1. Introduction
The aqueous solution of hypochlorous acid, HClO, is considered a powerful yet environmentally friendly disinfectant [
1,
2,
3]. It has been indicated for years as a biocide agent for enhancing cutaneous wound healing and in the safe treatment of eyes, nose, and ear infections. The effectiveness of hypochlorous acid is exerted by its capability of oxidizing several organic substances, including DNA and RNA, fatty acid groups, cholesterol, and proteins. For this, it can lead to the removal of many living and pathogenic microorganisms [
4]. Several studies have also demonstrated that HClO is effective in water, air, and surface sanitization or disinfection [
1].
Despite their excellent properties, these solutions can, from a chemical point of view, be aggressive for many materials, especially in the case of prolonged exposure. Reactivity is often also increased by the presence of secondary compounds. Sodium chloride can also show a synergistic action and make disinfectant solutions quite corrosive.
It should be taken into account that solutions of hypochlorites can be produced by electrolysis of aqueous sodium chloride solutions, event directly in situ, in both batch and flow processes [
5]. The HClO concentration is thus controlled by the NaCl concentration in the water; often, the amount naturally present in groundwater and drinking water (usually <200 ppm) is enough for this purpose. The main species formed by the electrolysis of an aqueous NaCl solution is HClO, an unstable compound in a pH range of 2 to 7.
Since the starting compound is sodium chloride, it is possible that residual traces of salt can be found in the sprayed product, thus leading to possible salt deposition over surfaces, in time causing subsequent eventual corrosion phenomena on many materials, particularly metals. Given the low relative acidity of the HClO solution (pK
a = 7.53 [
6]), the combined effects of NaCl presence and acidic environment could be the key factors for eventual corrosion mechanisms.
Salt solutions are an aggressive environment for many materials, including some such as stainless steels, which exhibit good corrosion resistance, but this normally occurs only at very high concentrations and for long periods and often in the presence of other substances that can act as catalysts or give rise to electrochemical processes. The corrosion rate actually depends on several factors, such as the concentration, the temperature, the pH, and the type of material. Hypochlorous acid, on the other hand, is too weak by itself to corrode most substances quickly. However, the synergistic action of these two compounds can give rise to corrosive phenomena, particularly in the case of prolonged exposure [
7].
Therefore, disinfectant solutions can represent aggressive agents for many materials, especially in the case of prolonged contact over time. Considering that they have applications in many different sectors, including hospital environments, the number of materials that can be subject to this type of corrosion is potentially very large; indeed, these solutions can reach numerous surfaces because they are often nebulized, and the resulting aerosols can have great mobility, possibly reaching points and areas normally not exposed to the action of solvents and liquids. This can lead to corrosion or at least to the alteration of materials and the degradation of their properties and functions over time.
However, these phenomena are still quite slow and can occur on the scale of years. This makes them difficult to study in the laboratory. In this case, it is possible to emulate the effects due to these mild solutions using moderately more concentrated solutions, in order to speed up the processes and be able to observe the evolution of their effects in reasonable times [
8,
9].
The aim of this work is to present an investigation, from a morphological and microscopic point of view, of the corrosion related to hypochlorous acid solutions on many different systems and materials that are used in very widespread and common activities, from the hospital environment to the electronic components in “touchscreen” systems, particularly with an in-depth study of the microstructures of such materials after interaction with these corrosive solutions.
3. Results
As stated in the introduction, hypochlorous acid (HClO) is a powerful oxidant that exhibits microbicidal activity towards a broad spectrum of microorganisms.
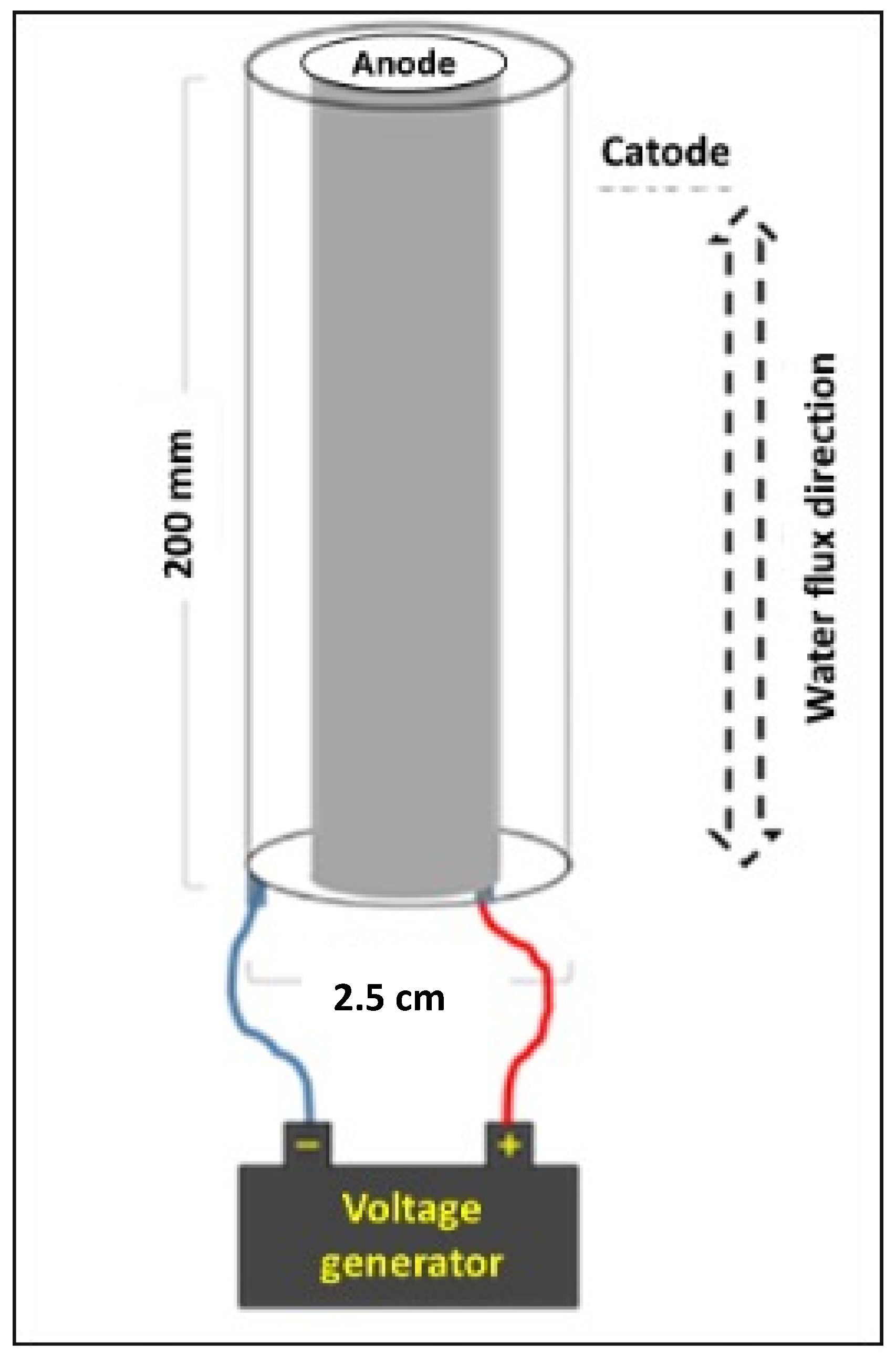
HClO can be produced as a water solution electrolyzed (EW). EW is produced by electrolysis of a sodium chloride solution. Even tap water can be used for this purpose. In the literature, different terms are reported to indicate solutions that are produced by the electrolysis of salts, such as electrochemically activated solutions (ECAs), electrolyzed oxidizing water (EOW) or simply electrolyzed water (EW).
During the electrolysis process, positively charged ions are formed, the sodium ion (Na
+) and the hydrogen ion (H
+), which move towards the cathode, where they receive electrons with the generation of sodium hydroxide (NaOH) and hydrogen (H
2). Charged negative ions that are formed, chloride ions (Cl
−) and hydroxide ions (OH
−), instead move towards the anode, where chlorine gas (Cl
2) is generated. From the hydrolysis of chlorine gas produced, they form hypochlorous acid and hypochlorite ion (OCl
−). The reactions occurring at the anode can be summarized as follows:
Figure 1 shows a schematic representation of a plant for the electrolytic production of hypochlorous acid.
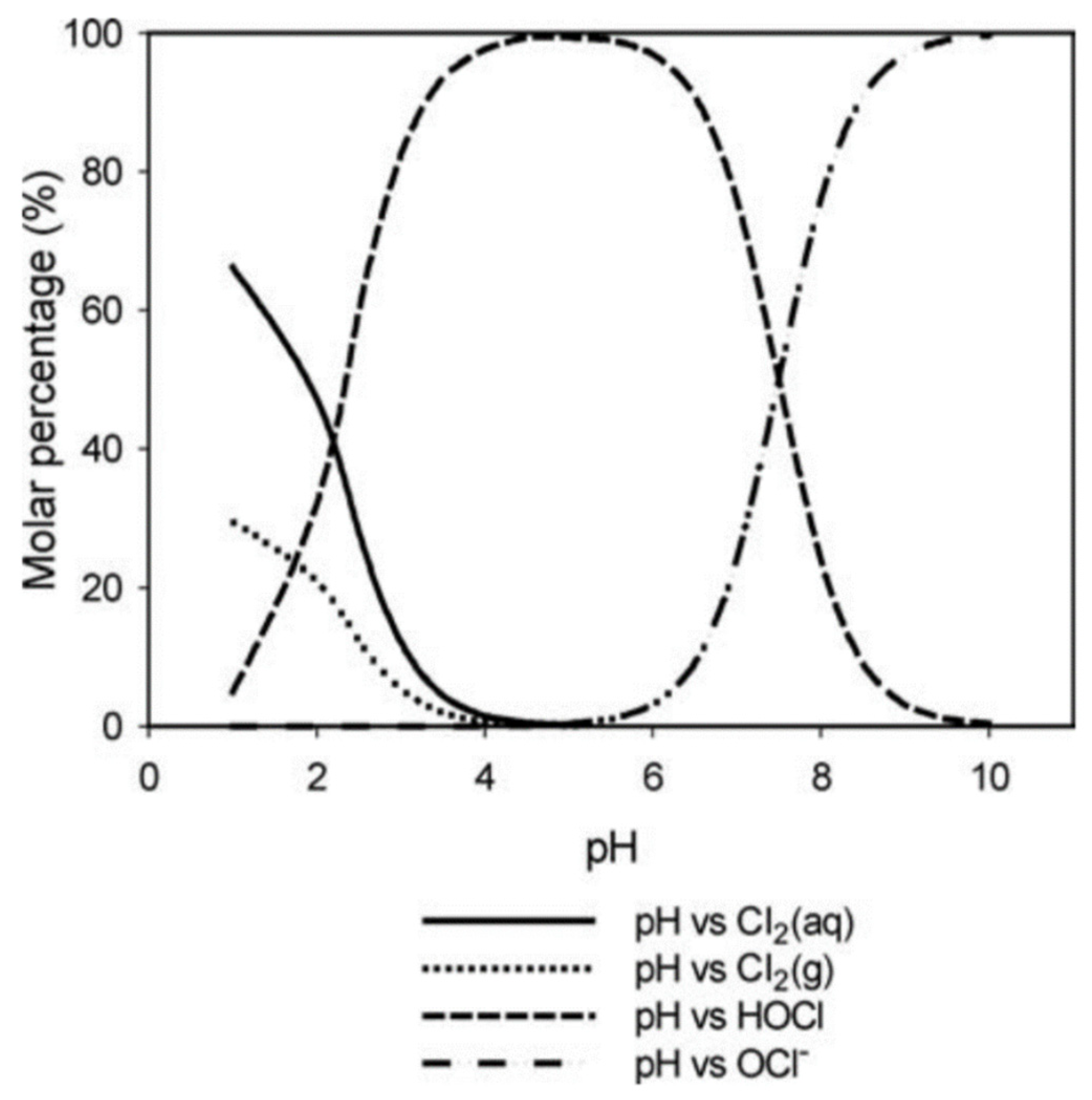
The ratio at which hypochlorous acid and hypochlorite ion are generated depends on the pH of the solution [
13]. If the solution has a pH between 3 and 7, HClO is the predominant species, while at higher pH values, the concentration of ClO
− gradually increases until it becomes the prevailing species, reaching 100% at pH 10. At pH less than 4, in addition to HClO, there is also chlorine in the aqueous phase and chlorine gas, the concentration of which increases greatly at pH values lower than 3. Hypochlorous acid at pH 6 turns out to be 98% free chlorine, and at pH 7.83%, it then decreases and is only 14% at pH 8.5 (
Figure 2).
Under exposure to the test solutions, the treated samples showed very different responses, which could reach very extensive and deep corrosion. This highlights that multiple chemical processes and mechanisms can be involved.
3.1. AISI 304
AISI 304 is the most common stainless steel and belongs to the austenitic stainless-steel family. The properties of this steel are excellent, and for this reason, it is among the most used in absolute terms. It is also very common in hospital contexts. Its corrosion resistance varies depending on the conditions to which it is exposed, but in this case, it has proven to be very stable [
14,
15].
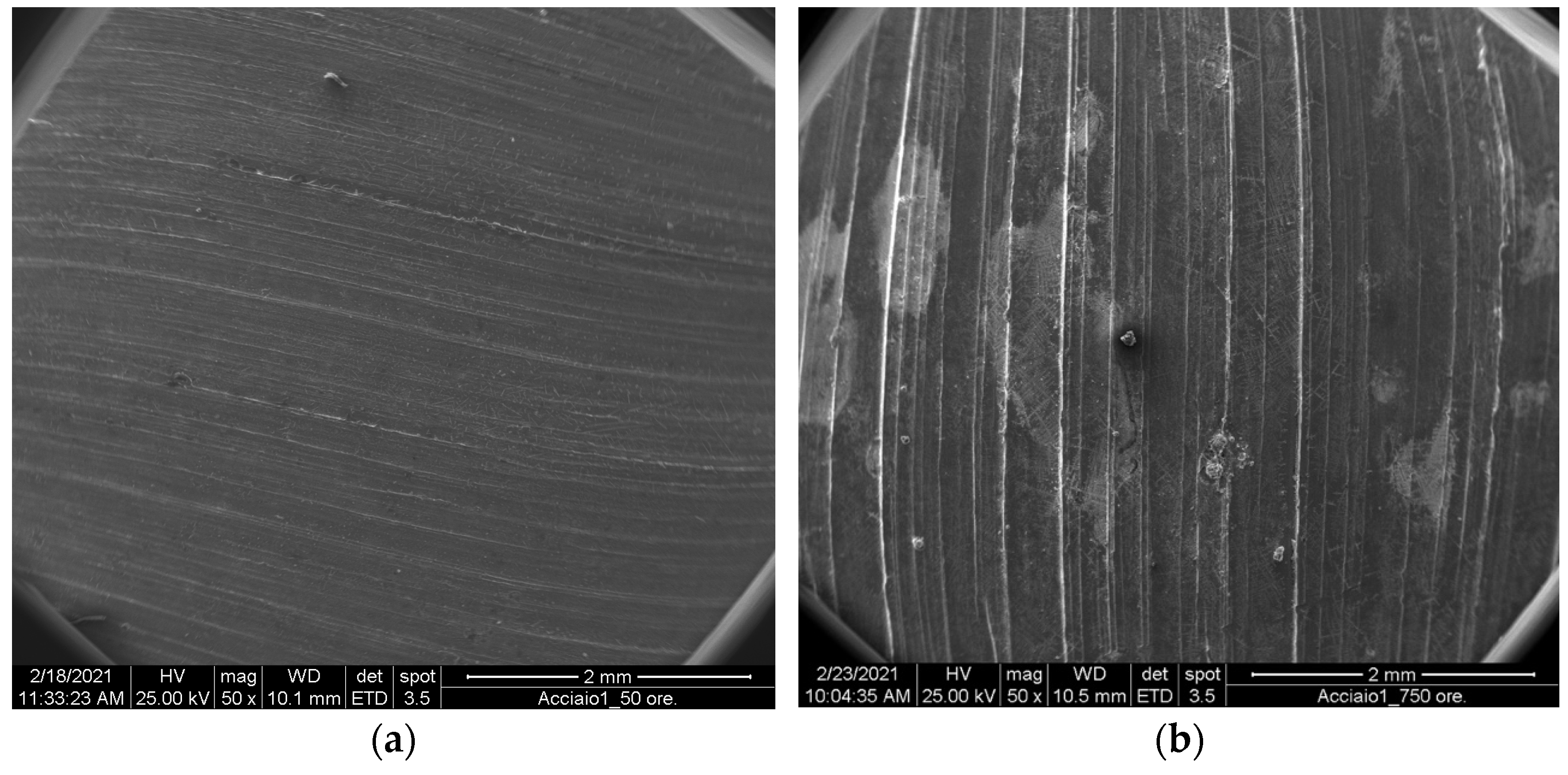
No corrosion phenomena were observed on the steel samples (
Figure 3), However, there were evident traces of salt deposits, which did not produce visible effects on the surface of the substrate. Even after washing in acetone, no damage from salt corrosion was apparent.
The appearance of a layer of salt on the surface of the samples was, however, rather interesting. Salt deposition on stainless steel AISI 304 is a complex phenomenon, influenced by multiple factors. It occurs through various mechanisms, such as hygroscopic growth, deliquescence, and efflorescence. Hygroscopic growth involves salt particles absorbing moisture from the air and adhering to the steel surface. Deliquescence occurs when salts absorb moisture, forming a solution that corrodes the steel. Efflorescence happens when salts crystallize on the steel surface due to evaporation. Several factors influence salt deposition, including environmental conditions (humidity, temperature, and pollution levels), surface roughness, and exposure duration [
16,
17].
3.2. Aluminum Alloy
Aluminum and aluminum-based alloys are materials very susceptible to corrosion by hypochlorous acid, and aluminum was certainly the material that showed the most macroscopic phenomena. After exposure, the samples appeared covered by a thick whitish and non-compact layer, probably a mixture of salt and alumina (Al2O3), a typical product of surface oxidation of non-anodized aluminum.
SEM observation allowed identification of the points at which the oxidative/corrosive phenomenon started and began to “scale” the surface of the metal. They were also visible after washing in acetone.
Corrosion mechanisms induced by salt (NaCl) could create structural damage, following different possible behaviors. Electrochemical processes: Aluminum corrosion in saltwater primarily occurs through electrochemical reactions. In a saline environment, water acts as an electrolyte, enabling the flow of electric current. The key reactions involved are the anodic oxidation of aluminum:
and the cathodic reduction of water and oxygen:
3.3. The Net Reaction Is the Formation of Aluminum Hydroxide (Al(OH)3) and the Release of Hydrogen Gas
The reduction reaction occurs at the cathode, and hypochlorous acid is a stronger oxidant than chlorine under standard conditions, having a standard potential E = 1.643 V:
pH increases the concentration of ClO−.
Microstructural observations indicate that several types of corrosion processes take place in this case, among which the most relevant appear to be pitting and galvanic corrosion, as can be observed in
Figure 4, showing the surface of an aluminum-based alloy exposed to HClO for up to 750 h.
Pitting corrosion is a severe form of localized corrosion that is particularly insidious for aluminum in salt environments. It initiates defects or imperfections on the aluminum surface that are accelerated by chloride ions, leading to the formation of small pits that can penetrate the material. The figure at a higher magnification shows some of these craters, which form in a very localized manner and, as a final consequence, show the detachment of some grains [
18,
19].
When aluminum instead comes into contact with other metals in a salt environment, galvanic corrosion can occur. This phenomenon results from the difference in the electrochemical potential between aluminum and the other metals, which drives the corrosion of the more anodic material (usually aluminum).
When considering the reference “operating room” where HClO is sprayed, Al alloys are typically not materials used in extremely critical components but rather, for example, in windows, small PC holders, etc. This is why simple operating instructions could be enough to avoid severe damage.
3.4. Copper Wire
As an element in electrical wires, connectors, and electronics, Cu was the most interesting material to test with HClO in this work. In salt-laden environments, copper is susceptible to corrosion, posing significant challenges in maintaining its integrity and functionality.
The corrosion processes can be summarized as follows:
Electrochemical Processes: Copper corrosion in saltwater primarily occurs through electrochemical reactions. In the presence of chloride ions, copper undergoes oxidation at the anode:
Meanwhile, water is reduced at the cathode, forming hydroxide ions:
These reactions lead to the formation of copper ions and corrosion products like copper oxide and copper hydroxide.
Figure 5 shows copper wires after being exposed to HClO for up to 750 h.
The final morphology that can be observed is very complex and, as in the case of aluminum, this strongly indicates that there are numerous phenomena that can take place.
Similarly to aluminum, which is, however, less noble, copper is susceptible to pitting corrosion and galvanic corrosion in salt environments. Localized imperfections or impurities on the copper surface facilitate the initiation of pits, which can lead to severe structural damage over time.
Galvanic Corrosion: When copper encounters dissimilar metals in a salt environment, galvanic corrosion can occur due to the difference in electrochemical potential. This process accelerates copper corrosion, especially when coupled with more anodic metals.
All the mechanisms mentioned above could lead to potential damage, both from a microstructural point of view and from physical and mechanic properties. Corroded copper surfaces impede electrical conductivity, affecting the performance of electrical systems and devices at the same time. The corrosion weakens the structural integrity of copper components, leading to material loss and potential failures in plumbing systems and architectural elements.
Accordingly, the observed copper specimens clearly showed a widespread layer of salt deposits, mixed with a probable layer of mixed oxides. The deposit progressively increased with exposure time, showing some lacerations/laminations in some places. NaCl crystals were easily found on the surface of corroded copper samples, as shown in
Figure 6 [
20,
21,
22,
23,
24].
3.5. Electronic Boards, Touchscreens, and Plastic Covers
The electronic board is an example of a composite sample. It is made of electronic supports, steel capacitors, electrical connection pins, and microchips [
25].
Electronic boards, also known as printed circuit boards (PCBs), are fundamental components in electronic devices, ranging from smartphones and laptops to industrial machinery. These boards consist of intricate networks of conductive pathways, which facilitate the smooth transmission of electrical signals. The choice of materials profoundly influences the efficiency, reliability, and environmental impact of electronic boards.
Types of common materials:
Substrate Materials:
FR-4 (Flame Retardant 4): The most used substrate material due to its excellent thermal and mechanical properties.
Flex PCBs: Made of flexible plastic substrates, suitable for applications requiring flexibility, such as wearable devices.
Metal-Core PCBs: Designed with metal core (aluminum or copper) for enhanced heat dissipation, ideal for high-power applications.
Conductive Materials:
Copper: Widely used for conductive traces due to its high electrical conductivity.
Gold Plating: Applied to critical connectors and switches for superior conductivity and corrosion resistance.
Silver Ink: Used in inkjet printing technologies for low-cost, flexible circuits.
Insulating Materials:
Solder Mask: Applied over conductive traces to prevent short circuits and provide mechanical support.
Silicones and Epoxies: Used for encapsulation and potting, providing insulation and protection against environmental factors.
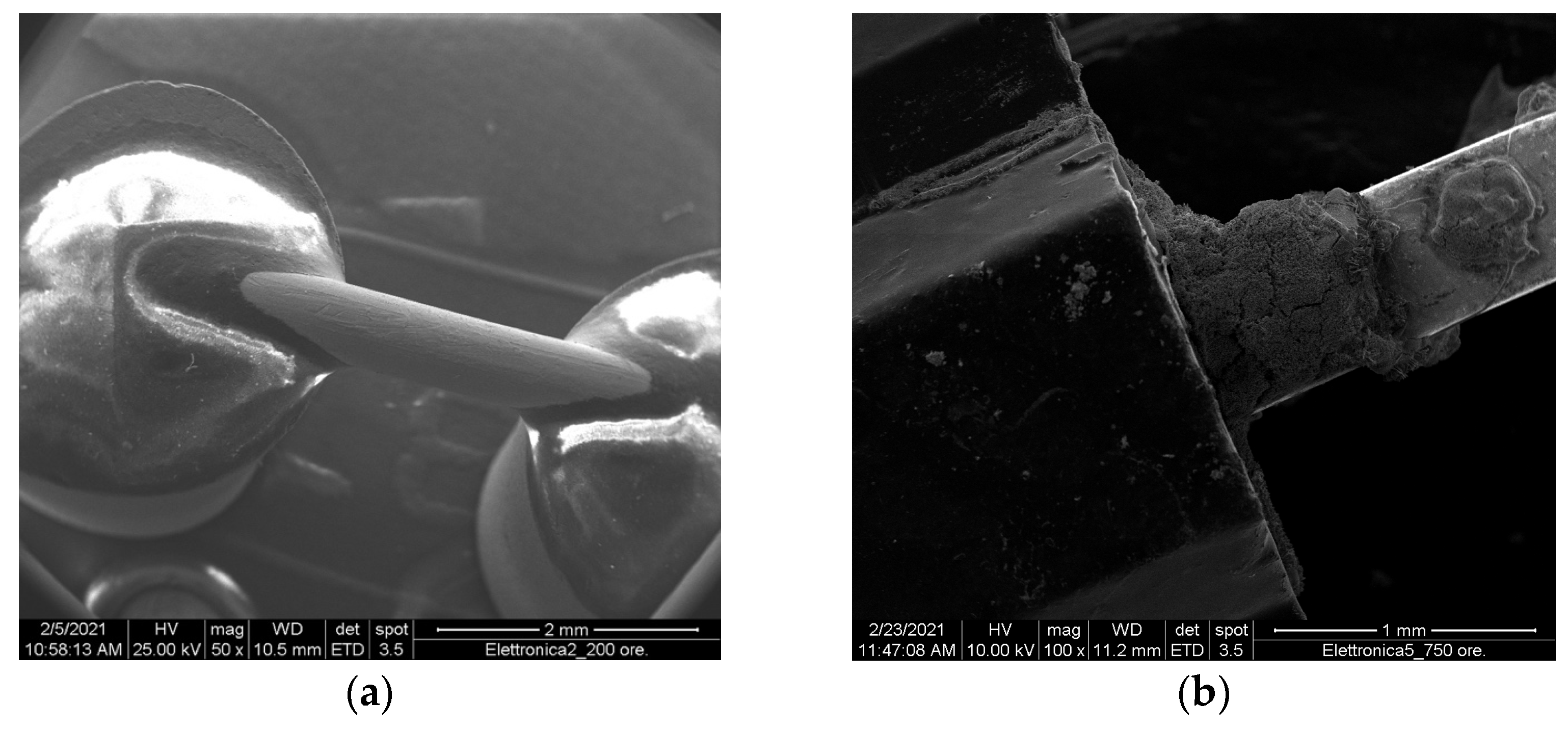
Exposure to HClO showed that the metallic connection pins were the parts that underwent salt deposition and oxidation phenomena, as expected (
Figure 7) [
26,
27].
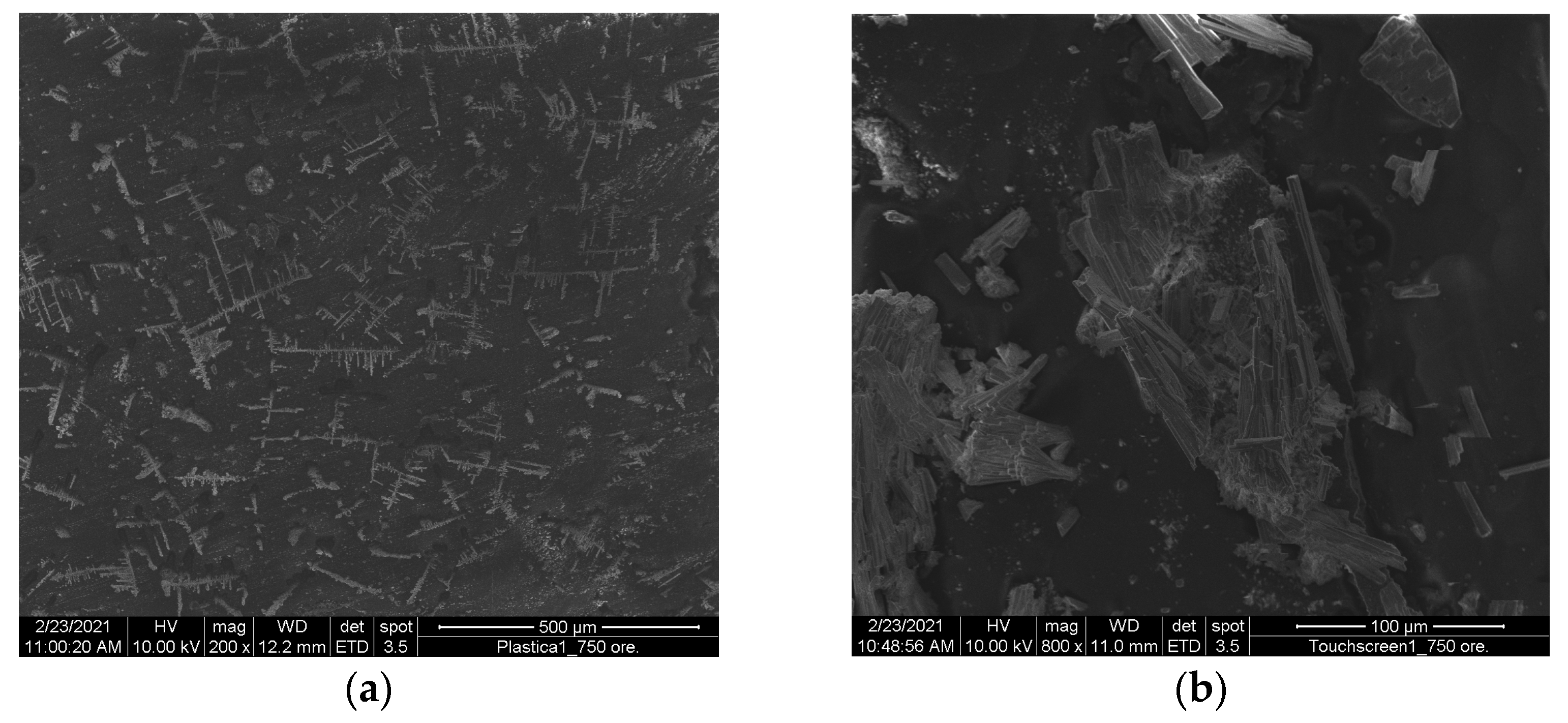
On the contrary, touchscreens are made of plastic, as are electric plug covers, and showed no signs of damage or corrosion other than sporadic salt deposits. This can be easily observed in
Figure 8.
4. Discussion
As stated before, copper represents the key problem when NaCl deposits form after HClO nebulization. It is the only reference material part of internal components inside the considered standard “operating room”.
Spray disinfectant involves a very common procedure: spray/wipe/dry. All the considered materials undergo this procedure; any unwanted NaCl unwanted deposition is then minimized. But, if the material is inside electronic equipment or electric plugs, no wiping or drying by the operator happens, and the accumulation of impurities starts.
Despite their excellent corrosion resistance in many environments, copper materials are vulnerable to corrosion when exposed to salt-containing media. Salt corrosion can compromise the structural integrity and aesthetic appeal of copper, making it imperative to understand the underlying mechanisms and devise effective corrosion mitigation strategies.
The experimental data shown in this chapter represent a screening test over reference materials [
28].
Some of the chosen materials can be regarded as “not critical”: i.e., aluminum, used for example, in windows and small tripods; stainless steel in solid structures; or plastic plates in walls. The HClO spray could indeed lead to possible damage, albeit still not troublesome in the chosen environment. On the other hand, bare metals, like Cu or connectors in electronics, can cause critical issues when corroded, leading to possible malfunctions of important instrumentation.
This is why the evidence of saline corrosion over Cu substrates is the most important information gathered from this experiment. Salt corrosion of copper occurs through electrochemical processes, primarily driven by the presence of chloride ions.
The primary mechanisms include the following:
Pitting corrosion: localized corrosion in the form of pits or holes on the copper surface.
Crevice corrosion occurs in confined spaces where stagnant electrolytes accumulate, facilitating localized corrosion [
29].
Stress corrosion cracking (SCC): A combination of tensile stress and the presence of chloride ions can induce SCC in copper.
There are several factors influencing the rate and severity of salt corrosion in copper:
Chloride concentration: Higher chloride levels in the environment increase the risk of corrosion [
13,
30].
Temperature: elevated temperatures can accelerate the corrosion rate.
Oxygen levels: The presence of oxygen is crucial for the formation of copper oxides, which protect against corrosion.
pH levels: The pH of the environment can significantly impact corrosion rates.
In the present case of HClO nebulization, the chlorine in an oxidizing acidic solution is the key factor in the depicted corrosion process over the surface of the bare metal. Pitting corrosion is the main mechanism observed.
Pitting corrosion often starts with a localized defect in the protective oxide layer on the metal’s surface. This defect can be caused by various factors, including surface contamination, mechanical damage, or imperfections in the metal’s crystal structure.
Several factors contribute to pitting corrosion:
Presence of chlorides (e.g., salt): Chloride ions, such as those found in salt (sodium chloride), are known to be particularly aggressive in causing pitting corrosion [
19,
31]. They can penetrate the protective oxide layer and facilitate the breakdown of the metal’s structure [
32].
Local variations in environment: Pitting corrosion can occur in areas where there are local variations in environmental conditions, such as differences in oxygen concentration, pH, or temperature.
Once a pit forms, it acts as a miniature electrochemical cell. In the presence of an electrolyte (e.g., saltwater, as in this case), anodic and cathodic areas are established within the pit. This leads to the dissolution of metal ions from the anodic areas and their migration to the cathodic areas, resulting in the continuous expansion of the pit. In the end, pitting corrosion can significantly compromise the structural integrity of the metal.
The corrosion process of copper in the presence of NaCl can be explained by the following electrochemical reactions:
In this process, copper (Cu) reacts with water (H2O) and oxygen (O2) in the presence of chloride ions (Cl−) to form copper ions (Cu2+) and hydroxide ions (OH−). Just like iron corrosion, copper corrosion involves the dissolution of the metal into the surrounding electrolyte.
The chloride ions play a crucial role in accelerating the corrosion of copper. They destabilize the passive film that typically forms on the copper surface, making the metal more susceptible to further corrosion. Additionally, chloride ions can catalyze the reduction of oxygen, which promotes the formation of copper ions and hydroxide ions.
Furthermore, copper corrosion products, such as copper chloride compounds, can form on the surface of the metal, leading to pitting corrosion. Pitting occurs when localized areas of the metal surface are attacked, leading to the formation of small pits or craters. This type of corrosion can significantly compromise the structural integrity of copper-based components.
On copper itself, even though not strictly part of this work, several mitigating strategies are commonly considered:
Copper can be alloyed with elements such as tin, aluminum, or nickel to improve its corrosion resistance. For example, copper-nickel alloys (Cu-Ni) have found extensive use in marine applications due to their exceptional resistance to saline corrosion.
Protective Coatings: The application of protective coatings, such as epoxy or polyurethane paints, can further shield copper from direct contact with saltwater and enhance its longevity.
Cathodic Protection: Cathodic protection systems, such as impressed current and sacrificial anodes, can be used to provide an external source of electrons, thereby preserving the integrity of copper structures by controlling the corrosion potential.
Design Considerations: Proper design and drainage systems can minimize the accumulation of stagnant saltwater, which can exacerbate corrosion.
Environmental Monitoring: Regular monitoring of environmental conditions and corrosion rates helps to identify and address issues before significant damage occurs.
“e” is the key point here: where impurities in the nebulized disinfectant cannot be forecasted or controlled well using production quality assessments, the only way to avoid damages is to physically protect the bare metals, using simple plastic covers.
Surface finishing plays a crucial role in protecting metals from corrosion, including the specific case of sodium chloride (NaCl) corrosion, which is commonly referred to as salt corrosion. The main experimental outcome of this experimental campaign was the evidence of pitting corrosion over bare metals, mostly on copper, enhanced by the presence of NaCl in the nebulized product.
The corrosion behavior, mainly consisting of the formation of mixed compositions of scales and layers over the surface, is thus strictly linked to the type of surface finish.
As an example, steel tripods or aluminum alloy fixtures are usually machined and polished into glossy materials. The corrosion resistance of such substrates is enhanced more using anodizing or chroming processes.
On the other hand, Cu electric wires or bare metal conductors/connectors inside electronics are not surface machined or modified, leaving the porous interface exposed to degradation and corrosion. Coupling this physical macroscopic fact with their electronegative properties, the occurrence of pitting corrosion must be regarded as expected.
Common approaches for the mitigation of such processes include the following:
Material Selection: Choosing corrosion-resistant alloys, such as stainless steels with higher chromium and molybdenum content, can significantly reduce the susceptibility to pitting corrosion.
Protective Coatings: Applied protective coatings, such as paints or specialized corrosion-resistant coatings, can act as a barrier against chloride ions.
Proper Design: Designing structures and components in a way that avoids crevices and areas where stagnant solutions can accumulate helps prevent pitting corrosion.
Controlled Environment: Limiting the exposure of susceptible metals to chloride-rich environments, especially in applications like industrial equipment and pipelines, can mitigate pitting corrosion [
33,
34].
Of course, in the present case, only the last of the above methods is applicable.
It has to be underlined, as stated in the introduction, that electrochemical processes enhance pitting corrosion. In the case of Cu electric wire, this is the case.
In this work, only the simple compatibility test was assessed. However, it is still a suitable screening method for inclusion in an operator manual of good practices.
Performing accelerated exposure tests with a water solution and a 220 v current attached is beyond the technical capability of our facilities.