Nitrogen-Doped Biocarbon Derived from Alginate-Extraction Residues of Sargassum spp.: Towards Low-Cost Electrocatalysts for Alkaline ORR
Abstract
1. Introduction
2. Materials and Methods
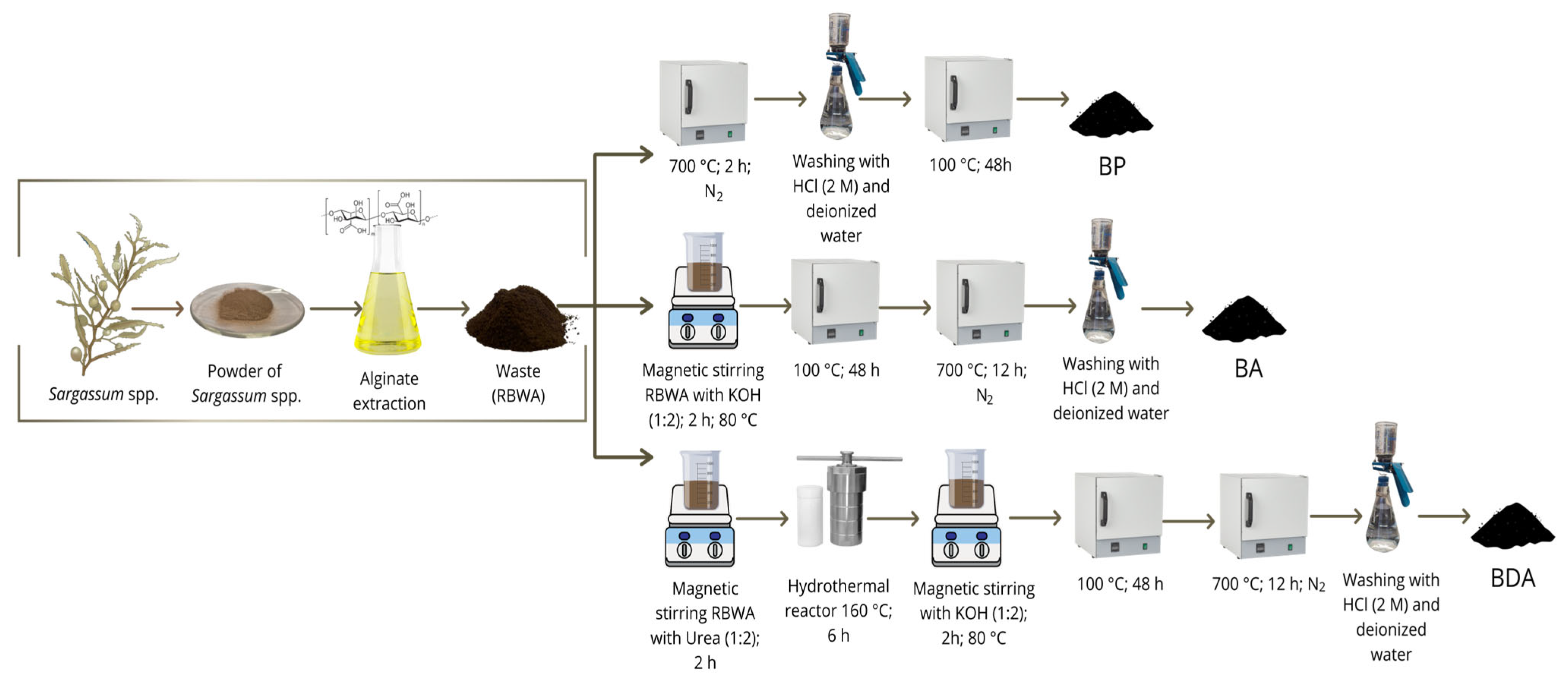
2.1. Synthesis of Biocarbon
2.2. Methods and Measurement Conditions
3. Results and Discussion
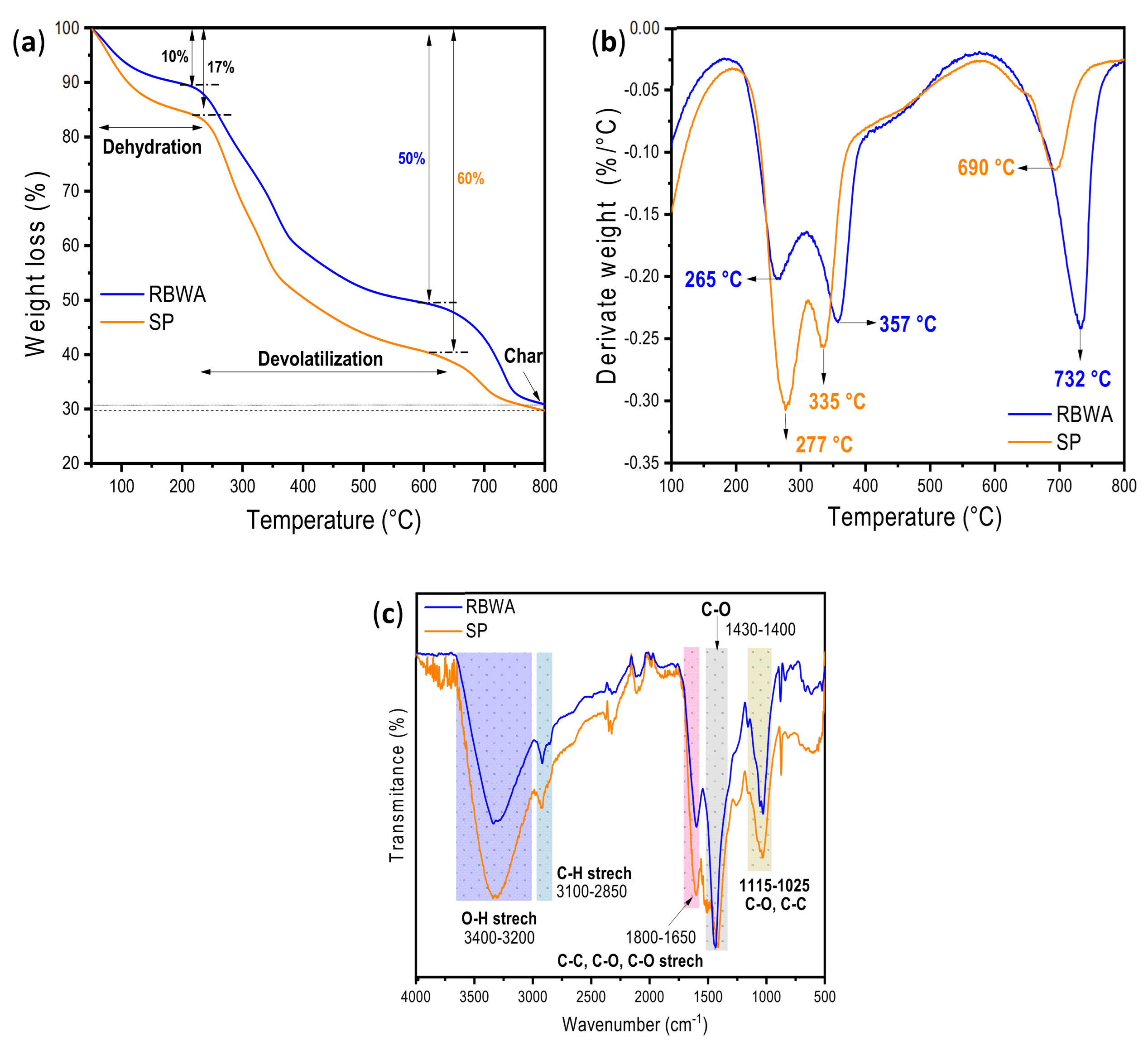
3.1. TGA and FTIR of Raw Materials
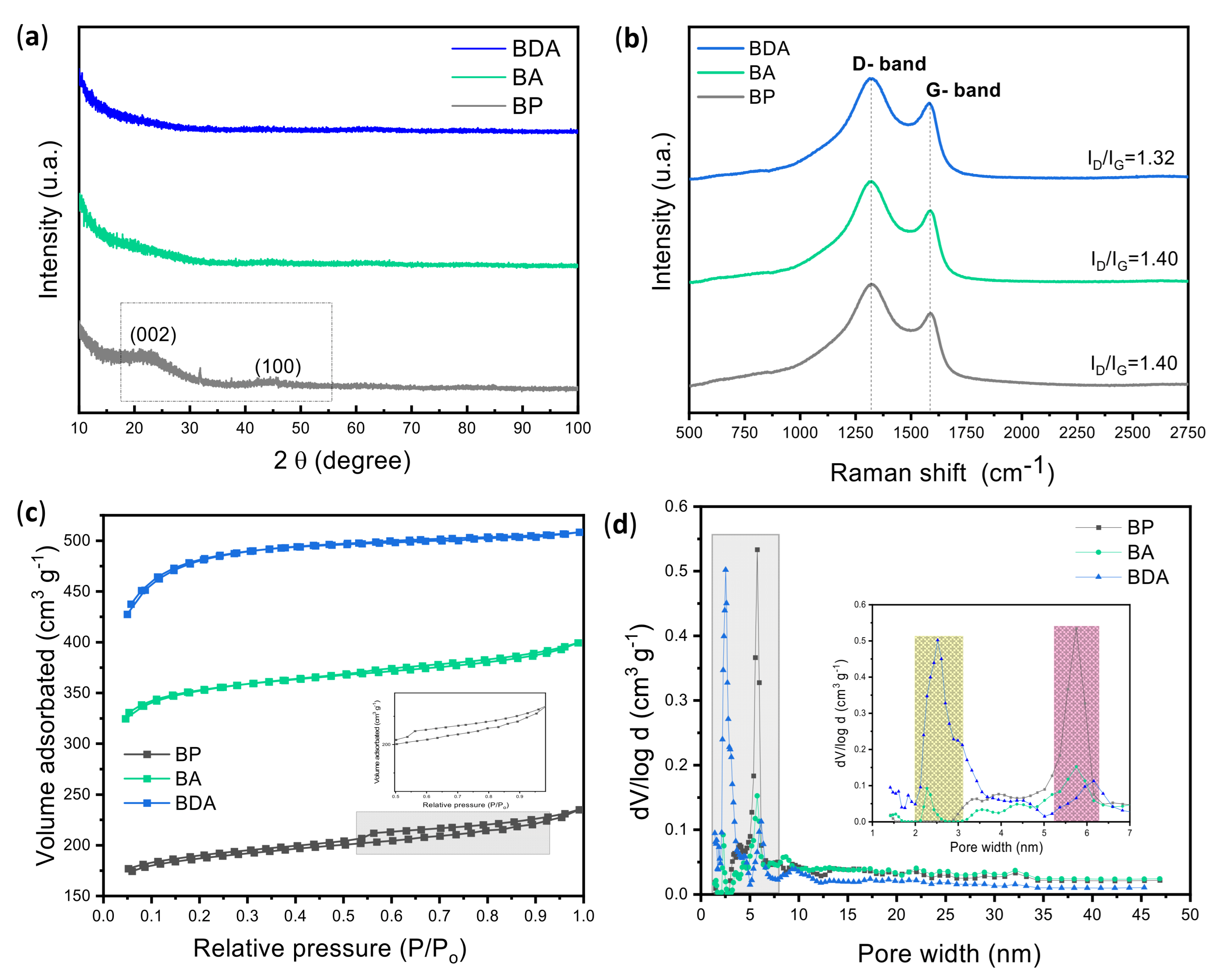
3.2. Physicochemical Characterization
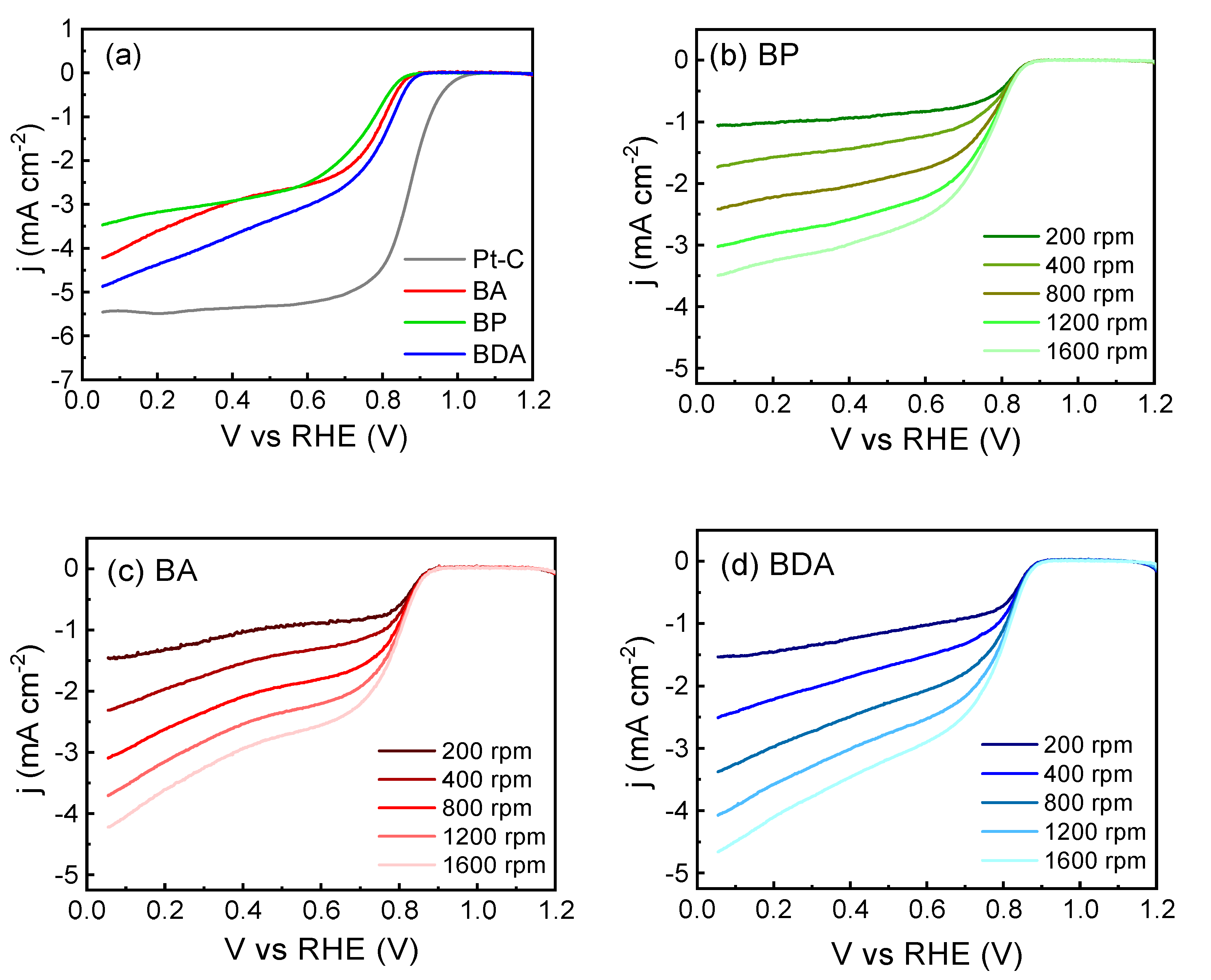
3.3. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paredes-Camacho, R.M.; González-Morales, S.; González-Fuentes, J.A.; Rodríguez-Jasso, R.M.; Benavides-Mendoza, A.; Charles-Rodríguez, A.V.; Robledo-Olivo, A. Characterization of Sargassum spp. from the Mexican Caribbean and its valorization through fermentation process. Processes 2023, 11, 685. [Google Scholar] [CrossRef]
- Amador-Castro, F.; García-Cayuela, T.; Alper, H.S.; Rodriguez-Martinez, V.; Carrillo-Nieves, D. Valorization of pelagic Sargassum biomass into sustainable applications: Current trends and challenges. J. Environ. Manag. 2021, 283, 112013. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M. Promising biorefinery products from marine macro and microalgal biomass: A review. Renew. Sustain. Energy Rev. 2024, 190, 114081. [Google Scholar] [CrossRef]
- López-Contreras, A.M.; Núñez, P.N.; García, B.C.; Driegen, J.; Lwanga, E.H.; Domin, P.; Gurrola, M.P. Sargassum in Mexico: From Environmental Problem to Valuable Resource; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Del-Río, P.G.; Gullón, B.; Romaní, A.; Garrote, G. Eco-friendly strategy for the joint valorization of invasive macroalgae and fast-growing wood to produce advanced biofuels. Renew. Energy 2023, 219, 119496. [Google Scholar] [CrossRef]
- Aparicio, E.; Rodríguez-Jasso, R.M.; Pinales-Márquez, C.D.; Loredo-Treviño, A.; Robledo-Olivo, A.; Aguilar, C.N.; Kostas, E.T.; Ruiz, H.A. High-pressure technology for Sargassum spp biomass pretreatment and fractionation in the third generation of bioethanol production. Bioresour. Technol. 2021, 329, 124935. [Google Scholar] [CrossRef]
- González-Gloria, K.D.; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; Shiva; Kostas, E.T.; Aparicio, E.; Sanchez, A.; López-Sandin, I.; Ruiz, H.A. Scale-up of hydrothermal processing: Liquid hot water and pilot-scale tubular steam explosion batch reactor for bioethanol production using macroalgae Sargassum spp. biomass. Bioresour. Technol. 2023, 369, 128448. [Google Scholar] [CrossRef]
- Seyedalhosseini, S.H.; Salati, A.P.; Mozanzadeh, M.T.; Parrish, C.C.; Shahriari, A. Effects of dietary seaweeds (Gracilaria spp. and Sargassum spp.) on growth, feed utilization, and resistance to acute hypoxia stress in juvenile Asian seabass (Lates calcarifer). Aquac. Rep. 2023, 31, 101663. [Google Scholar] [CrossRef]
- Grand View Research. Alginate Market Size, Share & Trends Analysis Report by Type (High M, High G), by Product (Sodium, Propylene Glycol), by Application (Pharmaceutical, Industrial), by Region, and Segment Forecasts, 2021–2028; Grand View Research: San Francisco, CA, USA, 2019. [Google Scholar]
- Ramírez-Partida, A.E.; García-Cayuela, T.; Amador-Castro, L.F.; Alper, H.S.; Carrillo-Nieves, D. Towards a biorefinery processing Sargassum seaweed: Techno-economic assessment of alginate and fucoidan production through SuperPro Designer® process simulation. Environ. Technol. Innov. 2024, 34, 103587. [Google Scholar] [CrossRef]
- Mazumder, A.; Holdt, S.L.; De Francisci, D.; Alvarado-Morales, M.; Mishra, H.N.; Angelidaki, I. Extraction of alginate from Sargassum muticum: Process optimization and study of its functional activities. J. Appl. Phycol. 2016, 28, 3625–3634. [Google Scholar] [CrossRef]
- Mohammed, A.; Rivers, A.; Stuckey, D.C.; Ward, K. Alginate extraction from Sargassum seaweed in the Caribbean region: Optimization using response surface methodology. Carbohydr. Polym. 2020, 245, 116419. [Google Scholar] [CrossRef]
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A brief review on the development of alginate extraction process and its sustainability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- Firouzjaie, H.A.; Mustain, W.E. Catalytic advantages, challenges, and priorities in alkaline membrane fuel cells. ACS Catal. 2020, 10, 225–234. [Google Scholar] [CrossRef]
- Men Truong, V.; Richard Tolchard, J.; Svendby, J.; Manikandan, M.A.; Miller, H.; Sunde, S.; Yang, H.; Dekel, D.R.; Barnett, A.O. Platinum and platinum group metal-free catalysts for anion exchange membrane fuel cells. Energies 2020, 13, 582. [Google Scholar] [CrossRef]
- Ng, W.K.; Wong, W.Y.; Rosli, N.A.; Loh, K.S. Commercial anion exchange membranes (AEMs) for fuel cell and water electrolyzer applications: Performance, durability, and materials advancement. Separations 2023, 10, 424. [Google Scholar] [CrossRef]
- Hossen, M.; Hasan, S.; Sardar, R.I.; Haider, J.; Mottakin, K.; Tammeveski, K.; Atanassov, P. State-of-the-art and developmental trends in platinum group metal-free cathode catalyst for anion exchange membrane fuel cell (AEMFC). Appl. Catal. B Environ. 2023, 325, 121733. [Google Scholar] [CrossRef]
- The, Y.Y.; Lee, K.T.; Chen, W.H.; Lin, S.C.; Sheen, H.K.; Tan, I.S. Dilute sulfuric acid hydrolysis of red macroalgae Eucheuma denticulatum with microwave-assisted heating for biochar production and sugar recovery. Bioresour. Technol. 2017, 246, 20–27. [Google Scholar] [CrossRef]
- Francavilla, M.; Manara, P.; Kamaterou, P.; Monteleone, M.; Zabaniotou, A. Cascade approach of red macroalgae Gracilaria gracilis sustainable valorization by extraction of phycobiliproteins and pyrolysis of residue. Bioresour. Technol. 2015, 184, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Méndez, A.; Gascó, G.; Ruiz, B.; Fuente, E. Hydrochars from industrial macroalgae “Gelidium Sesquipedale” biomass wastes. Bioresour. Technol. 2019, 275, 386–393. [Google Scholar] [CrossRef]
- del Río, P.G.; Gullón, B.; Pérez-Pérez, A.; Romaní, A.; Garrote, G. Microwave hydrothermal processing of the invasive macroalgae Sargassum muticum within a green biorefinery scheme. Bioresour. Technol. 2021, 340, 125733. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Salcedo, K.Y.; Alonso-Lemus, I.L.; Quintana, P.; Mena-Durán, C.J.; Barbosa, R.; Escobar, B. Self-doped Sargassum spp. derived biocarbon as electrocatalysts for ORR in alkaline media. Int. J. Hydrogen Energy 2019, 44, 12399–12408. [Google Scholar] [CrossRef]
- Rosas-Medellín, D.; Rodríguez-Varela, F.J.; Escobar, B. Sulfur doped biocarbon obtained from Sargassum spp. for the oxygen reduction reaction. Int. J. Hydrogen Energy 2022, 47, 30172–30177. [Google Scholar] [CrossRef]
- Chávez-Guerrero, L.; Toxqui-Terán, A.; Rivera-Haro, J.A.; Lozoya-Márquez, L.A.; Lara-Banda, M. Management of Pelagic Sargassum spp. Landings to atlantic coastlines through direct combustion and further synthesis of highly pure calcium carbonate using the residual ashes. Waste Biomass Valorization 2021, 12, 6591–6599. [Google Scholar] [CrossRef]
- Aragón-Vallejo, J.D.; Salazar-Cruz, B.A.; Chávez-Cinco, M.Y.; Rivera-Armenta, J.L.; Espindola-Flores, A.C. Novel Polypropylene–Sargassum Particles composites: Evaluation of thermal and thermomechanical properties. J. Compos. Sci. 2023, 7, 455. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on alginate-chitosan complex formed with different polymers ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- Li, L.; Ma, X.; Chen, R.; Wang, C.; Lu, M. Nitrogen-containing functional groups-facilitated acetone adsorption by ZIF-8-derived porous carbon. Materials 2018, 11, 159. [Google Scholar] [CrossRef]
- Chen, J.P.; Yang, L. Chemical modification of Sargassum sp. for prevention of organic leaching and enhancement of uptake during metal biosorption. Ind. Eng. Chem. Res. 2005, 44, 9931–9942. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Li, Y.; Li, X.; You, L.; Kulikouskaya, V.; Hileuskaya, K. Degradation of polysaccharides from Sargassum fusiforme using UV/H2O2 and its effects on structural characteristics. Carbohydr. Polym. 2020, 230, 115647. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Hammer, P.; Guibal, E.; Taulemesse, J.M.; Garcia, O. Characterization of metal-biomass interactions in the lanthanum (III) biosorption on Sargassum sp. using SEM/EDX, FTIR, and XPS: Preliminary studies. Chem. Eng. J. 2014, 239, 381–391. [Google Scholar] [CrossRef]
- Kannan, S. FT-IR and EDS analysis of the seaweeds Sargassum wightii and Gracilaria corticata (red algae). Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 341–351. [Google Scholar]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef]
- Wang, L.; Khalil, R.A.; Bakken, J.; Skreiberg, Ø. Production and characterization of biocarbon from woody biomasses produced at 1000 °C. Fuel 2024, 370, 131667. [Google Scholar] [CrossRef]
- Karbhal, I.; Chaturvedi, V.; Patrike, A.; Yadav, P.; Shelke, M.V. Honeycomb boron carbon nitride as high-performance anode material for Li-Ion batteries. ChemNanoMat 2022, 8, e202200056. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Waller, G.; Liu, Y.; Liu, M.; Wong, C.P. Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen-reduction reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, R.; Show, P.L.; Mahlknecht, J.; Wang, C. Degradation of tetracycline by nitrogen-doped biochar as a peroxydisulfate activator: Nitrogen doping pattern and non-radical mechanism. Sustain. Horizons 2024, 10, 100091. [Google Scholar] [CrossRef]
- Liang, K.; Chen, Y.; Wang, S.; Wang, D.; Wang, W.; Jia, S.; Mitsuzakic, N.; Chen, Z. Peanut shell waste derived porous carbon for high-performance supercapacitors. J. Energy Storage 2023, 70, 107947. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y. Efficient activation of persulfate for tetracycline hydrochloride degradation by biochar prepared from corn stover doped with organic phosphorus sources. Desalin. Water Treat. 2024, 319, 100559. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Grimm, A.; Fungaro, D.A.; Hu, T.; de Brum, I.A.S.; Lima, E.C.; Naushad, M.; Dotto, G.L.; Lassi, U. Synthesis of sustainable mesoporous sulfur-doped biobased carbon with superior performance sodium diclofenac removal: Kinetic, equilibrium, thermodynamic and mechanism. Environ. Res. 2024, 251, 118595. [Google Scholar] [CrossRef]
- Rodriguez Correa, C.; Otto, T.; Kruse, A. Influence of the biomass components on the pore formation of activated carbon. Biomass Bioenergy 2017, 97, 53–64. [Google Scholar] [CrossRef]
- López-Miranda, J.L.; Silva, R.; Molina, G.A.; Esparza, R.; Hernandez-Martinez, A.R.; Hernández-Carteño, J.; Estévez, M. Evaluation of a dynamic bioremediation system for the removal of metal ions and toxic dyes using Sargassum spp. J. Mar. Sci. Eng. 2020, 8, 899. [Google Scholar] [CrossRef]
- Watson, V.J.; Nieto Delgado, C.; Logan, B.E. Influence of chemical and physical properties of activated carbon powders on oxygen reduction and microbial fuel cell performance. Environ. Sci. Technol. 2013, 47, 6704–6710. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; dos Reis, G.S.; Khokarale, S.G.; Ekman, S.; Lima, E.C.; Xiong, S.; Hultberg, M. Shiitake spent mushroom substrate as a sustainable feedstock for developing highly efficient nitrogen-doped biochars for treatment of dye-contaminated water. J. Water Process Eng. 2023, 56, 104435. [Google Scholar] [CrossRef]
- Wang, X.; Zuo, L.; Wang, Y.; Zhen, M.; Xu, L.; Kong, W.; Shen, B. Electrochemical performance of nitrogen self-doping carbon materials prepared by pyrolysis and activation of defatted microalgae. Molecules 2023, 28, 7280. [Google Scholar] [CrossRef] [PubMed]
- Laisné, E.; Thivet, J.; Manavalan, G.; Petnikota, S.; Mikkola, J.P.; Thyrel, M.; Hu, T.; Lima, E.C.; Naushad, M.; Lassi, U.; et al. Box-Behnken design for the synthesis optimization of mesoporous sulfur-doped carbon-based materials from birch waste: Promising candidates for environmental and energy storage application. Colloids Surf. A Physicochem. Eng. Asp. 2024, 692, 133899. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Naeth, M.A.; Chang, S.X. Elemental composition of biochars is affected by methods used for its determination. J. Anal. Appl. Pyrolysis 2021, 156, 105174. [Google Scholar] [CrossRef]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Park, J.; Nabae, Y.; Hayakawa, T.; Kakimoto, M. Highly Selective Two-Electron Oxygen Reduction Catalyzed by Mesoporous Nitrogen-Doped Carbon. ACS Catal. 2014, 4, 3749–3754. [Google Scholar] [CrossRef]
- Olson, T.S.; Pylypenko, S.; Atanassov, P.; Asazawa, K.; Yamada, K.; Tanaka, H. Anion-exchange membrane fuel cells: Dual-site mechanism of oxygen reduction reaction in alkaline media on cobalt−polypyrrole electrocatalysts. J. Phys. Chem. C 2010, 114, 5049–5059. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Li, Y. A fundamental comprehension and recent progress in advanced Pt-based ORR nanocatalysts. SmartMat 2021, 2, 56–75. [Google Scholar] [CrossRef]
- Zhai, Q.; Huang, H.; Lawson, T.; Xia, Z.; Giusto, P.; Antonietti, M.; Jaroniec, M.; Chhowalla, M.; Baek, J.; Liu, Y.; et al. Recent advances on carbon-based metal-free electrocatalysts for energy and chemical conversions. Adv. Mater. 2024, 36, 2405664. [Google Scholar] [CrossRef]
- Mazzucato, M.; Facchin, A.; Parnigotto, M.; Durante, C. New and revised aspects of the electrochemical synthesis of hydrogen peroxide: From model electrocatalytic systems to scalable materials. ACS Catal. 2024, 14, 6369–6403. [Google Scholar] [CrossRef]
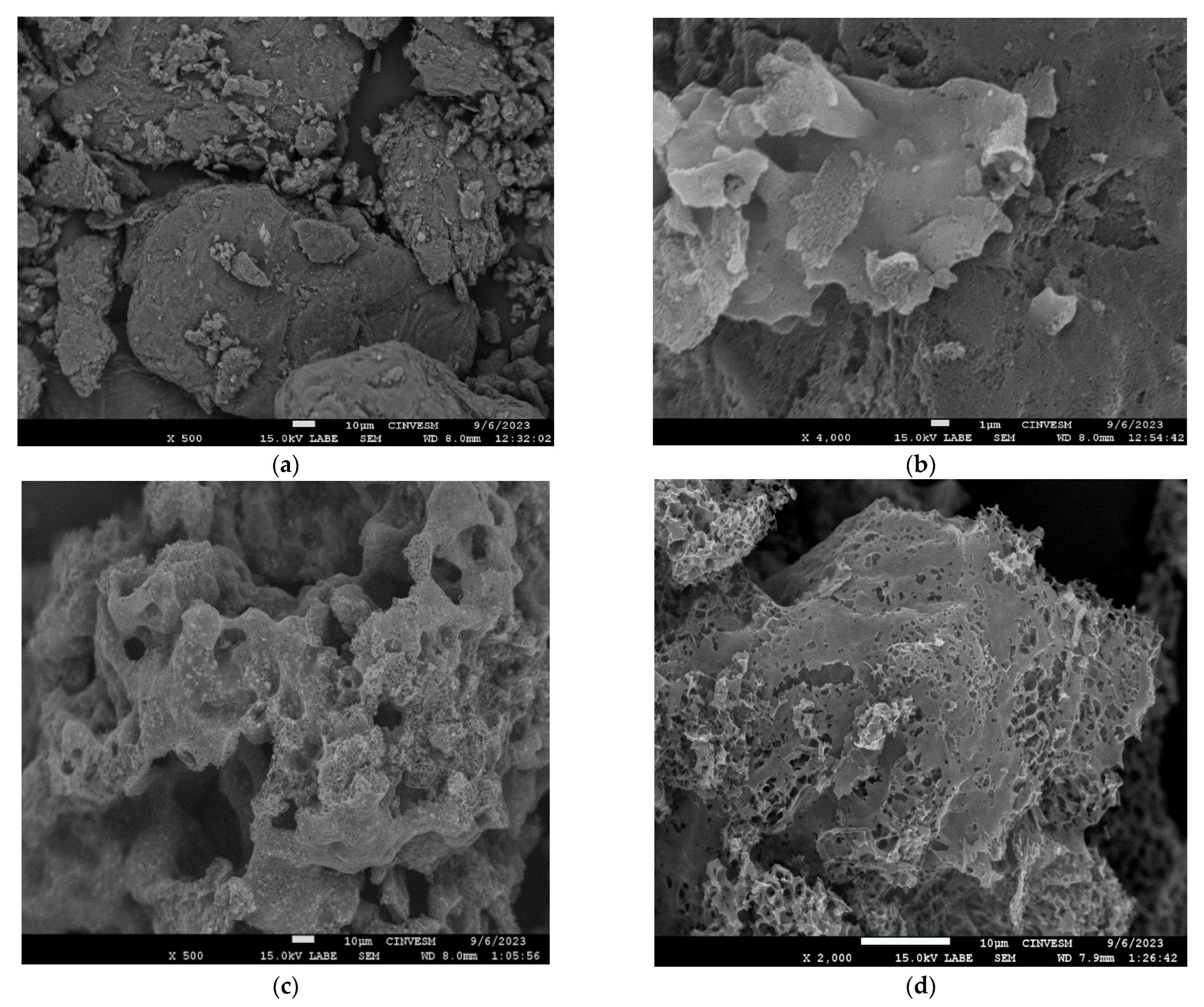
| Elemental Composition (%) | EDX Analysis wt.% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | SBET (m2 g−1) | Average Pore Diameter (nm) | C | H | N | S | C | N | S |
| SP | 1 | 4.0 | 34.85 | 3.74 | 0.60 | 1.50 | - | - | - |
| RBWA | 3 | 4.3 | 31.44 | 3.92 | 0.64 | 0.83 | 25.59 | 2.34 | 0.36 |
| BP | 682 | 4.5 | 75.87 | 1.09 | 0.18 | 1.57 | 75.42 | - | 1.69 |
| BA | 1306 | 3.1 | 77.07 | 0.63 | 0.59 | 2.04 | 88.23 | - | 1.34 |
| BDA | 1790 | 3.1 | 58.75 | 2.58 | 0.77 | 1.17 | 57.84 | 2.61 | 0.36 |
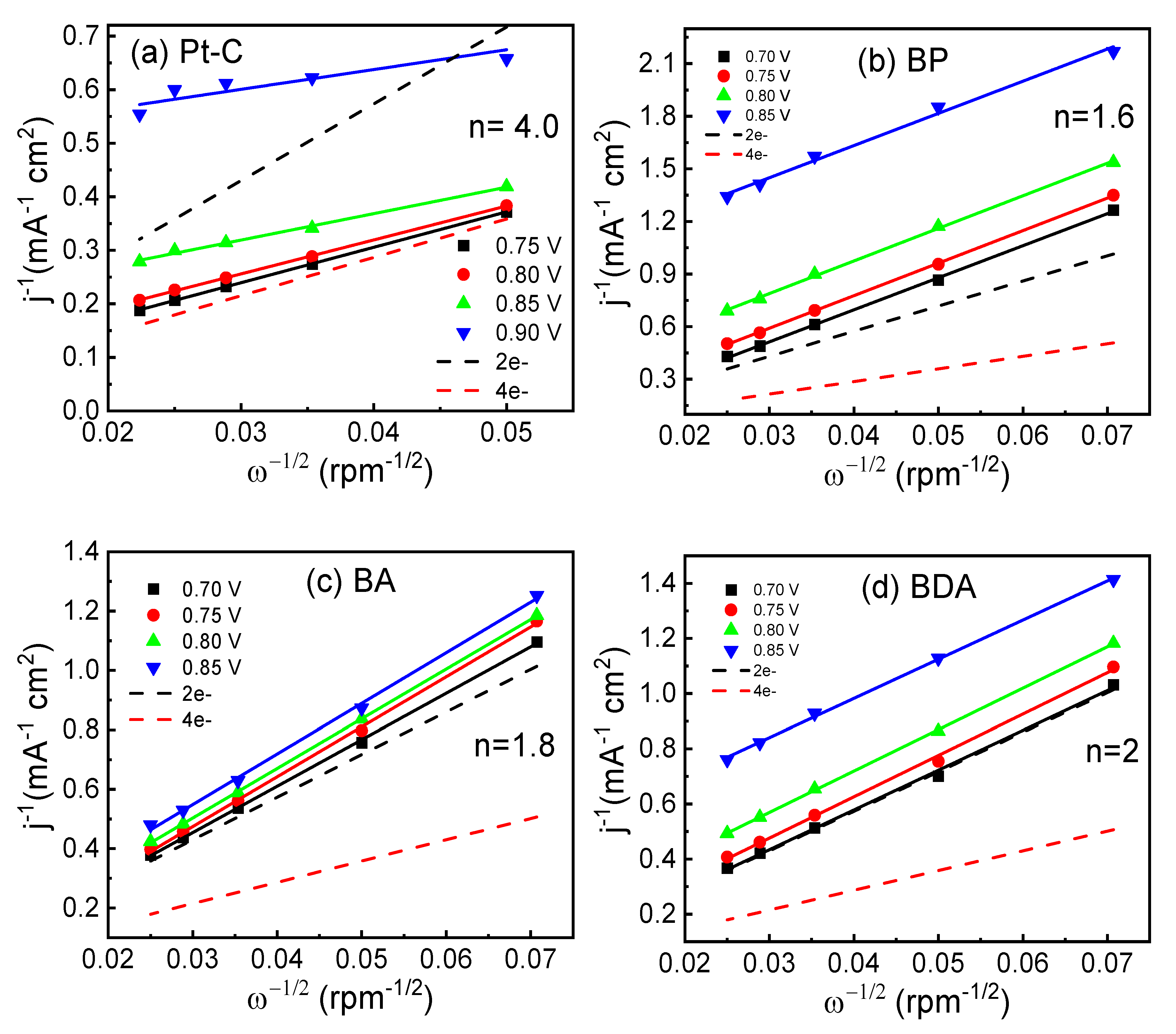
| Sample | Current Density a 0.2 E vs. RHE (mA cm−2) at 1600 RPM | Onset Potential (E vs. RHE) | Half-Wave Potential E1/2 (E vs. RHE) | Electron Transfer Number (n) |
|---|---|---|---|---|
| Pt-C | −5.48 | 1.059 | 0.870 | 4 |
| BP | −3.17 | 0.908 | 0.739 | 1.6 |
| BA | −3.60 | 0.896 | 0.772 | 1.8 |
| BDA | −4.37 | 0.922 | 0.775 | 2 |
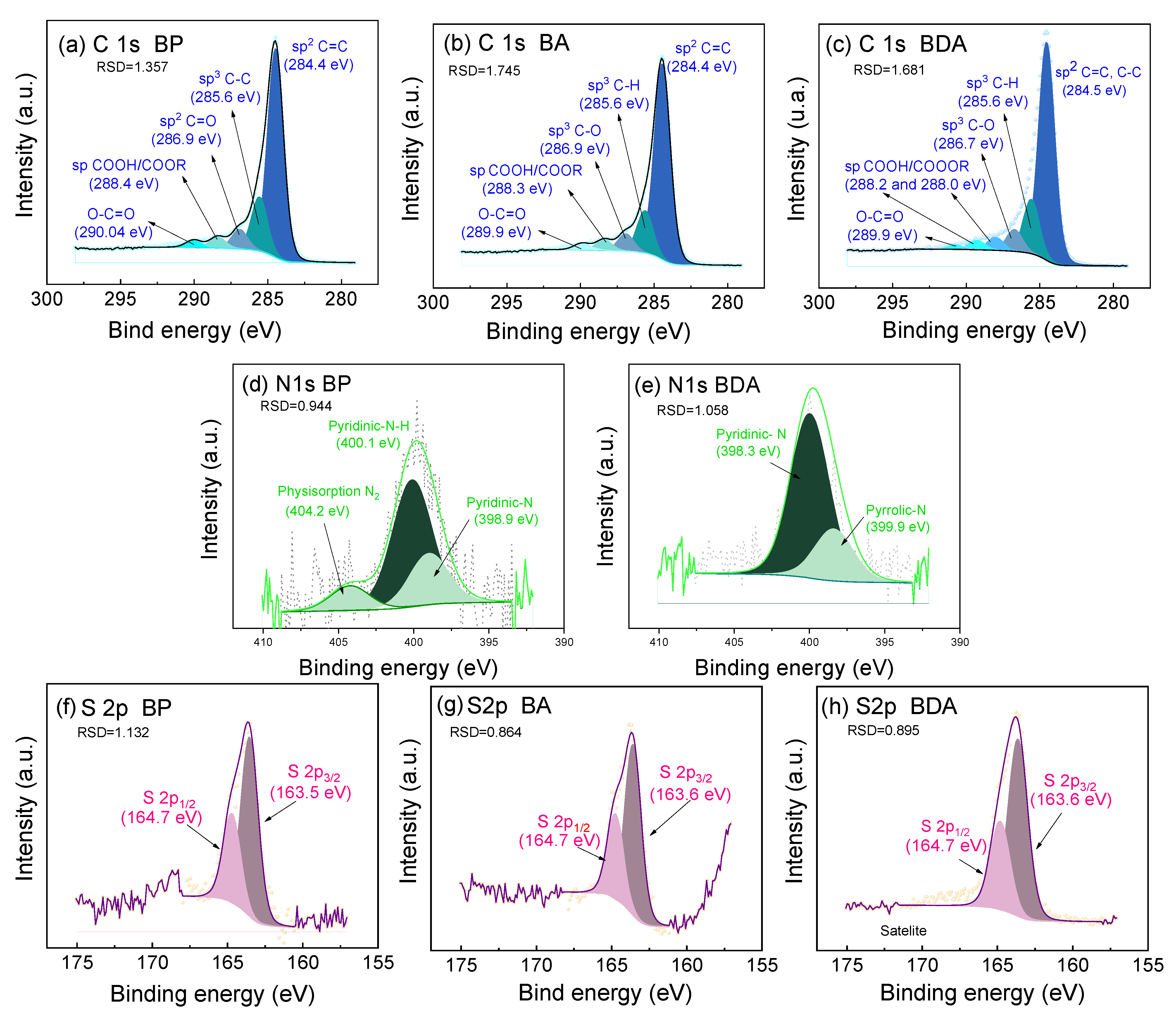
| Chemical Composition by XPS (at. %) | ||||||
|---|---|---|---|---|---|---|
| Sample | C1s | O1s | S2p | N1s | ||
| BP | 93.59 | 4.92 | 0.57 | 0.67 | ||
| Pyridinic N-H | Pyridinic N | Pyrrolic | ||||
| 62.61 | 25.31 | -- | ||||
| BA | 84.13 | 11.23 | 0.35 | -- | -- | -- |
| BDA | 92.98 | 4.40 | 1.77 | 0.85 | ||
| Pyridinic N-H | Pyridinic N | Pyrrolic N | ||||
| -- | 24.01 | 75.99 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldera, A.; Escobar, B.; Briceño, J.; Baas-López, J.M.; Barbosa, R.; Uribe, J. Nitrogen-Doped Biocarbon Derived from Alginate-Extraction Residues of Sargassum spp.: Towards Low-Cost Electrocatalysts for Alkaline ORR. Chemistry 2025, 7, 144. https://doi.org/10.3390/chemistry7050144
Caldera A, Escobar B, Briceño J, Baas-López JM, Barbosa R, Uribe J. Nitrogen-Doped Biocarbon Derived from Alginate-Extraction Residues of Sargassum spp.: Towards Low-Cost Electrocatalysts for Alkaline ORR. Chemistry. 2025; 7(5):144. https://doi.org/10.3390/chemistry7050144
Chicago/Turabian StyleCaldera, Aurora, Beatriz Escobar, Juan Briceño, José M. Baas-López, Romeli Barbosa, and Jorge Uribe. 2025. "Nitrogen-Doped Biocarbon Derived from Alginate-Extraction Residues of Sargassum spp.: Towards Low-Cost Electrocatalysts for Alkaline ORR" Chemistry 7, no. 5: 144. https://doi.org/10.3390/chemistry7050144
APA StyleCaldera, A., Escobar, B., Briceño, J., Baas-López, J. M., Barbosa, R., & Uribe, J. (2025). Nitrogen-Doped Biocarbon Derived from Alginate-Extraction Residues of Sargassum spp.: Towards Low-Cost Electrocatalysts for Alkaline ORR. Chemistry, 7(5), 144. https://doi.org/10.3390/chemistry7050144






