Synthesis, Dynamic NMR Characterization, and XRD Study of 2,4-Difluorobenzoyl-Substituted Piperazines
Abstract
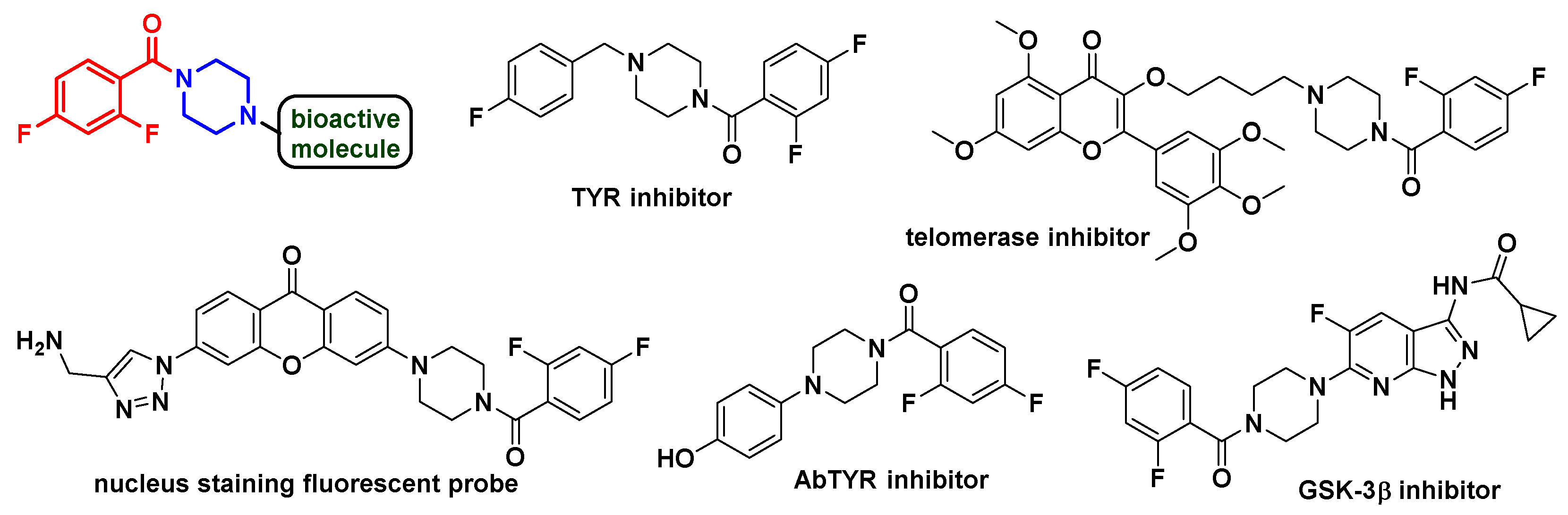
1. Introduction
2. Materials and Methods
2.1. General
2.2. VT-NMR Measurements
2.3. Single Crystal X-Ray Diffraction
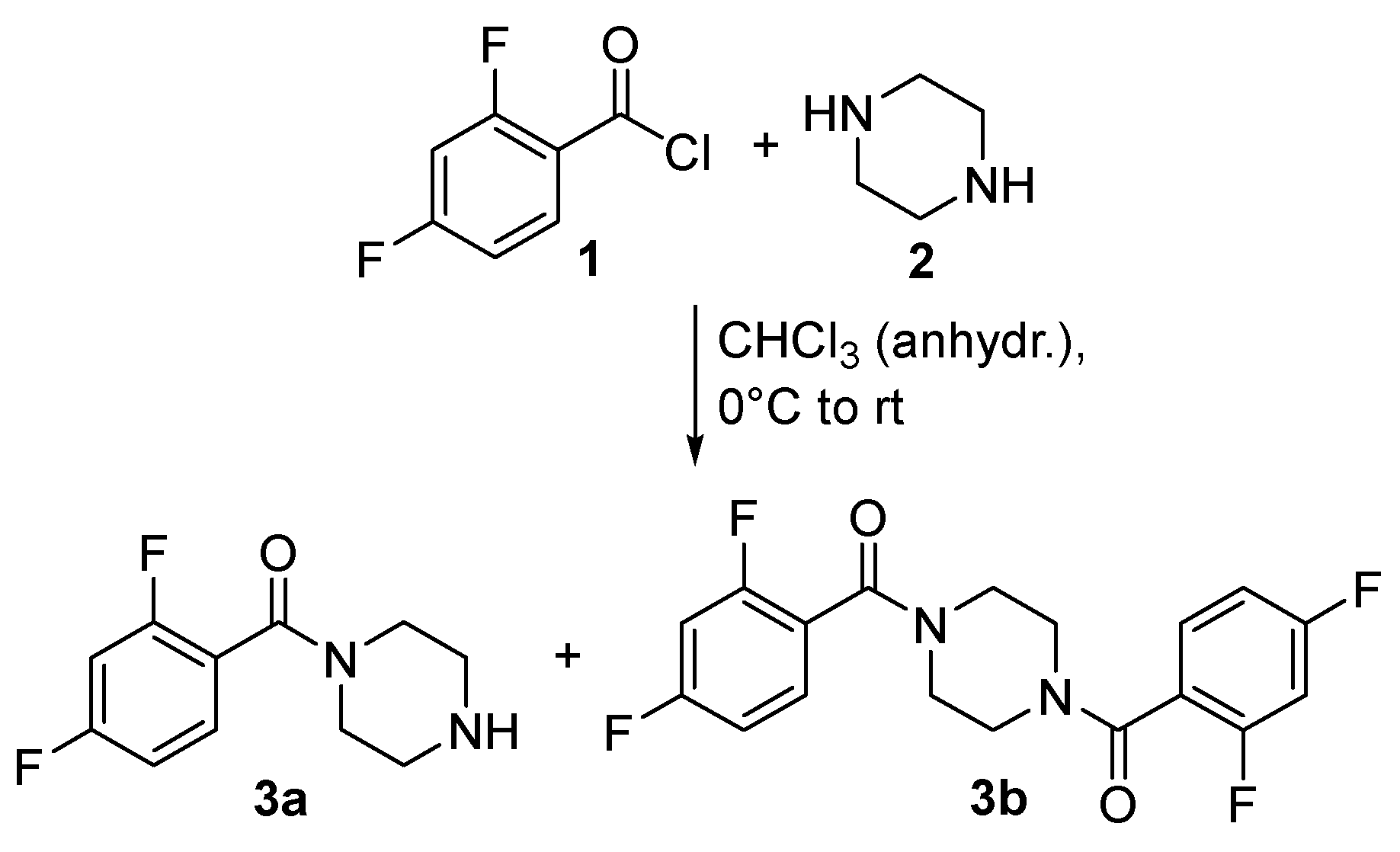
2.4. Synthesis of N-(2,4-Difluorobenzoyl)piperazine (3a)
2.5. Synthesis of N,N-Bis(2,4-difluorobenzoyl)piperazine (3b)
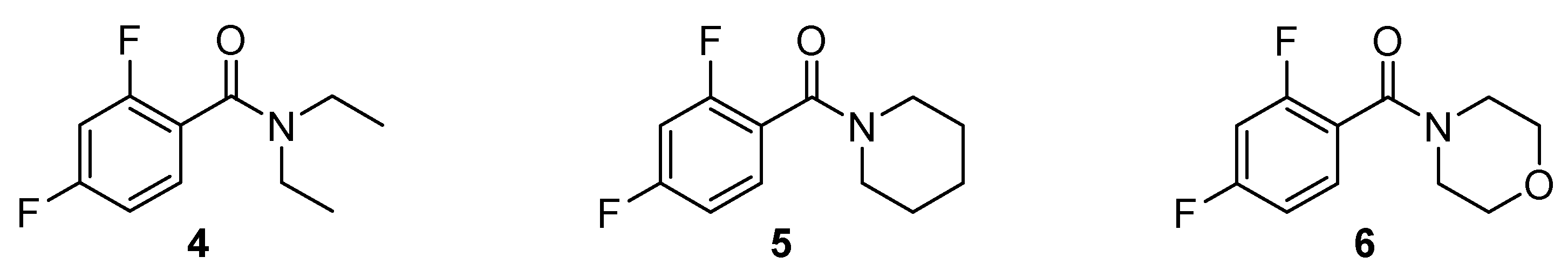
2.6. Synthesis of N,N-Diethyl-2,4-difluorobenzamide (4)
2.7. Synthesis of (2,4-Difluorophenyl)(piperidin-1-yl)methanone (5)
2.8. Synthesis of (2,4-Difluorophenyl)(morpholino)methanone (6)
3. Results and Discussion
3.1. Synthesis of the Piperazine Compounds
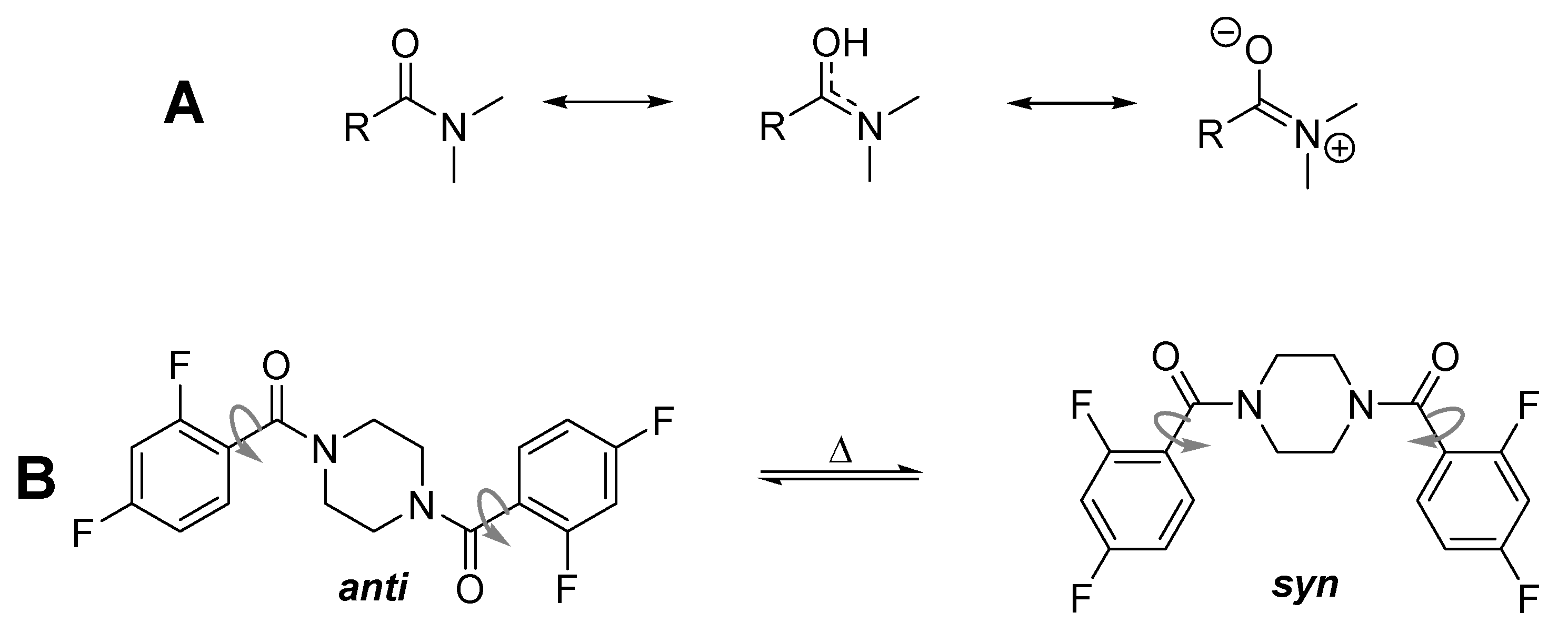
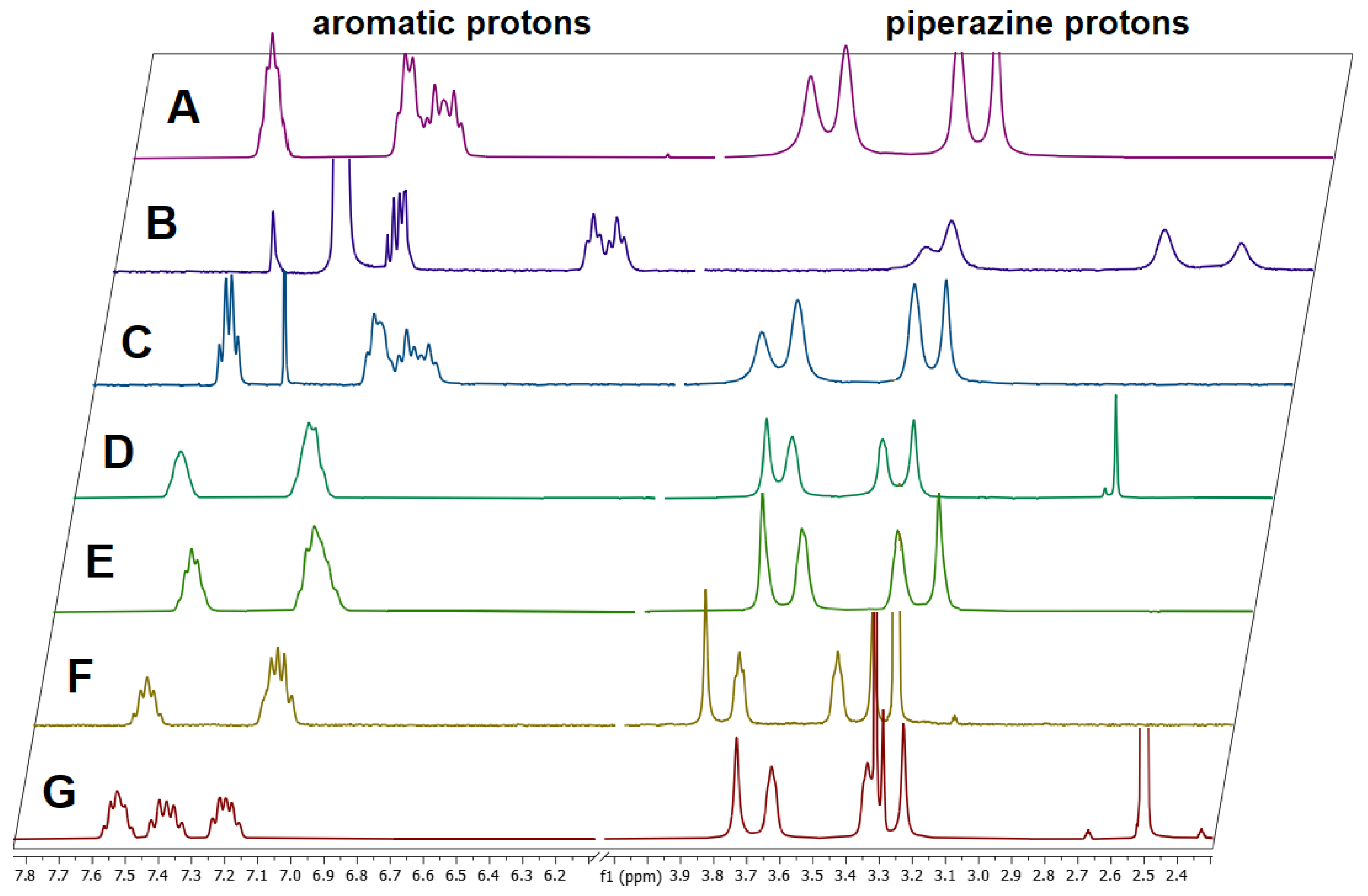
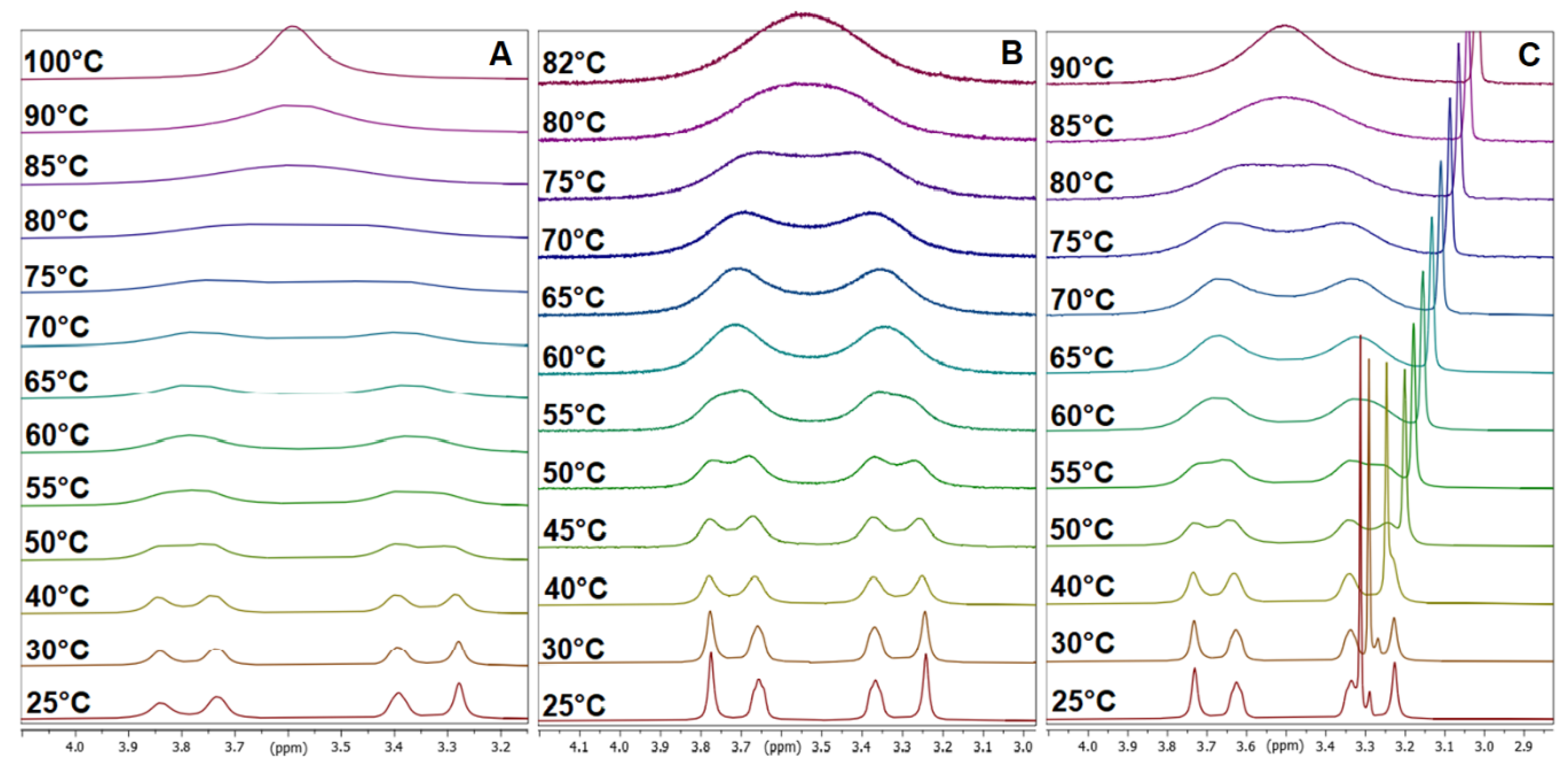
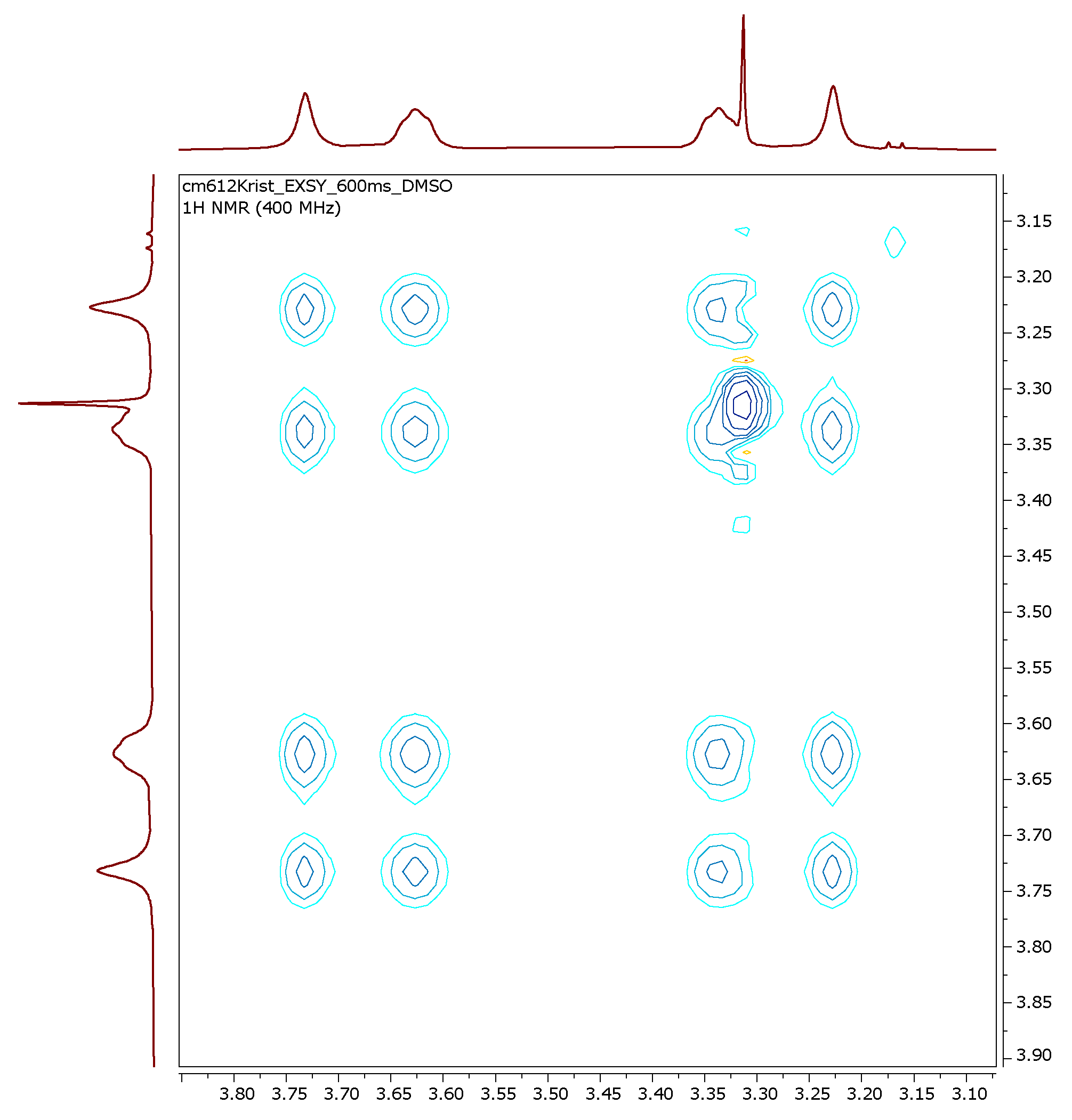
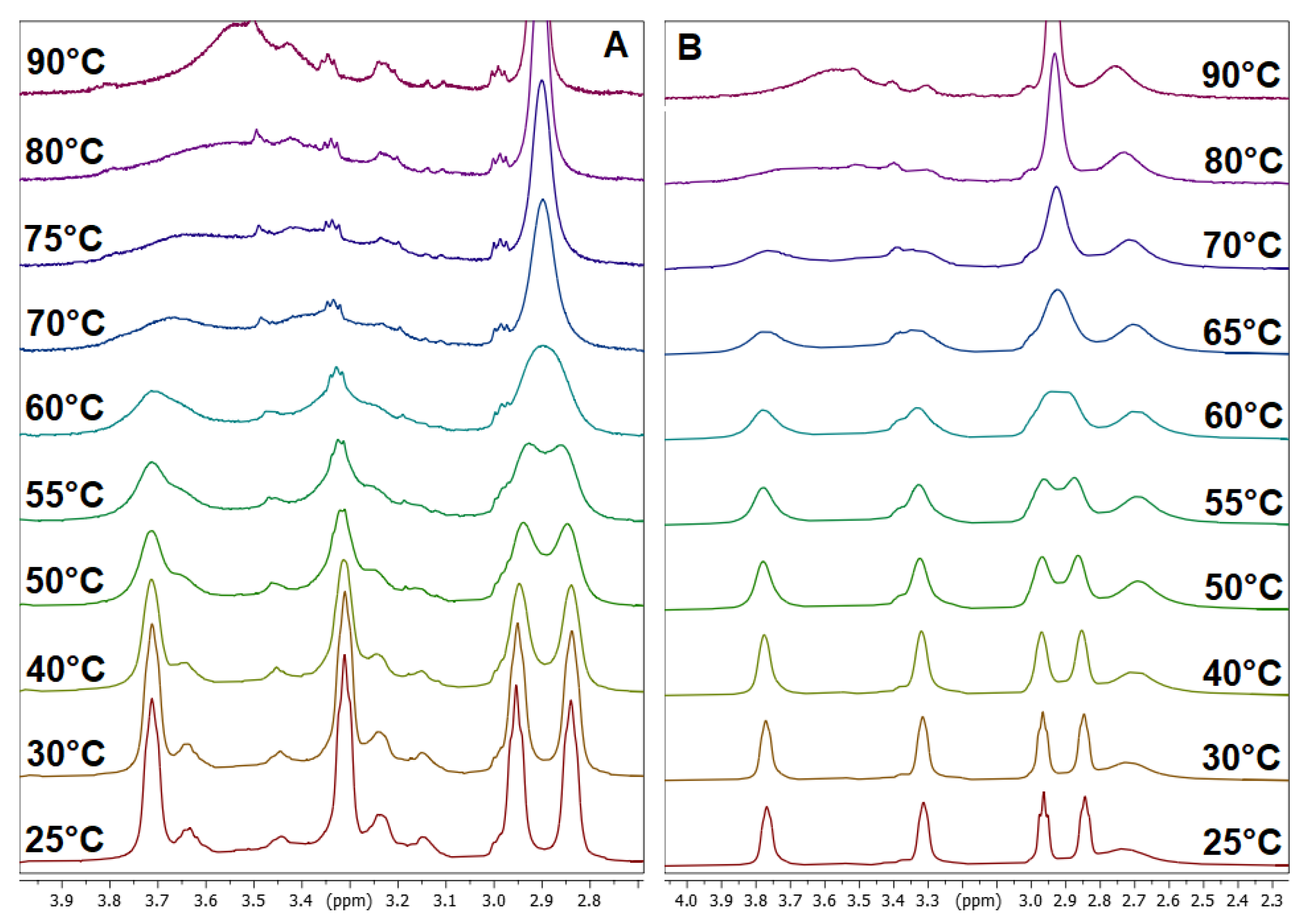
3.2. Dynamic VT-NMR Study for the Dimer 3b
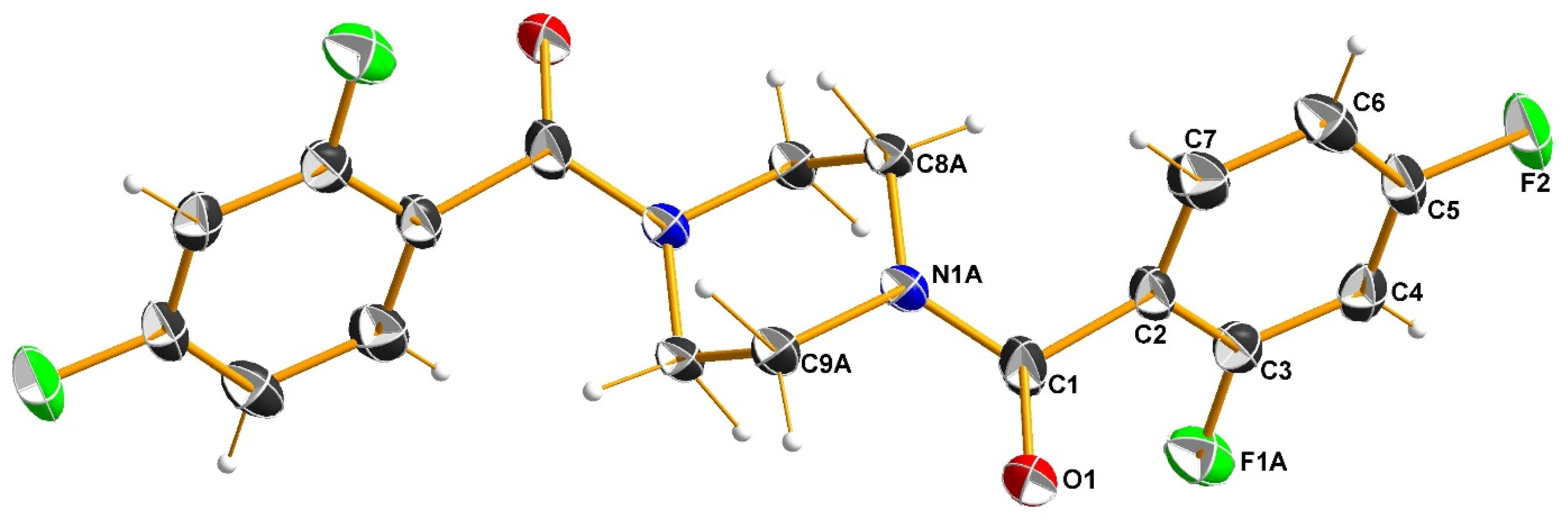
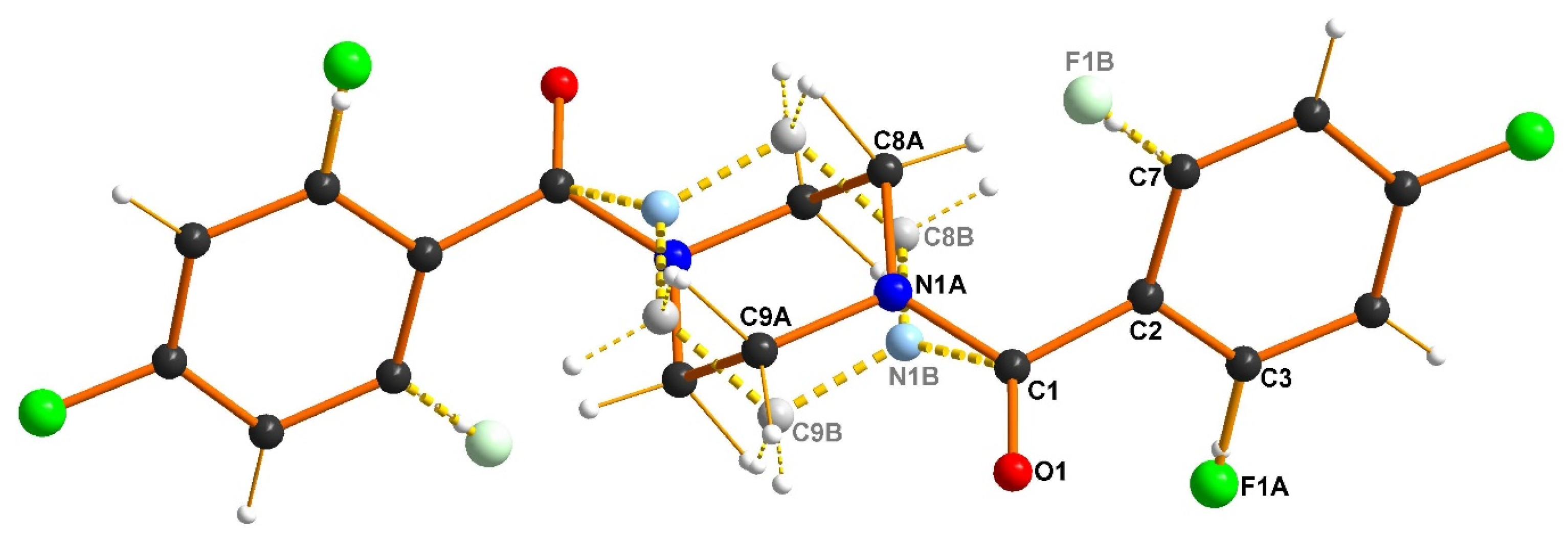
3.3. X-Ray Structure Analysis of 3b
3.4. Dynamic NMR Studies of Monomer 3a
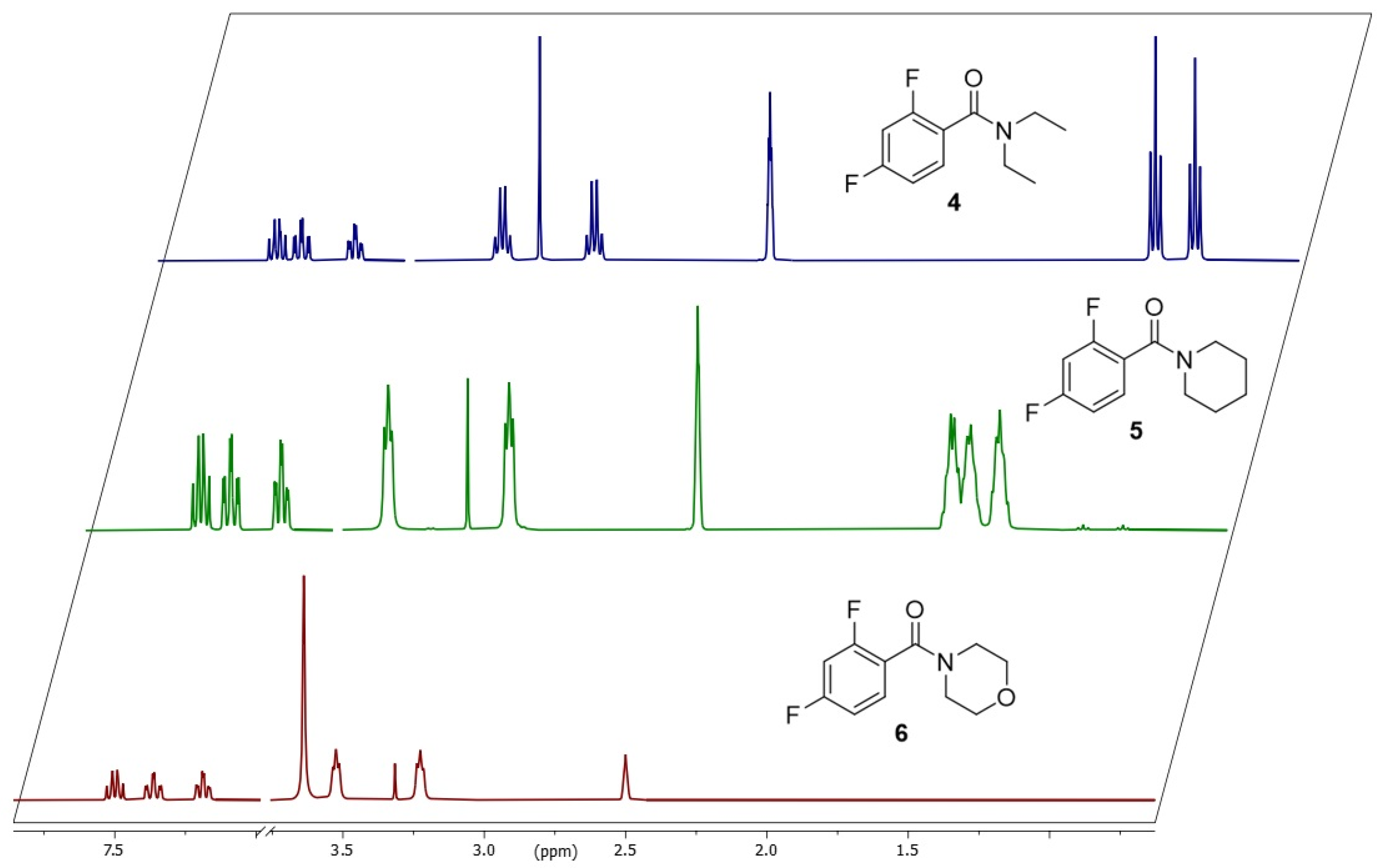
3.5. Comparison with Other 2,4-Difluorobenzamides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| COSY | Correlation spectroscopy |
| DMSO | Dimethyl sulfoxide |
| EXSY | Exchange spectroscopy |
| TCE | 1,1,2,2-Tetrachloroethane |
| TLC | Thin-layer chromatography |
| VT | Variable temperature |
| XRD | X-ray diffraction |
References
- Gettys, K.E.; Ye, Z.; Dai, M. Recent Advances in Piperazine Synthesis. Synthesis 2017, 49, 2589–2604. [Google Scholar] [CrossRef]
- Zhang, R.H.; Guo, H.Y.; Deng, H.; Li, J.; Quan, Z.S. Piperazine skeleton in the structural modification of natural products: A review. J. Enzyme Inhib. Med. Chem. 2021, 36, 1165–1197. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.M.; Federice, J.G.; Bell, C.N.; Cox, P.B.; Njardarson, J.T. An Update on the Nitrogen Heterocycle Compositions and Properties of U.S. FDA-Approved Pharmaceuticals (2013–2023). J. Med. Chem. 2024, 67, 11622–11655. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Kumar, R.; Mazumder, A.; Salahuddin; Kukreti, N.; Kumar, A.; Chaitanya, M.V.N.L. The medicinal chemistry of piperazines: A review. Chem. Biol. Drug Des. 2024, 103, e14537. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Dömling, A.; Huang, Y. Piperazine Scaffolds via Isocyanide-Based Multicomponent Reactions. Synthesis 2010, 17, 2859–2883. [Google Scholar] [CrossRef]
- Durand, C.; Szostak, M. Recent Advances in the Synthesis of Piperazines: Focus on C–H Functionalization. Organics 2021, 2, 337–347. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, W. UV curing behavior of hyperbranched polyphosphate acrylate/di(hydroxylpropyl methacrylate) piperazine and properties of the cured film. Prog. Org. Coat. 2007, 59, 312–317. [Google Scholar] [CrossRef]
- Zhao, B.; Xiao, Y.; Yuan, D.; Lu, C.; Yao, Y. Synthesis and characterization of bridged bis(amidato) rare earth metal amides and their applications in C–N bond formation reactions. Dalton Trans. 2016, 45, 3880–3887. [Google Scholar] [CrossRef]
- Sahoo, H.S.; Chand, D.K. Conformation of N,N′-bis(3-pyridylformyl)piperazine and spontaneous formation of a saturated quadruple stranded metallohelicate. Dalton Trans. 2010, 39, 7223–7225. [Google Scholar] [CrossRef]
- Barnes, D.J.; Chapman, R.L.; Vagg, R.S.; Watton, E.C. Synthesis of novel bis(amides) by means of triphenyl phosphite intermediates. J. Chem. Eng. Data 1978, 23, 349–350. [Google Scholar] [CrossRef]
- Andronati, S.A.; Karaseva, T.L.; Krysko, A.A. Peptidomimetics-antagonists of the fibrinogen receptors: Molecular design, structures, properties and therapeutic applications. Curr. Med. Chem. 2004, 11, 1183–1211. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. Exploring privileged structures: The combinatorial synthesis of cyclic peptides. Mol. Divers. 2000, 5, 289–304. [Google Scholar] [CrossRef]
- Wodtke, R.; König, J.; Pigorsch, A.; Köckerling, M.; Mamat, C. Evaluation of novel fluorescence probes for conjugation purposes using the traceless Staudinger Ligation. Dyes Pigment. 2015, 113, 263–273. [Google Scholar] [CrossRef]
- Heldt, J.-M.; Kerzendörfer, O.; Mamat, C.; Starke, F.; Pietzsch, H.-J.; Steinbach, J. Synthesis of Short and Versatile Heterobifunctional Linkers for Conjugation Purposes of Bioactive Molecules with (Radio-)Labels. Synlett 2013, 24, 432–436. [Google Scholar] [CrossRef]
- Lemoucheux, L.; Rouden, J.; Ibazizene, M.; Sobrio, F.; Lasne, M.C. Debenzylation of Tertiary Amines Using Phosgene or Triphosgene: An Efficient and Rapid Procedure for the Preparation of Carbamoyl Chlorides and Unsymmetrical Ureas. Application in Carbon-11 Chemistry. J. Org. Chem. 2003, 68, 7289–7297. [Google Scholar] [CrossRef]
- Grosse-Gehling, P.; Wuest, F.R.; Peppel, T.; Köckerling, M.; Mamat, C. [18F]1-(3-fluoropropyl)piperazines as model compounds for the radiofluorination of pyrido [2,3-d]pyrimidines. Radiochim. Acta 2011, 99, 365. [Google Scholar] [CrossRef]
- Pretze, M.; Mamat, C. Automated preparation of [18F]AFP and [18F]BFP: Two novel bifunctional 18F-labeling building blocks for Huisgen-click. J. Fluor. Chem. 2013, 150, 25–35. [Google Scholar] [CrossRef]
- Mamat, C.; Pretze, M.; Gott, M.; Köckerling, M. Synthesis, dynamic NMR characterization and XRD studies of novel N,N’-substituted piperazines for bioorthogonal labeling. Beilstein J. Org. Chem. 2016, 12, 2478–2489. [Google Scholar] [CrossRef]
- Wiemer, J.; Steinbach, J.; Pietzsch, J.; Mamat, C. Preparation of a novel radiotracer targeting the EphB4 receptor via radiofluorination using spiro azetidinium salts as precursor. J. Labelled Comp. Radiopharm. 2017, 60, 489–498. [Google Scholar] [CrossRef]
- Katz, D.P.; Deruiter, J.; Bhattacharya, D.; Ahuja, M.; Bhattacharya, S.; Clark, C.R.; Suppiramaniam, V.; Dhanasekaran, M. Benzylpiperazine: “A messy drug”. Drug Alcohol Depend. 2016, 164, 1–7. [Google Scholar] [CrossRef]
- Shaquiquzzaman, M.; Verma, G.; Marella, A.; Akhter, M.; Akhtar, W.; Khan, M.F.; Tasneem, S.; Alam, M.M. Piperazine scaffold: A remarkable tool in generation of diverse pharmacological agents. Eur. J. Med. Chem. 2015, 102, 487–529. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ye, Y.H.; Wang, X.T.; Zhu, H.L.; Xu, C.; Song, Y.C.; Li, H.; Tan, R.X. Structure and total synthesis of aspernigerin: A novel cytotoxic endophyte metabolite. Chem. Eur. J. 2006, 12, 4393–4396. [Google Scholar] [CrossRef] [PubMed]
- Irikura, T.; Masuzawa, K.; Nishino, K.; Kitagawa, M.; Uchida, H.; Ichinoseki, N.; Ito, M. New analgetic agents. V. 1-butyryl-4-cinnamylpiperazine hydrochloride and related compounds. J. Med. Chem. 1968, 11, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.K.; Syed, R.; Shin, H.S.; Patel, R.V. Piperazine derivatives for therapeutic use: A patent review (2010–present). Expert Opin. Ther. Pat. 2016, 26, 777–797. [Google Scholar] [CrossRef]
- Abou El-Ella, D.A.; Hussein, M.M.; Serya, R.A.; Abdel Naby, R.M.; Al-Abd, A.M.; Saleh, D.O.; El-Eraky, W.I.; Abouzid, K.A. Molecular design and synthesis of 1,4-disubstituted piperazines as α(1)-adrenergic receptor blockers. Bioorg. Chem. 2014, 54, 21–30. [Google Scholar] [CrossRef]
- Patel, R.V.; Park, S.W. An evolving role of piperazine moieties in drug design and discovery. Mini-Rev. Med. Chem. 2013, 13, 1579–1601. [Google Scholar] [CrossRef]
- Miniyar, P.B.; Murumkar, P.R.; Patil, P.S.; Barmade, M.A.; Bothara, K.G. Unequivocal role of pyrazine ring in medicinally important compounds: A review. Mini-Rev. Med. Chem. 2013, 13, 1607–1625. [Google Scholar] [CrossRef]
- Font, M.; Ardaiz, E.; Cordeu, L.; Cubedo, E.; Garcia-Foncillas, J.; Sanmartin, C.; Palop, J.A. Structural characteristics of novel symmetrical diaryl derivatives with nitrogenated functions. Requirements for cytotoxic activity. Bioorg. Med. Chem. 2006, 14, 1942–1948. [Google Scholar] [CrossRef]
- Radin, D.P.; Lippa, A.; Rana, S.; Fuller, D.D.; Smith, J.L.; Cerne, R.; Witkin, J.M. Amplification of the therapeutic potential of AMPA receptor potentiators from the nootropic era to today. Pharmacol. Biochem. Behav. 2025, 248, 173967. [Google Scholar] [CrossRef]
- Rana, S.; Fusco, A.F.; Witkin, J.M.; Radin, D.P.; Cerne, R.; Lippa, A.; Fuller, D.D. Pharmacological modulation of respiratory con-trol: Ampakines as a therapeutic strategy. Pharmacol. Ther. 2025, 265, 108744. [Google Scholar] [CrossRef]
- Czopek, A.; Kubacka, M.; Bucki, A.; Siwek, A.; Filipek, B.; Pawłowski, M.; Kołaczkowski, M. Novel serotonin 5-HT2A receptor antagonists derived from 4-phenylcyclohexane-5-spiro-and 5-methyl-5-phenyl-hydantoin, for use as potential antiplatelet agents. Pharmacol. Rep. 2021, 73, 1361–1372. [Google Scholar] [CrossRef]
- Liu, X.-K.; Ma, L.-X.; Wei, Z.-Y.; Cui, X.; Zhan, S.; Yin, X.-M.; Piao, H.-R. Synthesis and Positive Inotropic Activity of [1,2,4]Triazolo [4,3-a] Quinoxaline Derivatives Bearing Substituted Benzylpiperazine and Benzoylpiperazine Moieties. Molecules 2017, 22, 273. [Google Scholar] [CrossRef]
- Moorman, A.R. Adenosine a2a Receptor Antagonists. U.S. Patent 2008242672A1, 6 April 2010. [Google Scholar]
- Dong, H.; Zhang, L. Pyridino-Pyrimidine Compound and Application Thereof. CN Patent 109748914A, 25 May 2025. [Google Scholar]
- Arkinstall, S.; Halazy, S.; Church, D.; Camps, M.; Rueckle, T.; Gotteland, J.P.; Biamonte, M. Pharmaceutically Active Sulfonamide Derivatives. U.S. Patent 8012995B1, 6 September 2011. [Google Scholar]
- Ielo, L.; Deri, B.; Germanò, M.P.; Vittorio, S.; Mirabile, S.; Gitto, R.; Rapisarda, A.; Ronsisvalle, S.; Floris, S.; Pazy, Y.; et al. Exploiting the 1-(4-fluorobenzyl)piperazine fragment for the development of novel tyrosinase inhibitors as anti-melanogenic agents: Design, synthesis, structural insights and biological profile. Eur. J. Med. Chem. 2019, 178, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Yang, M.D.; Chen, X.; Wang, Y.; Chen, L.Z.; Cheng, X.; Liu, X.H. Discovery of new chromen-4-one derivatives as telomerase inhibitors through regulating expression of dyskerin. J. Enzyme. Inhib. Med. Chem. 2018, 33, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.K.; Jeong, Y.M.; Kang, N.Y.; Lee, J.; Diana, W.S.Y.; Kim, J.Y.; Yoo, J.; Kim, D.; Kim, Y.K.; Chang, Y.T. The development of a nucleus staining fluorescent probe for dynamic mitosis imaging in live cells. Chem. Commun. 2015, 51, 9336–9338. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.H.; Kim, J.S.; Kim, B.M. Novel heterocycle-substituted pyrimidines as inhibitors of NF-κB transcription regulation related to TNF-α cytokine release. Bioorg. Med. Chem. Lett. 2008, 18, 653–656. [Google Scholar] [CrossRef]
- Dugave, C.; Demange, L. Cis−Trans Isomerization of Organic Molecules and Biomolecules: Implications and Applications. Chem. Rev. 2003, 103, 2475–2532. [Google Scholar] [CrossRef]
- Jackman, L.M.; Kavanagh, T.E.; Haddon, R.C. Studies in nuclear magnetic resonance—IX. Rotational barriers in substituted N,N-dimethylbenzamides. Org. Magn. Reson. 1969, 1, 109–123. [Google Scholar] [CrossRef]
- Alberghina, G.; Bottino, F.A.; Fisichella, S.; Arnone, C. Solvent effects on the rotational barriers of the N,N-dimethylamides of 2- and 3-furoic and 2- and 3-thenoic acids. J. Chem. Res. (M) 1985, 1201–1217. [Google Scholar]
- Alberghina, G.; Bottino, F.A.; Fisichella, S.; Arnone, C. Solvent effects on the rotational barriers of the N,N-dimethylamides of 2- and 3-furoic and 2- and 3-thenoic acids. J. Chem. Res. (S) 1985, 108–109. [Google Scholar]
- Turnbull, M.M.; Nelson, D.J.; Lekouses, W.; Sarnov, M.L.; Tartarini, K.A.; Huang, T.-K. Rotational barriers in N,N-diethylbenzamides: Substituent and solvent effects. Tetrahedron 1990, 46, 6613–6622. [Google Scholar] [CrossRef]
- Wodtke, R.; Steinberg, J.; Köckerling, M.; Löser, R.; Mamat, C. NMR-based investigations of acyl-functionalized piperazines concerning their conformational behavior in solution. RSC Adv. 2018, 8, 40921–40933. [Google Scholar] [CrossRef]
- Friebolin, H. Basic One- and Two-Dimensional NMR Spectroscopy; Wiley-VCH: Weinheim, Germany, 2010; p. 319 ff. [Google Scholar]
- Gasparro, F.P.; Kolodny, N.H. NMR determination of the rotational barrier in N,N-dimethylacetamide. A physical chemistry experiment. J. Chem. Educ. 1977, 54, 258. [Google Scholar] [CrossRef]
- Jackman, L.M.; Cotton, F.A. (Eds.) Dynamic Nuclear Magnetic Resonance Spectroscopy; Academic Press: New York, NY, USA, 1975; p. 204 ff. [Google Scholar]
- Skoglof, A.; Nilsson, I.; Gustafsson, S.; Deinum, J.; Göthe, P.O. Cis-trans isomerization of an angiotensin I-converting enzyme inhibitor. An enzyme kinetic and nuclear magnetic resonance study. Biochim. Biophys. Acta 1990, 1041, 22–30. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL 2014/2; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
| Solvent | Ratio syn/anti | Δν [Hz] | kexc [Hz] | TC [K] | ΔG‡ [kJ/mol] |
|---|---|---|---|---|---|
| Benzene-d6 | 1.5:1 | 257 (a) 381 (s) | 313 (a) 494 (s) | n.d. | - - |
| CDCl3 | 1.2:1 | 141 (a) 223 (s) | 244 (a) 395 (s) | n.d. | - - |
| TCE-d2 | 1.1:1 | 136 (a) 224 (s) | 571 (a) 846 (s) | 356 | 70.8 69.3 |
| Acetone-d6 | 1.2:1 | 110 (a) 223 (s) | 302 (a) 498 (s) | n.d. | - - |
| ACN-d3 | 1.1:1 | 116 (a) 213 (s) | 258 (a) 473 (s) | 353 | 70.7 68.9 |
| CD3OD | 1.1:1 | 119 (a) 202 (s) | 264 (a) 448 (s) | n.d. | - - |
| DMSO-d6 | 1.2:1 | 116 (a) 202 (s) | 258 (a) 448 (s) | 353 | 70.7 69.0 |
| Compound | 3b |
|---|---|
| Formula | C18H14F4N2O2 |
| Formula weight (g·mol−1) | 366.31 |
| Temperature (K) | 123 |
| Crystal system | monoclinic |
| Space group | P21/c |
| Unit cell dimensions: | |
| a (Å) | 7.2687(3) |
| b (Å) | 17.2658(8) |
| c (Å) | 6.9738(3) |
| β (°) | 115.393(2) |
| Volume (Å3), Z | 790.65(6), 2 |
| Data/restraints/param. | 2955/0/157 |
| Measured reflections | 23,516 |
| 2θmax (°) | 66.0 |
| GoF on F2 | 1.08 |
| R1 [I > 2σ(I)] | 0.058 |
| wR2 (all data) | 0.154 |
| Larg. diff. peak/hole (e·Å3) | 0.36/−0.31 |
| Solvent | Δν [Hz] | kexc [Hz] | TC [K] | ΔG‡ [kJ/mol] |
|---|---|---|---|---|
| CDCl3 | 45 185 | 100 (amine) 411 (amide) | 330 (amine) n.d. (amide) | 68.5 - |
| TCE-d2 | 48 160 | 107 (amine) 355 (amide) | 336 (amine) 355 (amide) | 69.6 70.1 |
| DMSO-d6 | 46 160 | 102 (amine) 355 (amide) | 334 (amine) 352 (amide) | 69.3 69.5 |
| Compound | Δν [Hz] | kexc [Hz] | TC [K] | ΔG‡ [kJ/mol] |
|---|---|---|---|---|
| 3a | 116 (anti) 202 (syn) | 258 (anti) 448 (syn) | 353 | 70.7 69.0 |
| 3b | 48 (amine) 160 (amide) | 107 (amine) 355 (amide) | 336 (amine) 355 (amide) | 69.6 70.1 |
| 4 | 56 (CH3) 130 (CH2) | 124 (CH3) 289 (CH2) | 358 (CH3) 370 (CH2) | 73.9 73.8 |
| 5 | 171 (amide) 41 (CH2) | 380 (amide) 91 (CH2) | 371 348 | 73.2 72.6 |
| 6 | 119 | 264 | 361 | 72.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köckerling, M.; Mamat, C. Synthesis, Dynamic NMR Characterization, and XRD Study of 2,4-Difluorobenzoyl-Substituted Piperazines. Chemistry 2025, 7, 162. https://doi.org/10.3390/chemistry7050162
Köckerling M, Mamat C. Synthesis, Dynamic NMR Characterization, and XRD Study of 2,4-Difluorobenzoyl-Substituted Piperazines. Chemistry. 2025; 7(5):162. https://doi.org/10.3390/chemistry7050162
Chicago/Turabian StyleKöckerling, Martin, and Constantin Mamat. 2025. "Synthesis, Dynamic NMR Characterization, and XRD Study of 2,4-Difluorobenzoyl-Substituted Piperazines" Chemistry 7, no. 5: 162. https://doi.org/10.3390/chemistry7050162
APA StyleKöckerling, M., & Mamat, C. (2025). Synthesis, Dynamic NMR Characterization, and XRD Study of 2,4-Difluorobenzoyl-Substituted Piperazines. Chemistry, 7(5), 162. https://doi.org/10.3390/chemistry7050162







