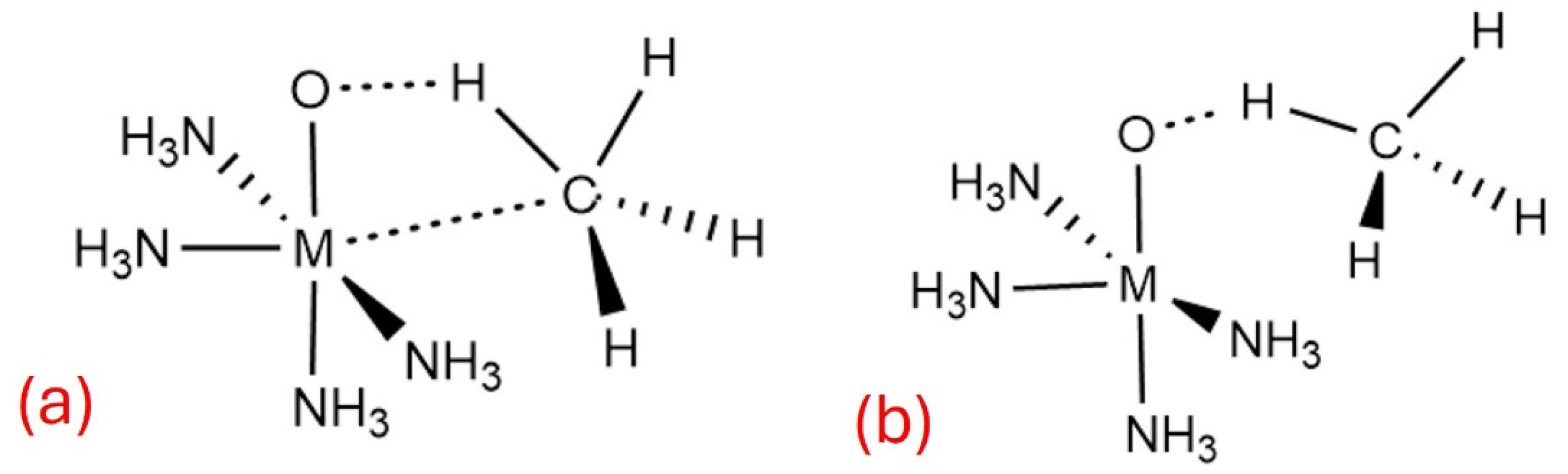
3.1. (NH3)4RhO2+
We will first refer to the electronic and geometric structure of the low-lying electronic states of [Rh]O
2+ and revisit the complete catalytic cycle of [Rh]O
2+ + CH
4 discussing important aspects not reported in our previous studies [
8,
12]. The ground state of [Rh]O
2+ is of doublet spin multiplicity and adopts trigonal bipyramidal geometry. The contours of the most relevant frontier orbitals to this discussion are shown in
Figure 2.
In the ground state, there are six electrons in the σ and π metal–oxygen bonding orbitals (
and
; see
Figure S1), four electrons in the
and
orbitals which are localized on the metal, and one electron in the anti-bonding
(see
Figure 2). The lowest quartet state is 8.3 kcal/mol higher and corresponds to the promotion of one electron from
to
. This change in the electronic structure induces the change in the geometry from trigonal bipyramidal to square pyramidal with one open metallic site (see
Figure 2). At this geometry, the doublet state is 9.1 kcal/mol higher and electronic configuration switches from
to
, i.e., the same as the quartet state but with the three unpaired electrons coupled into a doublet. In principle, this open site can accommodate the coordination of one methane molecule. Indeed, this happens, and one CH bond forms a σ-dative bond with the metal center, but according to our calculations, only after the remaining
electron is promoted to either the
or
. This implies that the formation of the (CH
4)[Rh]O
2+ complex is facilitated by excited doublet states of the open-site structure of [Rh]O
2+ with configurations
or
. This effect can be attributed to the space opening for the σ
CH electrons of methane to penetrate closer to the Rh
4+ center and populate the vacant
orbital (see σ
CH in
Figure 2).
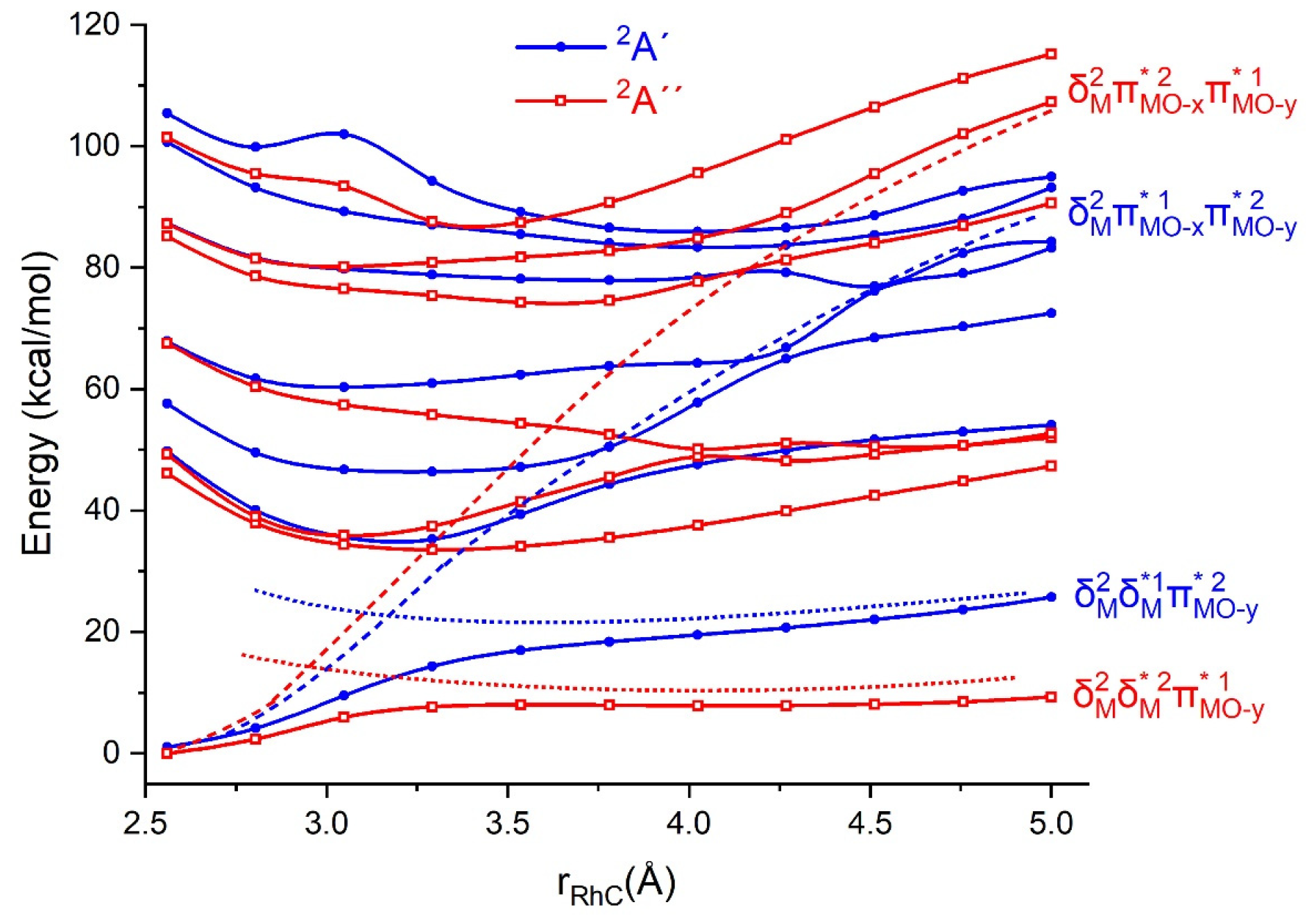
To further illustrate this mechanism, we performed multi-reference (CASSCF) calculations for multiple potential energy surfaces along the minimal energy path of the [Rh]O
2+ + CH
4 → (CH
4)[Rh]O
2+ reaction step (see
Figure 3). The geometries of the reaction coordinate were modified by rotating ammonia ligands to impose C
s symmetry and are listed in
Table S1. It is obvious that the approach of methane in the lowest potential energy curves of each symmetry is slightly (
2A′) or minimally (
2A″) attractive, but their slope becomes steeper at the distance of 3.3 Å. This results from avoided crossings with the potential energy curves of higher energy states with electronic configurations that facilitate the coordination of methane (see above and
Figure 3). Indeed, looking at the orbitals and coefficients for the major Slater determinant of the ground
2A″ and first excited
2A′ state, we see that the major coefficients remain constant for all Rh-C distances (0.90 ± 0.03 and 0.87 ± 0.05) but the composition of the 25a′ orbital changes gradually from
to
(see
Figure S2), which points to the
→
and
→
alterations.
Figure 4 shows the complete energy diagram for the oxidation of methane with ozone facilitated by [Rh]O
2+. Only the doublet state is considered, since all transition states and intermediate structures of the quartet state have higher energy (see
Figure S3). All structures are shown in
Figure S4, and their Cartesian coordinates are listed in
Table S2.
At first, methane forms an encounter complex with [Rh]O
2+, which is stable by 6.8 kcal/mol with respect to its fragments, but after a small activation barrier of 3.8 kcal/mol, it coordinates to the metal using the σ-electrons of one CH bond. As mentioned in [
8], the C-H bond and the Rh-O bond both elongate by 0.03 (1.091 to 1.125 Å) and 0.17 Å (1.697 to 1.866 Å), respectively, signaling (pre-)activation for both bonds, which facilitates the formation of CH
3[Rh]OH
2+ with a barrier of only 13.5 kcal/mol via a [2 + 2] mechanism. The elongation of the Rh-O bond can be attributed to the transfer of two electrons from
to
, induced by the coordination of methane (see above). CH
3[Rh]OH
2+ is quite stable requiring a reverse activation barrier of 44.9 kcal/mol towards (CH
4)[Rh]O
2+ or a smaller forward barrier of 22.6 kcal/mol towards the production of methanol. This forward step is highly exothermic and involves the transfer of two electrons to the metal center, the formal oxidation state of which changes from +4 to +2. The release of methanol entails 21.7 kcal/mol energy or 11.6 kcal/mol free energy at room temperature and 1 atm pressure due to the associated entropy increase.
The ground state of the remaining [Rh]2+ metal complex is still a doublet with a square planar structure (xy plane) well separated from the first quartet state with a square planar geometry as well (60.6 kcal/mol). The electronic configuration of the doublet is and the quartet state involves an excitation from to . The , , orbitals are polarized away from the Rh-N bonds, imitating the t2g orbitals of an octahedral structure, while the , imitate the eg orbitals.
The final step pertains to the oxidation of the metal center and the creation of the initial [Rh]O
2+ form of the catalyst. As an example, here we use ozone as an oxidant. O
3 and N
2O are commonly used experimentally and theoretically for molecular species, as opposed to O
2 which is employed in heterogeneous catalysis. Heterogeneous catalysts chemisorb and dissociate O
2 to form the catalytically active metal–oxygen units. The use of O
2 for molecular catalysts is more challenging since it makes the mechanism more involved and requires overcoming higher energy barriers [
21].
Ozone coordinates to the metal strongly with more than 20 kcal/mol and, after a barrier of 5.0 kcal/mol, creates the O2.[Rh]O2+ adduct. The two moieties are in their ground state, triplet for O2 and doublet for [Rh]O2+, with the three unpaired electrons coupled into an overall doublet state, which leads to large spin contamination (S = 0.9 instead of 0.5). The spin contamination does not affect the energy of this structure, considering that the corresponding quartet state (all three electrons with parallel spin) is only 0.01 kcal/mol lower in energy. The molecular oxygen is released spontaneously at room temperature and 1 atm pressure (required electronic energy is 4.9 kcal/mol and the free energy difference is −7.0 kcal/mol).
In conclusion, the doublet spin multiplicity is preserved along the full reaction path, and the undergoing electronic structure changes render the overall process energetically downhill with reasonable barriers for ambient conditions.
Another important feature of [Rh]O
2+ as a catalyst is that the activation of methane via a radical mechanism is less favorable. The activation barrier for the radical path is 14.9 kcal/mol, i.e., 1.4 kcal/mol higher than the [2 + 2] process. After correcting for zero-point vibrational energies or thermal contributions (free energy), this energy difference drops to 0.6 kcal/mol. In addition, the energy of the products for the radical mechanism is practically equal or slightly higher than that of the reactants (0.02 kcal/mol and 1.2 kcal/mol after thermal corrections). Therefore, the reverse reaction is more favorable, and for both kinetic and thermodynamic reasons, the [2 + 2] mechanism prevails over the radical one. The geometries and molecular orbital contours for the radical reaction step are given in
Table S3 and
Figure S5. It should be mentioned that in the radical mechanism, the methane molecule attacks the terminal oxygen atom at a perpendicular angle with respect to the RhO axis (end-on), compared to the [2 + 2] case (side-on), because the unpaired electron resides in the
orbital (see
Figure 2 and
Figure S5). The electronic state generated by promoting an electron from
to
lies 45.8 kcal/mol higher and is not expected to contribute to the radical process. During the hydrogen atom transfer, one proton is transferred to oxygen and one electron to the metal center (
; see
Figure S5). Therefore, the radical mechanism proceeds as proton-coupled electron transfer (PCET).
A final comment pertains to the activation of methanol. This is an important aspect since faster oxidation of methanol than methane leads to the production of overoxidized products and lower selectivity for the desired methanol as a major product. Here we confirm our previous CCSD(T) level results [
8] with the current methodology that the activation barriers for both the [2 + 2] and PCET mechanisms for methanol have larger barriers, 16.0 and 16.5 kcal/mol, respectively. The present free energy barriers are 13.3 and 13.7 kcal/mol, which are very close to that of the methane [2 + 2] mechanism, but our more accurate ring polymer instanton quantum dynamics predicted clearly larger rate constants for methane by an order of magnitude [
8]. This was attributed to the hydrogen bonding interactions between methanol and the ammonia ligands, which distort the transition states.
This is an example of the importance of the coordination environment and the metal–ligand co-operativity in improving selectivity. This is a common practice in organic chemistry, where hydrogen bonds allow the activation of specific C-H bonds (see for example [
9,
10,
11]) and is widely exploited by metalloenzymes (see for example [
22]).
3.2. (NH3)4CoO2+
Cobalt is the first-row transition metal counterpart of rhodium, and it would be reasonable to explore the reactivity of the corresponding (NH
3)
4CoO
2+, or [Co]O
2+, coordination complex. The possible replacement of Rh with the more Earth abundant Co can possibly provide lower-cost solutions. While ammonia ligands serve as model ligands, similar CoO
2+ complexes have been synthesized in the literature [
23,
24,
25,
26,
27].
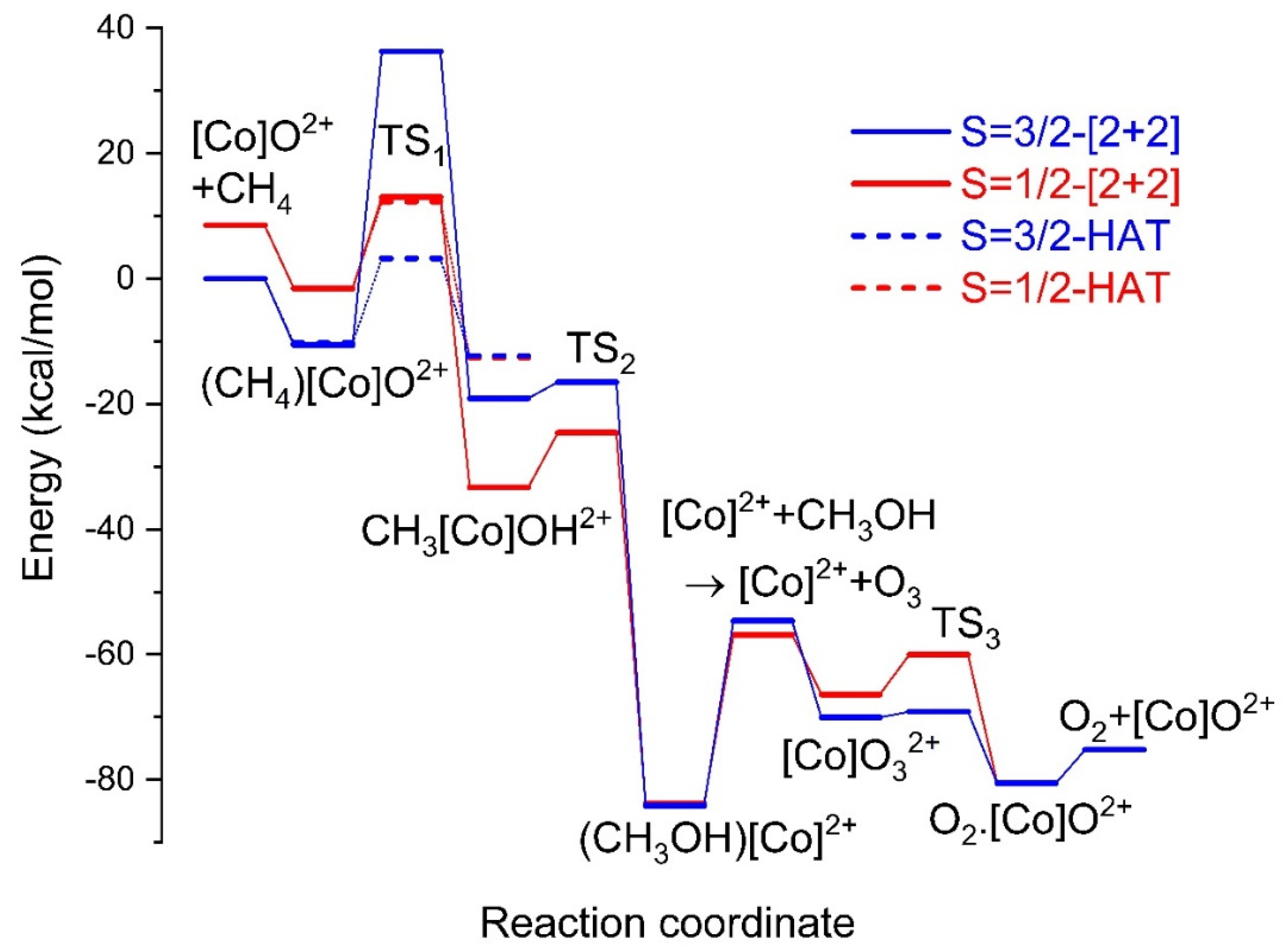
The doublet and quartet states of [Co]O2+ follow the inverse order compared to [Rh]O2+. The ground state of [Co]O2+ is quartet and has the same electron configuration as in [Rh]O2+, i.e., , while the doublet is 8.5 kcal/mol higher. Based on the natural orbital populations (), the electronic configuration of the doublet state is a mixture of a doublet electronic configuration made from the quartet state and flipping the spin of one electron (by 40%) and the electronic configuration (by 60%) that was found responsible for activating methane in [Rh]O2+. The optimal geometry for both the quartet and doublet states is square pyramidal, imitating that of the quartet state of [Rh]O2+. The sextet state lies 19.8 kcal/mol higher than the ground quartet state. After the coordination of methane to [Co]O2+, the composition of the doublet state changes to 80–20%, favoring further the combination. The coordinated CH bond of methane in (CH4)[Co]O2+ elongates less than in rhodium (0.02/0.01 Å for the doublet/quartet states), and for both states, the binding energy of methane is just over 10 kcal/mol.
As a result of the composition of the doublet state, the activation barrier for the doublet of [Co]O
2+ is three times smaller than the quartet (14.6 vs. 46.9 kcal/mol); thus, the transition state for the doublet is lower than that of the quartet. The overall energy diagram for both spin states is shown in
Figure 5. It is evident that the potential energy surfaces of the two states cross in the space between (CH
4)[Co]O
2+ and TS
1; thus, a two-state reactivity process may be enabled. Similar mechanisms have been observed for the activation of methane by bare FeO
+ and ligated FeO
2+ species [
28,
29]. Considering the energy difference between (CH
4)[Co]O
2+ (S = 3/2) and TS
1 (S = 1/2), the effective activation barrier for the two-state reactivity model is 23.6 kcal/mol, which is 10.1 kcal/mol higher than that of [Rh]O
2+. Overall, the replacement of rhodium by cobalt still leads to a reasonable energy barrier but slower activation of methane.
Another big difference between cobalt and rhodium pertains to the next reaction step. The activation barriers for the recombination of CH
3 and OH are only 8.6 (S = 1/2) and 2.6 (S = 3/2) kcal/mol for cobalt, which are minimal compared to 22.6 kcal/mol for rhodium. To comprehend this difference, we focus on the electronic structures of the reactants, CH
3[M]OH
2+, and products, (CH
3OH)[M]
2+, of this reaction step (M = Co, Rh). The oxidation state is formally M
4+ for the former and M
2+ for the latter, and thus the formation of methanol implies the two-electron reduction in the metal center. Looking at the third and fourth ionization energies of the two metals, we see that the Co
4+ → Co
2+ transition is more favorable than Rh
4+ → Rh
2+ by 11.7 eV (33.5 + 51.3 = 84.8 eV for Co and 31.1 + 42.0 = 73.1 eV for Rh) [
30]; thus, we believe the smaller barriers are due to the “easier” reduction of Co
4+. For both spin states and for both metals, the formation of methanol is highly exothermic. The quartet spin state of (CH
3OH)[Co]
2+ is slightly lower in energy (0.3 kcal/mol); thus, there is a second crossing of the potential energy surfaces during the formation of methanol. The energy required for the release of methanol is 29.5 kcal/mol for the ground quartet state, which is 7.8 kcal/mol larger than (CH
3OH)[Rh]
2+. The stronger binding of methanol to Co
2+ is because of its smaller ionic radius (since Rh
2+ is not reported—we refer to the ionic radii of Co
3+ vs. Rh
3+ = 0.55 vs. 0.67 Å) [
30].
Similarly to rhodium, the oxygen reload process using ozone is energetically favorable, with the product being 18.4 and 20.6 kcal/mol lower than the reactants for the doublet and quartet states, respectively. The activation barrier for the quartet is minimal (0.9 kcal/mol), and that for the doublet is small (6.4 kcal/mol). During the coordination of ozone, there is one more crossing between the doublet and the quartet potential energy surfaces, while after the formation of oxygen, only the quartet state of [Co]O2+ is produced. The reason is that the spin coupling of S = 3/2 (ground state of [Co]O2+) and S = 1 (ground state of O2) generates total S = 1/2, 3/2, 5/2 for the ground state of the O2.[Co]O2+ product, which includes both the doublet and quartet states of the reactants.
Regarding the PCET methane activation mechanism, the activation barriers for the two states are very similar (13.8 and 13.6 kcal/mol). This makes the [2 + 2] and PCET paths competitive for the doublet state but renders the PCET path of the quartet dominant (see
Figure 5). Therefore, it is essential to stabilize the doublet state of the cobalt complex to avoid the production of methyl radicals, which may lead to unfavorable side products. To this end, the replacement of ammonia with other, more proper ligands may be necessary. An alternative way to avoid the PCET mechanism can be the replacement of a Co
4+ with an Fe
3+. Both metal centers are 3d
5 ions with a
6S ground state, but the Fe
3+ is expected to favor less the transfer of an electron from methane.
3.3. (NH3)4FeO2+
The replacement of Co
4+ with Fe
3+ keeps the quartet state as the ground state and the doublet is 12.6 kcal/mol higher, i.e., the doublet is destabilized by 4.1 kcal/mol. The following electronic configurations for the two spin states remain practically intact:
and
. Comparing the energy landscapes for the reaction of methane with the two metals (see
Figure 5 and
Figure 6), we see that the quartet reaction path is always lower in energy for iron and no spin crossing is observed. This is different with what has been observed for the “naked” FeO
+ unit, which is a prototypical system for the spin crossover mechanism of the methane activation step [
29].
The most interesting observation is that for both spin states of [Fe]O
+, the PCET process bears higher energy transition states than 2 + 2 (see
Figure 6) by 4.5 (S = 3/2) and 7.6 (S = 1/2) kcal/mol, supporting our basic hypothesis that the lower oxidation state of Fe
3+ will disfavor the radical pathway. In addition, the energy of the products CH
3.[Fe]OH
+ is higher than the reactants (CH
4)[Fe]O
+ by 10.5 (S = 3/2) and 12.6 (S = 1/2) kcal/mol, signifying that the release of methyl radicals is also unfavorable.
The formation of the stable CH
3[Fe]OH
+ intermediate occurs after an activation energy barrier of 25.9 (S = 3/2) and 20.7 (S = 1/2) kcal/mol. As in the case of [Co]O
2+, the barrier is lower for the doublet state, but the barrier of the quartet state is comparable and not three times larger (see
Section 3.2). The production of methanol at the CH
3-OH recombination step is an energetically uphill process with relatively large activation barriers (45.9 and 58.1 for S = 3/2 and 1/2) but could be driven by the displacement of methanol by O
3 in the subsequent oxygen reload step. Two factors can explain why the recombination step is less favorable in the case of iron. First, the oxidation state (Fe
3+ → Fe
+) transition is the least favorable among the three metals (30.7 + 16.2 = 46.9 eV vs. 84.8 and 73.1 eV for cobalt and rhodium; see
Section 3.2). Second, the Fe
+ center is stabilized less by the ammonia ligands and the produced methanol compared to the Co
2+ and Rh
2+ centers, due mostly to the weaker Coulombic attraction (+ vs. 2+). For the latter reason, the energy required for the release of methanol from (CH
3OH)[Fe]
+ is 15.0 kcal/mol (S = 3/2), which is about half of the value found for (CH
3OH)[Co]
2+ (29.5 kcal/mol). The final metal oxidation step is facile (small activation barrier and stable products), closing the catalytic cycle resembling the rhodium and cobalt cases. As in cobalt, the two spin states end up in the quartet state of the metal oxide complex.
Regarding the oxidation of methanol, the dominant mechanism goes through the radical pathway via the quartet spin state (lower energy transition state than 2 + 2), but the activation barrier is found to be 29.3 kcal/mol (S = 3/2), which is 3.3 kcal/mol higher than the most favorable 2 + 2 route for the activation of methane. This difference promotes higher selectivity towards methanol [
8]. The energy diagram for the methanol activation process is given in
Figure S6 of the SI.
A last comment pertains to the comparison of the presently used [Fe]O
+ complex and the commonly used FeO
2+-containing complexes. The latter complexes bear a fully saturated first coordination sphere (see, for example, [
31,
32]), which disables the [2 + 2] mechanism. In addition, the overall 2+ charge of the metal oxygen unit enhances the radical character of oxygen [
1], promoting the radical mechanism (see the [Co]O
2+ case in
Section 3.2). Both factors favor the radical (hydrogen atom transfer or PCET) mechanism [
33], which leads to small selectivity values.
3.4. Comparison of Catalytic Efficiency
In this section, we compare the efficiency of converting methane to methanol for the three species by using the apparent activation energy of each chemical cycle. The apparent activation energy ΔE of a chemical cycle is not necessarily the largest activation energy barrier of the individual steps [
34]. ΔE can be predicted by implementing the energetic span model [
35,
36], which can identify the critical transition state (TDTS) and intermediate structure (TDI) that determine the turn-over frequency of a cycle. For all three metal oxides, TDI and TDTS turn out to belong to the same reaction step (see
Table S10 and
Figure S7). For [Rh]O
2+ and [Fe]O
+, the rate-determining step is the CH
3-OH recombination, while for [Co]O
2+, it is the release of methanol. Therefore, in none of the cases is the C-H activation step the limiting step. However, it is the step that determines the selectivity, since the C-H bond of methanol must be slower. Looking at the TOF values predicted by the energetic span model (see
Figure S7), we see that [Rh]O
2+ is the best option followed by [Co]O
2+ and [Fe]O
+. On the other hand, in terms of selectivity, [Fe]O
+ is the best option since it has the highest energy difference between the activation barriers for methanol and methane, and the production of methyl radical is unfavorable (see
Section 3.3). The second-best option is [Rh]O
2+, followed by [Co]O
2+, which finally promotes the radical mechanism. Considering both efficiency and selectivity, [Rh]O
2+ is the clear winner, implying that second-row transition metals are more appropriate. This analysis implies that future theoretical studies must focus on complete cycles (and not only methane activation) to assess efficiency and reactions with methanol to assess selectivity.