Abstract
An increase in the production of cationic dyes is expected over the next decade, which will have an impact on health and the environment. This work reports an adsorbent hydrogel composed of poly(acrylic acid) [poly(AA)] and Fe3O4 particles, prepared by radical polymerization and in situ co-precipitation of Fe3+ and Fe2+. This Fe3O4/poly(AA) composite hydrogel was used to evaluate its potential for removing the cationic dyes methylene blue (MB) and crystal violet (CV) from aqueous solutions. Instrumental characterization of the hydrogel was performed by FTIR, XRD, TGA, VSM, and physicochemical analysis (swelling and response to changes in pH). The results show that the incorporation of Fe3O4 particles improves the adsorption capacity of MB and CV dyes to a maximum adsorption of 571 and 321 mg/g, respectively, under the best conditions (pH 6.8, dose 1 g/L, time 24 h). The adsorption data best fit the pseudo-first order (PFO) kinetic model and the Freundlich isothermal model, indicating mass transfer via internal and/or external diffusion and active sites with different adsorption potentials. Moreover, the thermodynamic analysis confirmed that the adsorption process was spontaneous and exothermic, with physisorption as the dominant mechanism. In addition, the Fe3O4/poly(AA) hydrogel is capable of removing 95% of the dyes after ten consecutive adsorption–desorption cycles, demonstrating the potential of hydrogels loaded with Fe3O4 particles for the treatment of wastewater contaminated with dyes.
1. Introduction
Cationic dyes currently account for around 1% of global dye production. Their production is expected to increase over the next decade due to population growth and industrial development driven by the textile, paper, and leather sectors. However, between 10% and 15% of the dye used does not adhere to the fabric and ends up as waste in effluents [1]. Although cationic dyes represent a small proportion of total dye production, they pose a danger to human health and aquatic systems. Dyes such as methylene blue (MB) and crystal violet (CV) are of particular concern because they are toxic, persistent in the environment, and capable of bioaccumulation [2,3,4].
For this reason, various techniques have been developed to remove dyes from aqueous solutions, including coagulation–flocculation [5], chemical degradation [6], advanced oxidation processes [7], and adsorption [8]. Adsorption is the preferred technique due to its simplicity, low cost, and ease of design [9]. Several organic, inorganic, and hybrid adsorbent materials have been prepared, including hydrogels composed of inorganic materials such as SiO2, clay, Fe3O4, etc. [10,11]. These materials have great potential for dye removal due to the anionic groups, such as carboxylates (–COO−) or sulfonates (–SO3−), present in the hydrogel matrix, which promote electrostatic interactions with cationic dyes such as MB and CV. The large surface area and additional adsorption sites of the incorporated components contribute to a higher adsorption capacity [12].
Previous studies have demonstrated the efficacy of various modified hydrogels. For instance, Safarzadeh et al. developed poly(methacrylic acid-co-acrylamide)/Cloisite 30B hydrogels with a maximum MB adsorption capacity of 32.83 mg/g [13]. Roshini et al. obtained an adsorption capacity of up to 860 mg/g using magnetic hydrogels based on chitosan grafted with acrylamide and N-vinyl imidazole [14]. However, in both studies, the adsorption process was carried out with adsorbents in the form of fine powder, which presents several practical limitations such as difficulty in separation, loss of material, and column clogging [15]. In the present work, granular hydrogel has been used to overcome these limitations, improving handling, separation, and reuse.
In this context, and following the line of research we have developed in our laboratory on hydrogels composed of Fe3O4 particles using two strategies—(i) modifying the surface of the particles for their covalent incorporation into the polymer network, and (ii) incorporating the particles by in situ co-precipitation—we prepared Fe3O4/poly(AA) hydrogels using the second strategy, i.e., by coprecipitation of Fe2+/Fe3+ within the poly(AA) hydrogel matrix. The acrylic acid was used as a monomer due to its low cost, high hydrophilicity, fast reactivity, and wide industrial availability. In addition, its carboxylic groups (–COOH) favor electrostatic interaction with cationic dyes, promoting their adsorption. The composite hydrogel was characterized using instrumental techniques such as FTIR, XRD, TGA, and VSM, as well as physicochemical tests that included swelling, pH response, and zero charge point (pHpzc). Its performance in the adsorption of MB and CV dyes was evaluated, as well as its regeneration efficiency over multiple cycles.
2. Materials and Methods
2.1. Materials
Acrylic acid (AA, 99%), N,N′-methylenebisacrylamide (MBA, 99%), ammonium persulfate (APS, 98%), ferric chloride hexahydrate (FeCl3.6H2O, 99%), and ferrous chloride tetrahydrate (FeCl2.4H2O, 98%) were purchased from Sigma-Aldrich (Darmstadt, Germany). Sodium hydroxide (NaOH, 99%, Eka Chemicals, Marietta, GA, USA) and hydrochloric acid (HCl, 37%, RPE-ACS, Bernolsheim, France) were used as received. The solvent water (H2O) was bi-distilled before use. Crystal violet (CV) and methylene blue (MB) were of analytical grade (>96%,Central Drug House (CDH), New Delhi, India).
2.2. Preparation of Poly(AA) Hydrogel
The preparation of the poly(AA) hydrogel followed a protocol similar to that reported in previous studies [16]. First, 0.5 g of AA (100 mol%) and 0.034 g of MBA (3.5 mol%) were dissolved in 4 mL of water under constant stirring. The solution was then purged with nitrogen (N2) for 5 min, after which 1 mL of APS solution (15 mg/mL) was added and the mixture was homogenized in an ultrasonic bath. The resulting solution was poured into a cylindrical mold and maintained at 70 °C for 3 h. After polymerization, the hydrogel was removed from the mold and cut into discs. The discs were purified by immersion in distilled water for 10 h, with water renewed every 2 h. Finally, the hydrogels were dried at room temperature for 7 days, followed by drying at 70 °C for 4 h.
2.3. Preparation of Fe3O4/Poly (AA) Hydrogel Composite
For the preparation of the Fe3O4/poly(AA) composite hydrogel, the in situ co-precipitation method of Fe3+ and Fe2+ was followed [17]. The process began with the swelling of the poly(AA) hydrogel in distilled water for 24 h, after which it was transferred to a Fe3+/Fe2+ solution (2:1 molar ratio), where it remained for 3 h at room temperature. The amounts of FeCl3·6H2O and FeCl2·4H2O used were equivalent to the dry weight of the hydrogel, and the volume of water corresponded to 20 times the weight of the dry hydrogel. After 3 h, the hydrogel discs were removed from the solution, washed several times with distilled water, and immersed in a 0.5 mol/L NaOH solution for 24 h. They were then removed from the NaOH solution and washed repeatedly with distilled water until the pH was approximately 7. Finally, the composite hydrogels were dried at room temperature for 7 days, followed by oven-drying at 70 °C for 4 h.
2.4. Characterization of Hydrogels
Infrared spectroscopy (FTIR-Alpha II, Bruker, Ettlingen, Germany) was used to identify the different functional groups present in the hydrogel and to assess the interactions between its components in the composite hydrogel. Measurements were performed in the wavenumber range of 4000–400 cm−1 using attenuated total reflectance (ATR) at room temperature. The thermal stability of the hydrogel and the composite hydrogel was evaluated by thermogravimetric analysis (TGA) using a PerkinElmer STA 6000 (Waltham, MA, USA) instrument. The study was carried out from 30 to 600 °C under a nitrogen (N2) atmosphere, with a heating rate of 10 °C/min. X-ray diffraction (XRD) was employed to analyze the crystalline structure of the hydrogel after incorporation of the Fe3O4 particles. XRD patterns were recorded using a Bruker D8 ADVANCE, Karlsruhe, Germany powder diffractometer equipped with a Cu-Kα radiation source (λ = 1.5406 Å). Finally, the magnetic properties of the composite hydrogel were analyzed using a vibrating sample magnetometer (VSM, Lima, Peru).
2.5. Determination of the Point of Zero Charge (pHpzc)
The pHpzc of the Fe3O4/poly(AA) composite hydrogel was determined using the addition method [18]. The procedure began with the preparation of 0.1 mol/L NaCl solutions, whose pH values were adjusted from 2 to 10 using 0.1 mol/L HCl and 0.1 mol/L NaOH. Then, 10 mg of Fe3O4/poly(AA) was added to 10 mL of each solution. The suspensions were stirred at room temperature. After 24 h, the pH of each solution was measured. Since the experiment was carried out using composite hydrogel pieces, no separation steps such as filtration or centrifugation were necessary. To determine the pHpzc, a plot of the initial pH versus the ΔpH (initial pH—final pH) was constructed. The point where ΔpH equals zero was identified as the pHpzc of the composite hydrogel.
2.6. Swelling Study and pH-Response
The degree of swelling, or simply swelling (q), was evaluated by placing approximately 10 mg of dry hydrogel in contact with an excess of distilled water at room temperature for 24 h (sufficient time to reach equilibrium). After this period, the hydrogels were removed, and their surfaces were gently blotted with a paper towel to eliminate excess surface water. Their weight was immediately measured using an analytical balance. The swelling was calculated using the following Equation (1) [18]:
where Wd is the weight of the dry hydrogel samples, and Ws is the weight of the swollen hydrogel after 24 h of immersion in water. Additionally, swelling experiments were conducted in 0.01 mol/L NaOH and 0.01 mol/L HCl solutions through consecutive cycles to evaluate their behavior as a function of pH.
2.7. Adsorption Studies of MB and CV Dye
The adsorption process was carried out using the composite hydrogel, which had been swollen in distilled water at room temperature for 24 h. All adsorption tests were performed in a 50 mL round-bottom flask with 10 mL dye solution and were shaken on an orbital shaker. The first evaluated parameter was the effect of pH (ranging from 2 to 10) on the adsorption capacity of MB and CV. First, the pH of the MB and CV solutions was adjusted using 0.1 mol/L NaOH and 0.1 mol/L HCl. Then, 10 mg of the composite hydrogel was added to the solution. The mixture was stirred for 24 h at room temperature. After this period, the concentrations of MB and CV were determined using a UV-Vis spectrophotometer at 664 and 590 nm, respectively. For this, a calibration curve was previously constructed in the range of 0.5–7.5 mg/L. The linear regression equations obtained from the concentration versus absorbance plot were Abs = 0.2014X + 0.0394, R2 0.9999 for MB and Abs = 0.0518X − 0.0009, R2 0.9991 for CV, respectively. After establishing the optimum pH, the effects of other parameters were investigated, including adsorbent dose (5 to 50 mg), contact time (1 to 24 h), initial MB and CV concentrations (50 to 250 mg/L), and temperature (25 to 45 °C). All experiments were performed in triplicate. Adsorption capacity (Qe, mg/g) and removal efficiency (%R) were calculated using Equations (2) and (3), respectively [19].
Here, C0 (mg/L) is the initial concentration, Ce (mg/L) is the equilibrium concentration, m (g) is the hydrogel mass, and V (L) is the MB and CV solution volume used in the adsorption process.
2.8. Adsorption–Desorption Experiments
The adsorption–desorption experiment was conducted over ten consecutive cycles to evaluate the reusability of the hydrogel [20]. The adsorption step was carried out using 100 mg/L MB and 100 mg/L CV solutions under the optimal conditions previously determined. For the desorption process, the hydrogel was removed from the adsorption solution, rinsed with distilled water, and immersed in 10 mL of 0.1 mol/L HCl at room temperature for 24 h. Afterwards, it was taken out of the acidic solution, rinsed with 0.1 mol/L NaOH solution followed by distilled water, and subjected to a new adsorption cycle.
3. Results
The preparation of the Fe3O4/poly(AA) composite hydrogel adsorbent is schematically represented in Figure 1. The poly(AA) hydrogel was prepared by free radical polymerization using AA as the monomer, MBA as the crosslinker, and APS as the initiator. Then, Fe3O4 particles were synthesized in situ within the poly(AA) hydrogel matrix by coprecipitation of Fe3+ and Fe2+ ions. Initially, hydrogels based on hydroxyethyl methacrylate (HEMA) and varying proportions of AA were prepared. A screening evaluation was performed to assess their pH response (Figure S1) and MB and CV dye removal capacity (Figure S2). The results obtained show that the hydrogel with 100% AA has the best potential. Consequently, the poly(AA) system was selected as the matrix for preparing the Fe3O4/poly(AA) composite hydrogel.

Figure 1.
Schematic preparation of poly(AA) (a), Fe3O4/poly(AA) (b), and their macroscopic appearance (c).
3.1. Characterization
The FTIR spectroscopy was employed to characterize the functional groups present in both the poly(AA) and Fe3O4/poly(AA) hydrogels (Figure 2a). The FTIR spectrum of poly(AA) displays characteristic bands at 3049 cm−1 (–OH stretching), 2932 cm−1 (C–H stretching), and a sharp peak at 1693 cm−1 corresponding to C=O stretching vibrations. A broad band between 1160 cm−1 and 1230 cm−1 is associated with –CO stretching and –OH bending of carboxylic acid groups (–COOH), while the signal at 792 cm−1 is attributed to C–COOH stretching [21]. Notably, the absence of an absorption peak near 1633 cm−1 confirms the successful polymerization of AA via the opening of the C=C double bond. The presence of a peak at 1449 cm−1, associated with C–N stretching, indicates the effective crosslinking of AA by MBA, as also reported in previous studies [22,23]. In the spectrum of the Fe3O4/poly(AA) composite hydrogel, the –OH band appears at 3253 cm−1 with reduced intensity compared to that of pure poly(AA), suggesting hydrogen bonding between the carboxyl groups (–COOH) of the polymer and hydroxyl groups (–OH) on the surface of Fe3O4 particles [24]. The C=O stretching peak observed at 1693 cm−1 in the poly(AA) hydrogel shifts to 1536 cm−1 in the composite hydrogel, further supporting the formation of hydrogen bonds between the polymer and Fe3O4. Additionally, both spectra retain the characteristic C–H stretching peaks around 2930 cm−1. These spectral changes confirm the successful incorporation of Fe3O4 into the poly(AA) matrix and the establishment of intermolecular interactions.
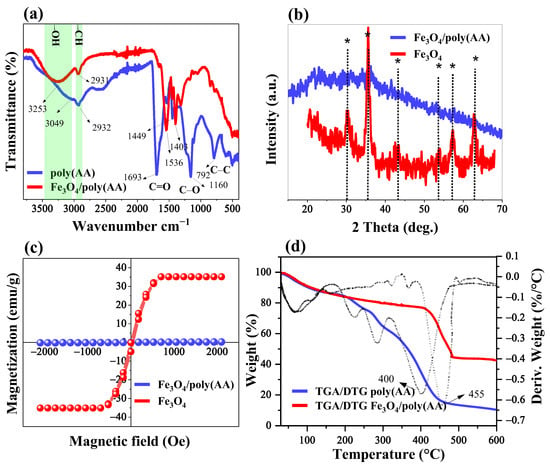
Figure 2.
Characterization of hydrogels. FTIR spectra of poly(AA) and Fe3O4/poly(AA) hydrogels (a), XRD pattern of Fe3O4 particles and Fe3O4/poly(AA) hydrogel (b), VSM of Fe3O4 particles and Fe3O4/poly(AA) hydrogel (c), and TGA/DTG analysis curves of poly(AA) and Fe3O4/poly(AA) hydrogels (d).
The XRD analysis was conducted to confirm the incorporation of Fe3O4 particles within the poly(AA) hydrogel matrix. As shown in Figure 2b, the diffractogram primarily displays features characteristic of an amorphous material, consistent with the structure of poly(AA). Unlike other Fe3O4-loaded hydrogels, such as Fe3O4/poly(HEMA-co-IA) [25] and Fe3O4/poly(HEMA-co-AMPS) [17], no XRD patterns corresponding to Fe3O4 (typically observed at 2θ = 30.30°, 35.48°, 43.01°, 53.80°, 57.31°, and 62.49°; marked with asterisks in Figure 2b) were observed in the Fe3O4/poly(AA) hydrogel. This absence suggests that the poly(AA) hydrogel has a relatively low affinity for Fe2+ and Fe3+ ions, leading to limited in situ formation of Fe3O4 particles. As a result, the low Fe3O4 content, along with the inherently diamagnetic nature of the hydrogel matrix, contributes to a reduced magnetic saturation of only 0.2 emu/g (Figure 2c). However, in their dry state, the hydrogel pieces can adhere to a magnet without much intensity, but when swollen, they are no longer attracted to the magnet because there is an increase in weight, making magnetic separation difficult. However, it should be noted that unlike other studies, which generally use the adsorbent in powder form, in our study, it was used in pieces, which facilitates separation without the need for an external magnetic field. It is important to mention that the small amount of Fe3O4 in the matrix has a significant effect on the adsorption capacity of MB and CV.
The TGA/DTG curves of poly(AA) and Fe3O4/poly(AA) hydrogels are shown in Figure 2d. Due to their highly hydrophilic nature, the initial weight loss observed in the TGA curve corresponds to water evaporation from the matrix. The thermal decomposition process occurs between 200–450 °C, with the maximum decomposition rate at 400 °C, consistent with previous reports [26]. The incorporation of additional materials into the poly(AA) hydrogel matrix has been reported to enhance thermal stability [27,28]. Similarly, the inclusion of Fe3O4 particles increases the thermal stability of the Fe3O4/poly(AA) hydrogel composite, shifting the decomposition temperature to 455 °C. This enhanced stability can be attributed to the formation of bonds between the Fe3O4 particles and the functional groups of the poly(AA) hydrogel.
It was decided to estimate the amount of Fe3O4 particles by calcining the sample and applying the corresponding conversion factor, taking into account that under these conditions, Fe3O4 is transformed into Fe2O3. During the process, it was observed that at 600 °C the poly(AA) sample decomposes almost completely, releasing mainly CO2. In contrast, a higher residue was observed in the Fe3O4/poly(AA) sample, which was attributed to carbon formation, meaning that Fe3O4 particles promote carbon formation. This explains the high percentage remaining in the TGA for Fe3O4/poly(AA) hydrogel. It was necessary to increase the temperature to 1000 °C to remove the residual carbon, and after 4 h of calcination, the amount decreased significantly, allowing an estimated content of approximately 9%. However, it should be recognized that this is only an imprecise estimate and that other more suitable methods need to be explored to quantify Fe3O4 in the hydrogel matrix.
3.2. Swelling Assessment
The swelling behavior of poly(AA) hydrogel and Fe3O4/poly(AA) composite hydrogel in different media is shown in Figure 3a. In acidic medium (0.01 mol/L HCl), only slight swelling is observed, which can be attributed to the protonation of the carboxylic groups (–COOH) of the hydrogel, promoting the formation of internal hydrogen bonds (–COOH·······HOOC–) that restrict the expansion of the polymeric network [29]. In neutral medium (distilled water), the Fe3O4/poly(AA) hydrogel exhibits a higher swelling degree, due to the presence of –OH groups on the surface of the Fe3O4 particles and the partial deprotonation of the –COOH groups in poly(AA), which enhances water absorption [25]. In contrast, under basic conditions (0.01 mol/L NaOH), the poly(AA) hydrogel shows a significant increase in swelling due to the complete deprotonation of the carboxylic groups, leading to electrostatic repulsion between polymer chains. However, in the Fe3O4/poly(AA) hydrogel, the swelling does not increase substantially, possibly due to the formation of hydrogen bonds between the –OH groups of the particles and the deprotonated carboxylate groups (–COO−·······HO–), acting as additional crosslinking points that restrict network expansion [30,31].

Figure 3.
Swelling of poly(AA) and Fe3O4/poly(AA) hydrogels in acidic (0.01 mol/L HCl), neutral (distilled water), and basic (0.01 mol/L NaOH) media (a) and in successive acid–base cycles (b).
Figure 3b shows the swelling behavior during consecutive acid–base cycles. In the poly(AA) hydrogel, an increase in swelling is observed with each cycle, attributable to the fragmentation of the hydrogel. In contrast, the Fe3O4/poly(AA) hydrogel maintains a more stable swelling profile throughout the cycles, suggesting that the incorporation of Fe3O4 particles enhances the structural integrity under repeated pH variations. These results indicate that the presence of Fe3O4 particles not only modifies the swelling behavior depending on the surrounding medium, but also imparts greater mechanical resistance to the hydrogel against the stress associated with successive swelling–deswelling cycles.
3.3. Adsorption Determination
A critical feature of adsorbents is pHpzc, which influences the performance of the adsorption process [32]. At pH values below this point, the hydrogel surface acquires a positive charge, which promotes the electrostatic adsorption of anionic species. On the contrary, at pH values above 6.8, the surface becomes negatively charged (Figure 4a), improving the interaction with cationic dyes such as MB and CV. In this study, the pHpzc of the poly(AA) and Fe3O4/poly(AA) hydrogels was determined from the ΔpH versus initial pH plot (Figure 4a). The results show that the poly(AA) hydrogel maintained a negative charge in the evaluated pH range (pH 2 to 10), reflecting extensive carboxylic deprotonation. In contrast, the pHpzc of Fe3O4/poly(AA) is at pH 6.8, which can be attributed to the effect of the interaction Fe–O····H····O–C.
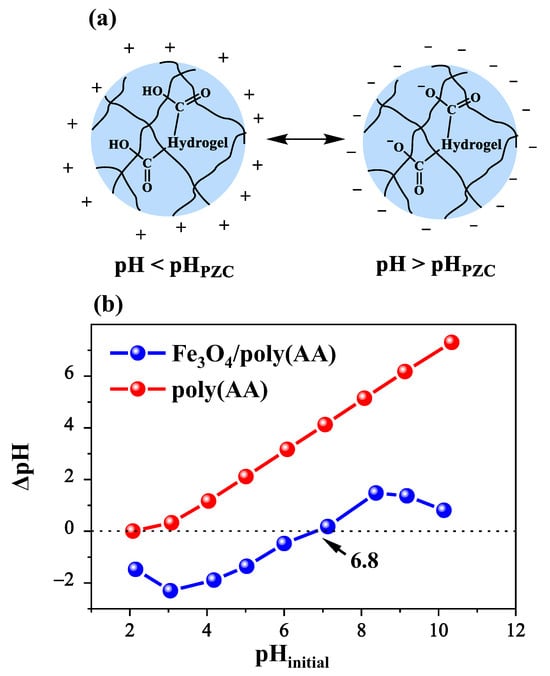
Figure 4.
Determination of the point of zero charge (pHpzc) of the poly(AA) and Fe3O4/poly(AA) hydrogels (a); schematic representation of the surface charge behavior of the hydrogel below and above the pHpzc (b).
Figure 5a shows the absorbance spectra corresponding to the adsorption of MB by the poly(AA) and Fe3O4/poly(AA) hydrogels, while Figure 5b presents the adsorption spectra for CV. In both cases, a significant decrease in the intensity of the characteristic dye peaks is observed in the presence of the hydrogels, indicating effective adsorption. When comparing both materials, the Fe3O4/poly(AA) hydrogel exhibits a much more pronounced reduction in the absorbance of MB and CV compared to the poly(AA) hydrogel.

Figure 5.
UV-Vis spectra of MB (a) and CV (b) solutions after adsorption by poly(AA) and Fe3O4/poly(AA) hydrogels (initial concentration 50 mg/L, contact time 24 h, pH 6.8, and room temperature).
This behavior demonstrates that the incorporation of Fe3O4 particles into the polymeric matrix notably enhances the adsorption capacity of the hydrogel toward cationic dyes. This improvement can be attributed to the increased number of active interaction sites (such as hydroxyl groups on the surface of Fe3O4) and a possible synergy between the functional groups of the polymer and the particles [12]. Therefore, due to its enhanced adsorption performance, the Fe3O4/poly(AA) hydrogel was chosen for further experimental analysis.
3.3.1. pH Assessment
The pH of the solution is a crucial parameter because it can influence the surface charge of the adsorbent and the ionization state of the dye molecules [33]. To evaluate the effect of pH, the adsorption capacity of the Fe3O4/poly(AA) composite hydrogel was studied in the pH range from 2 to 10. The experiment was performed with 10 mg of hydrogel in 10 mL of MB and CV solutions (50 mg/L), with a contact time of 24 h at room temperature. The adsorption capacity and the removal percentage are shown in Figure 6a,b. Both adsorption profiles show a strong dependence on the solution pH. The adsorption capacity decreased significantly under acidic conditions (pH 2–4). This behavior is attributed to the protonation of the carboxylic groups (–COOH), which decreases the negative surface charge density of the hydrogel, thus limiting electrostatic interactions with MB and CV [34]. As the pH increased towards neutral and slightly basic values (pH 6–8), a significant increase in the adsorption capacity was observed, reaching maximum values of approximately Qe = 45–48 mg/g and removal efficiencies above 90%. This increase is related to the deprotonation of the carboxylic groups (–COOH) to their anionic form (–COO−), which favors an electrostatic attraction with the dyes. At higher pH values (pH 10), the adsorption capacity remained high, but a slight decrease in the CV profile was observed. This decrease may be related to the competitive effects of ionic species (Na+ and OH−) in the solution, which could interfere with the dye–adsorbent interactions [35]. The similar adsorption behavior seen for both dyes indicates that electrostatic interaction is the main mechanism in this system, significantly influenced by the ionization state of the hydrogel depending on pH. Based on these findings, further adsorption experiments were carried out at pH 6.8, which is the pH of freshly prepared dye solutions without any need for adjustment.
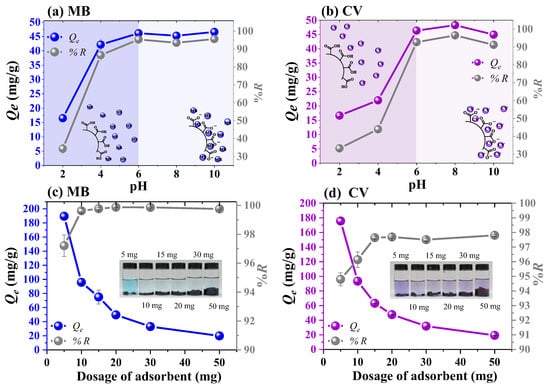
Figure 6.
Effect of pH (a,b) and adsorbent dosage (c,d) on the adsorption of MB and CV by Fe3O4/poly(AA) hydrogel.
3.3.2. Dose Assessment
Using the proper adsorbent amount helps lower operating costs and enhances process efficiency. In this experiment, different doses (5–50 mg) of the Fe3O4/poly(AA) composite hydrogel were placed in contact with 10 mL of MB (100 mg/L) and CV (100 mg/L) solutions for 24 h at pH 6.8. The results are presented in Figure 6c,d, which show the relationship between the amount of adsorbent and the adsorption capacity. It was observed that increasing the hydrogel dosage led to an improvement in dye removal. For MB, the removal increased from 97 to 99% when the adsorbent dose was increased from 5 to 10 mg, due to the large number of active adsorption sites provided with increasing adsorbent dose [13,34,36]. On the other hand, the CV removal efficiency increased from 94% to 96% and 97% as the adsorbent dosage increased from 5 to 10 and 15 mg, respectively. Therefore, 10 mg of Fe3O4/poly(AA) hydrogel was selected as the adsorbent dosage for MB and CV removal in subsequent experiments.
3.3.3. Time Assessment
The adsorption kinetics were examined to understand how contact time influences the adsorption capacity and to identify the possible limiting step of the process. Figure 7a,b show the effect of adsorption time on the adsorption capacity of the Fe3O4/poly(AA) composite hydrogel (previously swollen in distilled water for 24 h) for 10 mL of MB (100 mg/L) and CV (100 mg/L) solutions at pH 6.8 and room temperature. The adsorption of MB and CV was rapid during the first 5 h, followed by a slower phase until equilibrium was reached at approximately 10 h. To better understand the adsorption mechanism, the experimental data were fitted using pseudo-first order (PFO), pseudo-second order (PSO), Elovich, and intraparticle diffusion (IPD) kinetic models. These models are expressed as follows [37,38]:
where Qt (mg/g) is the dye adsorption capacity of the Fe3O4/poly(AA) hydrogel determined from experimental data, and Qe (mg/g) is the equilibrium adsorption capacity calculated from the kinetic models. K1 (1/h) and K2 (g/mg·h) represent the PFO and PSO constants. α is the initial adsorption value (mg/mg·h), and β is the desorption constant (g/mg). KIP (mg/g·h0.5) is the IPD rate constant, and C is the boundary layer thickness constant. These kinetic models are shown in Figure S3, and the parameters are summarized in Table 1.
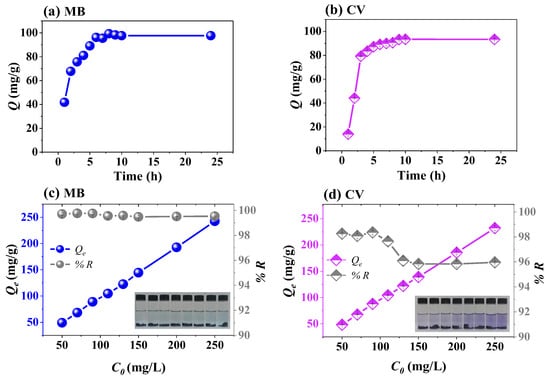
Figure 7.
Effect of contact time (a,b) and initial concentration (c,d) on the adsorption of MB and CV by Fe3O4/poly(AA) hydrogel.

Table 1.
Kinetic parameters for MB and CV adsorption onto Fe3O4/poly(AA) hydrogel.
The PFO model showed the best fit for both dyes (R2 = 0.97 for MB and 0.90 for CV), suggesting that the adsorption kinetics are governed by physical processes [39]. This implies that electrostatic interactions and hydrogen-bonding interactions between the Fe3O4/poly(AA) hydrogel and the MB/CV molecules were the main controlling factors during adsorption [20]. The PSO model also exhibited good fits, although slightly lower, and tended to overestimate the calculated adsorption capacity (Qcal) compared to the experimental values. The Elovich model, commonly associated with chemical or energetically heterogeneous mechanisms, showed a moderate fit (R2 = 0.82 for MB and 0.74 for CV). All data fit the intraparticle diffusion model and can be clearly divided into three linear regions with different slopes, from 47.15 to 0.22 for MB and from 88.39 to 0.10 for CV, i.e., the adsorption process is slower at each stage. Furthermore, C ≠ 0, indicating that the adsorption process is controlled by a multistep mechanism [20].
3.3.4. Concentration Assessment
Figure 7c,d show the effect of the initial concentration of MB and CV, in the range of 50–250 mg/L, on the adsorption efficiency. A maximum removal efficiency of 99% for MB and 97% for CV was achieved throughout this concentration range using 10 mg of the adsorbent. The results indicate that there was no significant decrease in adsorption efficiency with increasing initial concentration. Even at 250 mg/L, efficiency remained at 99%, suggesting that the active sites of the material were not saturated. Consequently, no plateau was observed in the adsorption curve, and it was not considered necessary to work with higher concentrations, as the material was specifically designed for use in the treatment of textile wastewater, where dye concentrations do not usually exceed 250 mg/L. Therefore, extending the concentration range solely to achieve adsorbent saturation would not be justified from a practical and environmental point of view. This approach prioritized reflecting actual contamination conditions rather than artificially establishing theoretical parameters that might not apply to real situations.
These results confirm that the adsorbent maintains a high number of active sites available within the range studied. To obtain more information about the adsorption mechanism, the experimental data were fitted to the Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich (DR) isotherm models [40,41].
where Qmax (mg/g) is the maximum adsorption capacity of the Fe3O4/poly(AA) hydrogel composite, KL (L/mg) is the Langmuir constant, KF (mg/g) and n are the Freundlich constants, KT (L/g) and bT (J/mol) represent the Temkin constants, ε is the Polanyi potential, β (mol2/kJ2) is the activity coefficient related to the Dubinin–Radushkevich model, E (kJ/mol) is the energy of activation, RL is the separation factor from the Langmuir model, R is the universal gas constant, and T is the absolute temperature. These kinetic models are shown in Figure S4, and the parameters are summarized in Table 2.

Table 2.
Isotherm parameters for MB and CV adsorption onto Fe3O4/poly(AA) hydrogel.
The Langmuir model, which assumes monolayer adsorption on a homogeneous surface [17], showed good correlation with R2 values of 0.91 for MB and 0.88 for CV. The theoretical maximum adsorption capacities (Qmax) reached 571 mg/g for MB and 321 mg/g for CV, indicating a high potential for the adsorbent. The Langmuir separation factor (RL) values ranged from 0.0073 to 0.0355 for MB and from 0.02 to 0.11 for CV, confirming the favorability of adsorption (0 < RL < 1). Although the Langmuir model provided a reasonable fit and high theoretical values of Qmax, the absence of a plateau in the adsorption isotherm suggests that saturation of the active sites was not reached within the tested concentration range (50–250 mg/L). Therefore, the estimated Qmax should be interpreted as a theoretical upper limit.
The Freundlich model, which describes adsorption on heterogeneous surfaces [37], provided the best fit to the experimental data, with the highest R2 values (0.94 for MB and 0.93 for CV). The 1/n values were below 1 (0.714 for MB and 0.58 for CV), also indicating favorable and potentially reversible adsorption. Additionally, the Freundlich constants KF (199 mg/g for MB and 55.06 mg/g for CV) corroborate the greater affinity of the adsorbent toward MB.
The Temkin model, which considers a linear decrease in the heat of adsorption with surface coverage, yielded moderate R2 values (0.85 for MB and 0.87 for CV). The bT parameters (32.8 and 39.2 J/mol) suggest that the adsorbate–adsorbent interactions are physical, although with different intensities for each dye [40].
Finally, the Dubinin–Radushkevich model showed the poorest fits, with R2 values of 0.81 for MB and 0.73 for CV. The low values of the mean free energy E (1.12 kJ/mol for MB and 0.40 kJ/mol for CV) indicate that the adsorption process is predominantly governed by physical interactions (since E < 8 kJ/mol) [42]. This agrees with the kinetic analysis, which also suggested a primarily physical mechanism.
Together, these results confirm that the Freundlich model best describes the adsorption process for both dyes, supporting the presence of a heterogeneous surface with different types of active sites and adsorption energies. Furthermore, the high Qmax values obtained with the Langmuir model further highlight the remarkable ability of the Fe3O4/poly(AA) hydrogel to remove cationic dyes from the aqueous solution.
3.3.5. Temperature Assessment
The effect of temperature on the adsorption capacity of the Fe3O4/poly(AA) composite hydrogel for MB and CV was evaluated in the range of 25–45 °C. A decrease in adsorption capacity was observed with increasing temperature, indicating that the process is exothermic. This reduction can be attributed to the enhanced kinetic energy of MB and CV molecules at higher temperatures, which promotes desorption and reduces the probability of stable interactions with the Fe3O4/poly(AA) hydrogel. Additionally, the increased thermal motion can disrupt weak interactions such as hydrogen bonding and electrostatic attractions between the dye molecules and the Fe3O4/poly(AA) hydrogel network.
To better understand the energetic nature of the adsorption process, the thermodynamic parameters ΔG°, ΔH°, and ΔS° were calculated using the van’t Hoff equation [42,43]:
where Kc is the equilibrium constant, Ce (mg/L) is the equilibrium concentration of MB and CV, Qe is the adsorption capacity at equilibrium (mg/g), T is the absolute temperature (K), and R is the universal gas constant (8.314 J/mol·K). The values of ΔH° and ΔS° were obtained from the linear plot of ln(Kc) versus 1/T, where the slope corresponds to ΔH° and the intercept to ΔS° (Figure 8a). Table 3 presents the thermodynamic parameters derived from the experimental results.
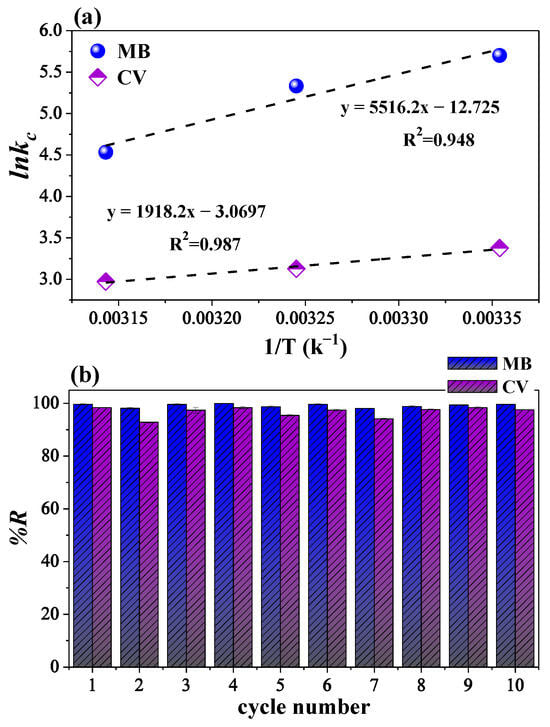
Figure 8.
Linear relationship of lnKc versus 1/T used to determine the thermodynamic parameters (a) and regeneration study over ten cycles using the Fe3O4/poly(AA) hydrogel (b).

Table 3.
Thermodynamic parameters for MB and CV adsorption onto Fe3O4/poly(AA) hydrogel.
Negative ΔG° values were obtained for both dyes at all tested temperatures, confirming the spontaneity of the adsorption process. However, the magnitude of ΔG° decreased slightly with increasing temperature, indicating that adsorption becomes less favorable at higher temperatures [15]. Furthermore, the calculated ΔG° values for MB and CV ranged between −8 and −14 kJ/mol, which falls within the typical range for physisorption processes (−20 to 0 kJ/mol). This suggests that the interaction between the dye molecules and the hydrogel is predominantly physical, likely governed by weak van der Waals forces, hydrogen bonding, or electrostatic attraction. In contrast, chemisorption typically involves much more negative free energy values (−80 to −400 kJ/mol) [44]. Thus, the thermodynamic data strongly support a physisorption mechanism for the dye adsorption process. The value of ΔH° was determined to be −45.86 kJ/mol for MB and −15.95 kJ/mol for CV, confirming the exothermic nature of adsorption in both cases [45]. The significantly more negative ΔH° value for MB suggests stronger interactions with the Fe3O4/poly(AA) hydrogel matrix, likely attributable to favorable electrostatic attractions, hydrogen bonding, or n–π interaction between MB and the network. The ΔS° changes were also negative (−105.80 J/mol·K for MB and −25.52 J/mol·K for CV), indicating a decrease in system randomness during adsorption [34]. This suggests that dye molecules become more ordered when immobilized within the polymeric network. The more pronounced entropy loss for MB may be associated with tighter binding or more structured interaction sites. The thermodynamic profile revealed that the hydrogel has a higher affinity for MB than for CV. This finding aligns with the observed greater spontaneity and stronger enthalpic contributions in MB adsorption, possibly linked to molecular size, charge density, and structural planarity.
3.4. Reuse Determination
Figure 8b shows the removal efficiency of MB and CV over ten consecutive adsorption–desorption cycles using the magnetic Fe3O4/poly(AA) hydrogel. The results indicate remarkable stability in the adsorptive capacity of the material, maintaining efficiencies above 95% even after the tenth cycle for both dyes. Notably, the hydrogel exhibits higher efficiency for MB than for CV across all cycles. This difference can be attributed to the distinct molecular characteristics of the dyes: MB, with its planar structure and lower molecular weight, diffuses more readily into the hydrogel matrix and forms stronger interactions with the AA functional groups. In contrast, CV, with a bulkier structure, likely hinders diffusion and limits access to active sites, particularly in later cycles. Overall, these results demonstrate that the Fe3O4/poly(AA) hydrogel exhibits excellent reusability, reinforcing its potential as a sustainable and economically viable adsorbent for wastewater treatment applications.
3.5. Mechanism Adsorption
To better understand the adsorption mechanism of MB and CV onto the Fe3O4/poly(AA) hydrogel, FTIR spectra were analyzed before and after dye adsorption (Figure 9a,b). The FTIR spectrum of the Fe3O4/poly(AA) hydrogel shows characteristic bands between ~1550 cm−1 and ~1300 cm−1, which correspond to C=O and –COO− vibrations. These functional groups play a crucial role in the adsorption process by providing active sites for electrostatic interactions with dye molecules. After MB and CV adsorption, slight shifts and intensity changes are observed in the stretching bands. Specifically, the decrease in intensity of the COO− band suggests an interaction between the negatively charged carboxylate groups and the cationic dye molecules. Additionally, the broad O–H stretching band (~3400 cm−1) becomes less intense, possibly due to hydrogen bonding. Importantly, no significant new peaks or major chemical shifts are observed, indicating that the adsorption process does not involve covalent bonding or chemical modification of the hydrogel structure. These findings support the conclusion that the interaction mechanism is primarily governed by physical adsorption. This is consistent with kinetic, isotherm, and thermodynamic results. Thus, the adsorption of MB and CV onto Fe3O4/poly(AA) is predominantly driven by electrostatic interactions, with secondary contributions from H-bonding and Yoshida H-bonding, as well as n–π interactions [14,17,18,25]. These multiple weak forces collectively contribute to the high efficiency and stability of the adsorption hydrogel. It is also important to mention that the –OH groups on the surface of the Fe3O4 particles in the Fe3O4/poly/(AA) hydrogel contribute to the formation of H-bonds and Yoshida H-bonds, which would also explain their greater capacity to adsorb MB and CV dyes compared to poly(AA) hydrogel (Figure 10).
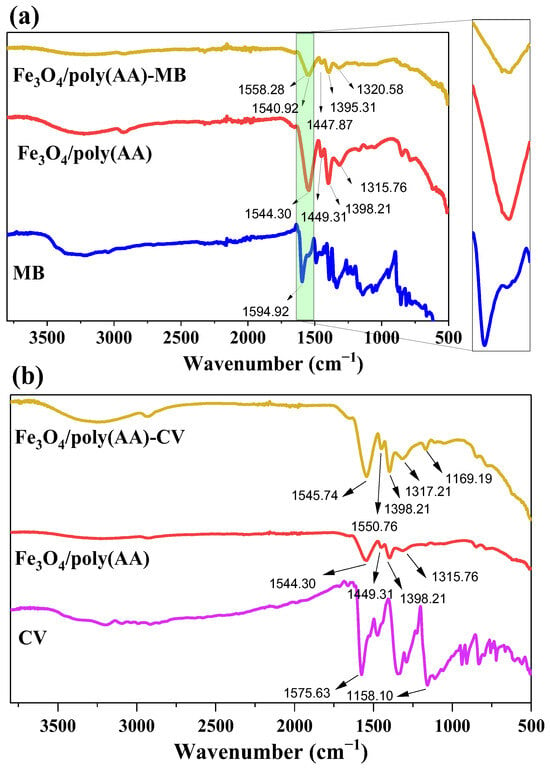
Figure 9.
FTIR spectra of Fe3O4/poly (AA) hydrogel before and after the adsorption of MB (a) and CV (b).
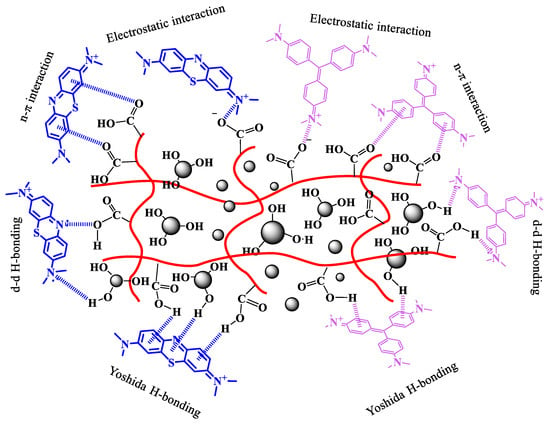
Figure 10.
Proposed removal mechanism of MB and CV by the Fe3O4/poly(AA) hydrogel.
3.6. Comparison with Other Adsorbents
The Fe3O4/poly(AA) hydrogel prepared in this study demonstrated high efficiency in the adsorption of cationic MB and CV dyes. This performance is attributed to the presence of carboxyl groups (–COOH) in the poly(AA) matrix, which promote electrostatic interactions with the dyes, and to the incorporation of Fe3O4 particles. In comparison with other adsorbents reported in the literature, the Fe3O4/poly(AA) hydrogel shows significant advantages in terms of adsorption capacity, ease of separation, and reusability (Table S1). Moreover, its flexible and pH-responsive polymeric structure provides superior adaptability in water treatment systems with variable environmental conditions. Overall, these results position the proposed hydrogel as an efficient, versatile, and reusable alternative to adsorbent materials.
4. Conclusions
In this study, poly(AA) hydrogels incorporating Fe3O4 particles were successfully prepared and applied to remove MB and CV from an aqueous solution. The composite hydrogel exhibited enhanced thermal and adsorption performance due to the incorporation of Fe3O4. Adsorption experiments demonstrated that the hydrogel exhibits optimal performance at pH 6.8. It achieves high adsorption capacities (Qmax = 571 mg/g for MB and 321 mg/g for CV) and excellent reusability (10 cycles with <5% efficiency loss for MB and CV). Kinetic studies followed the PFO model, suggesting that physisorption is the dominant mechanism, while the isotherm data fit the Freundlich model, indicating multilayer adsorption on a heterogeneous surface. The process is driven by synergistic mechanisms, including electrostatic attraction, hydrogen bonding, and n–π interactions. Thermodynamic parameters (ΔG < 0, ΔH < 0) confirmed spontaneous and exothermic adsorption. These results suggest the potential of Fe3O4/poly(AA) hydrogel as a reusable adsorbent for removing MB and CV dyes from aqueous media. Furthermore, the use of hydrogels in pieces offers an advantage over powdered hydrogels, such as easy handling and recovery. For future studies, it would be interesting to evaluate the reuse of the adsorbent under dynamic conditions (e.g., in column systems) and conduct experiments on its effectiveness in real water, where the presence of ions could affect its adsorption capacity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry7050156/s1, Figure S1: Acid–base response of hydrogel based on 2-hydroxyethyl methacrylate (HEMA) and acrylic acid (AA). Figure S2: Screening of MB and CV adsorption with hydrogel based on 2-hydroxyethyl methacrylate (HEMA) and acrylic acid (AA). (The hydrogel that showed the best potential to adsorb MB and CV dyes was the one prepared with 100% AA, Vial number 7). Figure S3: Adsorption kinetic models for MB and CV onto Fe3O4/poly(AA) hydrogel. Figure S4: Adsorption isotherm models for MB and CV onto Fe3O4/poly(AA) hydrogel. Table S1: Comparison of Fe3O4/poly(AA) hydrogel with other adsorbents reported in the literature. References [46,47] are cited in Table S1.
Author Contributions
Conceptualization, F.C.O. and M.A.L.H.; methodology, F.C.O. and M.A.L.H.; formal analysis, F.d.L.M.L., A.C.V.N., and M.A.L.H.; investigation, F.C.O. and M.A.L.H.; data curation, F.d.L.M.L. and A.C.V.N.; writing—original draft preparation, F.C.O. and M.A.L.H.; writing—review and editing, A.C.V.N. and M.A.L.H.; visualization, F.C.O., F.d.L.M.L., A.C.V.N., and M.A.L.H.; supervision, M.A.L.H.; project administration, M.A.L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research was carried out by the H2GEL team (Hidrogeles Híbridos para la Gestión Ambiental y Liberación) at the National University of San Antonio Abad del Cusco (UNSAAC). We thank Janet F. Gonzales Bellido of the Laboratory of Research, Analysis and Preparation of Organic Products-UNSAAC for the FT-IR analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bouras, H.D.; Isik, Z.; Arikan, E.B.; Yeddou, A.R.; Bouras, N.; Chergui, A.; Favier, L.; Amrane, A.; Dizge, N. Biosorption Characteristics of Methylene Blue Dye by Two Fungal Biomasses. Int. J. Environ. Stud. 2021, 78, 365–381. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene Blue Dye: Toxicity and Potential Elimination Technology from Wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Abdul Hameed, M.M.; Al-Aizari, F.A.; Thamer, B.M. Synthesis of a Novel Clay/Polyacrylic Acid-Tannic Acid Hydrogel Composite for Efficient Removal of Crystal Violet Dye with Low Swelling and High Adsorption Performance. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133130. [Google Scholar] [CrossRef]
- Periyasamy, A.P. Recent Advances in the Remediation of Textile-Dye-Containing Wastewater: Prioritizing Human Health and Sustainable Wastewater Treatment. Sustainability 2024, 16, 495. [Google Scholar] [CrossRef]
- Ihaddaden, S.; Aberkane, D.; Boukerroui, A.; Robert, D. Removal of Methylene Blue (Basic Dye) by Coagulation-Flocculation with Biomaterials (Bentonite and Opuntia Ficus Indica). J. Water Process Eng. 2022, 49, 102952. [Google Scholar] [CrossRef]
- El-Mas, S.M.; Hassaan, M.A.; El-Subruiti, G.M.; Eltaweil, A.S.; El Nemr, A. Box-Behnken Design Optimization of 2D Ti3C2Tx MXene Nanosheets as a Microwave-Absorbing Catalyst for Methylene Blue Dye Degradation. Chem. Eng. J. 2024, 500, 156969. [Google Scholar] [CrossRef]
- Rashid Ahmed, H.; Kayani, K.F. A Comparative Review of Fenton-like Processes and Advanced Oxidation Processes for Methylene Blue Degradation. Inorg. Chem. Commun. 2024, 170, 113467. [Google Scholar] [CrossRef]
- Sutar, S.; Jadhav, J. A Comparative Assessment of the Methylene Blue Dye Adsorption Capacity of Natural Biochar versus Chemically Altered Activated Carbons. Bioresour. Technol. Rep. 2024, 25, 101726. [Google Scholar] [CrossRef]
- Badawi, A.K.; Emam, H.E.; Hamad, H.N.; Idrus, S. Recent Developments in the Application of Bio-Waste-Derived Adsorbents for the Removal of Methylene Blue from Wastewater: A Review. Polymers 2022, 14, 783. [Google Scholar] [CrossRef]
- Visan, A.I.; Negut, I. Environmental and Wastewater Treatment Applications of Stimulus-Responsive Hydrogels. Gels 2025, 11, 72. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, M.; Nimesh, D.; Gupta, R. Exploring the Potential of Hydrogel Adsorbents for Antibiotic Removal from Water: A Review. J. Mol. Liq. 2025, 426, 127383. [Google Scholar] [CrossRef]
- Panda, S.K.; Aggarwal, I.; Kumar, H.; Prasad, L.; Kumar, A.; Sharma, A.; Vo, D.V.N.; Van Thuan, D.; Mishra, V. Magnetite Nanoparticles as Sorbents for Dye Removal: A Review. Environ. Chem. Lett. 2021, 19, 2487–2525. [Google Scholar] [CrossRef]
- Safarzadeh, H.; Peighambardoust, S.J.; Mousavi, S.H.; Foroutan, R.; Mohammadi, R.; Peighambardoust, S.H. Adsorption Ability Evaluation of the Poly(Methacrylic Acid-Co-Acrylamide)/Cloisite 30B Nanocomposite Hydrogel as a New Adsorbent for Cationic Dye Removal. Env. Res. 2022, 212, 113349. [Google Scholar] [CrossRef] [PubMed]
- Hingrajiya, R.D.; Patel, M.P. Fe3O4 Modified Chitosan Based Co-Polymeric Magnetic Composite Hydrogel: Synthesis, Characterization and Evaluation for the Removal of Methylene Blue from Aqueous Solutions. Int. J. Biol. Macromol. 2023, 244, 125251. [Google Scholar] [CrossRef]
- Noori, M.; Tahmasebpoor, M.; Foroutan, R. Enhanced Adsorption Capacity of Low-Cost Magnetic Clinoptilolite Powders/Beads for the Effective Removal of Methylene Blue: Adsorption and Desorption Studies. Mater. Chem. Phys. 2022, 278, 125655. [Google Scholar] [CrossRef]
- Oyarce, E.; Pizarro, G.D.C.; Oyarzún, D.P.; Martin-Trasanco, R.; Sánchez, J. Adsorption of Methylene Blue in Aqueous Solution Using Hydrogels Based on 2-Hydroxyethyl Methacrylate Copolymerized with Itaconic Acid or Acrylic Acid. Mater. Today Commun. 2020, 25, 101324. [Google Scholar] [CrossRef]
- Huaman, M.A.L.; Manco, A.E.Q.; de Liss Meza López, F.; Carrasco, R.L.A.; Chacón, A.M.L.; Khan, S. Removal of Methylene Blue Dye from Water with Fe3O4/Poly(HEMA-Co-AMPS) Magnetic Hydrogels. Results Chem. 2024, 7, 101454. [Google Scholar] [CrossRef]
- Ccoyo Ore, F.; María Lechuga Chacon, A.; Leonor Aranzábal Carrasco, R.; de Liss Meza López, F.; Cecilia Valderrama Negrón, A.; Azael Ludeña Huaman, M. Preparation of Nanocomposite Hydrogel Based on Fe3O4-TMSPM/Poly(HEMA-PEG6MA-IA) for the Removal of Methylene Blue Dye from Aqueous Solution. Results Chem. 2024, 12, 101888. [Google Scholar] [CrossRef]
- Zhang, W.; Lan, Y.; Ma, M.; Chai, S.; Zuo, Q.; Kim, K.H.; Gao, Y. A Novel Chitosan–Vanadium-Titanium-Magnetite Composite as a Superior Adsorbent for Organic Dyes in Wastewater. Env. Int. 2020, 142, 105798. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, Y.; Cui, Y.; Dai, R.; Shan, Z.; Chen, H. Fabrication of Starch-Based High-Performance Adsorptive Hydrogels Using a Novel Effective Pretreatment and Adsorption for Cationic Methylene Blue Dye: Behavior and Mechanism. Chem. Eng. J. 2021, 405, 126953. [Google Scholar] [CrossRef]
- Bui, T.Q.; Cao, V.D.; Wang, W.; Kjøniksen, A.L. Recovered Energy from Salinity Gradients Utilizing Various Poly(Acrylic Acid)-Based Hydrogels. Polymers 2021, 13, 645. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Xu, Z.; Liu, Y.; Guo, X.; Ou, M.; Xu, X. Highly Efficient Removal of Uranium (vi) from Wastewater by Polyacrylic Acid Hydrogels. RSC Adv. 2017, 7, 6278–6287. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, H.; Lan, W.; Miao, F.; Qin, M.; Wei, Y.; Hu, Y.; Liang, Z.; Huang, D. Fabrication of Adhesive Hydrogels Based on Poly (Acrylic Acid) and Modified Hyaluronic Acid. J. Mech. Behav. Biomed. Mater. 2022, 126, 105044. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Yadav, S.; Mehta, S.K.; Dan, A. In Situ Incorporation of Magnetic Nanoparticles within the Carboxymethyl Cellulose Hydrogels Enables Dye Removal. J. Macromol. Sci. Part. A 2022, 59, 271–284. [Google Scholar] [CrossRef]
- Ludeña, M.A.; Meza, F.d.L.; Huamán, R.I.; Lechuga, A.M.; Valderrama, A.C. Preparation and Characterization of Fe3O4/Poly(HEMA-Co-IA) Magnetic Hydrogels for Removal of Methylene Blue from Aqueous Solution. Gels 2023, 10, 15. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, D.; Wang, S.; Qi, S.; Zuo, H. Structure, Property Optimization, and Adsorption Properties of N,N′-Methylenebisacrylamide Cross-Linked Polyacrylic Acid Hydrogels under Different Curing Conditions. Polymers 2024, 16, 1990. [Google Scholar] [CrossRef]
- Ganguly, S.; Ray, D.; Das, P.; Maity, P.P.; Mondal, S.; Aswal, V.K.; Dhara, S.; Das, N.C. Mechanically Robust Dual Responsive Water Dispersible-Graphene Based Conductive Elastomeric Hydrogel for Tunable Pulsatile Drug Release. Ultrason. Sonochemistry 2018, 42, 212–227. [Google Scholar] [CrossRef]
- Cha, H.R.; Ramesh Babu, V.; Krishna Rao, K.S.V.; Kim, Y.H.; Mei, S.; Joo, W.H.; Lee, Y.I. Fabrication of Amino Acid Based Silver Nanocomposite Hydrogels from PVA- Poly(Acrylamide-Co-Acryloyl Phenylalanine) and Their Antimicrobial Studies. Bull. Korean Chem. Soc. 2012, 33, 3191–3195. [Google Scholar] [CrossRef]
- Huaman, M.A.L.; Vega-Chacón, J.; Quispe, R.I.H.; Negrón, A.C.V. Synthesis and Swelling Behaviors of Poly(2-Hydroxyethyl Methacrylate-Co-Itaconic Acid) and Poly(2-Hydroxyethyl Methacrylate-Co-Sodium Itaconate) Hydrogels as Potential Drug Carriers. Results Chem. 2023, 5, 100917. [Google Scholar] [CrossRef]
- Ozay, O.; Ekici, S.; Baran, Y.; Aktas, N.; Sahiner, N. Removal of Toxic Metal Ions with Magnetic Hydrogels. Water Res. 2009, 43, 4403–4411. [Google Scholar] [CrossRef]
- Paulino, A.T.; Belfiore, L.A.; Kubota, L.T.; Muniz, E.C.; Almeida, V.C.; Tambourgi, E.B. Effect of Magnetite on the Adsorption Behavior of Pb(II), Cd(II), and Cu(II) in Chitosan-Based Hydrogels. Desalination 2011, 275, 187–196. [Google Scholar] [CrossRef]
- Ahmadi, A.; Foroutan, R.; Esmaeili, H.; Peighambardoust, S.J.; Hemmati, S.; Ramavandi, B. Montmorillonite Clay/Starch/CoFe2O4 Nanocomposite as a Superior Functional Material for Uptake of Cationic Dye Molecules from Water and Wastewater. Mater. Chem. Phys. 2022, 284, 126088. [Google Scholar] [CrossRef]
- Goswami, K.; Ulaganambi, M.; Sukumaran, L.K.; Tetala, K.K.R. Synthesis and Application of Iron Based Metal Organic Framework for Efficient Adsorption of Azo Dyes from Textile Industry Samples. Adv. Sample Prep. 2023, 7, 100080. [Google Scholar] [CrossRef]
- Li, K.; Yan, J.; Zhou, Y.; Li, B.; Li, X. β-Cyclodextrin and Magnetic Graphene Oxide Modified Porous Composite Hydrogel as a Superabsorbent for Adsorption Cationic Dyes: Adsorption Performance, Adsorption Mechanism and Hydrogel Column Process Investigates. J. Mol. Liq. 2021, 335, 116291. [Google Scholar] [CrossRef]
- Faizan, S.; Bakhtawara; Ali Shah, L. Facile Fabrication of Hydrogels for Removal of Crystal Violet from Wastewater. Int. J. Environ. Sci. Technol. 2022, 19, 4815–4826. [Google Scholar] [CrossRef]
- Hu, X.S.; Liang, R.; Sun, G. Super-Adsorbent Hydrogel for Removal of Methylene Blue Dye from Aqueous Solution. J. Mater. Chem. A 2018, 6, 17612–17624. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Ahmadi, A.; Bikhabar, G.; Babaei, F.; Ramavandi, B. Impact of ZnO and Fe3O4 Magnetic Nanoscale on the Methyl Violet 2B Removal Efficiency of the Activated Carbon Oak Wood. Chemosphere 2022, 286, 131632. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Mohammadi, R.; Peighambardoust, S.H.; Ramavandi, B. Development of New Magnetic Adsorbent of Walnut Shell Ash/Starch/Fe3O4 for Effective Copper Ions Removal: Treatment of Groundwater Samples. Chemosphere 2022, 296, 133978. [Google Scholar] [CrossRef] [PubMed]
- Dibaji, Y.; Zilouei, H.; Bazarganipour, M. Removal of MTBE from Aqueous Solution Using Reduced Graphene Oxide/Fe3O4 Nanocomposite. Env. Nanotechnol. Monit. Manag. 2023, 20, 100842. [Google Scholar] [CrossRef]
- Pashaei-Fakhri, S.; Peighambardoust, S.J.; Foroutan, R.; Arsalani, N.; Ramavandi, B. Crystal Violet Dye Sorption over Acrylamide/Graphene Oxide Bonded Sodium Alginate Nanocomposite Hydrogel. Chemosphere 2021, 270, 129419. [Google Scholar] [CrossRef]
- Alsohaimi, I.H.; Alhumaimess, M.S.; Alqadami, A.A.; Hassan, H.M.A.; Chen, Q.; Alamri, M.S.; Alanzi, M.M.J.; Alraddadi, T.S. Chitosan-Carboxylic Acid Grafted Multifunctional Magnetic Nanocomposite as a Novel Adsorbent for Effective Removal of Methylene Blue Dye from Aqueous Environment. Chem. Eng. Sci. 2023, 280, 119017. [Google Scholar] [CrossRef]
- Hegazy, S.; Abdelwahab, N.A.; Ramadan, A.M.; Mohamed, S.K. Magnetic Fe3O4-Grafted Cellulose/Graphene Oxide Nanocomposite for Methylene Blue Removal from Aqueous Solutions: Synthesis and Characterization. Next Mater. 2024, 3, 100064. [Google Scholar] [CrossRef]
- Ilgin, P.; Onder, A.; Kıvanç, M.R.; Ozay, H.; Ozay, O. Adsorption of Methylene Blue from Aqueous Solution Using Poly(2-Acrylamido-2-Methyl-1-Propanesulfonic Acid-Co-2-Hydroxyethyl Methacrylate) Hydrogel Crosslinked by Activated Carbon. J. Macromol. Sci. Part A 2023, 60, 135–149. [Google Scholar] [CrossRef]
- Somsesta, N.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption Removal of Methylene Blue onto Activated Carbon/Cellulose Biocomposite Films: Equilibrium and Kinetic Studies. Mater. Chem. Phys. 2020, 240, 122221. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.Ü.; Brouers, F. Methylene Blue Adsorption on Magnetic Alginate/Rice Husk Bio-Composite. Int. J. Biol. Macromol. 2020, 154, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, M.; Ji, M.; Zhang, L.; Qin, Z.; Zhang, Y.; Gao, L.; Jiao, T. Magnetic graphene oxide-containing chitosan-sodium alginate hydrogel beads for highly efficient and sustainable removal of cationic dyes. Int. J. Biol. Macromol. 2021, 193, 2221–2231. [Google Scholar] [CrossRef]
- Kurdtabar, M.; Akhlaghi, F.; Marandi, G.B.; Nakhjiri, M.T.; Zargazi, M.H. Magnetic, Biocompatible and Biodegradable Carboxymethyl Cellulose-Based Hydrogel for Cationic Dye Adsorption. ChemistrySelect 2024, 9, e202303594. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).