In Silico Analysis of Curcumin and Its Analogs MS13 and MS17 Against HSF1 and HSP Family Proteins
Abstract
1. Introduction
2. Methodology
2.1. Selection and Preparation of Target Protein Structures
2.2. Generation and Preparation of Compound Structure
2.3. Molecular Docking Studies
2.4. Molecular Dynamics (MD) Simulations
2.5. Drug Likeness and ADMET Screening for MS13 and MS17
3. Results and Discussion
3.1. Analysis of MS13 Binding Affinity
3.2. Analysis of MS17 Binding Affinity
3.3. Comparison of Curcumin Binding Affinity with MS13 and MS17
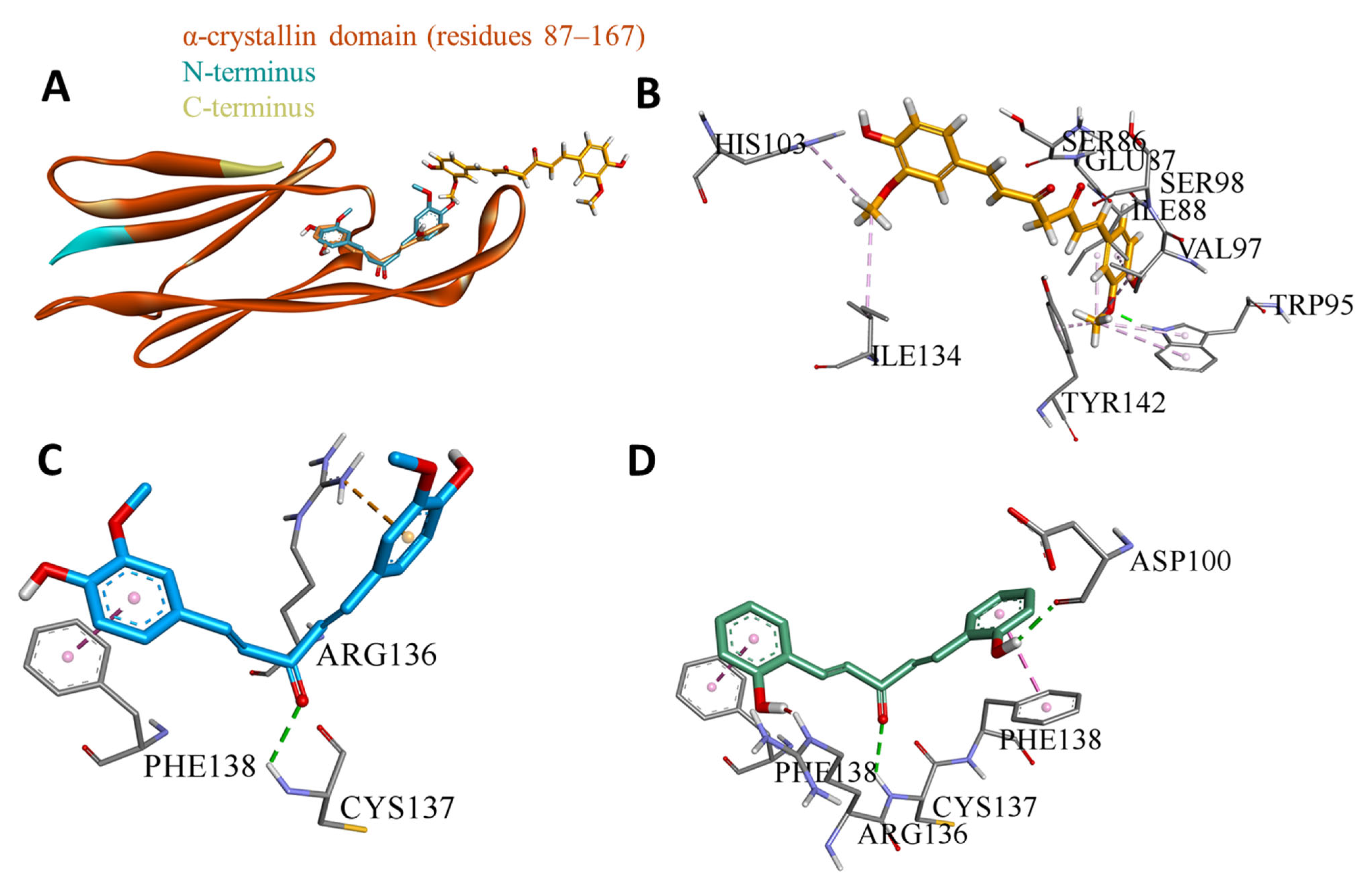
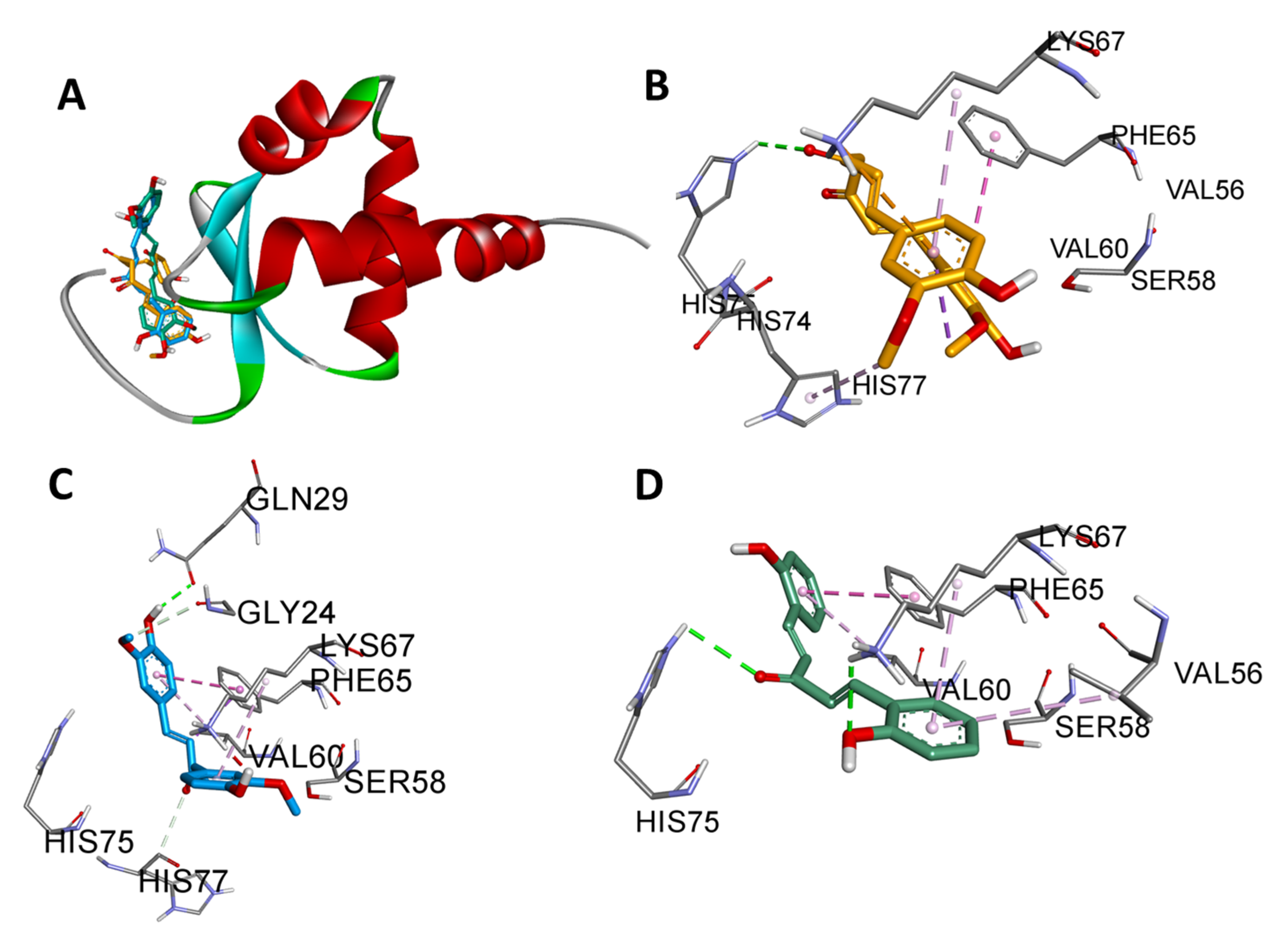
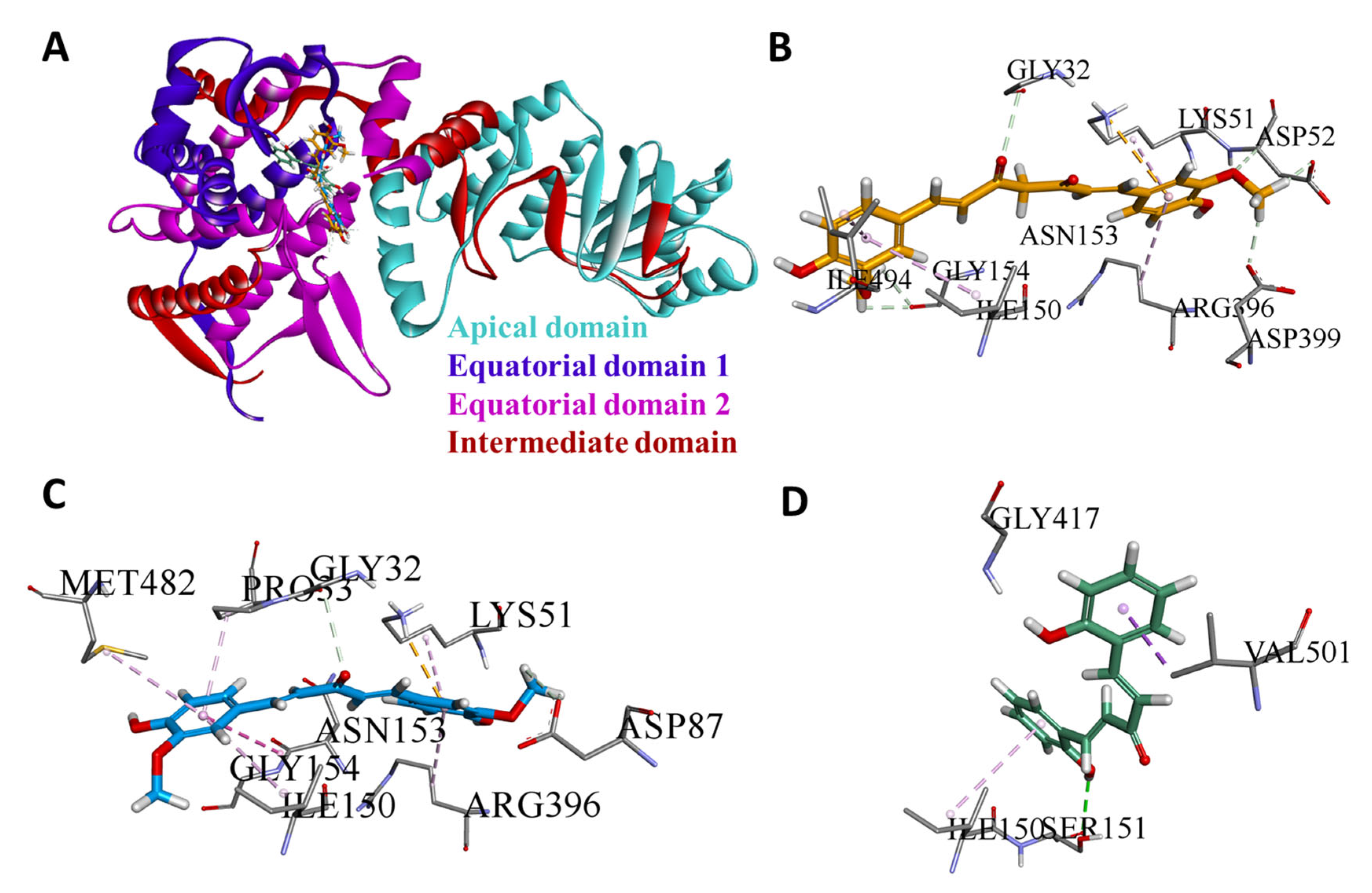
3.4. Domain-Wise Binding Orientation of MS13 and MS17 on Heat Shock Proteins
3.5. MD Simulation Analysis
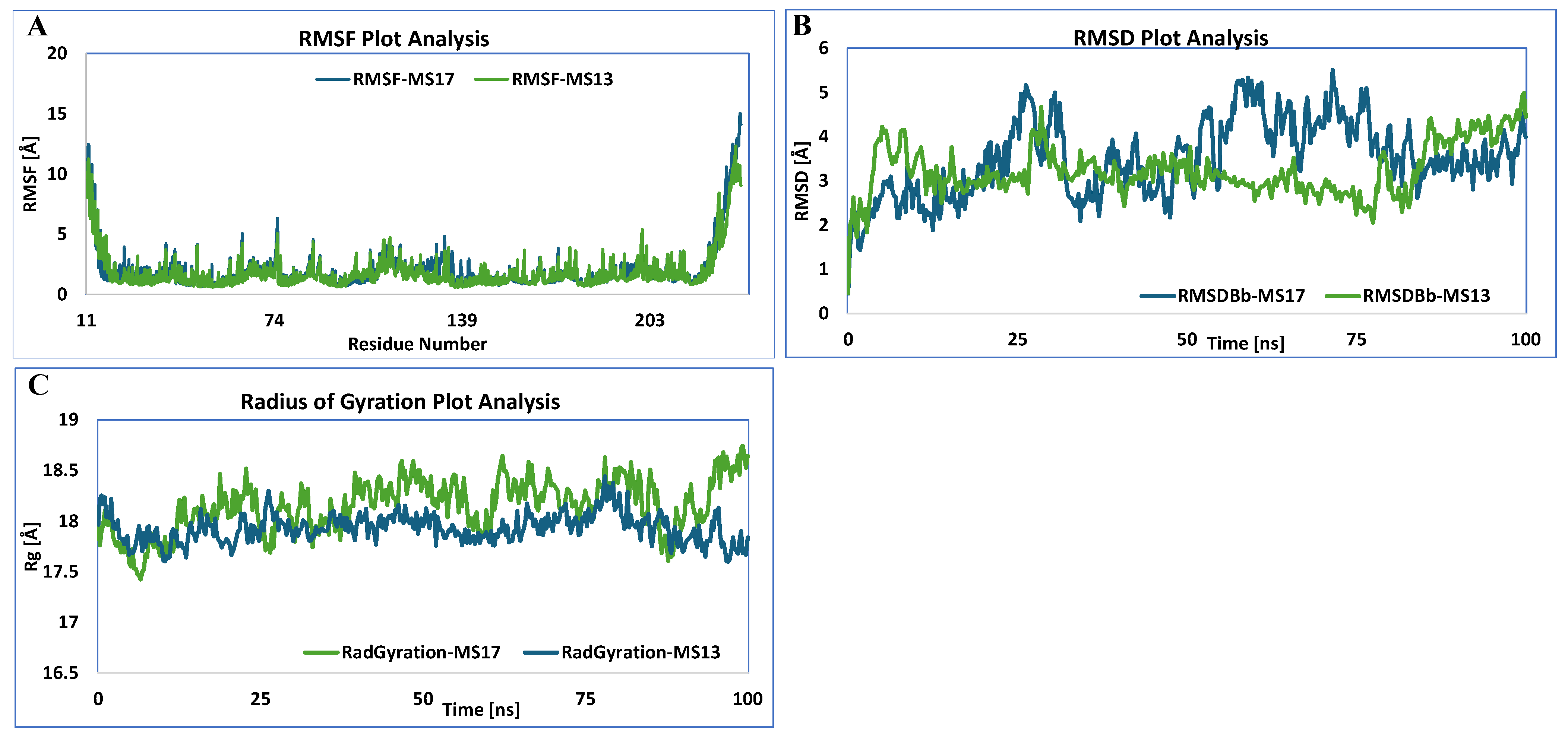
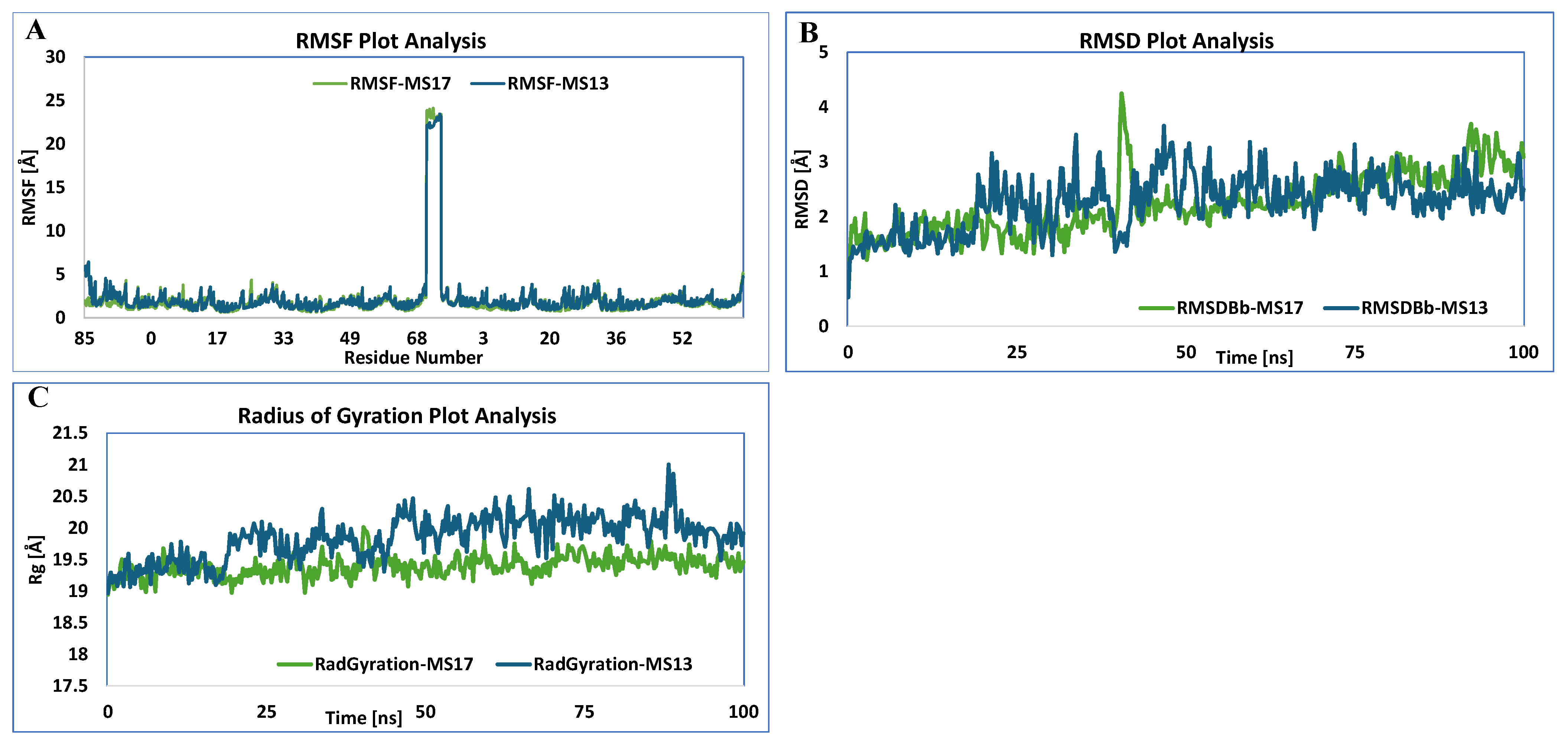
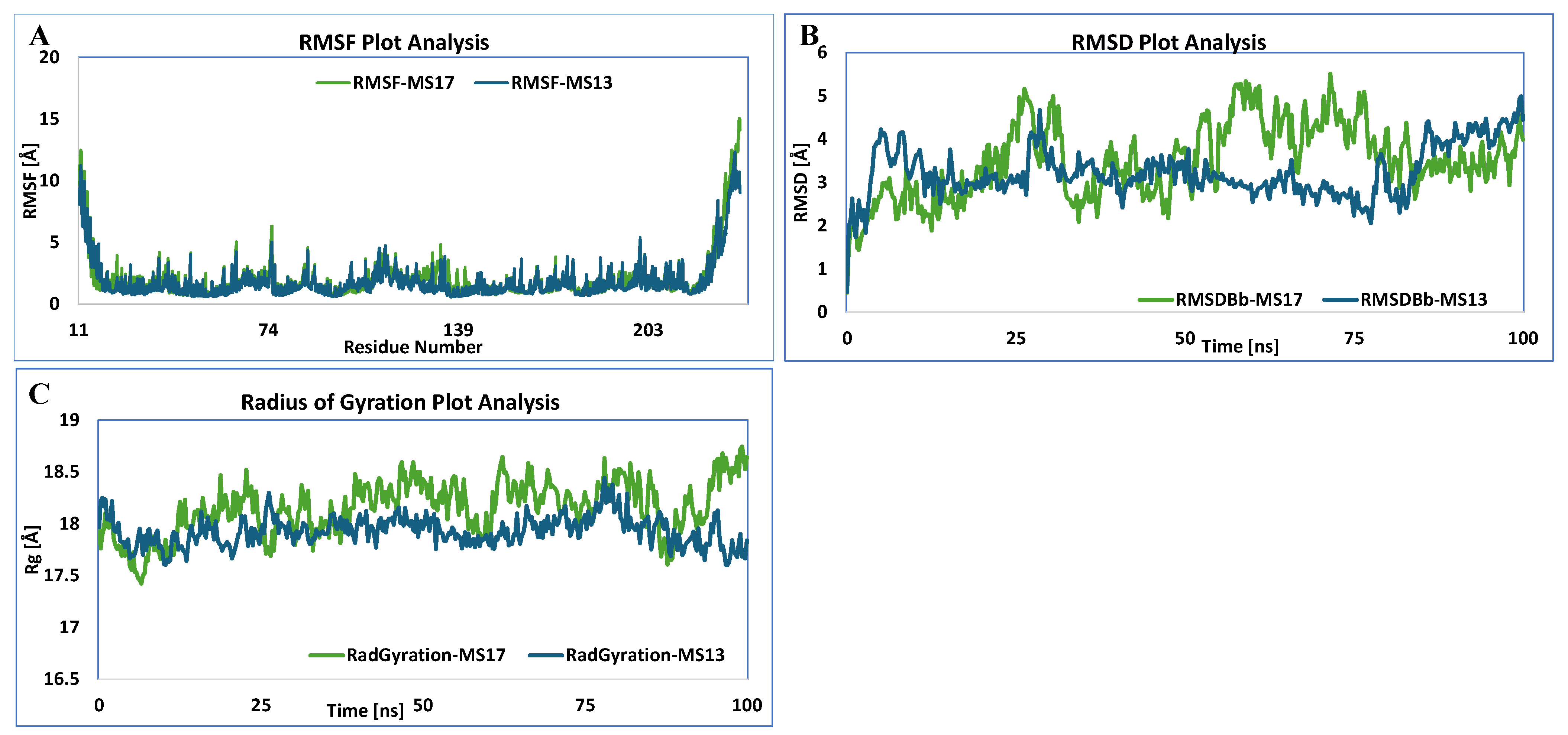
3.5.1. HSF1
3.5.2. HSP27
3.5.3. HSP40
3.5.4. HSP60
3.5.5. HSP70
3.5.6. HSP90
3.6. ADMET and Drug-likeness Properties of MS13, MS17, and Curcumin
3.7. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, B.; Li, G.; Yu, Q.; Liu, D.; Tang, X. HSP60 in cancer: A promising biomarker for diagnosis and a potentially useful target for treatment. J. Drug Target. 2022, 30, 31–45. [Google Scholar] [CrossRef]
- He, Y.; Wu, Y.; Mou, Z.; Li, W.; Zou, L.; Fu, T.; Zhang, A.; Xiang, D.; Xiao, H.; Wang, X. Proteomics-based identification of HSP60 as a tumor-associated antigen in colorectal cancer. Proteom. Clin. Appl. 2007, 1, 336–342. [Google Scholar] [CrossRef]
- Gao, G.; Liu, S.; Yao, Z.; Zhan, Y.; Chen, W.; Liu, Y. The Prognostic Significance of Hsp70 in Patients with Colorectal Cancer Patients: A PRISMA-Compliant Meta-Analysis. BioMed Res. Int. 2021, 2021, 5526327. [Google Scholar] [CrossRef]
- Jiang, W.; Pan, X.; Yan, H.; Wang, G. Prognostic Significance of the Hsp70 Gene Family in Colorectal Cancer. Med. Sci. Monit. 2021, 27, e928352. [Google Scholar] [CrossRef]
- Kai, M.; Nakatsura, T.; Egami, H.; Senju, S.; Nishimura, Y.; Ogawa, M. Heat shock protein 105 is overexpressed in a variety of human tumors. Oncol. Rep. 2003, 10, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Hrudka, J.; Jelínková, K.; Fišerová, H.; Matěj, R.; Mandys, V.; Waldauf, P. Heat Shock Proteins 27, 70, and 110: Expression and Prognostic Significance in Colorectal Cancer. Cancers 2021, 13, 4407. [Google Scholar] [CrossRef]
- Szczuka, I.; Wierzbicki, J.; Serek, P.; Szczęśniak-Sięga, B.M.; Krzystek-Korpacka, M. Heat Shock Proteins HSPA1 and HSP90AA1 Are Upregulated in Colorectal Polyps and Can Be Targeted in Cancer Cells by Anti-Inflammatory Oxicams with Arylpiperazine Pharmacophore and Benzoyl Moiety Substitutions at Thiazine Ring. Biomolecules 2021, 11, 1588. [Google Scholar] [CrossRef]
- Wang, G.; Cao, P.; Fan, Y.; Tan, K. Emerging roles of HSF1 in cancer: Cellular and molecular episodes. Biochim. Biophys. Acta (BBA) Rev. Cancer 2020, 1874, 188390. [Google Scholar] [CrossRef]
- Cyran, A.M.; Zhitkovich, A. Heat Shock Proteins and HSF1 in Cancer. Front. Oncol. 2022, 12, 860320. [Google Scholar] [CrossRef]
- Dai, C. The heat-shock, or HSF1-mediated proteotoxic stress, response in cancer: From proteomic stability to oncogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160525. [Google Scholar] [CrossRef]
- Barna, J.; Csermely, P.; Vellai, T. Roles of heat shock factor 1 beyond the heat shock response. Cell. Mol. Life Sci. 2018, 75, 2897–2916. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Chang, R.; Yang, L. Heat shock factor 1 promotes invasion and metastasis of hepatocellular carcinoma in vitro and in vivo. Cancer 2012, 118, 1782–1794. [Google Scholar] [CrossRef] [PubMed]
- Santagata, S.; Hu, R.; Lin, N.U.; Mendillo, M.L.; Collins, L.C.; Hankinson, S.E.; Schnitt, S.J.; Whitesell, L.; Tamimi, R.M.; Lindquist, S.; et al. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 18378–18383. [Google Scholar] [CrossRef] [PubMed]
- Mendillo Marc, L.; Santagata, S.; Koeva, M.; Bell, G.W.; Hu, R.; Tamimi, R.M.; Fraenkel, E.; Ince, T.A.; Whitesell, L.; Lindquist, S. HSF1 Drives a Transcriptional Program Distinct from Heat Shock to Support Highly Malignant Human Cancers. Cell 2012, 150, 549–562. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef]
- Lang, B.J.; Guerrero, M.E.; Prince, T.L.; Okusha, Y.; Bonorino, C.; Calderwood, S.K. The functions and regulation of heat shock proteins; key orchestrators of proteostasis and the heat shock response. Arch. Toxicol. 2021, 95, 1943–1970. [Google Scholar] [CrossRef]
- Tutar, Y.; Naureen, H.; Farooqi, A.A. Chapter 13—Heat shock proteins in tumor progression and metastasis. In Unraveling the Complexities of Metastasis; Farooqi, A.A., Qureshi, M.Z., Sabitaliyevich, U.Y., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 187–201. [Google Scholar]
- Liu, Z.; Liu, Y.; Long, Y.; Liu, B.; Wang, X. Role of HSP27 in the multidrug sensitivity and resistance of colon cancer cells. Oncol. Lett. 2020, 19, 2021–2027. [Google Scholar] [CrossRef]
- Tao, Y.; Messer, J.S.; Goss, K.H.; Hart, J.; Bissonnette, M.; Chang, E.B. Hsp70 exerts oncogenic activity in the Apc mutant Min mouse model. Carcinogenesis 2016, 37, 731–739. [Google Scholar] [CrossRef]
- Yamagishi, N.; Goto, K.; Nakagawa, S.; Saito, Y.; Hatayama, T. Hsp105 reduces the protein aggregation and cytotoxicity by expanded-polyglutamine proteins through the induction of Hsp70. Exp. Cell Res. 2010, 316, 2424–2433. [Google Scholar] [CrossRef]
- Berthenet, K.; Bokhari, A.d.; Lagrange, A.; Marcion, G.; Boudesco, C.; Causse, S.; De Thonel, A.; Svrcek, M.; Goloudina, A.R.; Dumont, S.; et al. HSP110 promotes colorectal cancer growth through STAT3 activation. Oncogene 2017, 36, 2328–2336. [Google Scholar] [CrossRef]
- Bhasin, N.; Dabral, P.; Senavirathna, L.; Pan, S.; Chen, R. Inhibition of TRAP1 Accelerates the DNA Damage Response, Activation of the Heat Shock Response and Metabolic Reprogramming in Colon Cancer Cells. Front. Biosci. (Landmark Ed.) 2023, 28, 227. [Google Scholar] [CrossRef]
- Lettini, G.; Sisinni, L.; Condelli, V.; Matassa, D.S.; Simeon, V.; Maddalena, F.; Gemei, M.; Lopes, E.; Vita, G.; Del Vecchio, L.; et al. TRAP1 regulates stemness through Wnt/β-catenin pathway in human colorectal carcinoma. Cell Death Differ. 2016, 23, 1792–1803. [Google Scholar] [CrossRef]
- Musiani, D.; Konda, J.D.; Pavan, S.; Torchiaro, E.; Erriquez, J.; Olivero, M.; Di Renzo, M.F. Heat Shock Protein 27 (HSP27, HSPB1) Is Up-Regulated by Targeted Agents and Confers Resistance to Both Targeted Drugs and Chemotherapeutics. In Heat Shock Protein-Based Therapies; Asea, A.A.A., Almasoud, N.N., Krishnan, S., Kaur, P., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 17–25. [Google Scholar]
- Tang, H.; Li, J.; Liu, X.; Wang, G.; Luo, M.; Deng, H. Down-regulation of HSP60 Suppresses the Proliferation of Glioblastoma Cells via the ROS/AMPK/mTOR Pathway. Sci. Rep. 2016, 6, 28388. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Long, T.-E.; Park, W.; Landry, J.C.; Taliaferro-Smith, L.; Farris, A.B.; Diaz, R.; El-Rayes, B.F. Heat shock protein 90 promotes epithelial to mesenchymal transition, invasion, and migration in colorectal cancer. Mol. Carcinog. 2015, 54, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-Q.; Chai, K.-Q.; Zhu, X.-M.; Jiang, H.; Wang, X.; Xue, Q.; Zheng, A.-H.; Zhou, H.-Y.; Chen, Y.; Chen, X.-C.; et al. Anti-cancer effects of curcumin on lung cancer through the inhibition of EZH2 and NOTCH1. Oncotarget 2016, 7, 26535. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Rokavec, M.; Huang, Z.; Hermeking, H. Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress colorectal cancer metastasis. Cell Death Differ. 2023, 30, 1771–1785. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, X.; Tong, Y.; Chen, S.; Liang, J. Curcumin against gastrointestinal cancer: A review of the pharmacological mechanisms underlying its antitumor activity. Front. Pharmacol. 2022, 13, 990475. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, S.; Shen, H.; Chen, W.; Xu, H.; Chen, X.; Sun, D.; Zhong, S.; Zhao, J.; Tang, J. Curcumin inhibits cancer progression through regulating expression of microRNAs. Tumor Biol. 2017, 39, 1010428317691680. [Google Scholar] [CrossRef]
- Pandya, N.; Khan, E.; Jain, N.; Satham, L.; Singh, R.; Makde, R.D.; Mishra, A.; Kumar, A. Curcumin analogs exhibit anti-cancer activity by selectively targeting G-quadruplex forming c-myc promoter sequence. Biochimie 2021, 180, 205–221. [Google Scholar] [CrossRef]
- Khudhayer Oglah, M.; Fakri Mustafa, Y. Curcumin analogs: Synthesis and biological activities. Med. Chem. Res. 2020, 29, 479–486. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Benton, L.; Bethi, S.R.; Shoji, M.; El-Rayes, B.F. Curcumin analogs: Their roles in pancreatic cancer growth and metastasis. Int. J. Cancer 2019, 145, 10–19. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Marino Gammazza, A.; Lo Cascio, F.; Mocciaro, E.; Vitale, A.M.; Vergilio, G.; Pace, A.; Cappello, F.; Campanella, C.; Palumbo Piccionello, A. Curcumin Affects HSP60 Folding Activity and Levels in Neuroblastoma Cells. Int. J. Mol. Sci. 2020, 21, 661. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yamamoto, Y.; Nishijima-Matsunobu, A.; Kawasaki, Y.; Shibata, H.; Omori, Y. A curcumin analogue GO-Y030 depletes cancer stem cells by inhibiting the interaction between the HSP70/HSP40 complex and its substrates. FEBS Open Bio 2023, 13, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhang, Y.; Akbari, A.; Wang, X.; Wei, J. EF-24, a Curcumin Analogue, Could Inhibit HSP90 and Induce Apoptosis-Mediated by Autophagy/Inflammation/Oxidative Stress in Papillary Thyroid Cancer Cell Lines. Nat. Prod. Commun. 2023, 18, 1934578X231166784. [Google Scholar] [CrossRef]
- Fan, Y.-j.; Zhou, Y.-x.; Zhang, L.-r.; Lin, Q.-f.; Gao, P.-z.; Cai, F.; Zhu, L.-p.; Liu, B.; Xu, J.-h. C1206, a novel curcumin derivative, potently inhibits Hsp90 and human chronic myeloid leukemia cells in vitro. Acta Pharmacol. Sin. 2018, 39, 649–658. [Google Scholar] [CrossRef]
- Ye, M.; Huang, W.; Wu, W.-W.; Liu, Y.; Ye, S.-N.; Xu, J.-H. FM807, a curcumin analogue, shows potent antitumor effects in nasopharyngeal carcinoma cells by heat shock protein 90 inhibition. Oncotarget 2017, 8, 15364. [Google Scholar] [CrossRef]
- Paulraj, F.; Abas, F.; Lajis, N.H.; Othman, I.; Hassan, S.S.; Naidu, R. The Curcumin Analogue 1,5-Bis(2-hydroxyphenyl)-1,4-pentadiene-3-one Induces Apoptosis and Downregulates E6 and E7 Oncogene Expression in HPV16 and HPV18-Infected Cervical Cancer Cells. Molecules 2015, 20, 11830–11860. [Google Scholar] [CrossRef]
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis, H.N.; Naidu, R. The Curcumin Analogue, MS13 (1,5-Bis(4-hydroxy-3- methoxyphenyl)-1,4-pentadiene-3-one), Inhibits Cell Proliferation and Induces Apoptosis in Primary and Metastatic Human Colon Cancer Cells. Molecules 2020, 25, 3798. [Google Scholar] [CrossRef]
- Abd Wahab, N.A.; Abas, F.; Othman, I.; Naidu, R. Diarylpentanoid (1,5-bis(4-hydroxy-3-methoxyphenyl)-1,4-pentadiene-3-one) (MS13) Exhibits Anti-proliferative, Apoptosis Induction and Anti-migration Properties on Androgen-independent Human Prostate Cancer by Targeting Cell Cycle-Apoptosis and PI3K Signalling Pathways. Front. Pharmacol. 2021, 12, 707335. [Google Scholar] [CrossRef]
- Citalingam, K.; Abas, F.; Lajis, N.; Othman, I.; Naidu, R. Identification of commonly regulated protein targets and molecular pathways in PC-3 and DU145 androgen-independent human prostate cancer cells treated with the curcumin analogue 1,5-bis(2-hydroxyphenyl)-1,4-pentadiene-3-one. Asian Pac. J. Trop. Biomed. 2018, 8, 436. [Google Scholar] [CrossRef]
- Lee, Y.Q.; Rajadurai, P.; Abas, F.; Othman, I.; Naidu, R. Proteomic Analysis on Anti-Proliferative and Apoptosis Effects of Curcumin Analog, 1,5-bis(4-Hydroxy-3-Methyoxyphenyl)-1,4-Pentadiene-3-One-Treated Human Glioblastoma and Neuroblastoma Cells. Front. Mol. Biosci. 2021, 8, 645856. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Tsai, S.-H.; Shen, S.-C.; Lin, J.-K.; Lee, W.-R. Alternative activation of extracellular signal-regulated protein kinases in curcumin and arsenite-induced HSP70 gene expression in human colorectal carcinoma cells. Eur. J. Cell Biol. 2001, 80, 213–221. [Google Scholar] [CrossRef]
- Guo, M.; Xu, W.; Yamamoto, Y.; Suzuki, T. Curcumin increases heat shock protein 70 expression via different signaling pathways in intestinal epithelial cells. Arch. Biochem. Biophys. 2021, 707, 108938. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.C.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. Conformer generation with OMEGA: Algorithm and validation using high quality structures from the Protein Databank and Cambridge Structural Database. J. Chem. Inf. Model. 2010, 50, 572–584. [Google Scholar] [CrossRef]
- McGann, M. FRED Pose Prediction and Virtual Screening Accuracy. J. Chem. Inf. Model. 2011, 51, 578–596. [Google Scholar] [CrossRef]
- Khan, S.U.; Ahemad, N.; Chuah, L.-H.; Naidu, R.; Htar, T.T. Natural bioactive compounds as a new source of promising G protein-coupled estrogen receptor (GPER) modulators: Comprehensive in silico approach. J. Biomol. Struct. Dyn. 2022, 40, 1617–1628. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. New ways to boost molecular dynamics simulations. J. Comput. Chem. 2015, 36, 996–1007. [Google Scholar] [CrossRef]
- Land, H.; Humble, M.S. YASARA: A Tool to Obtain Structural Guidance in Biocatalytic Investigations. In Protein Engineering: Methods and Protocols; Bornscheuer, U.T., Höhne, M., Eds.; Springer: New York, NY, USA, 2018; pp. 43–67. [Google Scholar]
- Krieger, E.; Dunbrack, R.L.; Hooft, R.W.W.; Krieger, B. Assignment of Protonation States in Proteins and Ligands: Combining pKa Prediction with Hydrogen Bonding Network Optimization. In Computational Drug Discovery and Design; Baron, R., Ed.; Springer: New York, NY, USA, 2012; pp. 405–421. [Google Scholar]
- Baildya, N.; Khan, A.A.; Ghosh, N.N.; Dutta, T.; Chattopadhyay, A.P. Screening of potential drug from Azadirachta Indica (Neem) extracts for SARS-CoV-2: An insight from molecular docking and MD-simulation studies. J. Mol. Struct. 2021, 1227, 129390. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An Open-Source Program for Chemistry Aware Data Visualization and Analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Xue, L.; He, Q. Exploring the therapeutic mechanism of curcumin in prostate cancer using network pharmacology and molecular docking. Heliyon 2024, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Labbadia, J.; Morimoto, R.I. Rethinking HSF1 in Stress, Development, and Organismal Health. Trends Cell Biol. 2017, 27, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Takii, R.; Nakai, A. Regulation of HSF1 transcriptional complexes under proteotoxic stress. BioEssays 2023, 45, 2300036. [Google Scholar] [CrossRef]
- Dong, B.; Jaeger, A.M.; Hughes, P.F.; Loiselle, D.R.; Hauck, J.S.; Fu, Y.; Haystead, T.A.; Huang, J.; Thiele, D.J. Targeting therapy-resistant prostate cancer via a direct inhibitor of the human heat shock transcription factor 1. Sci. Transl. Med. 2020, 12, eabb5647. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, R.; McAlpine, S.R. Heat Shock Protein 27: Structure, Function, Cellular Role and Inhibitors. In Heat Shock Protein Inhibitors: Success Stories; McAlpine, S.R., Edkins, A.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 221–234. [Google Scholar]
- Moens, U.; Kostenko, S. Hsp27 Phosphorylation Patterns and Cellular Consequences. In Cellular Trafficking of Cell Stress Proteins in Health and Disease; Henderson, B., Pockley, A.G., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 43–74. [Google Scholar]
- Rashmi, R.; Kumar, S.; Karunagaran, D. Ectopic expression of Hsp70 confers resistance and silencing its expression sensitizes human colon cancer cells to curcumin-induced apoptosis. Carcinogenesis 2004, 25, 179–187. [Google Scholar] [CrossRef]
- Rashmi, R.; Santhosh Kumar, T.R.; Karunagaran, D. Human colon cancer cells differ in their sensitivity to curcumin-induced apoptosis and heat shock protects them by inhibiting the release of apoptosis-inducing factor and caspases. FEBS Lett. 2003, 538, 19–24. [Google Scholar] [CrossRef]
- Thuringer, D.; Berthenet, K.; Cronier, L.; Solary, E.; Garrido, C. Primary tumor- and metastasis-derived colon cancer cells differently modulate connexin expression and function in human capillary endothelial cells. Oncotarget 2015, 6, 28800–28815. [Google Scholar] [CrossRef]
- Vahid, S.; Thaper, D.; Gibson, K.F.; Bishop, J.L.; Zoubeidi, A. Molecular chaperone Hsp27 regulates the Hippo tumor suppressor pathway in cancer. Sci. Rep. 2016, 6, 31842. [Google Scholar] [CrossRef]
- Lampros, M.; Vlachos, N.; Voulgaris, S.; Alexiou, G.A. The Role of Hsp27 in Chemotherapy Resistance. Biomedicines 2022, 10, 897. [Google Scholar] [CrossRef]
- Heinrich, J.C.; Donakonda, S.; Haupt, V.J.; Lennig, P.; Zhang, Y.; Schroeder, M. New HSP27 inhibitors efficiently suppress drug resistance development in cancer cells. Oncotarget 2016, 7, 68156. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Choi, S.-K.; Lee, J.; Park, S.H.; Park, Y.N.; Hwang, S.-Y.; Shin, J.-H.; Na, Y.; Kwon, Y.; Lee, H.J.; et al. Drug-Like Small Molecule HSP27 Functional Inhibitor Sensitizes Lung Cancer Cells to Gefitinib or Cisplatin by Inducing Altered Cross-Linked Hsp27 Dimers. Pharmaceutics 2021, 13, 630. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jung, Y.J.; Choi, B.; Lee, N.L.; Lee, H.J.; Kwak, S.Y.; Kwon, Y.; Na, Y.; Lee, Y.-S. Overcoming HSP27-mediated resistance by altered dimerization of HSP27 using small molecules. Oncotarget 2016, 7, 53178. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, F.; Thomas, C.; Yin, M.-J.; Fazli, L.; Zoubeidi, A.; Gleave, M.E. Suppression of heat shock protein 27 using OGX-427 induces endoplasmic reticulum stress and potentiates heat shock protein 90 inhibitors to delay castrate-resistant prostate cancer. Eur. Urol. 2014, 66, 145–155. [Google Scholar] [CrossRef]
- Gibert, B.; Hadchity, E.; Czekalla, A.; Aloy, M.T.; Colas, P.; Rodriguez-Lafrasse, C.; Arrigo, A.P.; Diaz-Latoud, C. Inhibition of heat shock protein 27 (HspB1) tumorigenic functions by peptide aptamers. Oncogene 2011, 30, 3672–3681. [Google Scholar] [CrossRef]
- Pesce, E.-R.; Blatch, G.L.; Edkins, A.L. Hsp40 Co-chaperones as Drug Targets: Towards the Development of Specific Inhibitors. In Heat Shock Protein Inhibitors: Success Stories; McAlpine, S.R., Edkins, A.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 163–195. [Google Scholar]
- Kaida, A.; Yamamoto, S.; Parrales, A.; Young, E.D.; Ranjan, A.; Alalem, M.A.; Morita, K.; Oikawa, Y.; Harada, H.; Ikeda, T.; et al. DNAJA1 promotes cancer metastasis through interaction with mutant p53. Oncogene 2021, 40, 5013–5025. [Google Scholar] [CrossRef]
- Stark, J.L.; Mehla, K.; Chaika, N.; Acton, T.B.; Xiao, R.; Singh, P.K.; Montelione, G.T.; Powers, R. Structure and Function of Human DnaJ Homologue Subfamily A Member 1 (DNAJA1) and Its Relationship to Pancreatic Cancer. Biochemistry 2014, 53, 1360–1372. [Google Scholar] [CrossRef]
- Hu, J.; Wu, Y.; Li, J.; Qian, X.; Fu, Z.; Sha, B. The crystal structure of the putative peptide-binding fragment from the human Hsp40 protein Hdj1. BMC Struct. Biol. 2008, 8, 3. [Google Scholar] [CrossRef]
- Jiang, Y.; Rossi, P.; Kalodimos, C.G. Structural basis for client recognition and activity of Hsp40 chaperones. Science 2019, 365, 1313–1319. [Google Scholar] [CrossRef]
- Tomiczek, B.; Delewski, W.; Nierzwicki, L.; Stolarska, M.; Grochowina, I.; Schilke, B.; Dutkiewicz, R.; Uzarska, M.A.; Ciesielski, S.J.; Czub, J.; et al. Two-step mechanism of J-domain action in driving Hsp70 function. PLoS Comput. Biol. 2020, 16, e1007913. [Google Scholar] [CrossRef]
- Nishikawa, S.; Kaida, A.; Parrales, A.; Ranjan, A.; Alalem, M.; Ren, H.; Schoenen, F.J.; Johnson, D.K.; Iwakuma, T. DNAJA1- and conformational mutant p53-dependent inhibition of cancer cell migration by a novel compound identified through a virtual screen. Cell Death Discov. 2022, 8, 437. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Alberti, G.; Vitale, A.M.; Paladino, L.; Campanella, C.; Rappa, F.; Gorska, M.; Conway de Macario, E.; Cappello, F.; Macario, A.J.L.; et al. Hsp60 Post-translational Modifications: Functional and Pathological Consequences. Front. Mol. Biosci. 2020, 7, 95. [Google Scholar] [CrossRef]
- Malik, J.A.; Lone, R. Heat shock proteins with an emphasis on HSP 60. Mol. Biol. Rep. 2021, 48, 6959–6969. [Google Scholar] [CrossRef]
- Gomez, C.R. Hsp60 in Cancer Immunity: Biological Basis, Diagnostic Potential and Therapeutic Opportunities. In Heat Shock Protein 60 in Human Diseases and Disorders; Asea, A.A.A., Kaur, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 117–134. [Google Scholar]
- Moré, S.H.; Breloer, M.; von Bonin, A. Eukaryotic heat shock proteins as molecular links in innate and adaptive immune responses: Hsp60-mediated activation of cytotoxic T cells. Int. Immunol. 2001, 13, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, Y.; Fan, S.; Wen, Q. The multiple roles and therapeutic potential of HSP60 in cancer. Biochem. Pharmacol. 2022, 201, 115096. [Google Scholar] [CrossRef] [PubMed]
- Basset, C.A.; Cappello, F.; Rappa, F.; Jurjus, A.R.; Conway de Macario, E.; Macario, A.J.L.; Leone, A. Chaperonin Hsp60 and Cancer Therapies. In Heat Shock Proteins in Human Diseases; Asea, A.A.A., Kaur, P., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 31–52. [Google Scholar]
- Tsai, Y.-P.; Yang, M.-H.; Huang, C.-H.; Chang, S.-Y.; Chen, P.-M.; Liu, C.-J.; Teng, S.-C.; Wu, K.-J. Interaction between HSP60 and β-catenin promotes metastasis. Carcinogenesis 2009, 30, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.S.-C.; Wong, V.W.-K.; Chan, C.M.-L.; Ma, B.B.-Y.; Hui, E.P.; Wong, M.C.-K.; Lam, M.Y.-Y.; Au, T.C.-C.; Chan, W.-H.; Cheuk, W.; et al. Identification of 5-fluorouracil response proteins in colorectal carcinoma cell line SW480 by two-dimensional electrophoresis and MALDI-TOF mass spectrometry. Oncol. Rep. 2008, 20, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Nitika; Zheng, B.; Ruan, L.; Kline, J.T.; Omkar, S.; Sikora, J.; Texeira Torres, M.; Wang, Y.; Takakuwa, J.E.; Huguet, R.; et al. Comprehensive characterization of the Hsp70 interactome reveals novel client proteins and interactions mediated by posttranslational modifications. PLoS Biol. 2022, 20, e3001839. [Google Scholar] [CrossRef]
- Yun, C.W.; Kim, H.J.; Lim, J.H.; Lee, S.H. Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy. Cells 2019, 9, 60. [Google Scholar] [CrossRef]
- Mayer, M.P. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem. Sci. 2013, 38, 507–514. [Google Scholar] [CrossRef]
- Schlecht, R.; Scholz, S.R.; Dahmen, H.; Wegener, A.; Sirrenberg, C.; Musil, D.; Bomke, J.; Eggenweiler, H.-M.; Mayer, M.P.; Bukau, B. Functional Analysis of Hsp70 Inhibitors. PLoS ONE 2013, 8, e78443. [Google Scholar] [CrossRef]
- Wen, W.; Liu, W.; Shao, Y.; Chen, L. VER-155008, a small molecule inhibitor of HSP70 with potent anti-cancer activity on lung cancer cell lines. Exp. Biol. Med. 2014, 239, 638–645. [Google Scholar] [CrossRef]
- Massey, A.J.; Williamson, D.S.; Browne, H.; Murray, J.B.; Dokurno, P.; Shaw, T.; Macias, A.T.; Daniels, Z.; Geoffroy, S.; Dopson, M.; et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother. Pharmacol. 2010, 66, 535–545. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Y.; Bai, J.; Yang, Y.; Wang, F.; Feng, Y.; Zhang, R.; Li, F.; Zhang, P.; Lv, N.; et al. Blockade of HSP70 by VER-155008 synergistically enhances bortezomib-induced cytotoxicity in multiple myeloma. Cell Stress Chaperones 2020, 25, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Slater, C.; de La Mare, J.A.; Edkins, A.L. In vitro analysis of putative cancer stem cell populations and chemosensitivity in the SW480 and SW620 colon cancer metastasis model. Oncol. Lett. 2018, 15, 8516–8526. [Google Scholar] [CrossRef] [PubMed]
- Javid, H.; Hashemian, P.; Yazdani, S.; Sharbaf Mashhad, A.; Karimi-Shahri, M. The role of heat shock proteins in metastatic colorectal cancer: A review. J. Cell. Biochem. 2022, 123, 1704–1735. [Google Scholar] [CrossRef]
- Niu, M.; Zhang, B.; Li, L.; Su, Z.; Pu, W.; Zhao, C.; Wei, L.; Lian, P.; Lu, R.; Wang, R.; et al. Targeting HSP90 Inhibits Proliferation and Induces Apoptosis Through AKT1/ERK Pathway in Lung Cancer. Front. Pharmacol. 2022, 12, 724192. [Google Scholar] [CrossRef]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef]
- Vaughan, C.K.; Gohlke, U.; Sobott, F.; Good, V.M.; Ali, M.M.U.; Prodromou, C.; Robinson, C.V.; Saibil, H.R.; Pearl, L.H. Structure of an Hsp90-Cdc37-Cdk4 Complex. Mol. Cell 2006, 23, 697–707. [Google Scholar] [CrossRef]
- Doyle, S.M.; Hoskins, J.R.; Kravats, A.N.; Heffner, A.L.; Garikapati, S.; Wickner, S. Intermolecular Interactions between Hsp90 and Hsp70. J. Mol. Biol. 2019, 431, 2729–2746. [Google Scholar] [CrossRef]
- Albakova, Z.; Mangasarova, Y.; Albakov, A.; Gorenkova, L. HSP70 and HSP90 in Cancer: Cytosolic, Endoplasmic Reticulum and Mitochondrial Chaperones of Tumorigenesis. Front. Oncol. 2022, 12, 829520. [Google Scholar] [CrossRef]
- Li, Z.-N.; Luo, Y. HSP90 inhibitors and cancer: Prospects for use in targeted therapies (Review). Oncol. Rep. 2023, 49, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qian, D. Hsp90α and cell death in cancers: A review. Discov. Oncol. 2024, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Schulte, T.W.; Akinaga, S.; Soga, S.; Sullivan, W.; Stensgard, B.; Toft, D.; Neckers, L.M. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress. Chaperones 1998, 3, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, C.E.; Russo, A.A.; Schneider, C.; Rosen, N.; Hartl, F.U.; Pavletich, N.P. Crystal Structure of an Hsp90–Geldanamycin Complex: Targeting of a Protein Chaperone by an Antitumor Agent. Cell 1997, 89, 239–250. [Google Scholar] [CrossRef]
- Bedin, M.; Gaben, A.-M.; Saucier, C.; Mester, J. Geldanamycin, an inhibitor of the chaperone activity of HSP90, induces MAPK-independent cell cycle arrest. Int. J. Cancer 2004, 109, 643–652. [Google Scholar] [CrossRef]
- Abd El-Fattah, E.E.; Zakaria, A.Y. Targeting HSP47 and HSP70: Promising therapeutic approaches in liver fibrosis management. J. Transl. Med. 2022, 20, 544. [Google Scholar] [CrossRef]
- Kosinsky, R.L.; Helms, M.; Zerche, M.; Wohn, L.; Dyas, A.; Prokakis, E.; Kazerouni, Z.B.; Bedi, U.; Wegwitz, F.; Johnsen, S.A. USP22-dependent HSP90AB1 expression promotes resistance to HSP90 inhibition in mammary and colorectal cancer. Cell Death Dis. 2019, 10, 911. [Google Scholar] [CrossRef]
- Tian, S.; Peng, P.; Li, J.; Deng, H.; Zhan, N.; Zeng, Z.; Dong, W. SERPINH1 regulates EMT and gastric cancer metastasis via the Wnt/β-catenin signaling pathway. Aging 2020, 12, 3574–3593. [Google Scholar] [CrossRef]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef]
- Yuan, J.; Dong, X.; Yap, J.; Hu, J. The MAPK and AMPK signalings: Interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.D.; Prince, T.; Gong, J.; Calderwood, S.K. mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis. PLoS ONE 2012, 7, e39679. [Google Scholar] [CrossRef] [PubMed]
- Su, K.-H.; Cao, J.; Tang, Z.; Dai, S.; He, Y.; Sampson, S.B.; Benjamin, I.J.; Dai, C. HSF1 critically attunes proteotoxic stress sensing by mTORC1 to combat stress and promote growth. Nat. Cell Biol. 2016, 18, 527–539. [Google Scholar] [CrossRef]
- Chen, N.-G.; Lu, C.-C.; Lin, Y.-H.; Shen, W.-C.; Lai, C.-H.; Ho, Y.-J.; Chung, J.-G.; Lin, T.-H.; Lin, Y.-C.; Yang, J.-S. Proteomic approaches to study epigallocatechin gallate-provoked apoptosis of TSGH-8301 human urinary bladder carcinoma cells: Roles of AKT and heat shock protein 27-modulated intrinsic apoptotic pathways. Oncol. Rep. 2011, 26, 939–947. [Google Scholar] [CrossRef]
- Shiota, M.; Bishop, J.L.; Nip, K.M.; Zardan, A.; Takeuchi, A.; Cordonnier, T.; Beraldi, E.; Bazov, J.; Fazli, L.; Chi, K.; et al. Hsp27 regulates epithelial mesenchymal transition, metastasis, and circulating tumor cells in prostate cancer. Cancer Res. 2013, 73, 3109–3119. [Google Scholar] [CrossRef]
- Cordonnier, T.; Bishop, J.L.; Shiota, M.; Nip, K.M.; Thaper, D.; Vahid, S.; Heroux, D.; Gleave, M.; Zoubeidi, A. Hsp27 regulates EGF/β-catenin mediated epithelial to mesenchymal transition in prostate cancer. Int. J. Cancer 2015, 136, E496–E507. [Google Scholar] [CrossRef]
- Qi, Y.; Cao, J.; Jiang, M.; Lin, Y.; Li, W.; Li, B. HSP27/IL-6 axis promotes OSCC chemoresistance, invasion and migration by orchestrating macrophages via a positive feedback loop. Cell Biol. Toxicol. 2025, 41, 36. [Google Scholar] [CrossRef]
- Asgharzadeh, F.; Moradi-Marjaneh, R.; Marjaneh, M.M. The Role of Heat Shock Protein 40 in Carcinogenesis and Biology of Colorectal Cancer. Curr. Pharm. Des. 2022, 28, 1457–1465. [Google Scholar] [CrossRef]
- Huang, Y.; Li, G.-M. Role of HSP40 proteins in genome maintenance, insulin signaling and cancer therapy. DNA Repair. 2025, 149, 103839. [Google Scholar] [CrossRef]
- Chun, J.N.; Choi, B.; Lee, K.W.; Lee, D.J.; Kang, D.H.; Lee, J.Y.; Song, I.S.; Kim, H.I.; Lee, S.-H.; Kim, H.S.; et al. Cytosolic Hsp60 Is Involved in the NF-κB-Dependent Survival of Cancer Cells via IKK Regulation. PLoS ONE 2010, 5, e9422. [Google Scholar] [CrossRef] [PubMed]
- Fucarino, A.; Pitruzzella, A. Role of HSP60/HSP10 in Lung Cancer: Simple Biomarkers or Leading Actors? J. Oncol. 2020, 2020, 4701868. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Kim, J.Y.; Cho, H.M.; Park, S.; Hwang, J.M.; You, H.; Chan Chae, Y.; Lee, W.-J.; Sun, W.; Kang, D.; et al. Heat shock protein 60 couples an oxidative stress-responsive p38/MK2 signaling and NF-κB survival machinery in cancer cells. Redox Biol. 2022, 51, 102293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Sun, H.; Zheng, C.; Gao, J.; Fu, Q.; Hu, N.; Shao, X.; Zhou, Y.; Xiong, J.; Nie, K.; et al. Oncogenic HSP60 regulates mitochondrial oxidative phosphorylation to support Erk1/2 activation during pancreatic cancer cell growth. Cell Death Dis. 2018, 9, 161. [Google Scholar] [CrossRef]
- Chatterjee, M.; Andrulis, M.; Stühmer, T.; Müller, E.; Hofmann, C.; Steinbrunn, T.; Heimberger, T.; Schraud, H.; Kressmann, S.; Einsele, H.; et al. The PI3K/Akt signaling pathway regulates the expression of Hsp70, which critically contributes to Hsp90-chaperone function and tumor cell survival in multiple myeloma. Haematologica 2013, 98, 1132–1141. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Liu, Y.; Zhao, K.; Wei, S.; Sugarman, E.T.; Liu, L.; Zhang, G. Targeting HSP90 as a Novel Therapy for Cancer: Mechanistic Insights and Translational Relevance. Cells 2022, 11, 2778. [Google Scholar] [CrossRef]
- Zhao, K.; Zhou, G.; Liu, Y.; Zhang, J.; Chen, Y.; Liu, L.; Zhang, G. HSP70 Family in Cancer: Signaling Mechanisms and Therapeutic Advances. Biomolecules 2023, 13, 601. [Google Scholar] [CrossRef]
- Acquaviva, J.; He, S.; Sang, J.; Smith, D.L.; Sequeira, M.; Zhang, C.; Bates, R.C.; Proia, D.A. mTOR Inhibition Potentiates HSP90 Inhibitor Activity via Cessation of HSP Synthesis. Mol. Cancer Res. 2014, 12, 703–713. [Google Scholar] [CrossRef]

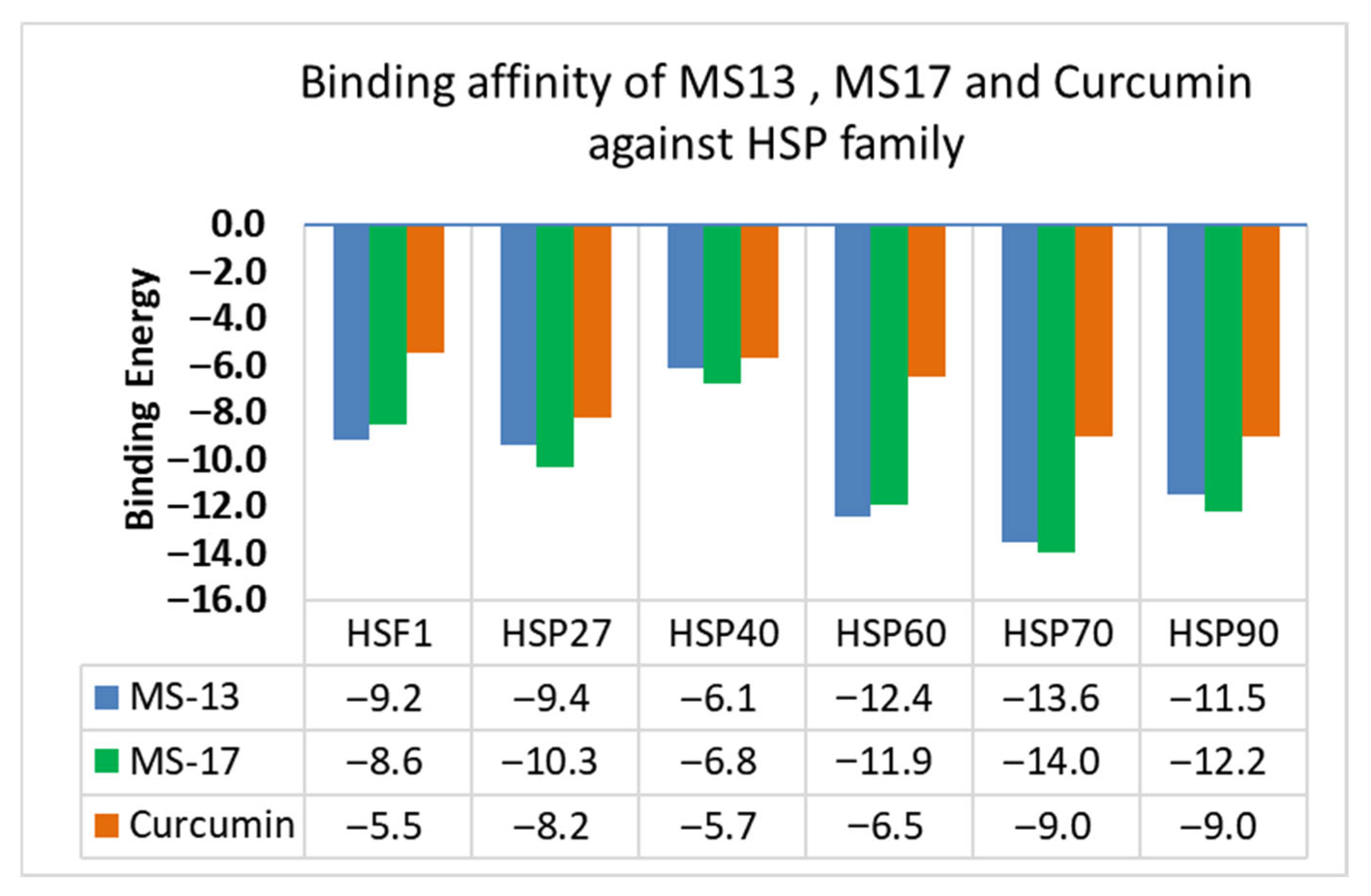






| Target Protein | Chemguass4 Score | ||
|---|---|---|---|
| MS13 | MS17 | Curcumin | |
| HSF1 | −9.2 | −8.6 | −5.5 |
| HSP27 | −9.4 | −10.3 | −8.2 |
| HSP40 | −6.1 | −6.8 | −5.7 |
| HSP60 | −12.4 | −11.9 | −6.5 |
| HSP70 | −13.6 | −14.0 | −9.0 |
| HSP90 | −11.5 | −12.2 | −9.0 |
| Target Protein | Amino Acid Residues at Binding Site | Implications |
|---|---|---|
| HSF1 (PDB ID:5d5v) | Residues 16–123 | This region corresponds to the N-terminal DNA-binding domain (DBD) of HSF1. MS13 and MS17 bind to this domain, which is responsible for recognizing and binding to heat shock elements (HSEs) in DNA, ultimately regulating the expression of HSPs. |
| HSP27 (PDB ID: 4mjh) | Residues 87–167 | This region represents the α-Crystallin Domain (ACD) of HSP27. The ACD is crucial for HSP27’s chaperone activity and dimerization. By binding to this domain, MS13 and MS17 could potentially disrupt HSP27 dimerization and impair its function. |
| HSP40 (PDB ID: 2l01) | Residues 1–80 | This region corresponds to the J-domain of HSP40. The J-domain interacts with HSP70 and is essential for stimulating ATP hydrolysis in HSP70, thereby influencing the chaperone activity of the HSP70-HSP40 complex. The binding of MS13 and MS17 to the J-domain might interfere with this interaction and potentially affect protein folding and quality control mechanisms. |
| HSP60 (PDB ID: 6mrd) | Residues 30–157 and 434–548 | These residues are located within the Equatorial Domain of HSP60. This domain contains the binding site for ATP and facilitates interactions critical for HSP60’s chaperone function. The binding of MS13 and MS17 to this domain may interfere with ATP binding and disrupt the stability of HSP60. |
| HSP70 (PDB ID: 1hjo) | Residues 3–382 | This region encompasses the N-terminal ATPase domain (NBD) of HSP70. The NBD is responsible for binding and hydrolyzing ATP, which provides the energy required for HSP70’s chaperone activity. The interaction of MS13 and MS17 with the NBD could inhibit HSP70’s ATPase activity, ultimately disrupting its chaperone function. |
| HSP90 (PDB ID: 2xjx) | Amino acids 11 to 236 | This region is found within the N-terminal ATPase domain (NBD) of HSP90. However, the source mentions that current data suggests preferential binding of MS13 and MS17 to the broader N-terminal domain (NTD), although specific residues for this interaction are not provided. NTD plays a critical role in HSP90’s function, including ATP binding and interaction with client proteins. Binding to this domain may disrupt HSP90’s chaperone activity and affect the stability of its client proteins. |
| Property | MS13 | MS17 | Curcumin |
|---|---|---|---|
| Consensus Log P | 3.05 | 3.08 | 3.03 |
| TPSA | 75.99 | 57.53 | 93.06 |
| #H-bond acceptors | 5 | 3 | 6 |
| #H-bond donors | 2 | 2 | 2 |
| GI absorption | High | High | High |
| BBB permeant | Yes | Yes | No |
| CYP1A2 inhibitor | Yes | Yes | No |
| CYP2C9 inhibitor | Yes | Yes | Yes |
| CYP3A4 inhibitor | Yes | Yes | Yes |
| Lipinski #violations | 0 | 0 | 0 |
| Bioavailability Score | 0.55 | 0.55 | 0.55 |
| PAINS #alerts | 0 | 0 | 0 |
| Brenk #alerts | 1 | 1 | 2 |
| Leadlikeness #violations | 0 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hon, K.W.; Khan, S.U.; Htar, T.T.; Naidu, R. In Silico Analysis of Curcumin and Its Analogs MS13 and MS17 Against HSF1 and HSP Family Proteins. Chemistry 2025, 7, 139. https://doi.org/10.3390/chemistry7050139
Hon KW, Khan SU, Htar TT, Naidu R. In Silico Analysis of Curcumin and Its Analogs MS13 and MS17 Against HSF1 and HSP Family Proteins. Chemistry. 2025; 7(5):139. https://doi.org/10.3390/chemistry7050139
Chicago/Turabian StyleHon, Kha Wai, Shafi Ullah Khan, Thet Thet Htar, and Rakesh Naidu. 2025. "In Silico Analysis of Curcumin and Its Analogs MS13 and MS17 Against HSF1 and HSP Family Proteins" Chemistry 7, no. 5: 139. https://doi.org/10.3390/chemistry7050139
APA StyleHon, K. W., Khan, S. U., Htar, T. T., & Naidu, R. (2025). In Silico Analysis of Curcumin and Its Analogs MS13 and MS17 Against HSF1 and HSP Family Proteins. Chemistry, 7(5), 139. https://doi.org/10.3390/chemistry7050139








