Coordination Chemistry of Polynitriles, Part XIII: Influence of 4,4′-Bipyridine on the Crystal and Molecular Structures of Alkali Metal Pentacyanocyclopentadienides
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Compounds
2.1.1. Reaction of [Li{C5(CN)5}(H2O)] with 4,4′-Bipyridine
2.1.2. Reaction of [Na{C5(CN)5}(MeOH)] with 4,4′-Bipyridine
2.1.3. Reaction of [KC5(CN)5] with 4,4′-Bipyridine
2.2. Crystallography
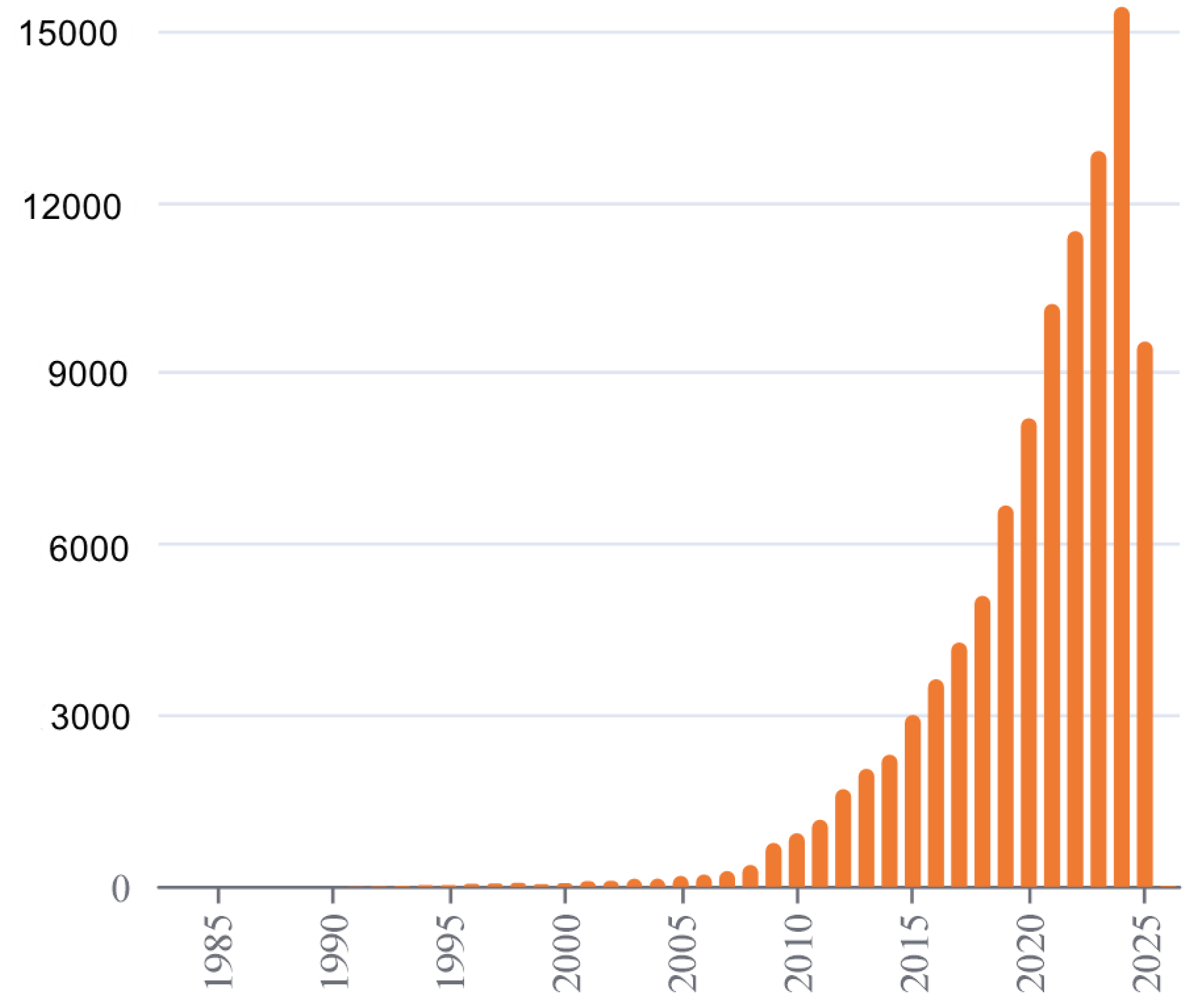
3. Results
3.1. Synthesis
3.2. Molecular Structures
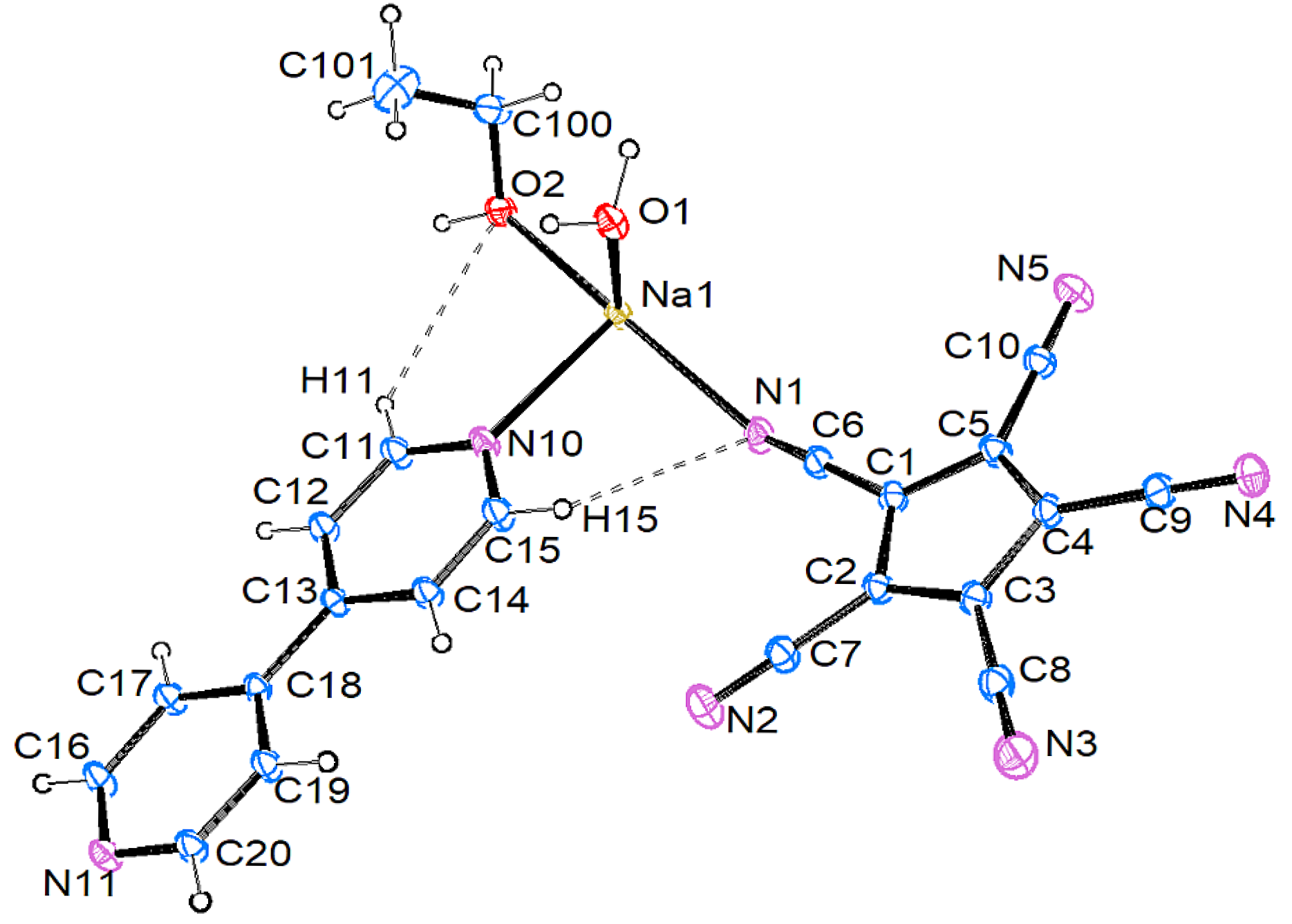
3.2.1. [Na{C5(CN)5}(EtOH)(H2O)(4,4′-C10H8N2)] (1)
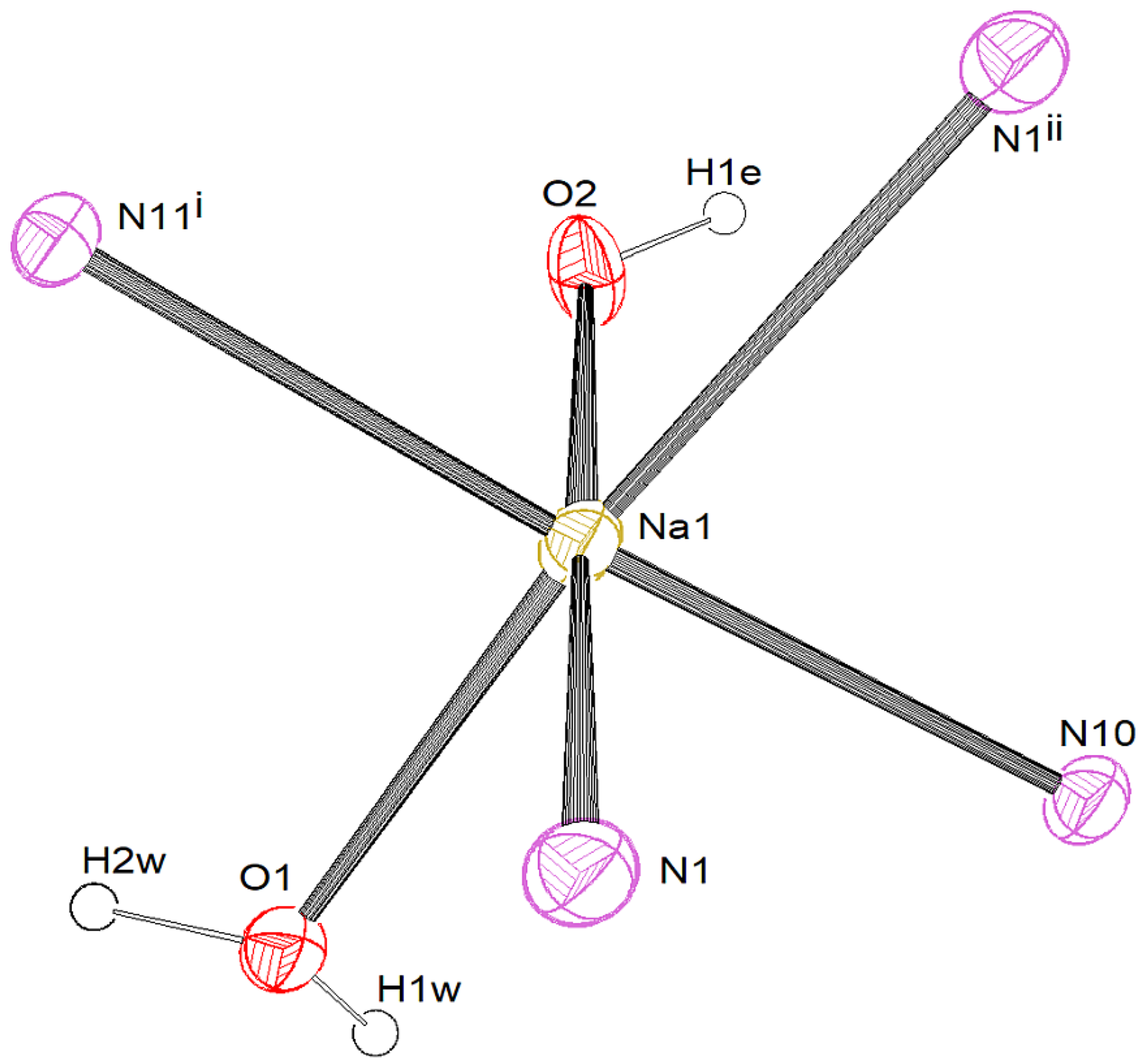
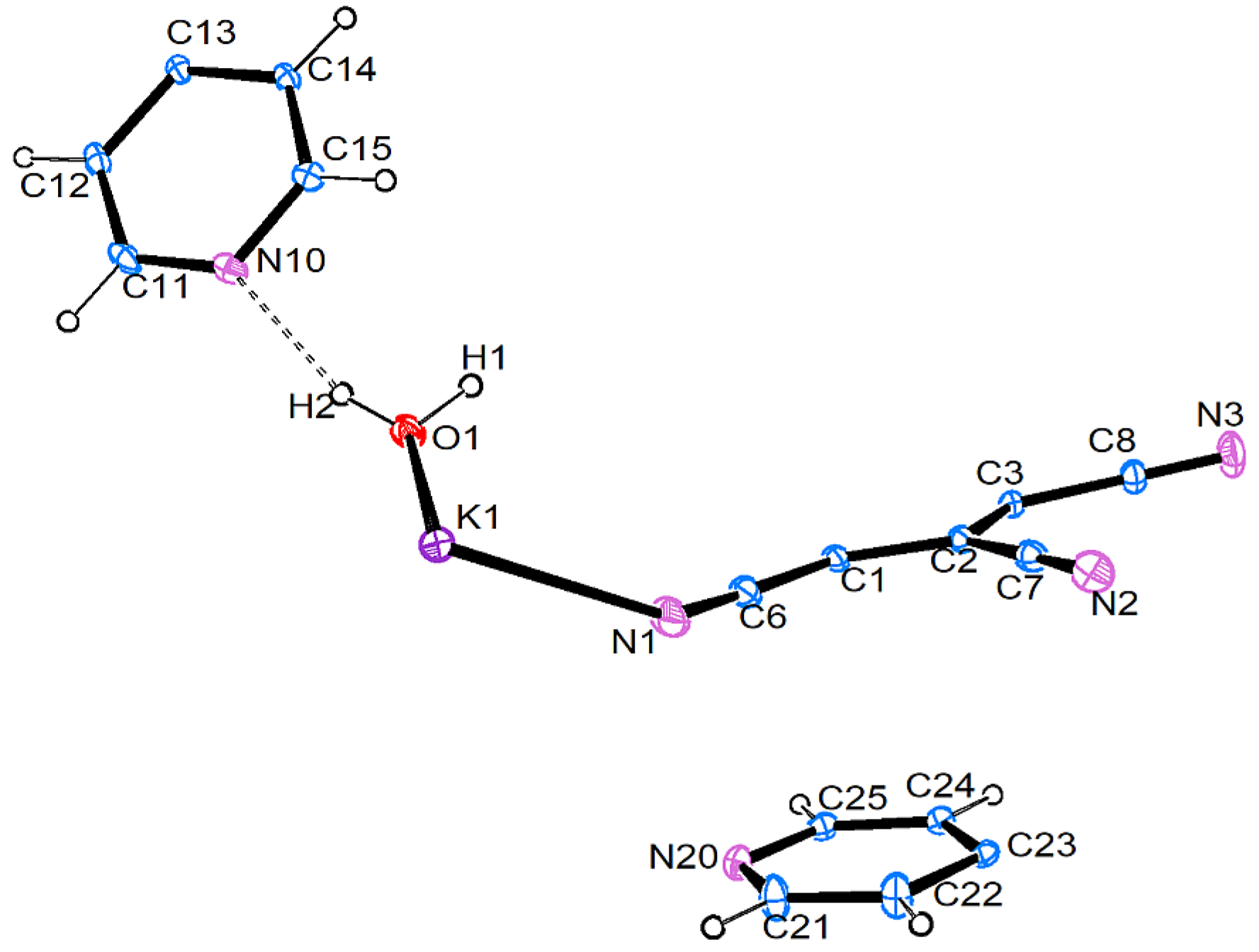
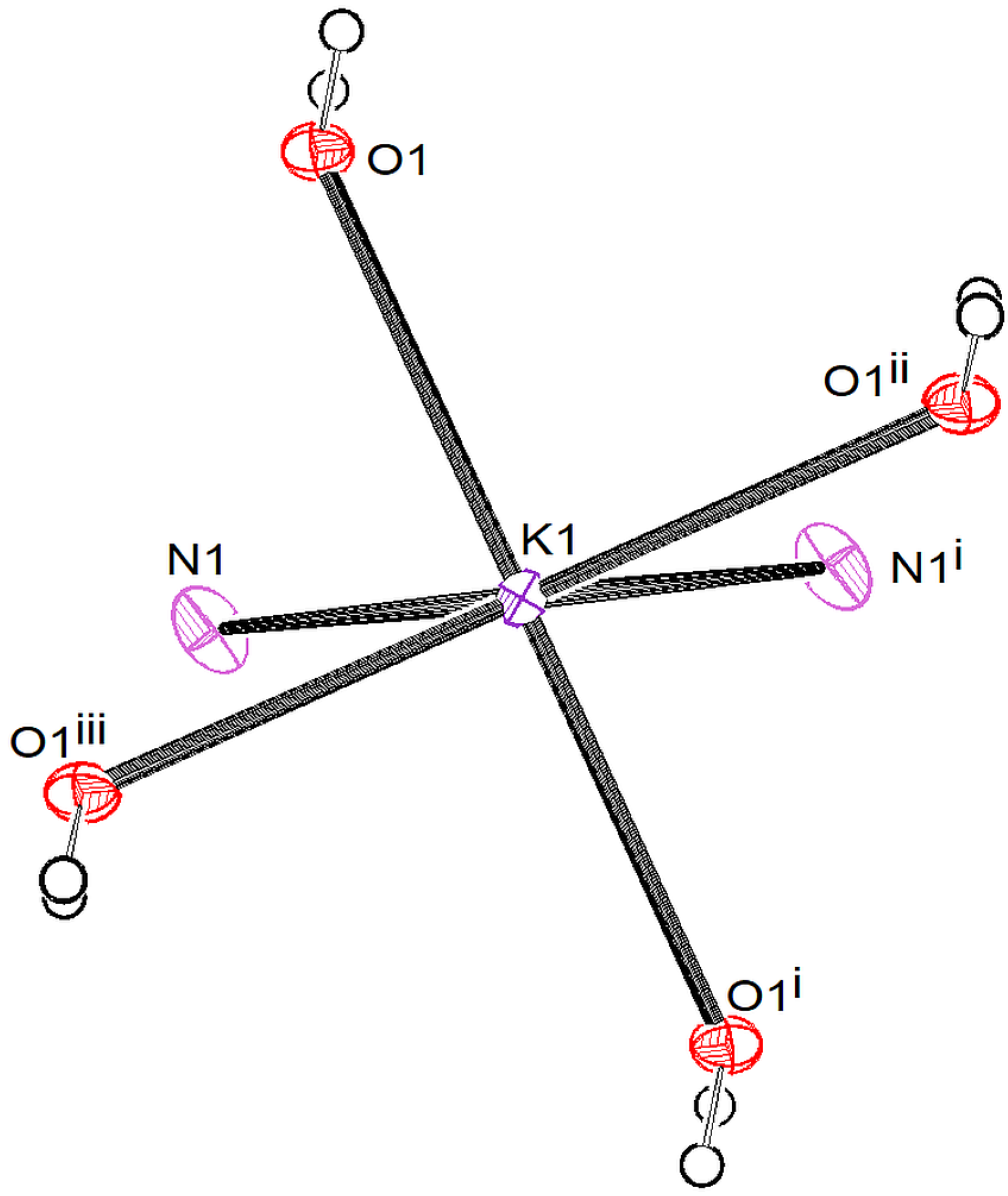
3.2.2. [K{C5(CN)5}(H2O)2] • 2 (4,4′-C10H8N2) (2)
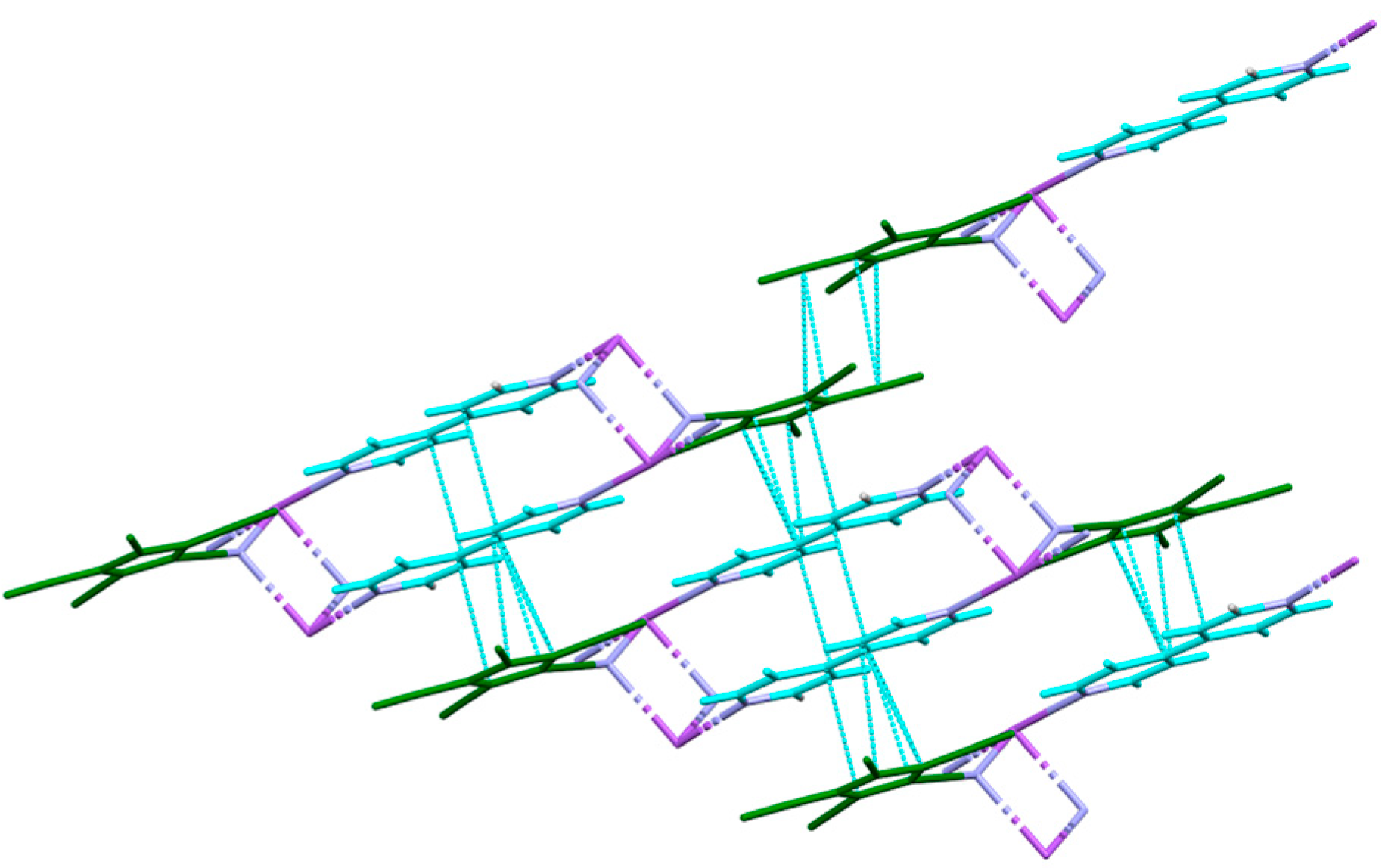
3.3. Crystal Structures and Intermolecular Interactions
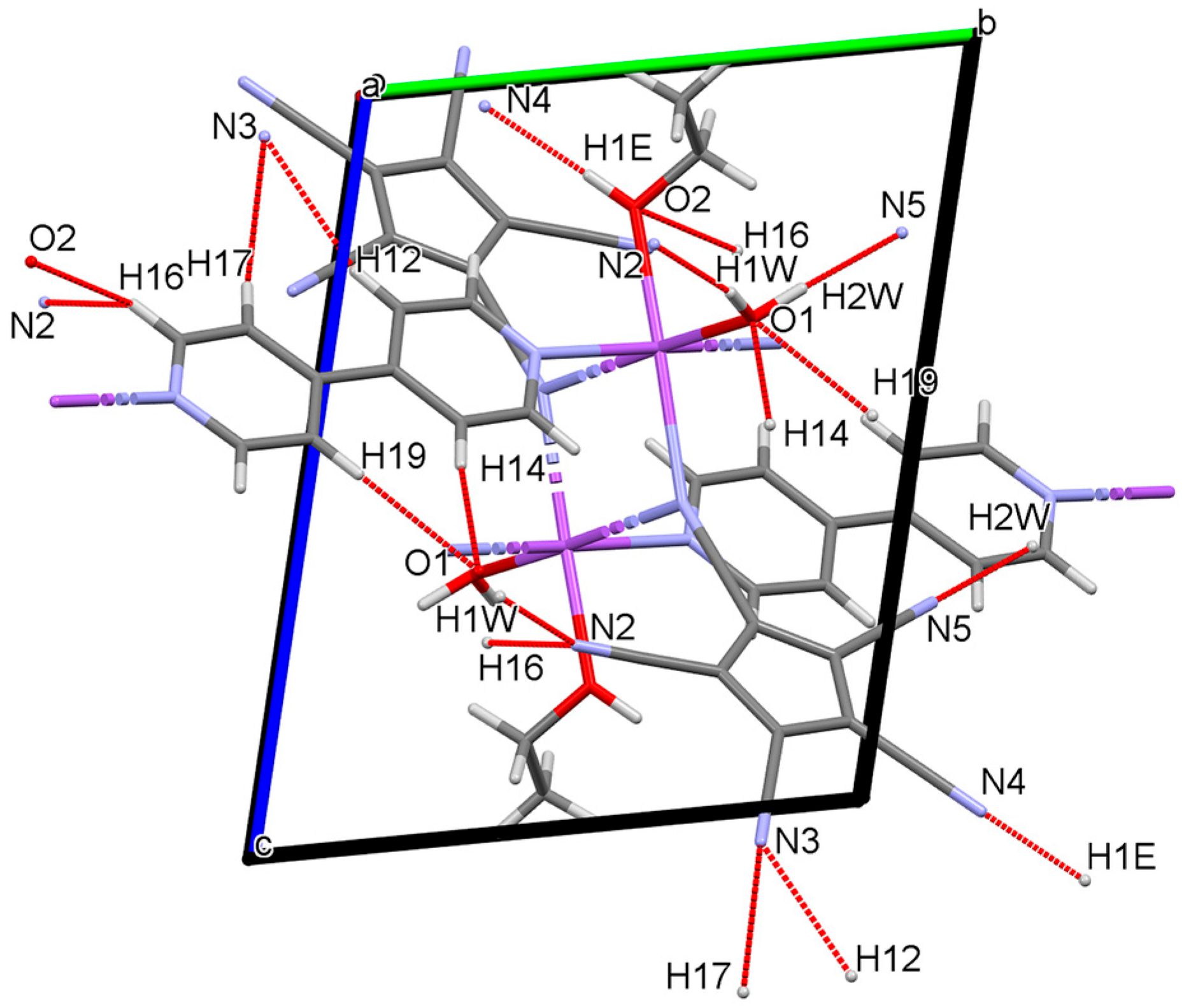
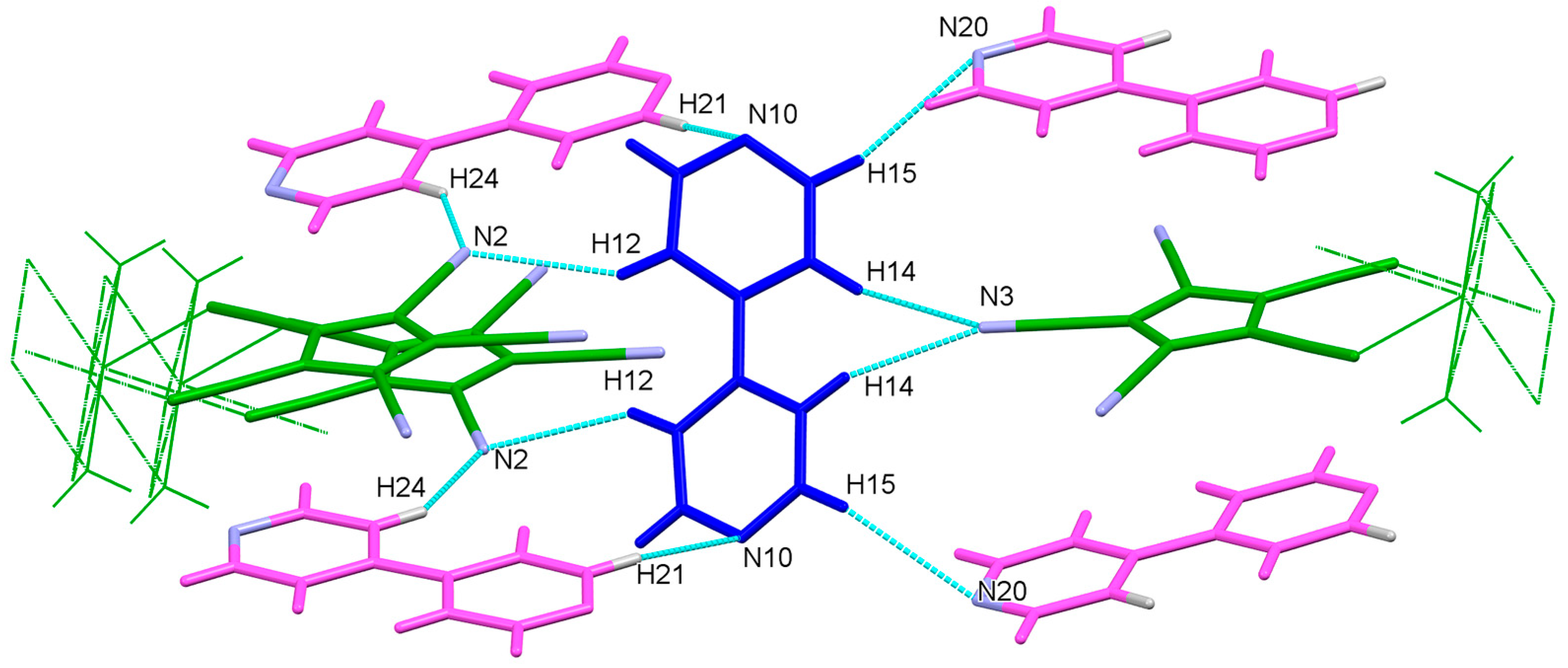
3.3.1. Hydrogen Bonding: O–H…X and C–H…X
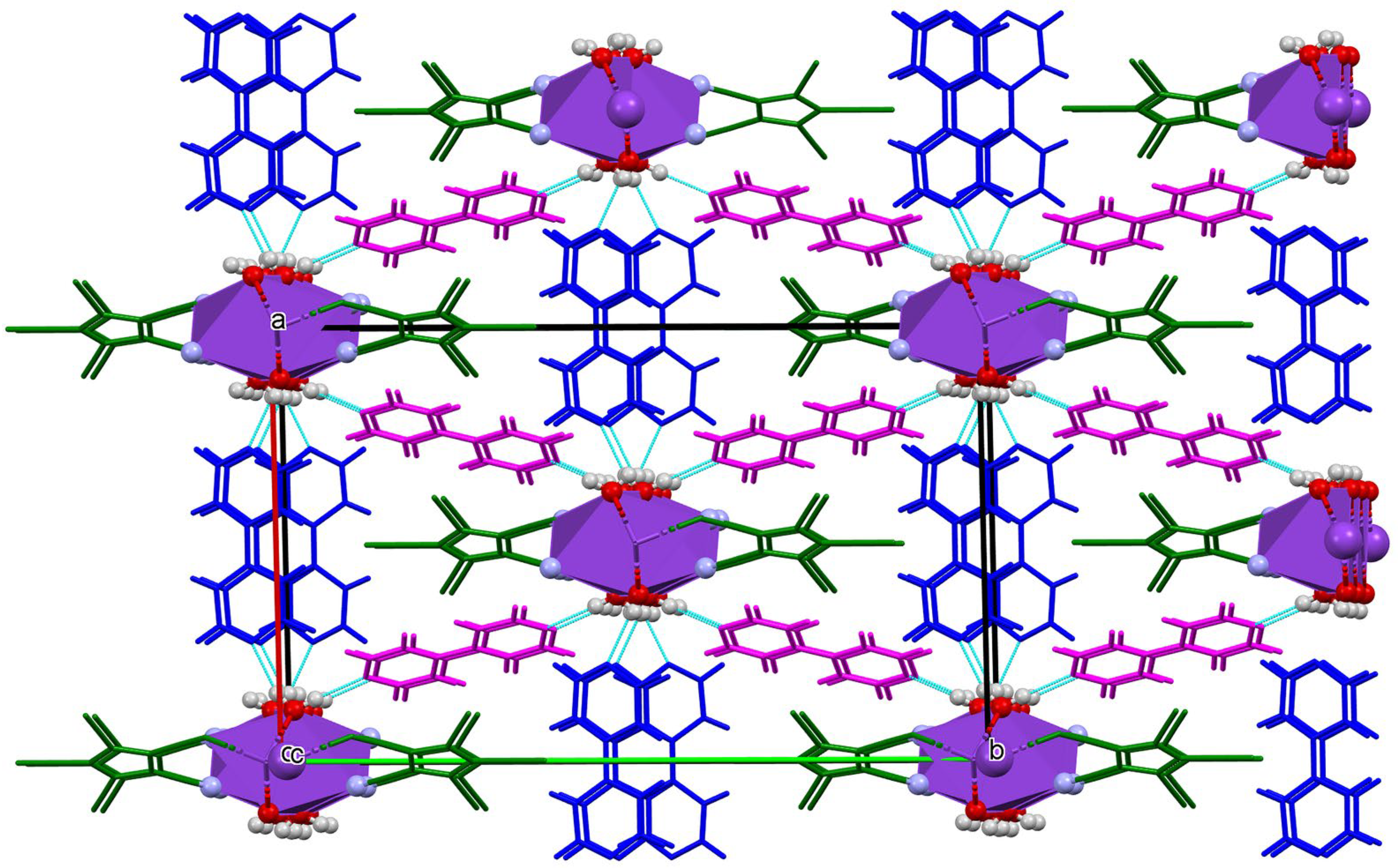
3.3.2. π Stacking Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Remya, V.R.; Kurian, M. Synthesis and catalytic applications of metal-organic frameworks: A review on recent literature. Int. Nano Lett. 2019, 9, 17–29. [Google Scholar] [CrossRef]
- Saboor, F.H.; Shahsavari, S.; Zandjou, M.; Asgari, M. From Structure to Catalysis: Advances in Metal-Organic Frameworks-Based Shape-Selective Reactions. ChemNanoMat 2024, 10, e202400049. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal-Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Jyoti; Dutta, T.; Kumar, P.; Jangra, R.; Sharma, A.K.; Singh, M.; Chaturvedi, P.; Sharma, S.; Garita, M.R.; Sharma, J.; et al. Recent advances in Metal-Organic Framework-Based fiber optic sensors and Photodetectors: Synthesis, Properties and applications. Chem. Eng. J. 2025, 507, 160543. [Google Scholar] [CrossRef]
- Jose, R.; Bangar, G.; Pal, S.; Rajaraman, G. Role of molecular modelling in the development of metal-organic framework for gas adsorption applications. J. Chem. Sci. 2023, 135, 19. [Google Scholar] [CrossRef]
- Gulati, S.; Choudhury, A.; Mohan, G.; Katiyar, R.; Kurikkal M P, M.A.; Kumar, S.; Varma, R.S. Metal-organic frameworks (MOFs) as effectual diagnostic and therapeutic tools for cancer. J. Mater. Chem. B 2023, 11, 6782–6801. [Google Scholar] [CrossRef]
- Ding, W.; Zhang, T.; Fu, R.; Yu, C.; Zhang, C.; Wang, K.; Zhang, P. Nano-sized coordination polymer particles (CPP) for synergetic application in antibacterial and anticancer therapeutics. Chem. Eng. J. 2025, 516, 163905. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Fromm, K.M. Coordination polymer networks with s-block metal ions. Coord. Chem. Rev. 2008, 252, 856–885. [Google Scholar] [CrossRef]
- Miao, J.; Graham, W.; Liu, J.; Hill, E.C.; Ma, L.-L.; Ullah, S.; Xia, H.-L.; Guo, F.-A.; Thonhauser, T.; Proserpio, D.M.; et al. An Octacarboxylate-Linked Sodium Metal-Organic Framework with High Porosity. J. Am. Chem. Soc. 2024, 146, 84–88. [Google Scholar] [CrossRef]
- Jyai, R.N.; Näther, C.; Bensch, W.; Srinivasan, B.R. Structural characterization of sodium and potassium 3-nitrohydrogenphthalate coordination polymers. Z. Naturforsch. 2022, 77b, 433–440. [Google Scholar] [CrossRef]
- Ye, G.; Chen, C.; Lin, J.; Peng, X.; Kumar, A.; Liu, D.; Liu, J. Alkali/ alkaline earth-based metal-organic frameworks for biomedical applications. Dalton Trans. 2021, 50, 17438–17454. [Google Scholar] [CrossRef]
- Hou, R.; Guo, Y.; Yi, Z.; Zhang, Z.; Zhang, C.; Xu, W. Construction and Structural Transformation of Metal-Organic Nanostructures Induced by Alkali Metals and Alkali Metal Salts. J. Phys. Chem. Lett. 2023, 14, 3636–3642. [Google Scholar] [CrossRef]
- Bilal, H.; Zahoor, S.; Choudhary, M.I.; Shaheen, F.; Dej-adisai, S.; Liang, H.; Chen, Z.-F. Carboxylate embedded metal organic frameworks for potential applications in drug delivery and controlled release. Coord. Chem. Rev. 2025, 538, 216688. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Samsonenko, D.G.; Fedin, V.P. Porous Coordination Polymers Based on Carboxylate Complexes of 3d Metals. Russ. J. Coord. Chem. 2016, 42, 557–573. [Google Scholar] [CrossRef]
- Biradha, K.; Sarkar, M.; Rajput, L. Crystal Engineering of coordination polymers using 4,4′-bipyridine as a bond between transition metal atoms. Chem. Commun. 2006, 42, 4169–4179. [Google Scholar] [CrossRef]
- Darwish, S.; Wang, S.-Q.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Comparison of Mechanochemistry vs Solution Methods for Synthesis of 4,4′-Bipyridine-Based Coordination Polymers. ACS Sustain. Chem. Eng. 2019, 7, 19505–19512. [Google Scholar] [CrossRef]
- Adarsh, N.N.; Dastidar, P. Coordination Polymers: What has been achieved in going from innocent 4,4′-bipyridine to bis-pyridyl ligands having a non-innocent backbone? Chem. Soc. Rev. 2012, 41, 3039–3060. [Google Scholar] [CrossRef]
- Verma, C.; Yaagoob, I.Y.; Goni, L.K.M.O.; Abdelkreem, S.S.E.; Mubarak, S.A.; Al-Mohsin, H.A.M.; Alfantazi, A.; Mazumder, M.A.J. Coordination complexes of Bipyridines (CCBs): Chemistry, bonding and applications. Coord. Chem. Rev. 2025, 529, 216433. [Google Scholar] [CrossRef]
- Bazyakina, N.L.; Moskalev, M.V.; Cherkasov, A.V.; Makarov, V.M.; Fedushkin, I.L. Coordination polymers derived from alkali metal complexes of redox-active ligands. CrystEngComm 2022, 24, 2297–2304. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Batten, S.R.; Hoskins, B.F.; Robson, R. AgC(CN)3-Based Coordination Polymers. Inorg. Chem. 2003, 42, 2654–2664. [Google Scholar] [CrossRef]
- Batten, S.R.; Deacon, G.B.; Murray, K.S.; Turner, D.R. Coordination Polymers of Small Cyano Anions. Chimia 2013, 67, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Less, R.J.; McPartlin, M.; Rawson, J.M.; Wood, P.T.; Wright, D.S. A Simple Approach to Coordination Compounds of the Pentacyanocyclopentadienide Anion. Chem. Eur. J. 2010, 16, 13723–13728. [Google Scholar] [CrossRef] [PubMed]
- Less, R.J.; Wilson, T.C.; Guan, B.; McPartlin, M.; Steiner, A.; Wood, P.T.; Wright, D.S. Solvent Direction of Molecular Architectures in Group I Metal Pentacyanocyclopentadienides. Eur. J. Inorg. Chem. 2013, 42, 1161–1169. [Google Scholar] [CrossRef]
- Sünkel, K.; Reimann, D.; Nimax, P. Synthesis, molecular, and crystal structures of 3d transition metal cyanocyclopentadienides [M(MeOH)n(H2O)4−n{C5(CN)4X}2] (M=Mn, Fe, Co, Ni, Cu, Zn; X=H, CN, NH2, NO2). Z. Naturforsch. 2019, 74B, 109–118. [Google Scholar] [CrossRef]
- Benmansour, S.; Atmani, C.; Setifi, F.; Triki, S.; Marchivie, M.; Gomez-Garcia, C.J. Polynitrile anions as ligands: From magnetic polymeric architectures to spin crossover materials. Coord. Chem. Rev. 2010, 254, 1468–1478. [Google Scholar] [CrossRef]
- Nimax, P.R.; Sünkel, K. Coordination Chemistry of Polynitriles, Part XI. Influence of 4,4′-Bipyridine and Solvent on the Crystal and Molecular Structures of Alkaline Earth Pentacyanocyclopentadienides. Chemistry 2022, 4, 1524–1545. [Google Scholar] [CrossRef]
- Nimax, P.R.; Sünkel, K. Sodium Salts of Substituted Tetracyanocyclopentadienides [C5(CN)4X]− (X= H, CN, NH2, NO2): Substituent Influence on the Crystal Structures of Potential Coordination Polymers. ChemistrySelect 2018, 3, 3330–3337. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Cryst. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WINGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Näther, C.; Greve, J.; Jeß, I. Poly[tetra-µ2-azido-di-µ2-4,4′-bipyridine-sodium(I)iron(III)]. Acta Cryst. 2005, E61, m317–m319. [Google Scholar]
- Reiß, A.; Göttlicher, J.; Vitova, T.; Feldmann, C. Semiconducting Manganese-Bipyridyl Coordination Polymer via a Manganese-Metal Nanoparticle Approach. Inorg. Chem. 2025, 64, 9469–9476. [Google Scholar] [CrossRef] [PubMed]
- Denning, M.S.; Irwin, M.; Goicoechea, J.M. Synthesis and Characterization of the 4,4′-Bipyridyl Dianion and Radical Monoanion. A Structural Study. Inorg. Chem. 2008, 47, 6118–6120. [Google Scholar] [CrossRef]
- Roesky, H.W.; Andruh, M. The interplay of coordinative, hydrogen bonding and π-π stacking interactions in sustaining supramolecular solid-state architectures. A study case of bis(4-pyridyl)- and bis(4-pyridyl-N-oxide) tectons. Coord. Chem. Rev. 2003, 236, 91–119. [Google Scholar] [CrossRef]
- Blake, A.J.; Baum, G.; Champness, N.R.; Chung, S.S.M.; Cooke, P.A.; Fenske, D.; Khlobystov, A.N.; Lemnovskii, D.A.; Li, W.-S.; Schröder, M. Long-range chain orientation in 1-D co-ordination polymers as a function of anions and intermolecular aromatic interactions. Dalton Trans. 2000, 4285–4291. [Google Scholar] [CrossRef]
- Carter-Fenk, K.; Herbert, J.M. Reinterpreting π stacking. Phys. Chem. Chem. Phys. 2020, 22, 24870–24886. [Google Scholar] [CrossRef]


| Comp. | D-H…A | D-H [Å] | H…A [Å] | D...A [Å] | <(DHA) [°] | Symm.op. |
|---|---|---|---|---|---|---|
| 1 | O1-H1W…N2 | 0.85(3) | 2.06(3) | 2.894(3) | 166(3) | −x,1−y, 1−z |
| O1-H2W…N5 | 0.94(4) | 2.01(5) | 2.932(3) | 167(4) | 1−x, 2−y, 1−z | |
| O2-H1E…N4 | 0.91(3) | 2.02(3) | 2.927(3) | 177(3) | x, y−1, z−1 | |
| C12-H12…N3 | 0.95 | 2.62 | 3.542(3) | 163 | x, y−1, z−1 | |
| C14-H14…O1 | 0.95 | 2.53 | 3.474(3) | 172 | −x, 1−y, 1−z | |
| C19-H19…O1 | 0.95 | 2.57 | 3.518(3) | 176 | −x, 1−y, 1−z | |
| 2 | C12-H12…N2 | 0.95 | 2.73 | 3.539(2) | 144 | x+1/2, y+1/2, z |
| C14-H14…N3 | 0.95 | 2.57 | 3.437(3) | 152 | −x−1/2, −y+1/2, −z+1 | |
| C15-H15…N20 | 0.95 | 2.81 | 3.647(2) | 148 | −x−1, y, −z+1/2 | |
| C21-H21…N10 | 0.95 | 2.65 | 3.546(2) | 157 | −x−1, −y+1, −z+1 | |
| C24-H24…N2 | 0.95 | 2.50 | 3.323(2) | 145 | x, y, z−1 | |
| O1-H1…N20 | 0.88(2) | 2.00(2) | 2.875(2) | 177(2) | −x−1, y, −z+1/2 | |
| O1-H2…N10 | 0.85(2) | 2.10(2) | 2.914(2) | 160(2) | x, y, z |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nimax, P.R.; Sünkel, K. Coordination Chemistry of Polynitriles, Part XIII: Influence of 4,4′-Bipyridine on the Crystal and Molecular Structures of Alkali Metal Pentacyanocyclopentadienides. Chemistry 2025, 7, 157. https://doi.org/10.3390/chemistry7050157
Nimax PR, Sünkel K. Coordination Chemistry of Polynitriles, Part XIII: Influence of 4,4′-Bipyridine on the Crystal and Molecular Structures of Alkali Metal Pentacyanocyclopentadienides. Chemistry. 2025; 7(5):157. https://doi.org/10.3390/chemistry7050157
Chicago/Turabian StyleNimax, Patrick R., and Karlheinz Sünkel. 2025. "Coordination Chemistry of Polynitriles, Part XIII: Influence of 4,4′-Bipyridine on the Crystal and Molecular Structures of Alkali Metal Pentacyanocyclopentadienides" Chemistry 7, no. 5: 157. https://doi.org/10.3390/chemistry7050157
APA StyleNimax, P. R., & Sünkel, K. (2025). Coordination Chemistry of Polynitriles, Part XIII: Influence of 4,4′-Bipyridine on the Crystal and Molecular Structures of Alkali Metal Pentacyanocyclopentadienides. Chemistry, 7(5), 157. https://doi.org/10.3390/chemistry7050157






