Subcritical Extraction of Rosa alba L. in Static and Dynamic Modes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Analysis
2.4. Olfactory Evaluation
2.5. Statistics
3. Results and Discussion
3.1. Effect of the Extraction Mode on the Yield
3.2. Effect of the Extraction Mode on the Chemical Composition
3.3. Effect of the Extraction Mode on the Aroma Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dobreva, A.; Dincheva, I.; Nenov, N. On the Subcritical Extraction of Rosa Damascena Mill. Bulg. J. Agric. Sci. 2021, 27, 785–791. [Google Scholar]
- Kovacheva, N.; Rusanov, K.; Atanassov, I. Industrial Cultivation of Oil Bearing Rose and Rose Oil Production in Bulgaria During 21st Century, Directions and Challenges. Biotechnol. Biotechnol. Equip. 2010, 24, 1793–1798. [Google Scholar] [CrossRef]
- Rusanov, K.; Kovacheva, N.; Rusanova, M.; Atanassov, I. Flower Phenotype Variation, Essential Oil Variation and Genetic Diversity among Rosa alba L. Accessions Used for Rose Oil Production in Bulgaria. Sci. Hortic. 2013, 161, 76–80. [Google Scholar] [CrossRef]
- Jovtchev, G.; Stankov, A.; Georgieva, A.; Dobreva, A.; Bakalova, R.; Aoki, I.; Mileva, M. Cytotoxic and Genotoxic Potential of Bulgarian Rosa alba L. Essential Oil—In Vitro Model Study. Biotechnol. Biotechnol. Equip. 2018, 32, 513–519. [Google Scholar] [CrossRef]
- Verma, A.; Srivastava, R.; Sonar, P.K.; Yadav, R. Traditional, Phytochemical, and Biological Aspects of Rosa alba L.: A Systematic Review. Futur. J. Pharm. Sci. 2020, 6, 114. [Google Scholar] [CrossRef]
- Vilhelmova-Ilieva, N.; Dobreva, A.; Doynovska, R.; Krastev, D.; Mileva, M. Antiviral Activity of Rosa Damascena Mill. and Rosa alba L. Essential Oils against the Multiplication of Herpes Simplex Virus Type 1 Strains Sensitive and Resistant to Acyclovir. Biology 2021, 10, 746. [Google Scholar] [CrossRef]
- Nedkov, N.; Dobreva, A.; Kovacheva, N.; Bardarov, V.; Velcheva, A. Bulgarian Rose Oil of White Oil-Bearing Rose. Bulg. J. Agric. Sci. 2009, 15, 318–322. [Google Scholar]
- Gerasimova, T.; Jovtchev, G.; Gateva, S.; Topashka-Ancheva, M.; Stankov, A.; Angelova, T.; Dobreva, A.; Mileva, M. Study on Cytotoxic and Genotoxic Potential of Bulgarian Rosa Damascena Mill. and Rosa alba L. Hydrosols—In Vivo and In Vitro. Life 2022, 12, 1452. [Google Scholar] [CrossRef]
- Georgieva, A.; Ilieva, Y.; Kokanova-Nedialkova, Z.; Zaharieva, M.M.; Nedialkov, P.; Dobreva, A.; Kroumov, A.; Najdenski, H.; Mileva, M. Redox-Modulating Capacity and Antineoplastic Activity of Wastewater Obtained from the Distillation of the Essential Oils of Four Bulgarian Oil-Bearing Roses. Antioxidants 2021, 10, 1615. [Google Scholar] [CrossRef]
- Shishkova, M.; Ivanova, B.; Beluhova-Uzunova, R.; Harizanova, A. Opportunities and Challenges for Sustainable Production and Processing of Rosa Damascena in Bulgaria. Ind. Crops Prod. 2022, 186, 115184. [Google Scholar] [CrossRef]
- Babu, K.G.D.; Singh, B.; Joshi, V.P.; Singh, V. Essential Oil Composition of Damask Rose (Rosa Damascena Mill.) Distilled under Different Pressures and Temperatures. Flavour Fragr. J. 2002, 17, 136–140. [Google Scholar] [CrossRef]
- Alborzi, S.S.; Roosta, A. The Effect of Different Solvents on the Production of Rose Concrete and Rose Absolute, Experimental Study and Thermodynamic Aspects Using the UNIFAC Model. Chem. Eng. Res. Des. 2022, 184, 326–337. [Google Scholar] [CrossRef]
- Antonova, D.V.; Medarska, Y.N.; Stoyanova, A.S.; Nenov, N.S.; Slavov, A.M.; Antonov, L.M. Chemical Profile and Sensory Evaluation of Bulgarian Rose (Rosa Damascena Mill.) Aroma Products, Isolated by Different Techniques. J. Essent. Oil Res. 2021, 33, 171–181. [Google Scholar] [CrossRef]
- Aycı, F.; Aydınlı, M.; Bozdemir, Ö.A.; Tutaş, M. Gas Chromatographic Investigation of Rose Concrete, Absolute and Solid Residue. Flavour Fragr. J. 2005, 20, 481–486. [Google Scholar] [CrossRef]
- Moates, G.K.; Reynolds, J. Comparison of Rose Extracts Produced by Different Extraction Techniques. J. Essent. Oil Res. 1991, 3, 289–294. [Google Scholar] [CrossRef]
- Sofiya, K.; Kumar, G.B. Study on the Effect of Dual Solvent Proportions on Composition ofRosa x Damascena Concrete Oil Obtained Using Soxhlet Extraction Method. Asian J. Chem. 2021, 34, 78–84. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A Review of Sustainable and Intensified Techniques for Extraction of Food and Natural Products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical Fluid Extraction and Fractionation of Natural Matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Hedayati, A.; Mousavi, S.O. Quercetin Extraction from Rosa Damascena Mill via Supercritical CO2: Neural Network and Adaptive Neuro Fuzzy Interface System Modeling and Response Surface Optimization. J. Supercrit. Fluids 2016, 112, 57–66. [Google Scholar] [CrossRef]
- Reverchon, E.; Delta Porta, G. Rose Concrete Fractionation by Supercritical CO2. J. Supercrit. Fluids 1996, 9, 199–204. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G.; Gorgoglione, D. Supercritical CO2 Extraction of Volatile Oil from Rose Concrete. Flavour Fragr. J. 1997, 12, 37–41. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Natolino, A. Application of a Supercritical CO2 Extraction Procedure to Recover Volatile Compounds and Polyphenols from Rosa Damascena. Sep. Sci. Technol. 2015, 50, 1175–1180. [Google Scholar] [CrossRef]
- Li, S.; Guo, L.; Liu, C.; Zhang, Y. Application of Supercritical Fluid Extraction Coupled with Counter–Current Chromatography for Extraction and Online Isolation of Unstable Chemical Components from Rosa Damascena. J. Sep. Sci. 2013, 36, 2104–2113. [Google Scholar] [CrossRef]
- Dobreva, A.; Nedeltcheva-Antonova, D.; Nenov, N.; Getchovska, K.; Antonov, L. Subcritical Extracts from Major Species of Oil-Bearing Roses—A Comparative Chemical Profiling. Molecules 2021, 26, 4991. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Kurkcuoglu, M.; Özek, T. Turkish Rose Oil Research: Recent Results. Perfum. Flavorist 2003, 28, 34–42. [Google Scholar]
- Kurkcuoglu, M.; Baser, K.H.C. Studies on Turkish Rose Concrete, Absolute, and Hydrosol. Chem. Nat. Compd. 2003, 39, 457–464. [Google Scholar] [CrossRef]
- Seify, Z.; Yadegari, M.; Pirbalouti, A. Essential Oil Composition of Rosa Damascena Mill. Produced With Different Storage Temperatures and Durations. Korean J. Hortic. Sci. Technol. 2018, 36, 552–559. [Google Scholar] [CrossRef]
- Solkane®134a Pharma; SOLVAY FLUOR: Bad Wimpfen, Germany. Available online: https://www.solvay.com/sites/g/files/srpend221/files/2021-01/PSS-Tetrafluoroethane.pdf (accessed on 15 July 2025).
- Barao, T.; de Castro, C.A.N.; Mardolcar, U.V.; Okambawa, R.; St-Arnaud, J.M. Dielectric Constant, Dielectric Virial Coefficients, and Dipole Moments of 1,1,1,2-Tetrafluoroethane. J. Chem. Eng. Data 1995, 40, 1242–1248. [Google Scholar] [CrossRef]
- Solkane®134a Thermodynamics; SOLVAY FLUOR GMBH: Bad Wimpfen, Germany. Available online: http://chlazeni.kovosluzbaots.com/chlazeni/pdf402/chladiva/r134a.pdf (accessed on 15 July 2025).
- Antonova-Nedeltcheva, D.; Dobreva, A.; Gechovska, K.; Antonov, L. Volatile Compounds Profiling of Fresh R. Alba L. Blossom by Headspace—Solid Phase Microextraction and Gas Chromatography. Molecules 2025, 30, 3102. [Google Scholar] [CrossRef]
- Acree, T.; Arn, H. Flavornet and Human Odor Space. Available online: http://www.flavornet.org/flavornet.html (accessed on 15 July 2025).
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, 1st ed.; Wiley: Hoboken, NJ, USA, 2016; ISBN 978-3-527-33160-4. [Google Scholar]
- Dobreva, A. Aromatic Products of the White Oil-Bearing Rose (Rosa alba L.). Sci. Work. Univ. Food Technol. 2010, LVII, 354–358. [Google Scholar]
- Supanivatin, P.; Siriwattanayotin, S.; Thipayarat, A.; Ekkaphan, P.; Wongwiwat, J. Effect of Overfilled Solvent and Storage Time of Subcritical Extraction of Jasminum Sambac on Yield, Antioxidant Activity, Antimicrobial Activity and Tentative Volatile Compounds. Plants 2023, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, T.; Nenov, N.; Lazarov, A.; Takov, T.; Stoyanova, A. Low Temperature Extraction with Liquefied Gases of Essential Oil Raw Materials. 3. Hyacinth Flowers (Hyacinthus orientalis L.). Sci. Work. Russe Univ. Bulg. 2009, 48, 131–135. [Google Scholar]
- Merdjanov, P.; Denkova, V.; Janakieva, V.; Nenov, N.; Atanasova, T.; Stoyanova, A. Low Temperature Extraction with Liquefied Gases of Essential Oil Raw Materials. 13. White Lilium (Lilium candidum L.). J. Voronezh State Agrar. Univ. 2013, 3, 116–120. [Google Scholar]
- Feng, D.; Jian, H.; Zhang, H.; Qiu, X.; Wang, Z.; Du, W.; Xie, L.; Wang, Q.; Zhou, N.; Wang, H.; et al. Comparison of Volatile Compounds between Wild and Cultivated Roses. HortScience 2022, 57, 657–663. [Google Scholar] [CrossRef]
- Hadian, Z.; Maleki, M.; Feizollahi, E.; Alibeyk, S.; Saryazdi, M. Health Aspects of Geraniol as a Main Bioactive Compound of Rosa Damascena Mill: A Systematic Review. Electron. Physician 2020, 12, 7724–7735. [Google Scholar] [CrossRef] [PubMed]
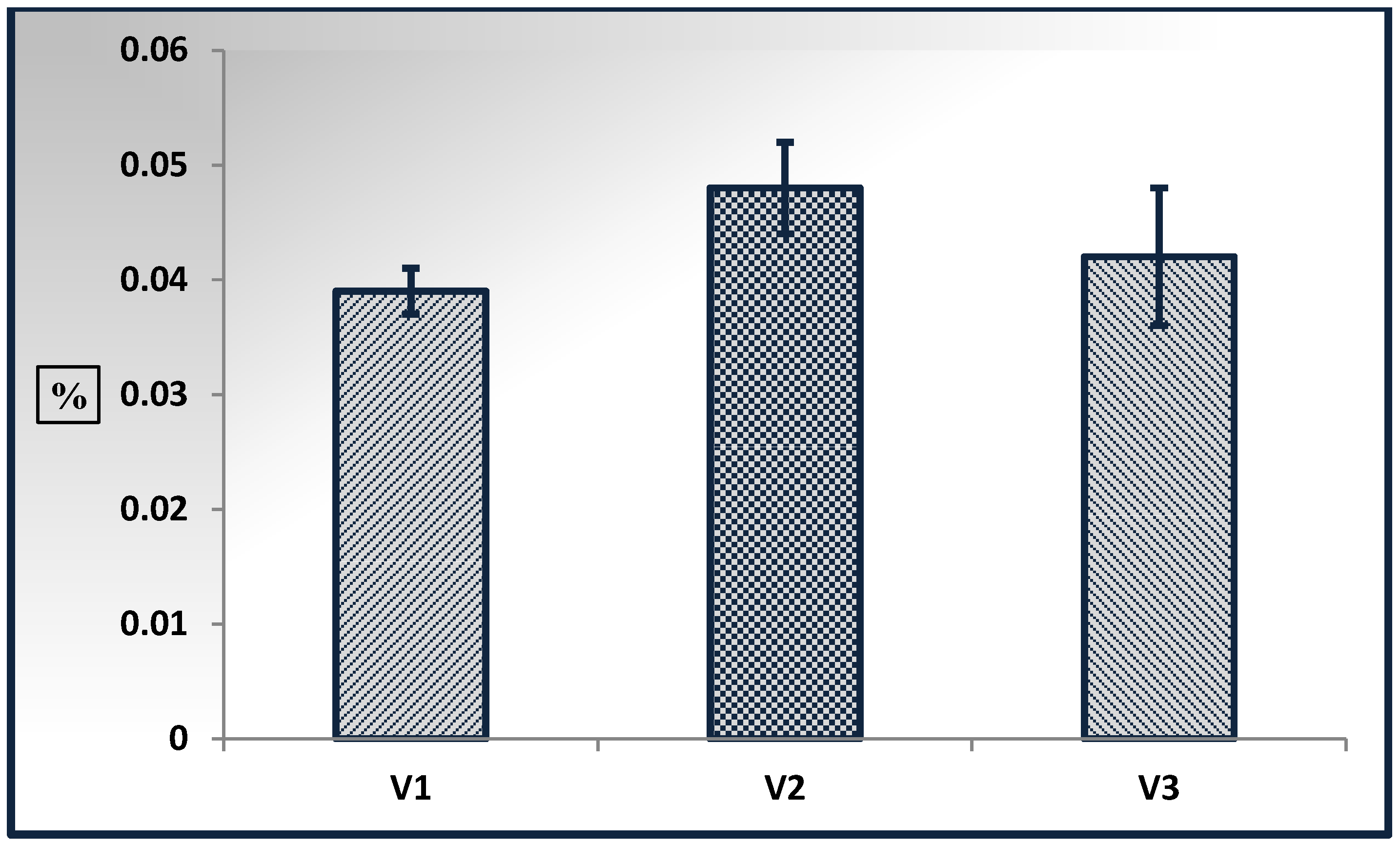
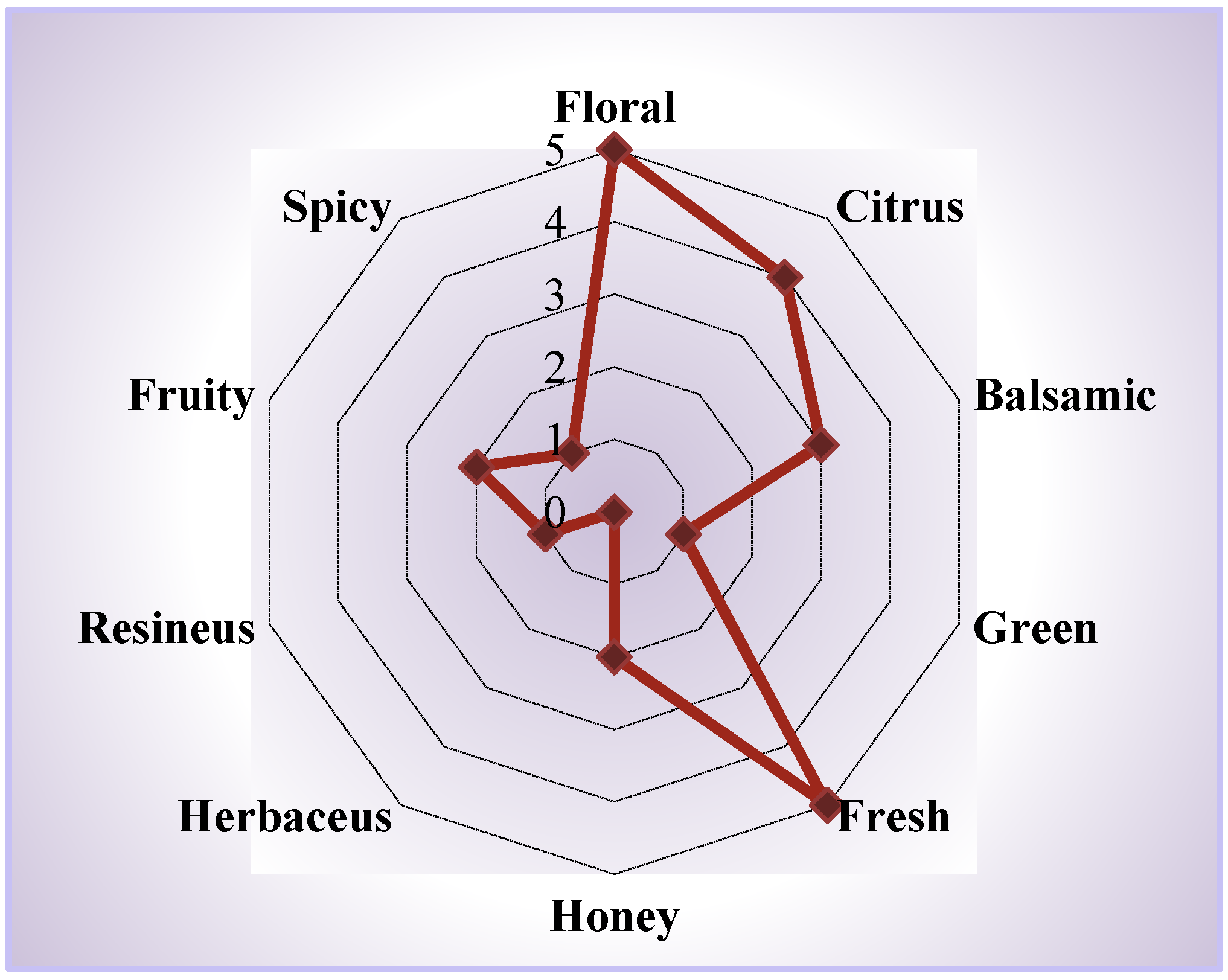

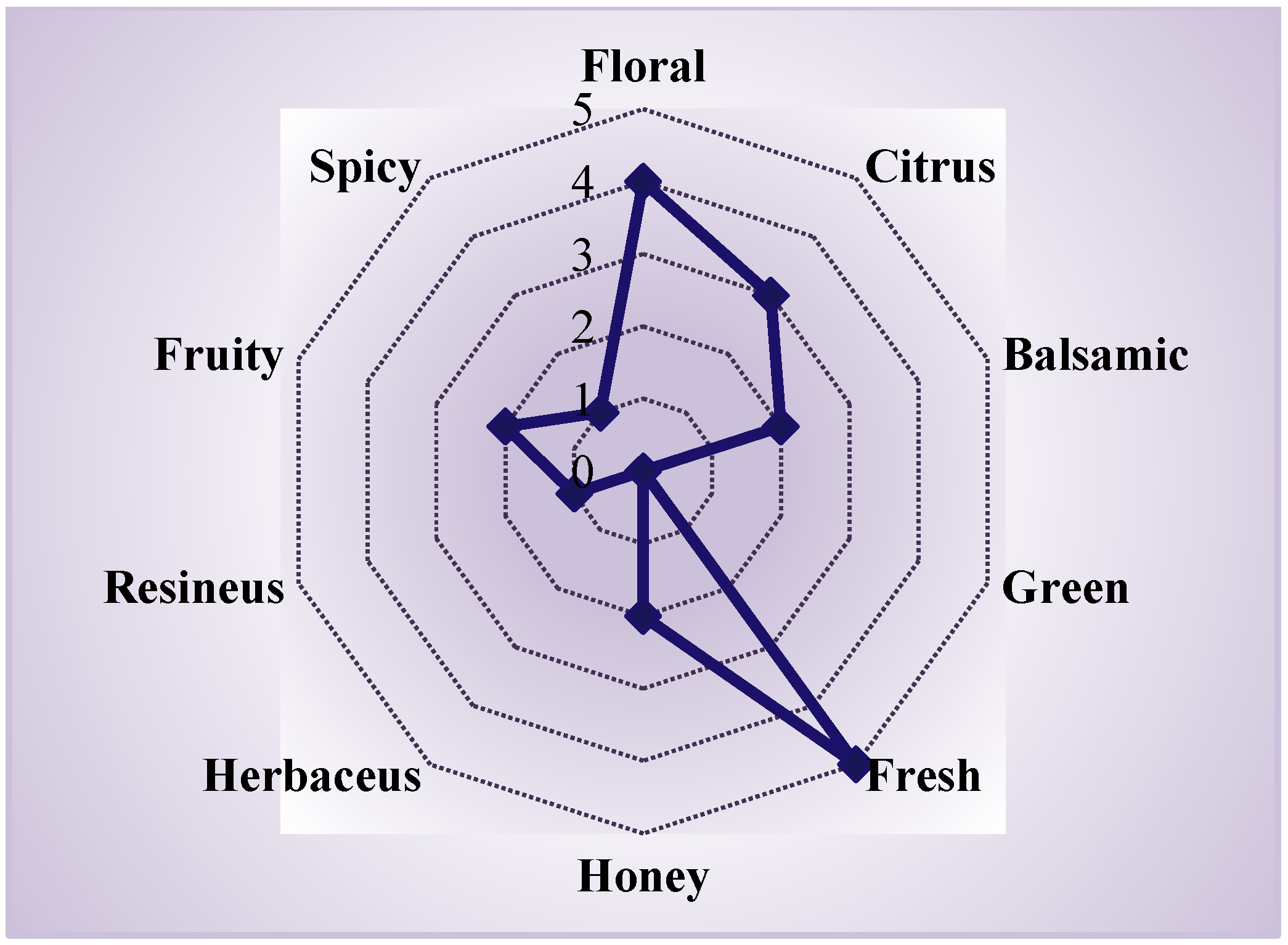
| Variation | Mode | Extraction Time, min |
|---|---|---|
| Variant 1 (V1) | Static | 60 |
| Variant 2 (V2) | Dynamic | 60 |
| Variant 3 (V3) | Static double-staged | 5–30 |
| No | Compound | LRIexp DB-17HT | Rel. %, as Determined by GC-FID | Scent Description | ||
|---|---|---|---|---|---|---|
| Variant 1 | Variant 2 | Variant 3 | ||||
| 1. | α-Pinene | 845 | 0.13 | n.d. | 0.05 | Herbal type |
| 2. | β-Pinene | 944 | 0.01 | n.d. | n.d. | Woody-green, pine-like |
| 3. | β-Myrcene | 968 | 0.04 | n.d. | 0.02 | Sweet-balsamic-resinous |
| 4. | Limonene | 1003 | 0.08 | n.d. | 0.03 | lemon-like |
| 5. | Cymene | 1026 | 0.01 | n.d. | n.d. | Harsh chemical, woody and terpy-like |
| 6. | Benzaldehyde | 1051 | n.d. | n.d. | 0.07 | Sharp, sweet, bitter almond cherry |
| 7. | Linalool | 1201 | 0.01 | n.d. | 0.05 | Floral, spicy wood, |
| 8. | Benzyl alcohol | 1223 | 5.34 | 3.63 | 4.05 | Slightly sweet, floral |
| 9. | Octanoic acid | 1298 | 0.02 | n.d. | n.d. | Pungent |
| 10. | Phenyl ethyl Alcohol | 1317 | 14.97 | 12.57 | 13.11 | Rose note, very lasting/mild and warm rose honey |
| 11. | Citronellol | 1366 | 4.04 | 3.21 | 3.16 | Sweet, rose-like |
| 12. | Nerol | 1374 | 6.10 | 5.90 | 6.39 | Rose-like, fresh green note |
| 13. | Phenyl ethyl formate | 1381 | 0.09 | n.d. | n.d. | Green floral rose-like |
| 14. | Neral | 1392 | 0.08 | n.d. | n.d. | Citrus, milder, and sweeter |
| 15. | Geraniol | 1414 | 14.82 | 12.09 | 13.62 | Sweet, floral, rose-like |
| 16. | Geranial | 1440 | 0.92 | 1.12 | 0.84 | Strong, lemon-like |
| 17. | Phenyl ethyl acetate | 1469 | 0.06 | n.d. | n.d. | Sweet, rosy-fruity, honey-like |
| 18. | β-Elemene | 1472 | 0.01 | n.d. | n.d. | Herbal type |
| 19. | Cytronellyl acetate | 1475 | 0.10 | n.d. | n.d. | Fresh, rosy, fruity |
| 20. | Anethole | 1482 | 0.25 | n.d. | 0.14 | Fresh, green, spicy |
| 21. | Pentadecane (C15) | 1500 | 0.09 | n.d. | n.d. | - |
| 22. | β-Caryophyllene | 1506 | 0.98 | 0.25 | 0.53 | Softly spicy, woody |
| 23. | Geranic acid | 1522 | 0.26 | n.d. | 0.19 | Soft, fresh, green-floral |
| 24. | Geranyl acetate | 1540 | 0.03 | n.d. | 0.09 | Floral, fruity, rose-like |
| 25. | α-Caryophyllene | 1552 | n.d. | n.d. | 0.03 | Sweet, woody spice |
| 26. | Hydroxy linalool | 1568 | 0.03 | n.d. | n.d. | - |
| 27. | Eugenol | 1574 | 0.08 | n.d. | 0.03 | Spicy, clove-like |
| 28. | β-Cubebene | 1598 | 0.25 | n.d. | n.d. | Herbal type |
| 29. | α-Muurolene | 1607 | 0.04 | n.d. | 0.07 | - |
| 30. | δ-Guaiene | 1611 | 0.21 | n.d. | n.d. | Spicy, powdery, balsamic |
| 31. | β-Copaene | 1616 | 0.04 | n.d. | n.d. | - |
| 32. | β-Cadinene | 1646 | n.d. | n.d. | 0.06 | Woody |
| 33. | Heptadecane (C17) | 1700 | 1.65 | 0.48 | 0.68 | - |
| 34. | Hedycaryol | 1703 | 0.25 | n.d. | 0.28 | - |
| 35. | Heptadecene (C17:1) | 1710 | 0.01 | n.d. | n.d. | - |
| 36. | Benzyl tiglate + Heptadecadiene (C17:2) | 1714 | 0.07 | n.d. | 0.07 | - |
| 37. | γ-Eudesmol | 1796 | 0.02 | n.d. | n.d. | Waxy, sweet |
| 38. | Octadecane (C18) | 1800 | n.d. | n.d. | 0.05 | - |
| 39. | τ-Cadinol | 1805 | 0.09 | n.d. | 0.07 | Balsamic |
| 40. | α-Eudesmol | 1819 | 0.02 | n.d. | 0.07 | - |
| 41. | β-Eudesmol | 1826 | 0.14 | n.d. | 0.14 | Woody green |
| 42. | Nonadecane+Nonadecene (C19+ C19:1) | 1900 | 15.66 | 16.85 | 15.21 | - |
| 43. | Hexadecanal | 1936 | 0.04 | n.d. | 0.03 | Cardboard-like |
| 44. | Eicosane (C20) | 2000 | 1.50 | 1.66 | 1.43 | - |
| 45. | Unknown | 2054 | 0.47 | 0.39 | 0.58 | |
| 46. | Unknown sesquiterpene derivative | 2079 | n.d. | 0.08 | 0.08 | - |
| 47. | Heneicosane(C21) | 2100 | 11.83 | 13.78 | 11.81 | - |
| 48. | Heneicosene (C21:1) | 2105 | 1.31 | 1.41 | 1.34 | - |
| 49. | Heneicosene (C21:1), isomer | 2121 | 0.25 | 0.28 | 0.27 | - |
| 50. | Docosane (C22) | 2200 | n.d. | 0.45 | 0.39 | - |
| 51. | Docosene (C22:1) | 2211 | n.d. | 0.12 | 0.11 | - |
| 52. | Tricosane (C23) | 2300 | 2.46 | 2.96 | 2.62 | - |
| 53. | Tricosene (C23:1) | 2318 | 1.86 | 2.13 | 1.91 | - |
| 54. | Tricosene (C23:1), isomer | 2334 | 0.33 | 0.28 | 0.34 | - |
| 55. | 1,1,9-Eicosadiene | 2348 | 0.12 | 0.06 | 0.11 | - |
| 56. | Tetracosane (C24) | 2400 | n.d. | 0.16 | 0.15 | - |
| 57. | Cyclotetracosane | 2408 | n.d. | 0.24 | 0.20 | - |
| 58. | Farnesol, isomer | 2423 | n.d. | 0.06 | 0.07 | Floral, green, milky, muguet, waxy, oily, dairy, fatty, soapy |
| 59. | Hexanoic acid, 2-ethyl, tetradecyl ester | n.d. | 0.10 | 0.09 | - | |
| 60. | Pentacosane (C25) | 2500 | 0.48 | 0.56 | 0.57 | - |
| 61. | Pentacosene (C25:1) | 2511 | 0.21 | 0.15 | 0.18 | - |
| 62. | Pentacosene (C25:1) | 2524 | 0.97 | 1.26 | 1.05 | - |
| 63. | Pentacosene (C25:1) | 2532 | 0.32 | 0.44 | 0.35 | - |
| 64. | Unknown | 2541 | 0.29 | 0.13 | 0.26 | - |
| 65. | Nonanoic acid, tetradecyl ester | 2548 | 0.04 | 0.06 | 0.05 | Waxy, fatty type |
| 66. | Hexacosane (C26) | 2600 | 0.05 | 0.07 | 0.06 | - |
| 67. | Hexacosene (C26:1) | 2612 | 0.20 | 0.27 | 0.23 | - |
| 68. | Hexadecyl octanoate | 2668 | n.d. | 0.17 | n.d. | - |
| 69. | Heptacosane +Heptacosene (C27 + C27:1) | 2700 | 0.21 | 0.30 | 0.25 | - |
| 70. | Heptacosene (C27:1), isomer | 2708 | 0.27 | 0.40 | 0.34 | - |
| 71. | Heptacosene (C27:1), isomer | 2721 | 1.19 | 1.75 | 1.45 | - |
| 72. | Stearic acid, citronellyl ester | 2843 | n.d. | 0.06 | 0.05 | - |
| 73. | Unknown geranyl ester | 2876 | n.d. | 0.08 | 0.09 | |
| 74. | Nonacosene (C29:1) | 2911 | 0.49 | 0.74 | 0.68 | - |
| 75. | Unknown phenyl ethyl ester | 0.52 | 0.54 | 0.66 | - | |
| 76. | Dodecanoic acid, phenyl methyl ester | 2939 | n.d. | 0.17 | 0.15 | - |
| 77. | Unknown citronellyl ester | 2942 | n.d. | 0.13 | 0.12 | - |
| 78. | Unknown phenyl ethyl ester | 2956 | n.d. | 0.08 | 0.07 | - |
| 79. | Unknown neryl ester | 2961 | 0.29 | 0.28 | 0.30 | - |
| 80. | Unknown phenyl ethyl ester | 2964 | n.d. | 0.08 | 0.09 | - |
| 81. | Olean-12-en-3-one | 3468 | n.d. | 0.15 | 0.15 | - |
| 82. | α-Amyrin + Unindentified triterpene | 3476 | n.d. | 0.10 | 0.07 | - |
| 83. | Lupeol | 3616 | n.d. | 0.14 | 0.15 | - |
| Number of detected compounds | 64 | 49 | 68 | |||
| Monoterpene hydrocarbons, % | 0.26 | 0.10 | 0 | |||
| Oxygenated monoterpenes, % | 26.29 | 24.57 | 22.81 | |||
| Sesqiterpene hydrocarbones, % | 1.80 | 1.00 | 0.25 | |||
| Phenylpropanoids, % | 21.31 | 18.22 | 16.90 | |||
| Paraffins, % | 41.46 | 41.78 | 46.97 | |||
| Others, % | 1.58 | 2.70 | 1.10 | |||
| Total identified, % | 92.70 | 88.37 | 88.00 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobreva, A.; Nedeltcheva-Antonova, D.; Gechovska, K.; Nenov, N.; Antonov, L. Subcritical Extraction of Rosa alba L. in Static and Dynamic Modes. Chemistry 2025, 7, 149. https://doi.org/10.3390/chemistry7050149
Dobreva A, Nedeltcheva-Antonova D, Gechovska K, Nenov N, Antonov L. Subcritical Extraction of Rosa alba L. in Static and Dynamic Modes. Chemistry. 2025; 7(5):149. https://doi.org/10.3390/chemistry7050149
Chicago/Turabian StyleDobreva, Ana, Daniela Nedeltcheva-Antonova, Kamelia Gechovska, Nenko Nenov, and Liudmil Antonov. 2025. "Subcritical Extraction of Rosa alba L. in Static and Dynamic Modes" Chemistry 7, no. 5: 149. https://doi.org/10.3390/chemistry7050149
APA StyleDobreva, A., Nedeltcheva-Antonova, D., Gechovska, K., Nenov, N., & Antonov, L. (2025). Subcritical Extraction of Rosa alba L. in Static and Dynamic Modes. Chemistry, 7(5), 149. https://doi.org/10.3390/chemistry7050149







