[1,2,5]Oxadiazolo[3,4-b]dithieno[2,3-f:2′,3′-h]quinoxaline as a Versatile Scaffold for the Construction of Various Polycyclic Systems as Potential Organic Semiconductors
Abstract
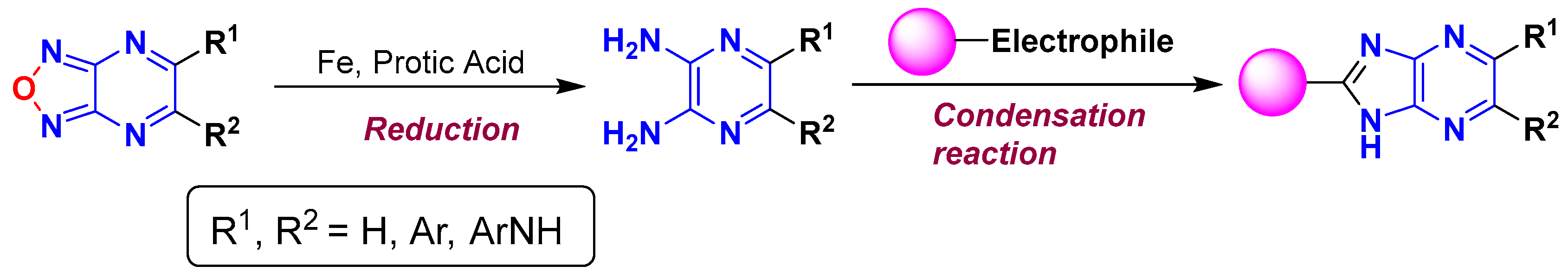
1. Introduction
2. Experimental
3. Results and Discussion
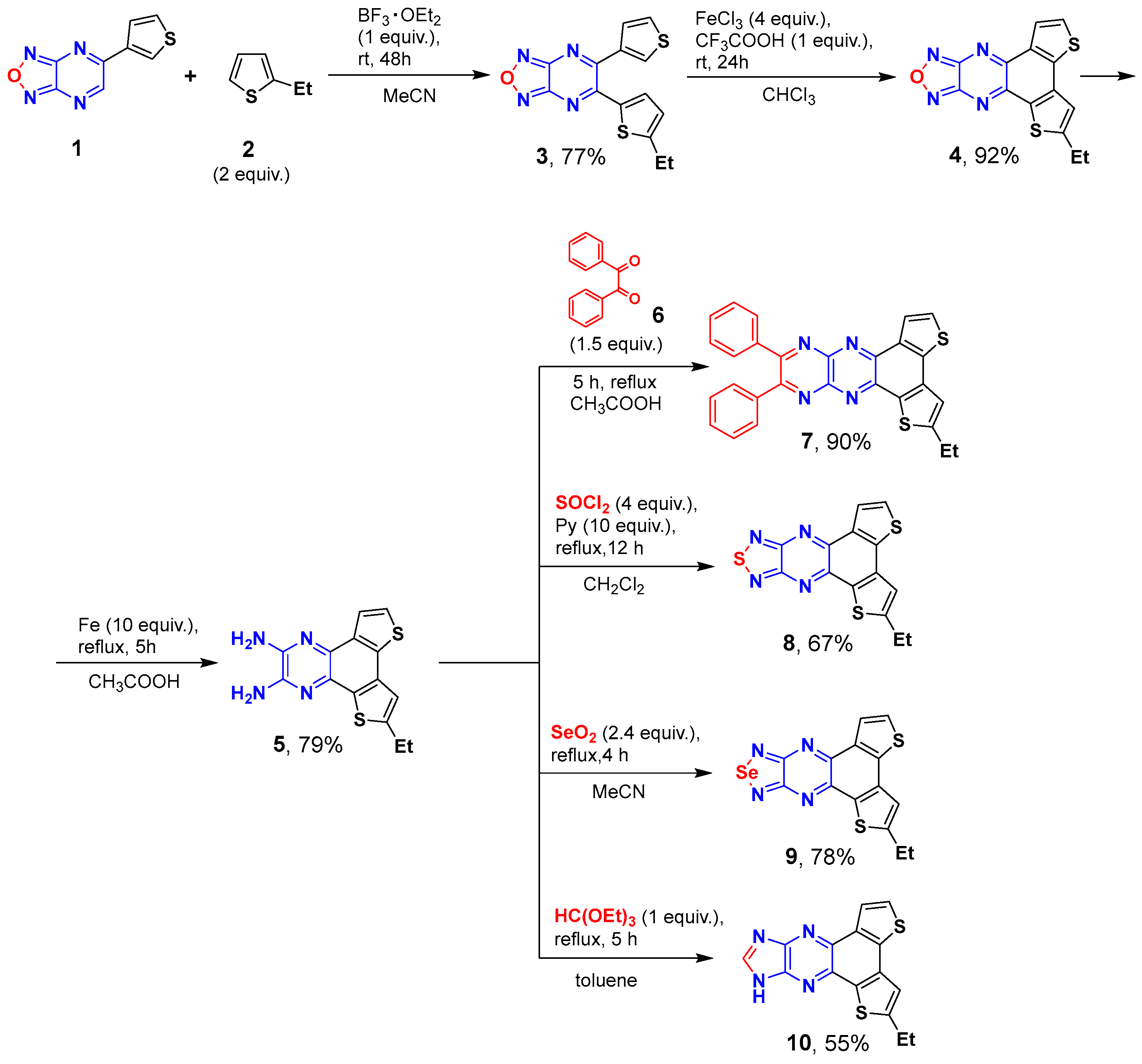
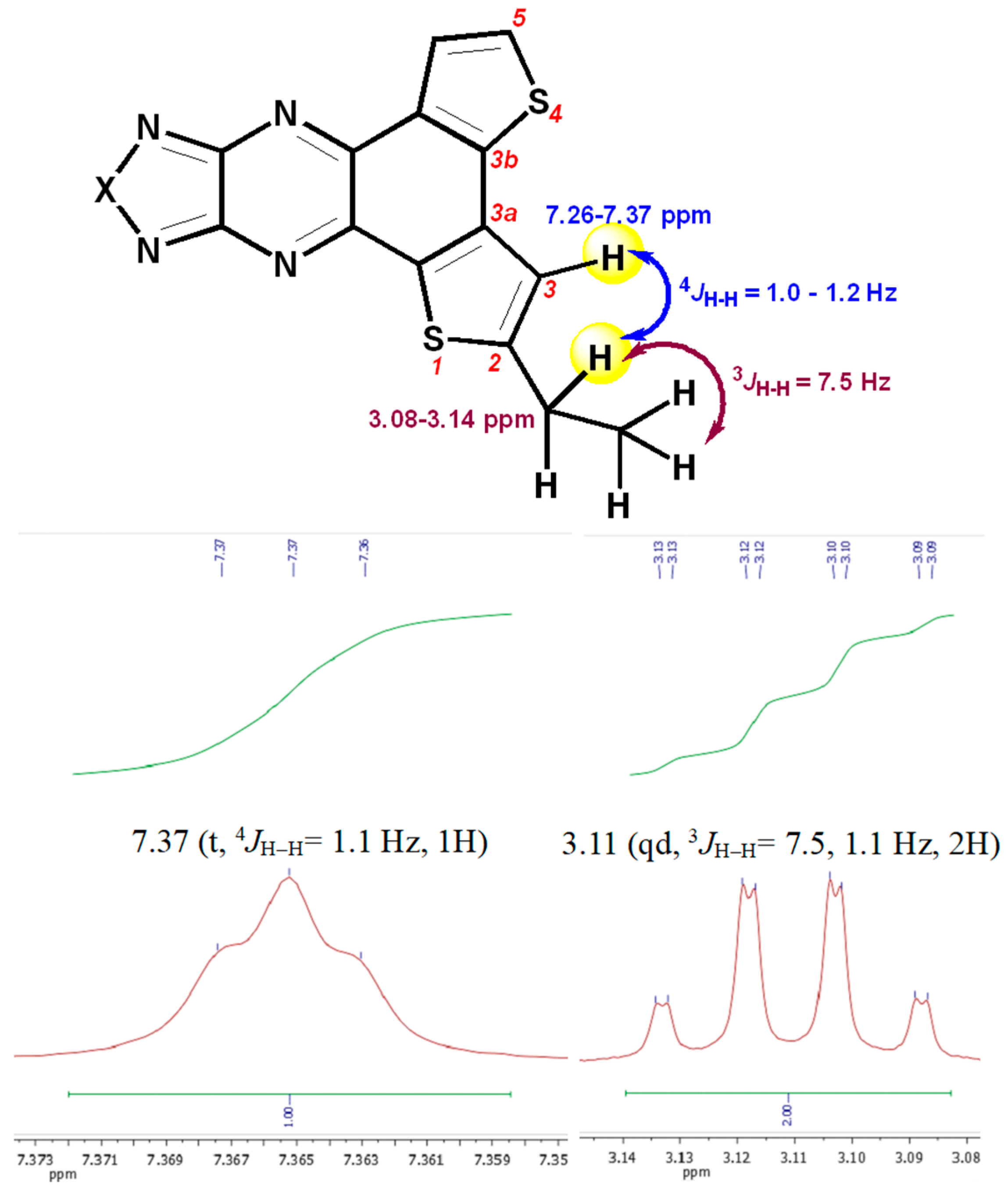
3.1. Synthesis and Thermal Properties
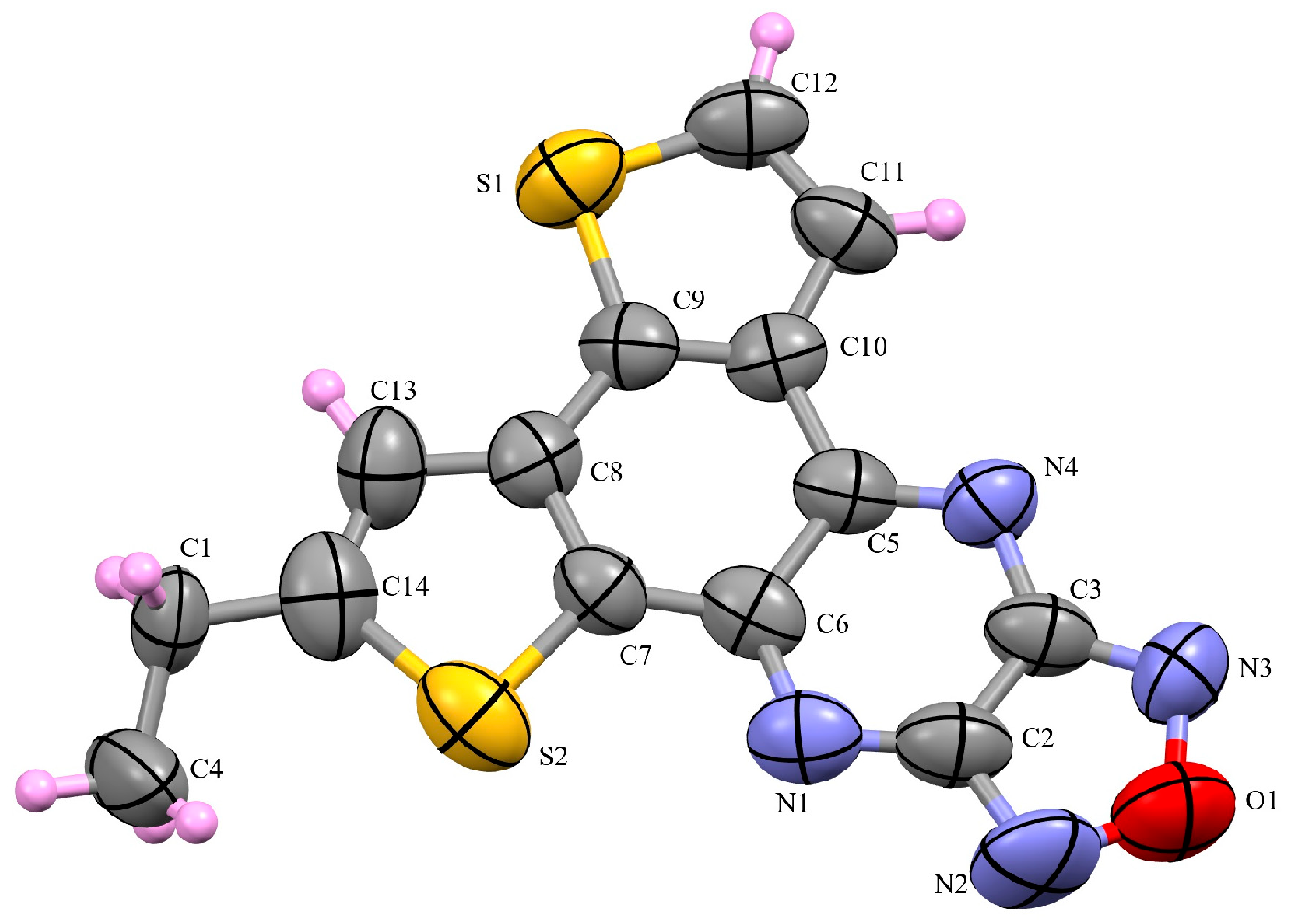
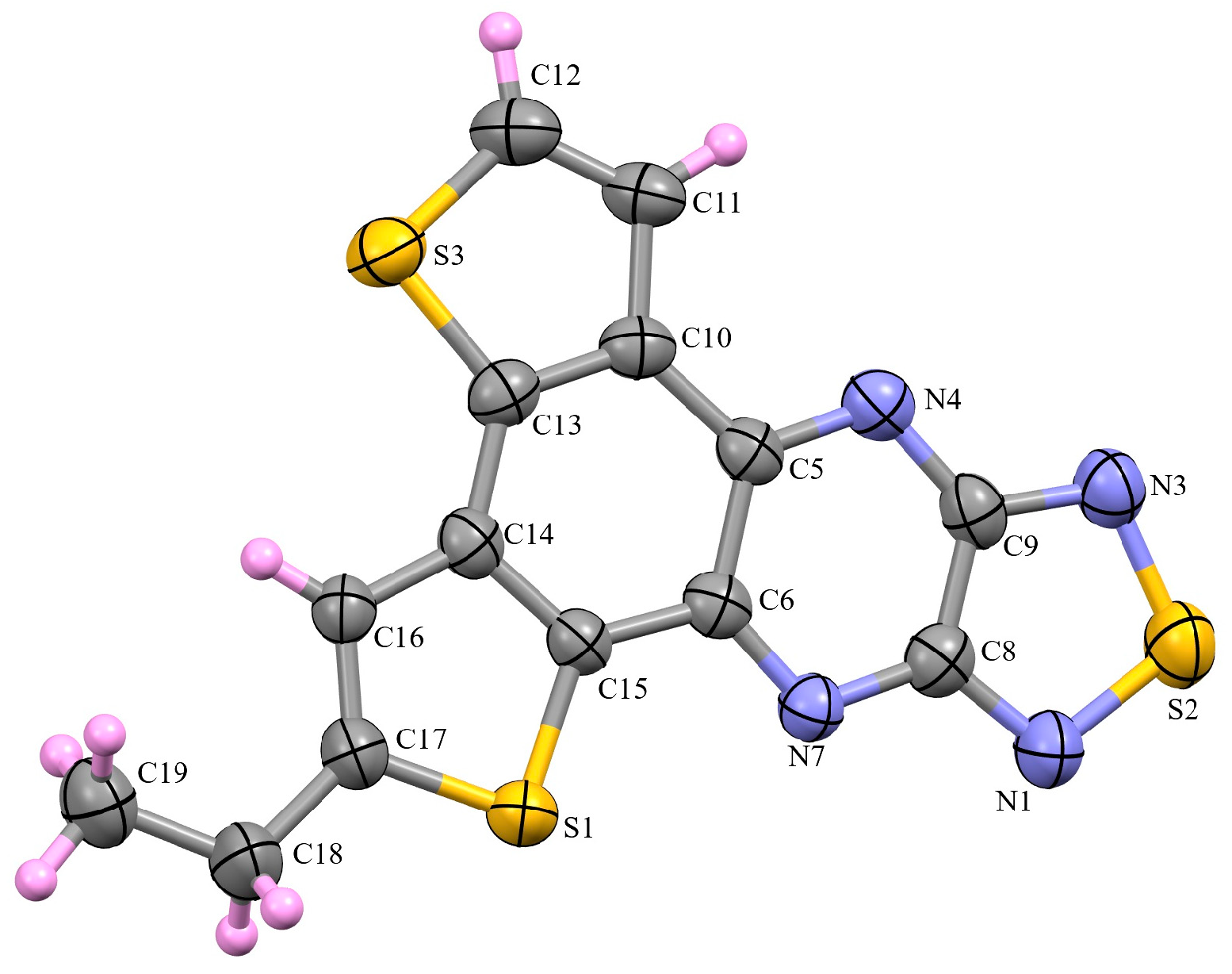
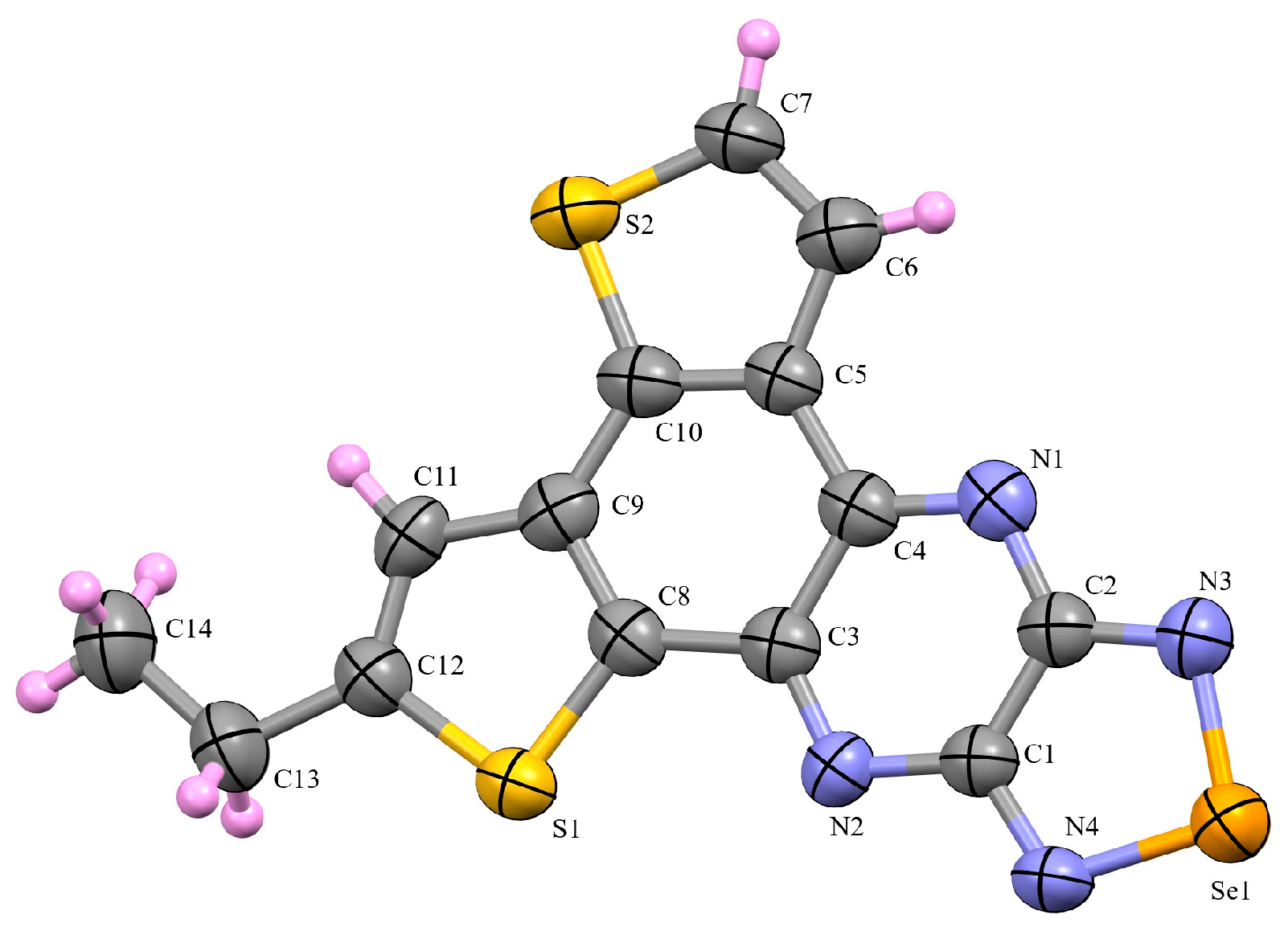
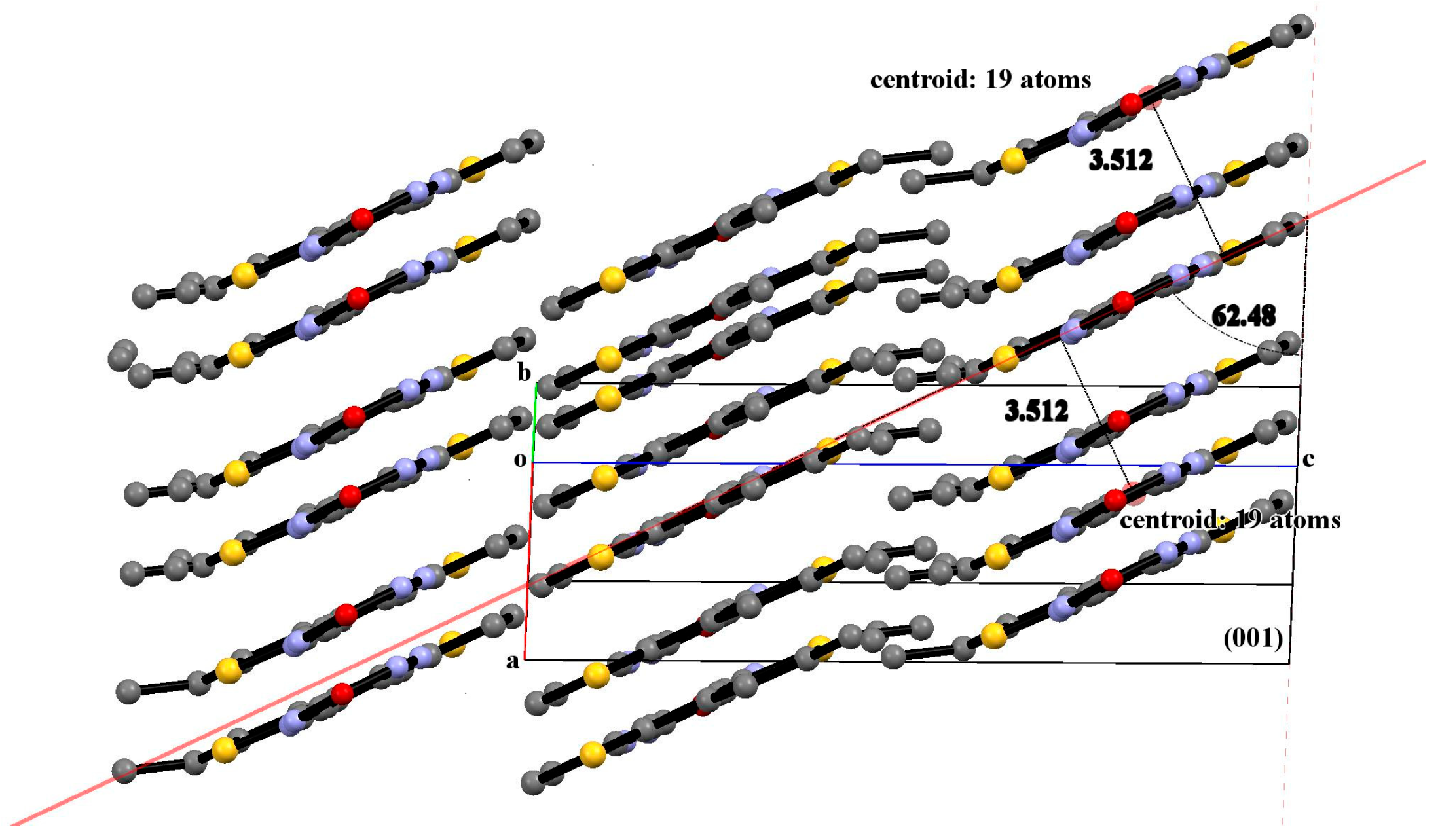
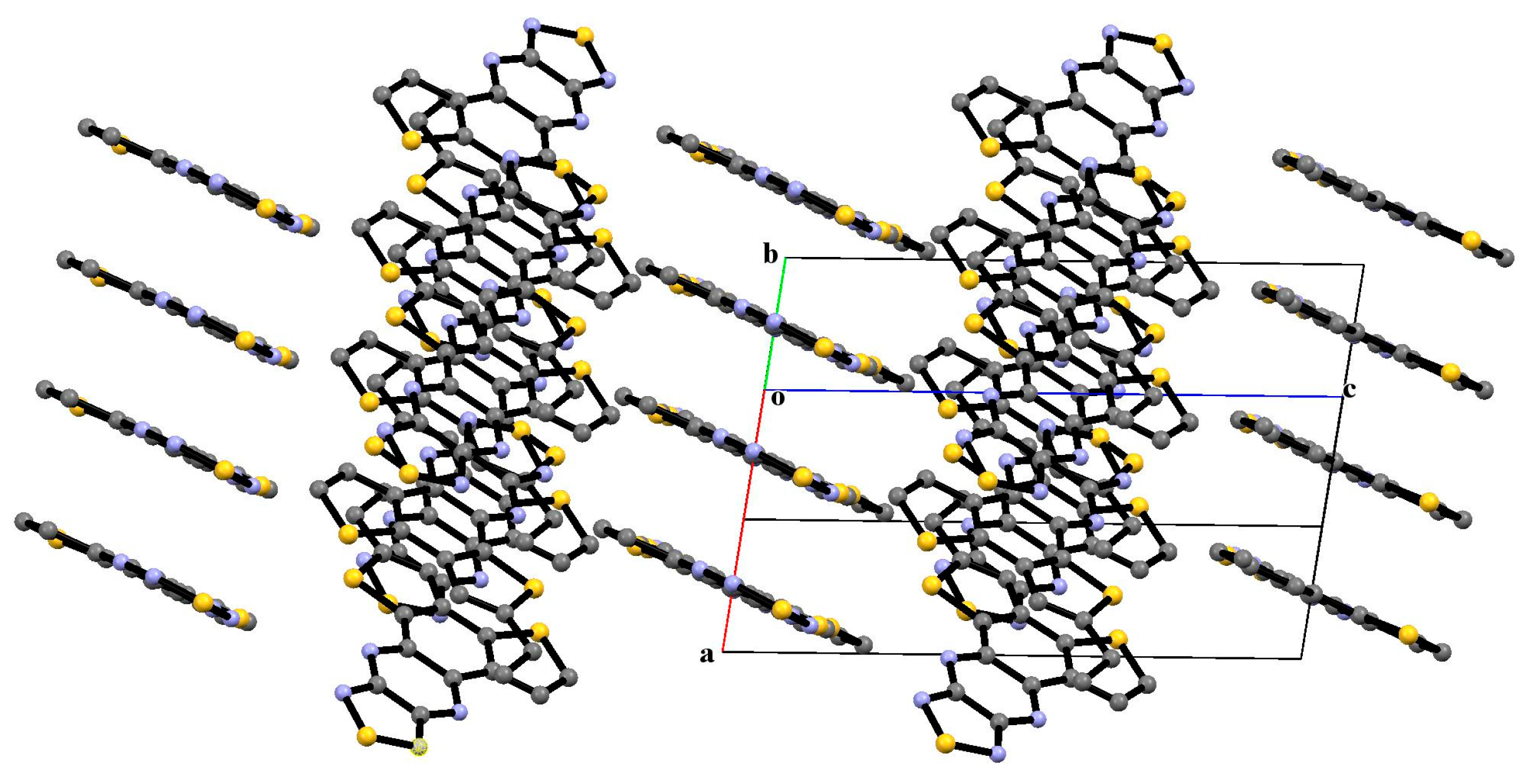
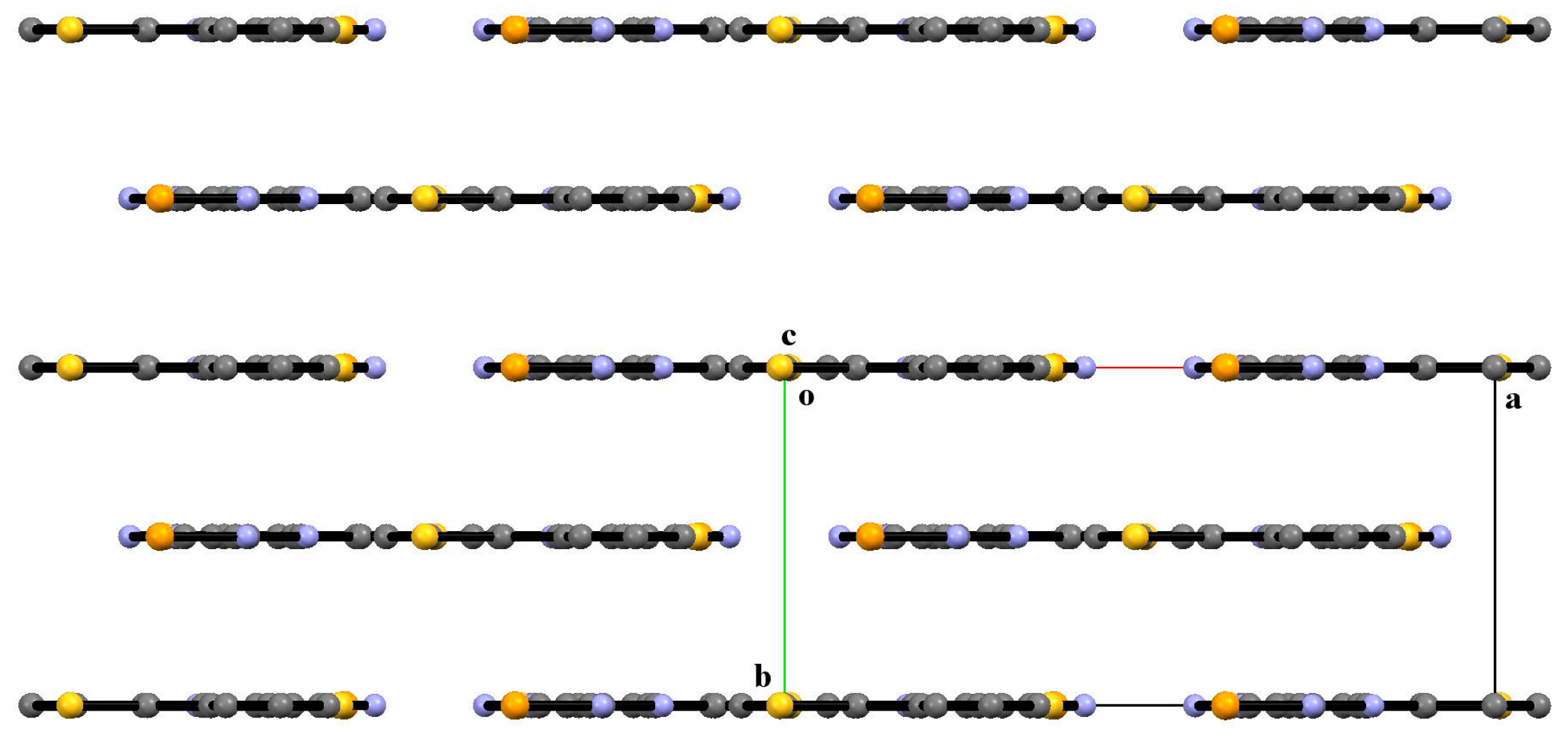
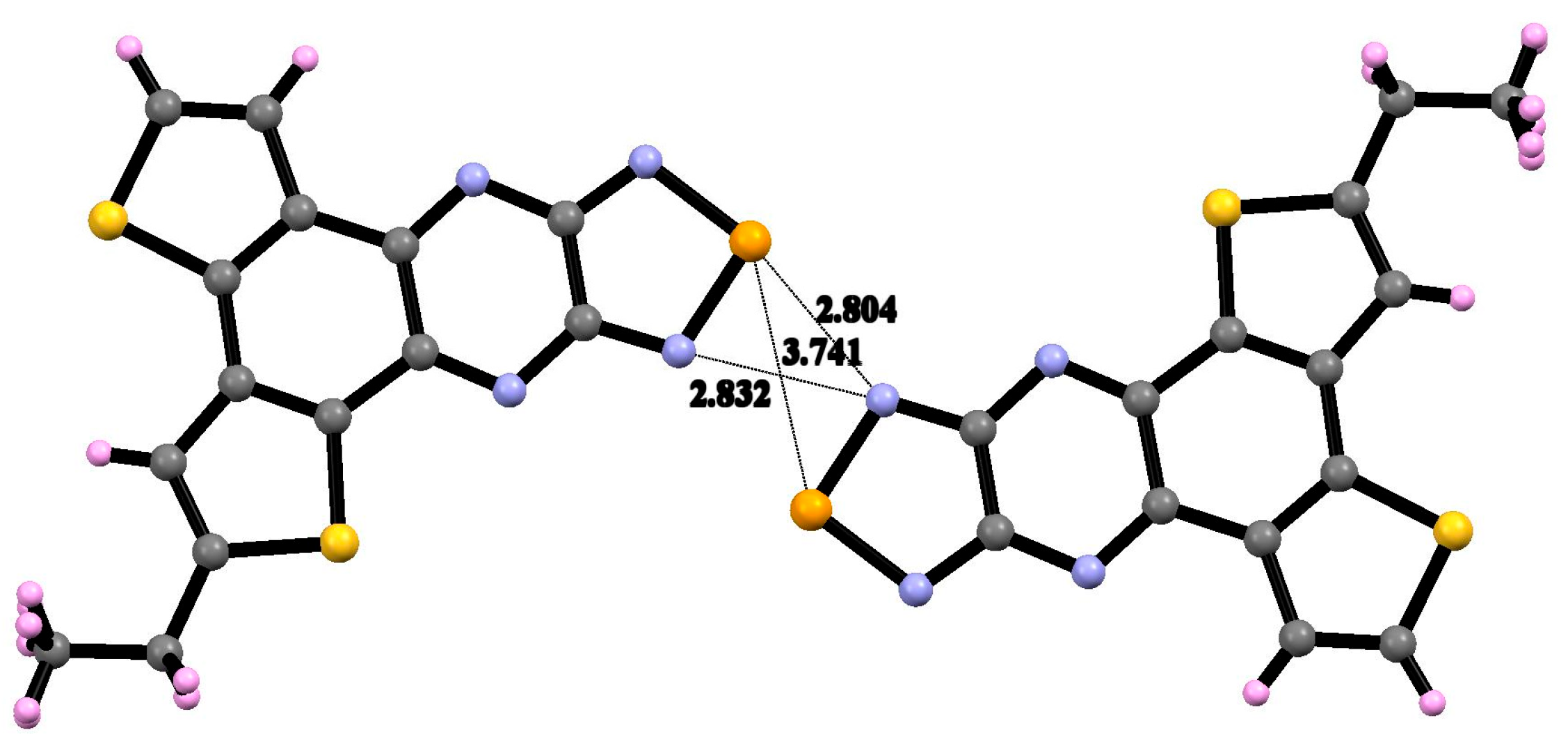
3.2. X-Ray Diffraction Analysis of the Polycyclic Compounds 4, 8, and 9
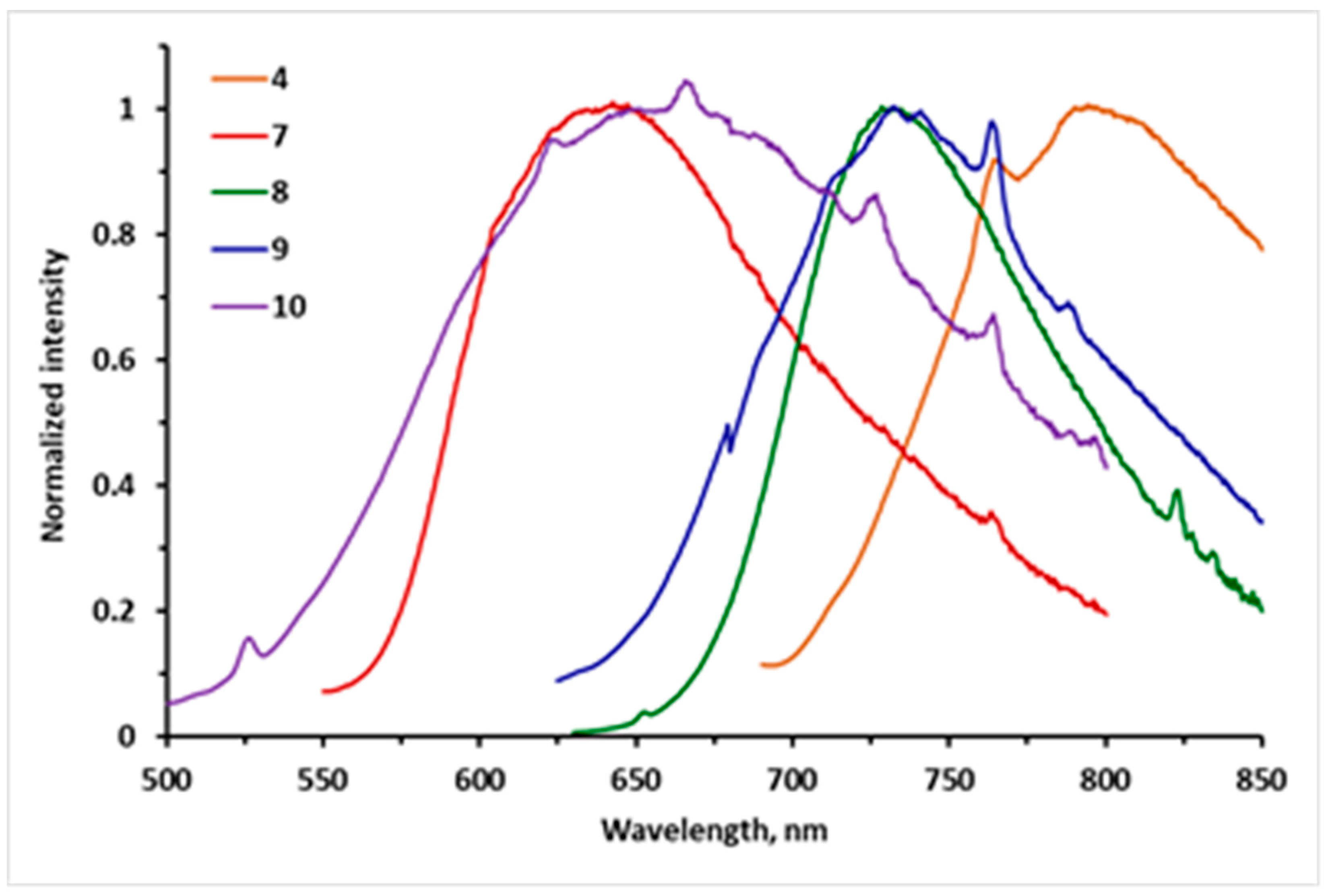
3.3. Photophysical Studies of the Polycyclic Compounds
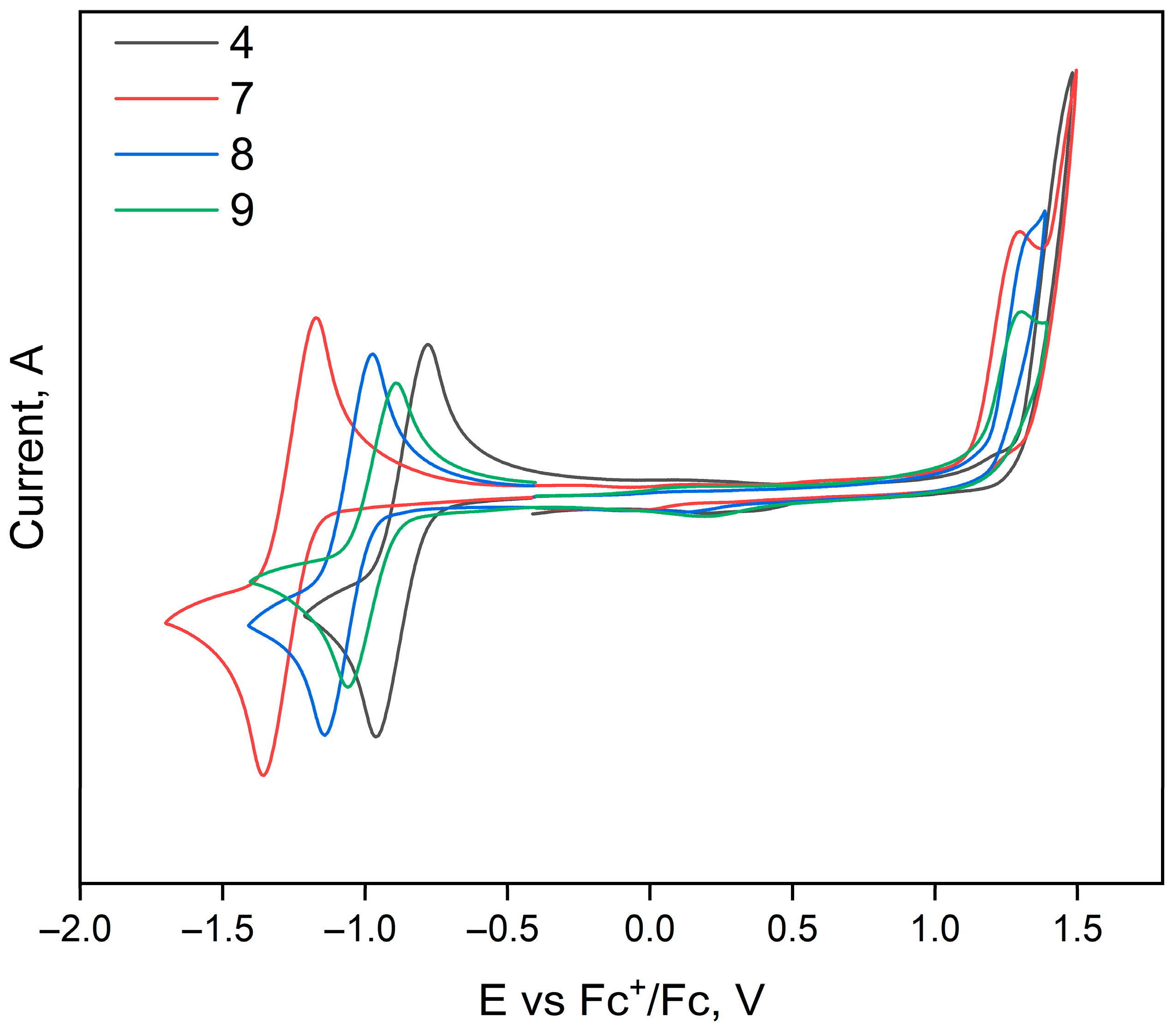
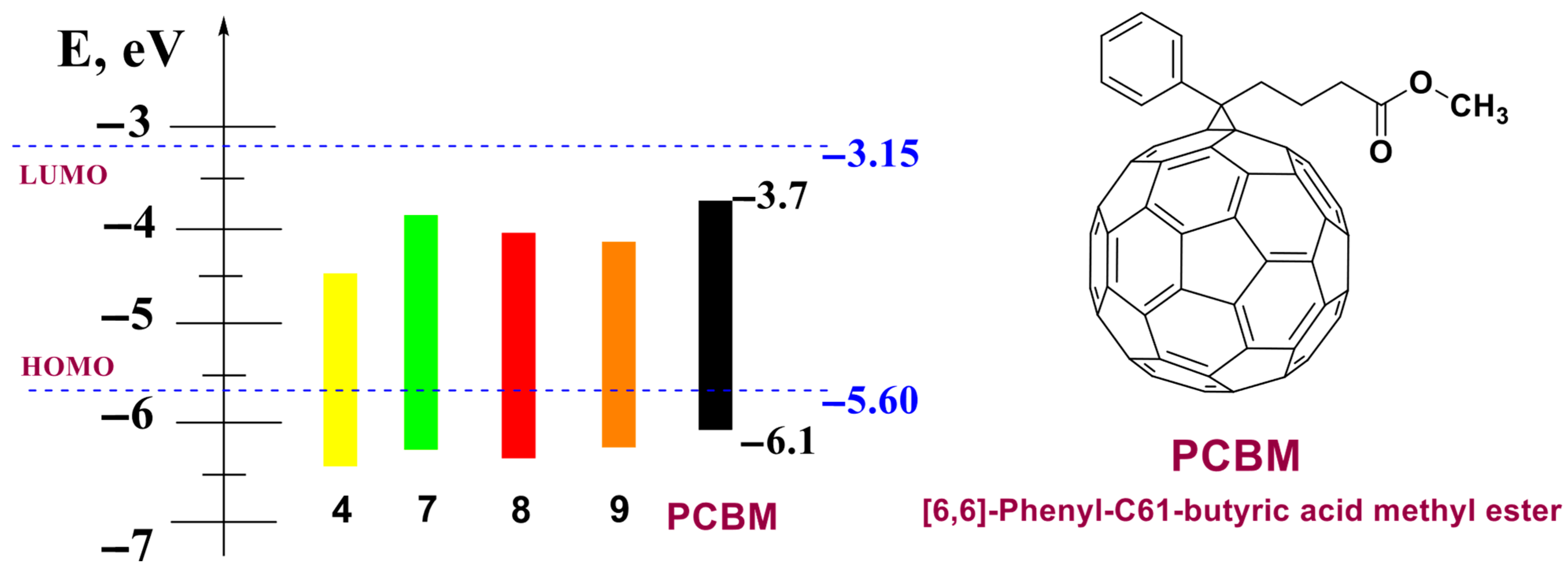
3.4. Electrochemical Properties of the Polycyclic Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Zhang, Y.; Xie, Z.; Zhen, Y.; Hud, W.; Dong, H. Polycyclic aromatic hydrocarbon-based organic semiconductors: Ring-closing synthesis and optoelectronic properties. J. Mater. Chem. C 2022, 10, 2411–2430. [Google Scholar] [CrossRef]
- Borissov, A.; Maurya, Y.K.; Moshniaha, L.; Wong, W.-S.; Zyła-Karwowska, M.; Stępien, M. Recent Advances in Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds. Chem. Rev. 2022, 122, 565–788. [Google Scholar] [CrossRef] [PubMed]
- Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds: Synthetic Routes, Properties, and Applications. Chem. Rev. 2017, 117, 3479–3716. [Google Scholar] [CrossRef] [PubMed]
- Mamada, M.; Yamashita, Y. S-Containing Polycyclic Heteroarenes: Thiophene-Fused and Thiadiazole-Fused Arenes as Organic Semiconductors. In Polycyclic Arenes and Heteroarenes; John Wiley and Sons: Hoboken, NJ, USA, 2015; pp. 277–308. [Google Scholar] [CrossRef]
- Mehta, H.K.; Pathan, S.K.; Trivedi, S.M. A Mini-Review on Emerging Trends in the Design and Synthesis of Heterocyclic Compounds for Organic Electronics Applications. Russ. J. Org. Chem. 2023, 59 (Suppl. S1), S123–S129. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Charushin, V.N. The strategy of combined application of nucleophilic aromatic substitution of hydrogen (SNH) and transition metal-catalyzed cross-coupling reactions. Mendeleev Commun. 2025, 35, 493–502. [Google Scholar] [CrossRef]
- Lu, N.; Li, L.; Geng, D.; Liu, M. A review for polaron dependent charge transport in organic semiconductor. Org. Electron. 2018, 61, 223–234. [Google Scholar] [CrossRef]
- Kvashnin, Y.A.; Verbitskiy, E.V.; Rusinov, G.L.; Charushin, V.N. Modification and application of 1,2,5-oxadiazolo[3,4-b]pyrazine derivatives: Highlights and perspectives. Russ. Chem. Bull. 2022, 71, 1342–1362. [Google Scholar] [CrossRef]
- Sheremetev, A.B.; Yudin, I.L. Advances in the chemistry of furazano[3,4-b]pyrazines and their analogues. Russ. Chem. Rev. 2003, 72, 87–100. [Google Scholar] [CrossRef]
- Efanov, D.E.; Tolstikov, S.E.; Romanenko, G.V.; Letyagin, G.A.; Smirnova, K.A.; Chernavin, P.A.; Veber, S.L.; Romashev, N.F.; Osik, N.A.; Bogomyakov, A.S. Stable anion radicals based on a triazole-fused furazano[3,4-b]pyrazine scaffold. New J. Chem. 2025, 49, 3869–3876. [Google Scholar] [CrossRef]
- Ovcharenko, V.I.; Sheremetev, A.B.; Strizhenko, K.V.; Fokin, S.V.; Romanenko, G.V.; Bogomyakov, A.S.; Morozov, V.A.; Syroeshkin, M.A.; Kozmenkova, A.Y.; Lalov, A.V.; et al. Novel organic magnet derived from pyrazine-fused furazans. Mendeleev Commun. 2021, 31, 784–788. [Google Scholar] [CrossRef]
- Ovcharenko, V.I.; Fokin, S.V.; Sheremetev, A.B.; Strizhenko, K.V.; Romanenko, G.V.; Bogomyakov, A.S.; Egorov, M.P. Cesium salts with the difurazanopyrazine radical anion. J. Struct. Chem. 2022, 63, 1697–1707. [Google Scholar] [CrossRef]
- Tolstikov, S.E.; Efanov, D.E.; Romanenko, G.V.; Egorov, M.P.; Ovcharenko, V.I. Structures of reaction products of 5,6-dichlorofurazano[3,4-b]pyrazine with R-hydrazines. Russ. Chem. Bull. 2022, 71, 1821–1825. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Kvashnin, Y.A.; Baranova, A.A.; Yakovleva, Y.A.; Khokhlov, K.O.; Rusinov, G.L.; Charushin, V.N. 9-Ethyl-3-{6-(het)aryl-[1,2,5]oxadiazolo[3,4-b]pyrazin-5-yl}-9H-carbazoles: Synthesis and study of sensitivity to nitroaromatic compounds. Russ. Chem. Bull. 2018, 67, 1078–1082. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Kvashnin, Y.A.; Baranova, A.A.; Khokhlov, K.O.; Chuvashov, R.D.; Yakovleva, Y.A.; Makarova, N.I.; Vetrova, E.V.; Metelitsa, A.V.; Rusinov, G.L.; et al. Novel fluorophores based on imidazopyrazine derivatives: Synthesis and photophysical characterization focusing on solvatochromism and sensitivity towards nitroaromatic compounds. Dye. Pigment. 2019, 168, 248–256. [Google Scholar] [CrossRef]
- Kvashnin, Y.A.; Zhilina, E.F.; Dubovik, A.I.; Gazizov, D.A.; Mekhaev, A.V.; Krynina, E.M.; Rusinov, G.L.; Verbitskiy, E.V.; Charushin, V.N. Conversion of tetraphenylethylene-substituted oxadiazolo[3,4-b]pyrazines into the corresponding imidazo[4,5-b]-and pyrazino[2,3-b]pyrazines, as chemosensors for the selective detection of nitroaromatics in aqueous media. Dyes Pigment. 2024, 228, 112253. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Lipunova, G.N.; Nosova, E.V.; Charushin, V.N. Advances in the design of functionalized 1,4-diazines as components for photo- or/and electroactive materials. Dyes Pigment. 2023, 220, 111763. [Google Scholar] [CrossRef]
- Kvashnin, Y.A.; Verbitskiy, E.V.; Eltsov, O.S.; Slepukhin, P.A.; Tameev, A.R.; Nekrasova, N.V.; Rusinov, G.L.; Nunzi, J.-M.; Chupakhin, O.N.; Charushin, V.N. Dibenzo[f,h]furazano[3,4-b]quinoxalines: Synthesis by intramolecular cyclization through direct transition metal-free C–H functionalization and electrochemical, photophysical, and charge mobility characterization. ACS Omega 2020, 5, 8200–8210. [Google Scholar] [CrossRef] [PubMed]
- Steparuk, A.S.; Kvashnin, Y.A.; Rusinov, G.L.; Verbitskiy, E.V.; Aleksandrov, A.E.; Lypenko, D.A.; Tameev, A.R.; Charushin, V.N. The first application of push-pull systems based on 1,2,5-oxadiazolo[3,4-b]pyrazine in organic light-emitting diodes and perovskite solar cells. Russ. Chem. Bull. 2023, 72, 527–533. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Kvashnin, Y.A.; Medvedeva, M.V.; Svalova, T.S.; Kozitsina, A.N.; Eltsov, O.S.; Rusinov, G.L.; Charushin, V.N. First synthesis of new polycyclic systems from ortho-di(heteroaryl)-substituted furazanopyrazine derivatives by the Scholl reaction. Mendeleev Commun. 2022, 32, 722–725. [Google Scholar] [CrossRef]
- Krynina, E.M.; Kvashnin, Y.A.; Gazizov, D.A.; Kodess, M.I.; Ezhikova, M.A.; Rusinov, G.L.; Verbitskiy, E.V.; Charushin, V.N. Two-step synthesis of new fused systems based on [1,2,5]oxadiazolo[3,4-b]quinoxaline by a combination of the Scholl reaction and nucleophilic aromatic substitution of hydrogen (SNH). Russ. Chem. Bull. 2024, 73, 1647–1658. [Google Scholar] [CrossRef]
- Kvashnin, Y.A.; Krynina, E.M.; Medvedeva, M.V.; Svalova, T.S.; Kozitsina, A.N.; Eltsov, O.S.; Rusinov, G.L.; Verbitskiy, E.V.; Charushin, V.N. Synthesis of new polycyclic systems based on [1,2,5]chalcogenodiazolo[3,4-b]thieno[3,2-h]quinoxalines. Russ. Chem. Bull. 2023, 72, 939–947. [Google Scholar] [CrossRef]
- Dai, Y.; Santiago-Rivera, J.A.; Hargett, S.; Salamoun, J.M.; Hoehn, K.L.; Santos, W.L. Conversion of oxadiazolo[3,4-b]pyrazines to imidazo[4,5-b]pyrazines via a tandem reduction-cyclization sequence generates new mitochondrial uncouplers. Bioorg. Med. Chem. Lett. 2022, 73, 128912. [Google Scholar] [CrossRef]
- Rakitin, O.A.; Zibarev, A.V. Synthesis and applications of 5-membered chalcogen-nitrogen π-heterocycles with three heteroatoms. Asian J. Org. Chem. 2018, 7, 2397–2416. [Google Scholar] [CrossRef]
- Chulanova, E.A.; Semenov, N.A.; Pushkarevsky, N.A.; Gritsan, N.P.; Zibarev, A.V. Charge-transfer chemistry of chalcogen-nitrogen π-heterocycles. Mendeleev Commun. 2018, 28, 453–460. [Google Scholar] [CrossRef]
- Rakitin, O.A. Fused 1,2,5-thia- and 1,2,5-selenadiazoles: Synthesis and applications in materials chemistry. Tetrahedron Lett. 2020, 61, 152230. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; le Poul, P.; Bureš, F.; Achelle, S.; Barsella, A.; Kvashnin, Y.A.; Rusinov, G.L.; Charushin, V.N. Push–Pull Derivatives Based on 2,4′-Biphenylene Linker with Quinoxaline, [1,2,5]Oxadiazolo[3,4-b]Pyrazine and [1,2,5]Thiadiazolo[3,4-b]Pyrazine Electron Withdrawing Parts. Molecules 2022, 27, 4250. [Google Scholar] [CrossRef]
- Radiush, E.A.; Korshunov, V.M.; Chulanova, E.A.; Konstantinova, L.S.; Ferulev, A.I.; Irtegova, I.G.; Shundrina, I.K.; Frank, E.A.; Semenov, N.A.; Taidakov, I.V.; et al. Polycyclic 1,2,5-chalcogenadiazole dyes: Structural, optical, and redox properties in neutral and radical-ion states (chalcogen = S, Se). Dyes Pigment. 2025, 242, 112922. [Google Scholar] [CrossRef]
- Komin, A.P.; Carmack, M. The chemistry of 1,2,5-thiadiazoles V. Synthesis of 3,4-diamino-1,2,5-thiadiazole and [1,2,5]thiadiazolo[3,4-b]pyrazines. J. Heterocycl. Chem. 1976, 13, 13–22. [Google Scholar] [CrossRef]
- Sharma, K.S.; Kumari, S.; Singh, R.P. Condensed Heterocycles; Xl. Synthesis of 1,2,5-Thia(selena)diazolo[3,4-b]quinolines and 1,2,5-Thia(selena)diazolo[3,4-h]quinolines. Synthesis 1981, 4, 316–318. [Google Scholar] [CrossRef]
- Muehlmann, F.L.; Day, A.R. Metabolite Analogs. V. Preparation of Some Substituted Pyrazines and Imidazo[b]pyrazines. J. Am. Chem. Soc. 1956, 78, 242–244. [Google Scholar] [CrossRef]
- Prima, D.O.; Vorontsova, E.V.; Makarov, A.G.; Makarov, A.Y.; Bagryanskaya, I.Y.; Mikhailovskaya, T.F.; Slizhov, Y.G.; Zibarev, A.V. Halogenated (F, Cl) 1,3-benzodiazoles, 1,2,3-benzotriazoles, 2,1,3-benzothia(selena)diazoles and 1,4-benzodiazines inducing Hep2 cell apoptosis. Mendeleev Commun. 2017, 27, 439–442. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Bobkova, I.E.; Nelyubina, Y.V.; Chulanova, E.A.; Irtegova, I.G.; Vasilieva, N.V.; Camacho, P.S.; Ashbrook, S.E.; Hua, G.; Slawin, A.M.Z.; et al. [1,2,5]Selenadiazolo[3,4-b]pyrazines: Synthesis from 3,4-Diamino-1,2,5-selenadiazole and Generation of Persistent Radical Anions. Eur. J. Org. Chem. 2015, 5585–5593. [Google Scholar] [CrossRef]
- Michalczyk, M.; Malik, M.; Zierkiewicz, W.; Scheiner, S. Experimental and Theoretical Studies of Dimers Stabilized by Two Chalcogen Bonds in the Presence of a N···N Pnicogen Bond. J. Phys. Chem. A 2021, 125, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Appleton, A.L.; Brombosz, S.M.; Barlow, S.; Sears, J.S.; Bredas, J.-L.; Marder, S.R.; Bunz, U.H.F. Effects of electronegative substitution on the optical and electronic properties of acenes and diazaacenes. Nat. Commun. 2020, 1, 91. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Tian, H.; Li, R.; Huang, Z.; Zhu, D.; Xiao, F.; Zhao, Y.; Xu, J. Role of Substitution Patterns in Four Regioisomeric Tetraphenylethylene–Thiophene Derivatives. Molecules 2025, 30, 2953. [Google Scholar] [CrossRef]
- Purushothama, U.; Narahari Sastry, G. Conjugate acene fused buckybowls: Evaluating their suitability for p-type, ambipolar and n-type air stable organic semiconductors. Phys. Chem. Chem. Phys. 2013, 15, 5039–5048. [Google Scholar] [CrossRef]
- Online Catalog of Chemical Reagents Sigma-Aldrich ([6,6]-Phenyl-C61-butyric acid methyl ester). Available online: https://www.ossila.com/products/pcbm (accessed on 28 August 2025).
- Hu, L.; Li, S.; Zhang, L.; Liu, Y.; Zhang, C.; Wu, Y.; Sun, Q.; Cui, Y.; Zhu, F.; Hao, Y.; et al. Unravelling the role of C60 derivatives as additives into active layers for achieving high-efficiency planar perovskite solar cells. Carbon 2020, 167, 160–168. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Rusinov, G.L.; Charushin, V.N. Diazatriphenylenes and their thiophene analogues: Synthesis and applications. Arkivoc 2017, I, 356–401. [Google Scholar] [CrossRef]


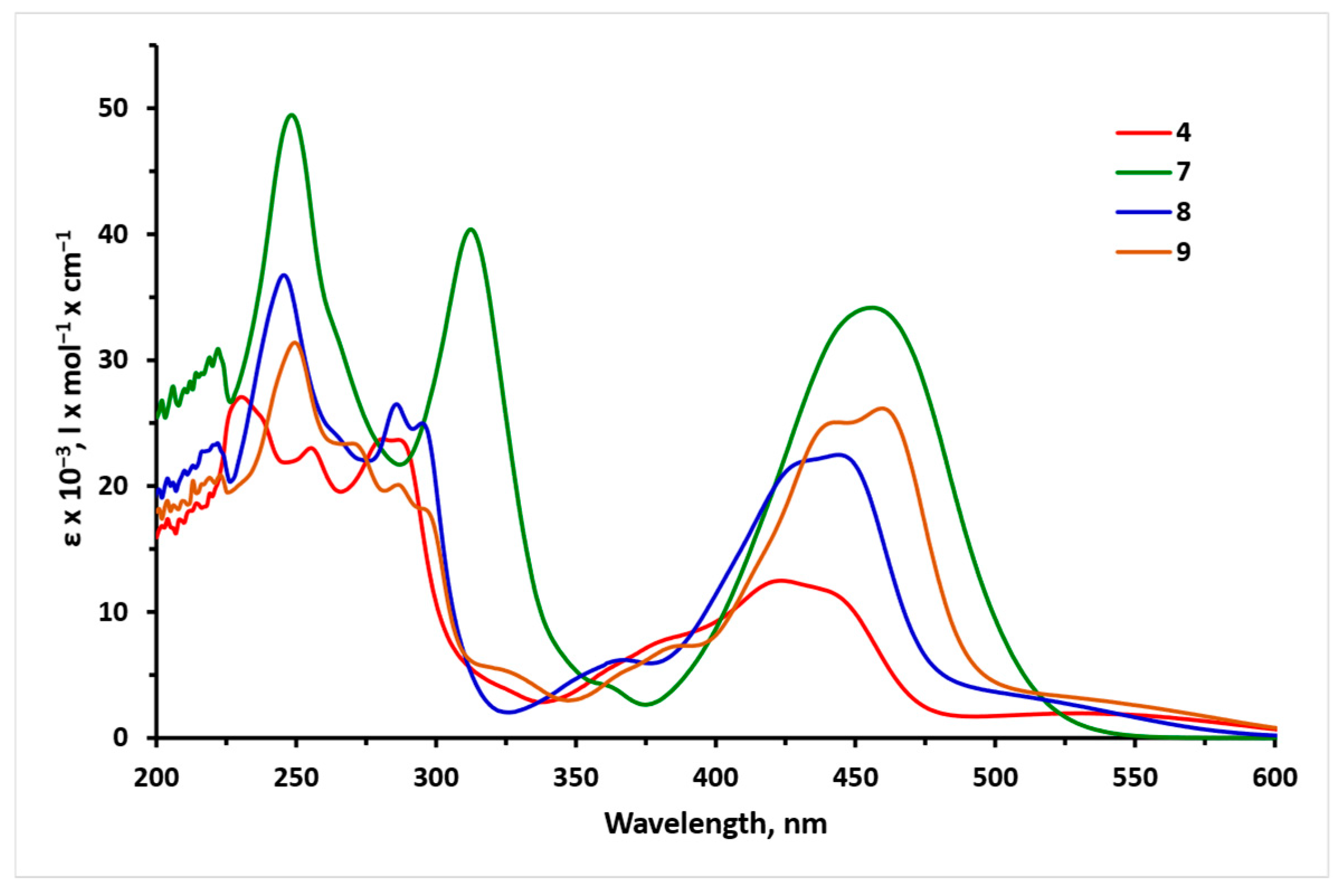
| Sample | Absorption | Fluorescence | ||||||
|---|---|---|---|---|---|---|---|---|
| Solution in CH2Cl2 | Solid | |||||||
| λabsmax (nm)/ ε (M−1·cm−1) | λex (nm) | λem (nm) | τavg, [ns]/χ2 | ΦF | λem (nm) | τavg, [ns]/χ2 | ΦF | |
| 4 | 423/12,300 281/23,600 255/2300 230/27,100 | 288, 433, 540 | 740 | 0.57/1.267 | <0.01 | 800 | 0.71/1.228 | <0.01 |
| 7 | 456/34,400 312/40,900 248/50,600 | 313, 458 | 611 | 2.04/1.151 | 0.02 | 640 | 2.11/1.051 | 0.02 |
| 8 | 443/22,400 368/6100 295/24,900 286/26,500 245/37,100 | 305, 464 | 695 | 1.68/1.051 | <0.01 | 732 | 2.76/1.033 | 0.03 |
| 9 | 459/26,300 443/25,200 286/20,000 249/31,800 | 460 | 730 | 0.79/1.188 | <0.01 | 733 | 0.36/1.327 | <0.01 |
| 10 | - | - | - | - | - | 650 | 1.31/1.180 | <0.01 |
| Compound | EOxonset, V | ERedonset, V | EHOMO, eV | ELUMO, eV | Egel, eV |
|---|---|---|---|---|---|
| 4 | 1.28 | −0.78 | −6.38 | −4.32 | 2.06 |
| 7 | 1.11 | −1.17 | −6.21 | −3.93 | 2.28 |
| 8 | 1.17 | −0.97 | −6.27 | −4.13 | 2.14 |
| 9 | 1.12 | −0.88 | −6.22 | −4.22 | 2.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krynina, E.M.; Kvashnin, Y.A.; Zhilina, E.F.; Gazizov, D.A.; Slepukhin, P.A.; Rusinov, G.L.; Verbitskiy, E.V.; Charushin, V.N. [1,2,5]Oxadiazolo[3,4-b]dithieno[2,3-f:2′,3′-h]quinoxaline as a Versatile Scaffold for the Construction of Various Polycyclic Systems as Potential Organic Semiconductors. Chemistry 2025, 7, 158. https://doi.org/10.3390/chemistry7050158
Krynina EM, Kvashnin YA, Zhilina EF, Gazizov DA, Slepukhin PA, Rusinov GL, Verbitskiy EV, Charushin VN. [1,2,5]Oxadiazolo[3,4-b]dithieno[2,3-f:2′,3′-h]quinoxaline as a Versatile Scaffold for the Construction of Various Polycyclic Systems as Potential Organic Semiconductors. Chemistry. 2025; 7(5):158. https://doi.org/10.3390/chemistry7050158
Chicago/Turabian StyleKrynina, Elizaveta M., Yuriy A. Kvashnin, Ekaterina F. Zhilina, Denis A. Gazizov, Pavel A. Slepukhin, Gennady L. Rusinov, Egor V. Verbitskiy, and Valery N. Charushin. 2025. "[1,2,5]Oxadiazolo[3,4-b]dithieno[2,3-f:2′,3′-h]quinoxaline as a Versatile Scaffold for the Construction of Various Polycyclic Systems as Potential Organic Semiconductors" Chemistry 7, no. 5: 158. https://doi.org/10.3390/chemistry7050158
APA StyleKrynina, E. M., Kvashnin, Y. A., Zhilina, E. F., Gazizov, D. A., Slepukhin, P. A., Rusinov, G. L., Verbitskiy, E. V., & Charushin, V. N. (2025). [1,2,5]Oxadiazolo[3,4-b]dithieno[2,3-f:2′,3′-h]quinoxaline as a Versatile Scaffold for the Construction of Various Polycyclic Systems as Potential Organic Semiconductors. Chemistry, 7(5), 158. https://doi.org/10.3390/chemistry7050158






