatives of non-precious metals are metals such as Ni, Zn, Fe, and Cu. Due to the extremely low prices of non-precious metals, they are suitable for large-scale applications. Therefore, the development of Ni as a low-cost metal has become a widespread trend. Table 6 lists common Ni metal catalysts, their reaction conditions, and related product parameters.

Table 6.
Ni-based catalyst for hydrogenation deoxygenation of bio-oil and its model compounds.
Marinič et al. [62] employed a sulfide-type NiMo/γ-Al2O3 catalyst in their experiments to perform one-step liquefaction and hydrogenation of microalgal biomass, successfully converting it into alkane biofuels within the diesel range. Under optimal reaction conditions (350 °C, 50 bar hydrogen pressure), the product primarily consisted of C15–C18 alkanes, with specific compositions of 5.1% C15, 10.7% C16, 10.4% C17, and 26.1% C18. The high yields of C18 and C16 indicate that hydrogenation-deoxygenation (HDO) is the primary deoxygenation pathway, while the formation of C17 and C15 stems from decarboxylation and decarbonylation reactions. This catalyst exhibits outstanding hydrogenation-deoxygenation activity and selectivity, significantly reducing oxygen content in the products and enhancing fuel quality, making it suitable for the efficient production of microalgae-based biofuels.
Haider et al. [65] employed a pre-sulfurized commercial NiMo/Al2O3 catalyst for the hydrogenation of biorefinery crude oil. Under optimized conditions (375 °C, 70 bar initial hydrogen pressure, 3 h), the catalyst demonstrated outstanding performance, achieving complete oxygen removal (100% deoxygenation rate) and significant nitrogen removal (60% denitrogenation rate). Furthermore, the upgraded oil yielded a 33% aviation kerosene fraction (190–290 °C), demonstrating its substantial potential as a precursor for sustainable aviation biofuels. These results highlight the NiMo/Al2O3 catalyst’s effectiveness in heteroatom removal and fuel fraction distribution under moderate hydrogenation conditions.
Compared to other non-precious metals, iron (Fe) has advantages such as high abundance and low cost. For a long time, iron has been regarded as an inert transition metal for aromatic ring hydrogenation. However, it has been confirmed that iron exhibits extremely high selectivity for the hydrogenation-deoxygenation (HDO) of phenols, thereby demonstrating significant potential for iron-based catalysts in the HDO reaction of phenols. Although iron-based catalysts exhibit high selectivity toward complete deoxygenation products, their activity is relatively low. Additionally, iron is prone to surface oxidation or carbon deposition, leading to catalyst deactivation. Therefore, research on iron-based catalysts often involves combining them with another metal to form bimetallic catalysts, thereby overcoming these drawbacks [67].
As such, in current research, the stability of various catalysts is primarily influenced by two factors: the active metal and the support [67]. Regarding the metal, catalyst deactivation occurs because the active metal phase undergoes sintering during the hydrogenation-deoxygenation reaction, and carbon deposition on the metal surface covers some of the HDO active sites, thereby affecting the catalyst’s catalytic performance. Regarding the support, changes in the density of acidic sites on the support are typically the primary cause of catalyst deactivation. Therefore, prior to catalyst preparation, it is essential not only to consider the impact of impurities in the raw materials but also to enhance the catalyst’s inherent stability, prevent metal particle sintering, and minimize the influence of coke and certain metal oxides generated during calcination on catalyst activity [53].
5. Current Status of Industrialization of Green Aviation Fuel at Home and Abroad
The catalytic hydrogenation process for bio-based oils is relatively mature, with numerous projects successfully implemented worldwide. For instance, the research initiative “Hydrogenation-Based Production Technology for Bio-Jet Fuel,” led by multiple Sinopec subsidiaries including its Petrochemical Research Institute, passed a technical appraisal organized by the corporation’s Science and Technology Department. In another case, China National Petroleum Corporation’s (CNPC) Liaoyang Petrochemical Company developed an in-house method for producing low-temperature flow improvers for diesel derived from Russian crude oil, which received a national invention patent in 2014 [68].
In recent years, China has seen a diversified development trend in biojet fuel technology, particularly in the large-scale production of biojet fuel using waste resources. It is expected that in 2022, Sinopec will commission China’s first large-scale industrialized 100,000-ton-per-year biojet fuel facility at its Ningbo Zhenhai Refining and Chemical Base [69]. The facility employs its independently developed HEFA (hydrogenated esters and fatty acids) technology to produce biojet fuel using used cooking oil (UCO, commonly known as “gutter oil”) as feedstock. In terms of application, China International Airlines has conducted commercial flight trials (such as the Beijing-Chengdu route) using biojet fuel produced by China National Petroleum Corporation (CNPC) and has deepened cooperation with Airbus to jointly promote the development of the biojet fuel industry chain. China Eastern Airlines has partnered with Sinopec and China National Cereals, Oils and Foodstuffs Corporation (COFCO) to explore a full-industry-chain demonstration project for biojet fuel production using cellulose-based biomass feedstocks such as corn stover. Hainan Airlines is collaborating with Boeing to promote a regional industrial chain pilot project covering the production and application of biojet fuel in the Hainan Free Trade Port.
Internationally, the large-scale production and application of biojet fuel are more widespread. Neste, a global leader in renewable fuel production, operates production facilities in Finland, the Netherlands, and Singapore, with an annual SAF production capacity exceeding 1 million tons, supplying international airlines such as Lufthansa and Delta Air Lines on a long-term basis. In terms of microalgae-based SAF production technology, Japanese company Euglena is actively advancing the development of its microalgae biotechnology platform, aiming for commercialization by 2025. This project has received strong support from Japan Airlines and the Japan Aerospace Exploration Agency (JAXA).
6. Summary and Outlook
This paper provides a systematic review of research progress in the use of microalgal oil as a renewable feedstock for the production of biojet fuel through catalytic hydrogenation technology. Against the backdrop of global urgency to achieve carbon neutrality in response to climate change and environmental pollution, microalgal oil has emerged as an ideal pathway for developing biojet fuel due to its unique non-competitive nature with food crops, high carbon capture efficiency, and potential. The research focuses on the core process of “oil-to-oil” conversion, delving into the key mechanisms of hydrogenation-deoxygenation (HDO) and cracking/isomerization reactions, as well as strategies for designing efficient catalysts. Analysis indicates that the HDO-based technical route, with its superior economic viability and product yield, has become the mainstream conversion pathway. Additionally, the study indicates that the development of bifunctional catalysts (particularly nickel-based non-precious metal catalysts characterized by high activity, low cost, and excellent stability) and the optimization of material systems such as mesoporous molecular sieve carriers and nickel-cobalt alloys demonstrate significant advantages in enhancing overall reaction efficiency and the selectivity of key isomerized alkanes. Although domestic and international policy support and demonstration projects are actively promoting the commercialization of microalgae-based jet fuel, the key focus of future research remains on developing advanced catalyst systems with higher conversion efficiency, superior selectivity, and long-term stability. This will further reduce production costs, accelerate the transition of this technology from the laboratory to large-scale industrial application, and provide critical technological support for achieving a green and low-carbon transformation in the aviation transportation industry.
Author Contributions
J.L.: writing—review and editing, supervision, resources, investigation. C.M.: writing—review and editing, writing—original draft, supervision, investigation, funding acquisition, formal analysis, conceptualization. H.L.: writing—original draft, formal analysis, data curation, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Binzhou Institute of Technology, Weiqiao-UCAS Science and Technology Park (project number: GYY-NYHJ-2023-ZY-001, project name: Microalgae-based aviation kerosene refining project), also supported by the Fundamental ResearchFunds for the Central Universities (PTYX202449), National NaturalScience Foundation of China (21808014) and Fundamental Research Funds for the Central Universities, China (2021ZY25).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Nomenclature
| Abbreviations | |
| AC | Activated carbon |
| ATJ | Alcohol-To-Jet |
| CNPC | China National Petroleum Corporation |
| CO2 | Carbon dioxide |
| COFCO | China National Cereals, Oils and Foodstuffs Corporation |
| DCO | Decarbonylation |
| DCO2 | Decarboxylation |
| FAME | Fatty acid methyl esters |
| FCC | Fluid catalytic cracking |
| FT | Fischer-Tropsch |
| GTJ | Gas-to-Jet |
| HDO | Hydrogenation-deoxygenation |
| HEFA | Hydroprocessed Esters and Fatty Acids |
| HEFA-SPK | microalgae-based synthetic paraffin kerosene |
| HTL | Hydrothermal liquefaction |
| JAXA | Japan Aerospace Exploration Agency |
| RED II | Renewable Energy Directive Ⅱ |
| OTJ | Oil-to-Jet |
| SAF | Sustainable Aviation Fuel |
| STJ | Sugar-to-Jet |
| TGA | Triglycerides |
| UCO | Used cooking oil |
References
- Nema, P.; Nema, S.; Roy, P. An overview of global climate changing in current scenario and mitigation action. Renew. Sustain. Energy Rev. 2012, 16, 2329–2336. [Google Scholar] [CrossRef]
- Davidson, M.; Karplus, V.J.; Zhang, D.; Zhang, X. Policies and institutions to support carbon neutrality in China by 2060. Econ. Energy Environ. Policy 2021, 10, 7–24. [Google Scholar] [CrossRef]
- Chen, L.; Msigwa, G.; Yang, M. Strategies to achieve a carbon neutral society: A review. Environ. Chem. Lett. 2022, 20, 2277–2310. [Google Scholar] [CrossRef]
- Zou, C. The role of new energy in carbon neutral. Adv. Pet. Explor. Dev. 2021, 48, 480–491. [Google Scholar] [CrossRef]
- Li, B.; Ding, S.; Guo, H.J. Research Progress of Bio-Oil Hydrodeoxygenation Catalysts. Adv. New Renew. Energy 2021, 9, 524–532. [Google Scholar]
- Zhao, Q.Y.; Han, F.; Shi, X.X. Research on Carbon Sequestration, Emission Reduction and Economic Benefit of Microalgae Biodiesel. Ind. Water Treat. 2023, 43, 1–17. [Google Scholar]
- Guo, B.W.; Li, X.; Zong, B.N. Carbon Fixation by Microalgae to Achieve CO2 Emission Reduction and Biomass Valorization. Acta Pet. Sin. Pet. Process. Sect. 2023, 39, 668–678. [Google Scholar]
- Wei, H.; Liu, W.; Chen, X. Renewable Bio-jet Fuel Production for Aviation:Areview. Fuel 2019, 254, 115599. [Google Scholar] [CrossRef]
- Dooley, S.; Won, S.H.; Heyne, J. The experimental evaluation of a methodology for surrogate fuel formulation to emulate gas phase combustion kinetic phenomena. Combust. Flame 2012, 159, 1444–1466. [Google Scholar] [CrossRef]
- Wang, F.; Rijal, D. Sustainable aviation fuels for clean skies: Exploring the potential and perspectives of strained hydrocarbons. Energy Fuels 2024, 38, 4904–4920. [Google Scholar] [CrossRef]
- Checa, R.; Lorentz, C. Catalytic hydroconversion of HTL micro-algal bio-oil into biofuel over NiWS/Al2O3. Algal Res. 2023, 71, 103012. [Google Scholar]
- Liu, H.C.; Li, Y.M. Chapter 9: Catalytic Conversion of Biomass. Ind. Catal. 2016, 24, 81–136. [Google Scholar]
- Neupane, D. Biofuels from renewable sources, a potential option for biodiesel production. Bioengineering 2022, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Soni, V.K.; Dhara, S.; Krishnapriya, R. Highly selective Co3O4/silica-alumina catalytic system for deoxygenation of triglyceride-based feedstock. Fuel 2020, 266, 117065. [Google Scholar] [CrossRef]
- Gong, M.Y.; Jiang, W.; Xin, Y. Research Progress on Nickel-based Biomass Oil Hydrogenation Deoxygenation Catalysts. Spec. Petrochem. 2022, 39, 66–70. [Google Scholar]
- Almomani, F.; Al, K.A.; Judd, S.; Shurair, M. Impact of CO2 concentration and ambient conditions on microalgal growth and nutrient removal from wastewater by a photobioreactor. Sci. Total Environ. 2019, 662, 662–671. [Google Scholar] [CrossRef]
- Almomani, F.; Omar, A. Application of microalgae in wastewater treatment: Simultaneous nutrient removal and carbon dioxide bio-fixation for biofuel feedstock production. Pet. Ind. Wastewater 2022, 18, 87–101. [Google Scholar]
- Judd, S.J.; Al Momani, F.A.O.; Znad, H. The cost benefit of algal technology for combined CO2 mitigation and nutrient abatement. Renew. Sustain. Energy Rev. 2017, 71, 379–387. [Google Scholar] [CrossRef]
- Al Ketife, A.M.D.; Almomani, F.; Muftah, E.L.N. A technoeconomic assessment of microalgal culture technology implementation for combined wastewater treatment and CO2 mitigation in the Arabian Gulf. Process Saf. Environ. Prot. 2019, 127, 90–102. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Yasin, N.H.M.; Derek, C.J.C. Microalgae as a sustainable energy source for biodiesel production: A review. Renew. Sustain. Energy Rev. 2011, 15, 584–593. [Google Scholar] [CrossRef]
- Khan, A.A.; Gul, J.; Naqvi, S.R. Recent progress in microalgae-derived biochar for the treatment of textile industry wastewater. Chemosphere 2022, 306, 135565. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Mathimani, T.; Anto, S. Cell density, Lipidomic profile, and fatty acid characterization as selection criteria in bioprospecting of microalgae and cyanobacterium for biodiesel production. Bioresour. Technol. 2020, 304, 123061. [Google Scholar] [CrossRef]
- Rony, Z.I.; Mofijur, M.; Hasan, M.M. Unanswered issues on decarbonizing the aviation industry through the development of sustainable aviation fuel from microalgae. Fuel 2023, 334, 126553. [Google Scholar] [CrossRef]
- Lim, J.H.K.; Gan, Y.Y.; Ong, H.C. Utilization of microalgae for bio-jet fuel production in the aviation sector: Challenges and perspective. Renew. Sustain. Energy Rev. 2021, 149, 111396. [Google Scholar] [CrossRef]
- Ma, C.; Dai, Y.; Zhuang, G. Advances in hydrodeoxygenation techniques and catalysts for the sustainable conversion of microalgal oil into biofuels. Chem. Eng. Sci. 2025, 318, 122168. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, F.; Su, X. Direct extraction of lipids from wet microalgae slurries by super-high hydrostatic pressure. Algal Res. 2021, 58, 102412. [Google Scholar] [CrossRef]
- Liu, X.Q.; Chen, Y.; Zhou, Y. Large-scale Power-hydrogen Coupling Systems: Technical Perspective Analysis and Prospect of Large Energy Companies in China and Europe. Proc. CSEE 2023, 43, 7003–7009. [Google Scholar]
- Gan, C.Y.; Ding, S.T.; Qiu, T. History and Trends in the Development of Safety Standards for Sustainable Aviation Fuels. J. Aerosp. Power 2025, 40, 20230201. [Google Scholar]
- Bhatt, A.H.; Zhang, Y.; Milbrandt, A. Evaluation of performance variables to accelerate the deployment of sustainable aviation fuels at a regional scale. Energy Convers. Manag. 2023, 275, 116441. [Google Scholar] [CrossRef]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Chong, W.T.; Kee Lam, M.; Kwan Loh, P.; Vellayan, V. Microalgae biofuels as an alternative to fossil fuel for power generation. Renew. Sustain. Energy Rev. 2016, 58, 180–197. [Google Scholar] [CrossRef]
- Mao, W.W.; Zhang, L.; Yin, Q.R. Strategies and Prospect of Photosynthesis Mechanism Intensification of Microalgae CO2 Fixation. Clean Coal Technol. 2022, 28, 30–43. [Google Scholar]
- Zheng, Y.Q.; Chen, D.H.; Ren, L. The Strategic Significance and International Cooperation of Blue Economy. Pac. J. 2023, 31, 66–78. [Google Scholar]
- Mansy, A.E.; Daniel, S.; Fonzeu Monguen, C.K. Catalytic production of aviation jet biofuels from biomass: A review. Environ. Chem. Lett. 2025, 23, 419–461. [Google Scholar] [CrossRef]
- Zhang, J.T.; Wu, R.R.; Wu, X.H. Research Advances in Hydrodeoxygenation Catalysts for Phenolic Compounds. Mod. Chem. Ind. 2021, 41, 57–62. [Google Scholar]
- Vicerich, M.A.; Sánchez, M.A.; Benítez, C.F. Influence of the Activation Method of Rh-Sn-B/γ-Al2O3 Catalysts on the Selective Hydrogenation of Oleic Acid to Oleyl Alcohol. Catal. Lett. 2024, 154, 5806–5816. [Google Scholar]
- Xiong, Y.; Miao, W.; Wang, N. Solid alcohol based on waste cooking oil: Synthesis, properties, micromorphology and simultaneous synthesis of biodiesel. Waste Manag. 2019, 85, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Chen, Y.B.; Liu, Y. One-step Hydrogenation of Castor Oil Catalyzed by Pt-La/SAPO-11 Catalyst for Preparing of Aviation Kerosene. Chem. Ind. For. Prod. 2021, 41, 65–71. [Google Scholar]
- Žula, M.; Grilc, M.; Likozar, B. Hydrocracking, hydrogenation and hydro-deoxygenation of fatty acids, esters and glycerides: Mechanisms, kinetics and transport phenomena. Chem. Eng. J. 2022, 444, 136564. [Google Scholar] [CrossRef]
- Wang, Z.C.; Xie, F.L.; Duan, P.G. Hydro-upgrading of Crude Algal Bio-oil: Influence of External Hydrogen Sources. Clean Coal Technol. 2024, 30, 21–28. [Google Scholar]
- Nie, H.; Meng, X.K.; Zhang, Z.M. Development of Technology for Producing Bio-jet Fuel from Several Feedstocks. Sci. Sin. Chim. 2014, 44, 46–54. [Google Scholar] [CrossRef]
- Jing, L.; Can, L.; Gang, Z.; Shituan, S.; Long, R. Hydrotreatment of Jatropha Oil over NiMoLa/Al2O3 Catalyst. Green Chem. 2012, 14, 2499–2505. [Google Scholar]
- Verma, D.; Kumar, R.; Rana, B.S.; Sinha, A.K. Aviation fuel producti on from lipids by asingle-steproute using hierarchi calm esoporous zeolites. Energy Environ. Sci. 2011, 4, 1667. [Google Scholar] [CrossRef]
- Zainal, N.A.; Zulkifli, N.W.M.; Gulzar, M. A review on the chemistry, production, and technological potential of bio-based lubricants. Renew. Sustain. Energy Rev. 2018, 82, 80–102. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Yusup, S. Catalytic hydrodeoxygenation of triglycerides: An approach to clean diesel fuel production. Renew. Sustain. Energy Rev. 2017, 80, 1072–1088. [Google Scholar] [CrossRef]
- Doliente, S.S.; Narayan, A.; Tapia, J.F.D. Bio-aviation fuel: A comprehensive review and analysis of the supply chain components. Front. Energy Res. 2020, 8, 110. [Google Scholar] [CrossRef]
- Li, B.; Liu, J.H.; Song, Y.K. Recent Advances in Application of Metal-Organic Frameworks for Hydrogen Generation by Catalytic Hydrolysis of Ammonia Borane. Chin. J. Appl. Chem. 2023, 10, 2300726. [Google Scholar]
- Gao, X.H.; Du, X.H. Overview of Synthesis of Mesoporous Zeolitic Materials. Pet. Sci. Bull. 2016, 1, 164–170. [Google Scholar]
- Wang, C.; Cao, Q.; Zhang, Y.Q. Application Progress of Activated Carbon Material as Carrier. New Chem. Mater. 2019, 47, 23–27. [Google Scholar]
- Rostami, S.; Pour, A.N.; Veiskarami, S. Effect of sulfur-containing organic molecules on the structural stability of Ni–Y zeolite adsorbent. Sep. Purif. Technol. 2025, 377, 134253. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, H.; Yan, W. Strategies to enhance the catalytic performance of ZSM-5 zeolite in hydrocarbon cracking: A review. Catalysts 2017, 7, 367. [Google Scholar] [CrossRef]
- Mihalcik, D.J.; Mullen, C.A.; Boateng, A.A. Screening acidic zeolites for catalytic fast pyrolysis of biomass and its components. J. Anal. Appl. Pyrolysis 2011, 92, 224–232. [Google Scholar] [CrossRef]
- 51Altalhi, A.A.; Morsy, S.M.; Abou Kana, M.T.H. Pyrolytic conversion of waste edible oil into biofuel using sulphonated modified alumina. Alex. Eng. J. 2022, 61, 4847–4861. [Google Scholar] [CrossRef]
- Tong, L.; Zhang, L.; Pan, H. Research Progress of Hydrodeoxygenation Catalysts for Bio-oil Model Compounds. Chem. Ind. For. Prod. 2021, 41, 130–140. [Google Scholar]
- Qichang, G.; Jingbo, M.; Chunyu, L. Hydrodeoxygenation of Guaiacol as a Model Compound of Lignin: A Review. J. Liaoning Univ. Pet. Chem. Technol. 2022, 42, 1. [Google Scholar]
- Biller, P.; Sharma, B.K.; Kunwar, B. Hydroprocessing of bio-crude from continuous hydrothermal liquefaction of microalgae. Fuel 2015, 159, 197–205. [Google Scholar] [CrossRef]
- Duan, P.; Savage, P.E. Hydrothermal liquefaction of a microalga with heterogeneous catalysts. Ind. Eng. Chem. Res. 2011, 50, 52–61. [Google Scholar] [CrossRef]
- Poddar, M.K.; Anand, M.; Farooqui, S.A. Hydroprocessing of lipids extracted from marine microalgae Nannochloropsis sp. over sulfided CoMoP/Al2O3 catalyst. Biomass Bioenergy 2018, 119, 31–36. [Google Scholar] [CrossRef]
- Baik, Y.; Lee, K.; Choi, M. Catalytic conversion of triglycerides into diesel, jet fuel, and lube base oil. Chin. J. Catal. 2024, 58, 15–24. [Google Scholar] [CrossRef]
- Rahmawati, Z.; Santoso, L.; McCue, A. Selectivity of reaction pathways for green diesel production towards biojet fuel applications. RSC Adv. 2023, 13, 13698–13714. [Google Scholar] [CrossRef]
- Pongsiriyakul, K.; Kiatkittipong, W.; Adhikari, S. Effective Cu/Re promoted Ni-supported γ-Al2O3 catalyst for upgrading algae bio-crude oil produced by hydrothermal liquefaction. Fuel Process. Technol. 2021, 216, 106670. [Google Scholar] [CrossRef]
- Peng, B.; Yao, Y.; Zhao, C. Towards quantitative conversion of microalgae oil to diesel-range alkanes with bifunctional catalysts. Angew. Chem.-Int. Ed. 2012, 51, 2072. [Google Scholar] [CrossRef] [PubMed]
- Marinič, D.; Grilc, M.; Hočevar, B. Liquefaction, cracking and hydrogenation of microalgae biomass resources to CO2 negative advanced biofuels: Mechanisms, reaction microkinetics and modelling. Renew. Energy 2023, 203, 382–393. [Google Scholar] [CrossRef]
- Guo, B.; Walter, V.; Hornung, U. Hydrothermal liquefaction of Chlorella vulgaris and Nannochloropsis gaditana in a continuous stirred tank reactor and hydrotreating of biocrude by nickel catalysts. Fuel Process. Technol. 2019, 191, 168–180. [Google Scholar] [CrossRef]
- Castello, D.; Haider, M.S.; Rosendahl, L.A. Catalytic upgrading of hydrothermal liquefaction biocrudes: Different challenges for different feedstocks. Renew. Energy 2019, 141, 420–430. [Google Scholar] [CrossRef]
- Haider, M.S.; Castello, D.; Michalski, K.M. Catalytic hydrotreatment of microalgae biocrude from continuous hydrothermal liquefaction: Heteroatom removal and their distribution in distillation cuts. Energies 2018, 11, 3360. [Google Scholar] [CrossRef]
- Marangon, B.B.; De Siqueira Castro, J.; Calijuri, M.L. Aviation fuel based on wastewater-grown microalgae: Challenges and opportunities of hydrothermal liquefaction and hydrotreatment. J. Environ. Manag. 2024, 354, 120418. [Google Scholar] [CrossRef]
- Gong, S.F.; Gong, J.Y.; Lei, W.Q. Review of Noble Metal Catalyst for the Hydrodeoxygenation of Animal Fats and Vegetable Oils. China Oils Fasts 2022, 47, 82–89. [Google Scholar]
- Dong, K.; Dai, L.-S.; Jia, Y.-Z. Study on Hydrotreating Reactivity of Yichang Residue II. catalyst and operation conditions. Pet. Process. Petrochem. 2015, 46, 7. [Google Scholar]
- Shi, X.X.; Song, H.C.; Huang, Y. Current Situation and Countermeasures of Hydroprocessing Biofuels Development in Large Petrochemical Corporation. Biomass Chem. Eng. 2019, 53, 59–66. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).