Abstract
Parasitic nematodes, such as the zoonotic rat lungworm Angiostrongylus cantonensis, pose a significant global health burden, with current anthelmintics like albendazole showing limited efficacy. Here, we report the isolation of piplartine from Piper truncatum Vell. (Piperaceae) and its potent in vitro activity against A. cantonensis larvae. Piplartine demonstrated superior efficacy to albendazole, with EC50 values of 8.3 µM for first-stage larvae (L1) and 10.4 µM for infective third-stage larvae (L3), compared to 14.2 µM (L1) and 15.6 µM (L3) for albendazole. Notably, piplartine exhibited no toxicity in the Caenorhabditis elegans model at therapeutic concentrations, underscoring its selective antiparasitic action. In silico profiling further revealed favorable drug-likeness and pharmacokinetic properties, including high gastrointestinal absorption and blood–brain barrier permeability, which are critical for targeting neurotropic infections. As the first study to characterize the activity of piplartine against A. cantonensis, our work highlights its potential as a structurally novel anthelmintic lead. Based on the obtained results, piplartine may be considered a promising and accessible candidate for combating angiostrongyliasis and related helminthic infections.
1. Introduction
Parasitic nematodes impose a staggering burden on global health, infecting nearly one billion people worldwide and disproportionately impacting marginalized communities in tropical and subtropical regions [1]. Among these pathogens, Angiostrongylus cantonensis—the rat lungworm—has emerged as a zoonotic threat of growing concern [2,3]. This parasite has a complex life cycle involving rats as definitive hosts and gastropods (e.g., snails and slugs) as intermediate hosts. Adult worms reside in the pulmonary arteries of rats, where females lay eggs that hatch into first-stage larvae (L1) within the pulmonary vasculature. These larvae migrate to the pharynx, are swallowed, and then excreted in the feces. After being ingested by or penetrating gastropod intermediate hosts, the larvae undergo two molts, developing into third-stage larvae (L3), which are infective to mammals. In accidental hosts, including humans, L3 migrate to the central nervous system, causing eosinophilic meningitis—a severe neurotropic disease that can lead to permanent neurological damage or death [2]. Although L3 are the principal infective stage, larvae may continue to develop to L4 or even L5 in humans, although they are unable to reach full maturity. Paratenic hosts, such as freshwater shrimp and crabs, can also harbor L3. Human infections primarily occur through the ingestion of raw or undercooked gastropods or contaminated produce, a route amplified by climate change and globalized food distribution networks [2,4]. Despite its rising public health significance, treatment remains suboptimal. Current anthelmintics such as albendazole, a benzimidazole derivative, exhibit limited efficacy against larval stages of A. cantonensis and pose risks of resistance development in helminths [5]. This unmet therapeutic need underscores the urgency of discovering novel anthelmintics with distinct mechanisms of action [6,7].
Natural products have long served as a cornerstone of antiparasitic drug discovery, exemplified by ivermectin, a bacterial-derived macrocyclic lactone isolated from Streptomyces sp., and artemisinin, an endoperoxide sesquiterpenoid isolated from Artemisia annua [8]. The genus Piper (Piperaceae), widely used in traditional medicine to treat parasitic infections, is a prolific source of bioactive compounds including benzoic acids, lignoids, terpenoids, flavonoids, and amides [9]. Among these compounds, piplartine (piperlongumine)—a secondary metabolite abundant in different Piper species including P. tuberculatum [10], P. pseudoarboreum [11], P. chaba [12], and P. retrofractum [13]—has garnered attention for its anticancer, anti-inflammatory, and antiparasitic properties [14]. Studies report that piplartine is among the major constituents in some Piper species, with concentrations reaching up to 0.5–1.5% of dry weight depending on the species and part used [15]. While the activity of piplartine against protozoans (e.g., Leishmania spp. [16] and Plasmodium falciparum [10]) and flatworms (e.g., Schistosoma mansoni [17,18]) is well-documented, its potential against parasitic nematodes remains largely unexplored.
Here, we report the first investigation of piplartine efficacy against A. cantonensis. Isolated from P. truncatum, piplartine was evaluated for in vitro activity against first-stage (L1) and infective third-stage (L3) larvae. Its potency was benchmarked against albendazole, and its selectivity was assessed using the free-living nematode Caenorhabditis elegans as a toxicity model. Computational profiling further revealed favorable drug-likeness properties, including compliance with Lipinski’s Rule of Five, high gastrointestinal absorption, and blood–brain barrier permeability. Our findings demonstrate that piplartine outperformed albendazole in targeting both larval stages of A. cantonensis while exhibiting no toxicity in C. elegans, positioning it as a structurally novel, sustainable, and selective lead compound for combating angiostrongyliasis.
2. Materials and Methods
2.1. General Experimental Procedures
Silica gel (Merck, 230–400 mesh) and Sephadex LH-20 (GE Healthcare, Chicago, IL, USA) were used for column chromatography (CC), whereas silica gel 60 PF254 (Merck, Darmstadt, Germany) was used for analytical TLC separations. HPLC chromatography separations were performed on an Ultimate 3000 system (Dionex, Sunnyvale, CA, USA) equipped with a quaternary pump system, a PDA detector, and a Phenomenex reversed-phase C18 column (250 mm × 10.0 mm, 5 μm). NMR spectra were recorded on a Varian (Palo Alto, CA, USA) INOVA spectrometer, operating at 500 MHz and 125 MHz to 1H and 13C nuclei, respectively. CDCl3 and TMS (both from Sigma-Aldrich, St. Louis, MI, USA) were used as the solvent and as the internal standard, respectively.
2.2. Plant Material
The twigs of Piper truncatum Vell. were collected in Petropolis, Rio de Janeiro State, Brazil, in September/2020 (registration code at SISGEN A4123E4). The plant material was identified by Prof. Dr. Guilherme M. Antar and a voucher specimen (Antar 3098) was deposited in the Herbarium of the Institute of Biosciences, University of São Paulo, SP, Brazil.
2.3. Extraction and Isolation of Piplartine
Dried and powdered twigs of P. truncatum (254 g) were exhaustively extracted using hexane at room temperature. After removal of the solvent under reduced pressure, 1.5 g of crude extract was obtained. Part of this material (1.4 g) was subjected to a silica gel column eluted with increasing amounts of EtOAc in hexane to obtain eight groups (A–H). Part of group F (150 mg) was chromatographed over Sephadex LH-20, eluted with hexane:CH2Cl2 (1:4), CH2Cl2:acetone (3:2 and 1:4), and 100% acetone to afford nine groups (F1–F9). Group F4 (41 mg) was purified by semi-preparative C18 HPLC, eluted with MeOH:H2O 7:3, to afford 35 mg of pure piplartine.
Piplartine. White amorphous solid. 1H NMR (CDCl3, 500 MHz) δ 7.67 (d, J = 15.5 Hz, H-3), 7.43 (d, J = 15.5 Hz, H-2), 6.94 (m, H-2′), 6.80 (s, H-5 and H-9), 6.05 (dt, J = 9.8 and 1.9 Hz, H-3′), 4.04 (t, J = 6.5 Hz, H-5′), 3.89 (s, 6-OMe and 8-OMe), 3.87 (s, 7-OMe), 2.48 (ddt, J = 6.3, 4.1 and 1.9 Hz, H-4′), 13C NMR (CDCl3, 125 MHz) δ 168.9 (C-1), 165.8 (C-1′), 153.3 (C-6 and C-8), 145.5 (C-3′), 143.8 (C-4), 139.9 (C-7), 130.6 (C-3), 125.8 (C-2′), 121.0 (C-2), 105.5 (C-5 and C-9), 60.9 (7-OMe), 56.1 (6-OMe and 8-OMe), 41.6 (C-5′), 24.8 (C-4′).
2.4. Parasite and Animal Maintenance
The Angiostrongylus cantonensis (NPDN-AC strain) life cycle was sustained in laboratory conditions at the Research Center for Neglected Diseases, Guarulhos University. Definitive hosts (Wistar rats, Rattus norvegicus) and intermediate hosts—freshwater snails (Biomphalaria glabrata) or giant African land snails (Achatina fulica)—were housed in controlled environments (22 ± 1 °C, 50–60% humidity) with unrestricted access to food and water. The free-living nematode Caenorhabditis elegans (Bristol N2 strain) was cultured on nematode growth medium (NGM) agar plates seeded with Escherichia coli OP5047 as a nutrient source [19].
2.5. Antiparasitic Activity Against A. cantonensis L1
First-stage larvae (L1) were collected from fecal samples of infected Wistar rats using the Rugai sedimentation method [20]. Larvae were washed three times in RPMI 1640 medium supplemented with 100 U/mL penicillin and 100 µg/mL streptomycin. Approximately 50 L1 were allocated into individual wells of a 96-well plate containing 200 µL of final volume of RPMI medium [21]. Piplartine and albendazole were dissolved in DMSO (final concentration ≤ 0.5%) and tested at serial dilutions starting from 100 µM. Plates were incubated at 21 °C for 24 h, and larval motility was assessed microscopically using a four-tiered classification system: immobile, intermittent, slow, or highly active. A compound was deemed effective if ≥60% of larvae showed complete immobility [22].
2.6. Antiparasitic Activity Against A. cantonensis L3
Infective third-stage larvae (L3) were harvested from experimentally infected A. fulica snails through pepsin-HCl digestion (1% pepsin in 0.7% HCl, 37 °C, 2 h) [23], followed by Rugai sedimentation [20]. Approximately 50 L3 were transferred to each well of a 96-well plate containing RPMI 1640 medium. Piplartine and albendazole were tested as described for L1. Larval viability was monitored at 0 h and 24 h post-treatment using an inverted microscope (100× magnification). Motility scoring mirrored the L1 assay criteria.
2.7. Selectivity Assessment in C. elegans
Synchronized L4-stage C. elegans larvae were distributed into 96-well plates containing M9 buffer (60 larvae/well). Piplartine was evaluated at concentrations up to 1000 µM, with albendazole as the positive control and 0.5% DMSO as the vehicle control [24]. After 24 h incubation at 21 °C, larval survival was determined by motility: larvae unresponsive to gentle mechanical stimulation were classified as nonviable. Toxicity was defined as ≥ 60% immobility [25].
2.8. Computational ADME Profiling
Piplartine drug-likeness and pharmacokinetic properties were predicted using the SwissADME web tool [26]. The compound structure was drawn in Marvin JS (ChemAxon), exported as a SMILES string, and analyzed for compliance with Lipinski’s Rule of Five, Veber’s bioavailability criteria, and pan-assay interference (PAINS) alerts. Key parameters included gastrointestinal absorption and blood–brain barrier permeability.
2.9. Statistical Analysis
Dose–response curves were generated in GraphPad Prism 8.0 (GraphPad Software, Boston, MA, USA) using a four-parameter logistic regression model to calculate EC50 (antiparasitic activity) and LD50 (toxicity) values [27]. Statistical significance between treatment groups was determined by one-way ANOVA with Tukey’s post hoc test (p < 0.05).
2.10. Ethical Compliance
All animal experiments were approved by the Ethics Committee of Guarulhos University (Protocol #064/24) and conducted in accordance with Brazilian national guidelines for animal welfare (CONCEA).
3. Results
3.1. Chemical Characterization of Piplartine
Piplartine was isolated as a white amorphous solid (100% purity by HPLC) from P. truncatum, and its structure, shown in Figure 1, was proposed by analysis of 1H and 13C NMR spectral data (see Supplementary Materials) followed by comparison with those previously reported [10].
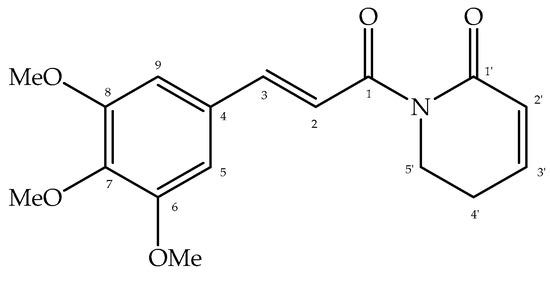
Figure 1.
Structure of piplartine, isolated from twigs of P. truncatum.
3.2. Anthelmintic Activity Against A. cantonensis Larvae
Piplartine demonstrated potent in vitro efficacy against both larval stages of A. cantonensis, outperforming the reference drug albendazole (Table 1 and Figure 2). For first-stage larvae (L1), piplartine exhibited an EC50 of 8.3 ± 0.9 µM, significantly lower than albendazole’s EC50 of 14.2 ± 1.1 µM (p < 0.01). Similarly, against third-stage larvae (L3), piplartine achieved an EC50 of 10.4 ± 0.7 µM, nearly 1.5-fold more potent than albendazole (EC50 = 15.6 ± 1.2 µM; p < 0.01) (Table 1). These results underscore piplartine’s superior anthelmintic activity across critical developmental stages of A. cantonensis.

Table 1.
In vitro anthelmintic activity of piplartine and albendazole against Angiostrongylus cantonensis larvae (L1 and L3) and toxicity in Caenorhabditis elegans.
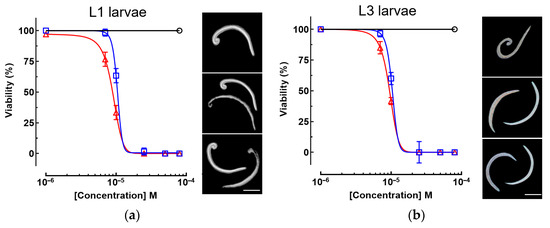
Figure 2.
In vitro anthelmintic activity of piplartine and albendazole against Angiostrongylus cantonensis larvae. (a) L1: dose-dependent viability and morphological alterations. (b) L3: dose-dependent viability and morphological alterations. Viability curves represent piplartine (red line), albendazole (blue line), and untreated control (black line). Morphological images (right panels) display untreated control, albendazole-exposed, and piplartine-exposed larvae (top to bottom, respectively). L1 are excreted in the feces of the definitive host and initiate infection in the intermediate host, whereas L3 represent the infective stage for humans. Data are expressed as mean ± standard deviation (SD) of three independent experiments.
Consistent with these potency metrics, piplartine induced a concentration-dependent reduction in larval viability, with near-complete immobility observed at concentrations as low as 10 µM for L1 (Figure 2a). In contrast, albendazole required higher concentrations to achieve comparable effects, further highlighting piplartine enhanced efficacy. Similar trends were observed for L3, for which piplartine dose–response curve demonstrated steeper declines in viability compared to albendazole (Figure 2b). In both stages, larvae exposed to piplartine or albendazole exhibited pronounced morphological alterations—particularly in the caudal region—including shrinkage and surface disruption (Figure 2).
3.3. Selectivity and Safety Profile
Piplartine displayed remarkable selectivity, showing no toxicity toward the free-living nematode C. elegans at concentrations up to 500 µM (LD50 > 500 µM). In contrast, albendazole exhibited significant toxicity in C. elegans (LD50 = 14.8 ± 1.5 µM), highlighting piplartine’s > 34-fold wider therapeutic window (LD50/EC50 ratio) compared to the reference drug (Table 1). This selectivity suggests that the mechanism of action of piplartine preferentially targets parasitic nematodes.
3.4. In Silico ADME and Drug-Likeness Profiling
Computational analysis using SwissADME positioned piplartine within ideal ranges for key physicochemical parameters, including lipophilicity (LogP = 1.96), polarity (TPSA = 65.07 Å2), and solubility (LogS = −2.91), as visualized in the bioavailability radar (Figure 3a). These properties align with high oral bioavailability, further supported by predicted gastrointestinal absorption (94%) and blood–brain barrier (BBB) permeability (Figure 3b), a critical feature for targeting neurotropic parasites like A. cantonensis. Piplartine was not identified as a substrate for P-glycoprotein (P-gp), reducing risks of drug efflux and resistance.
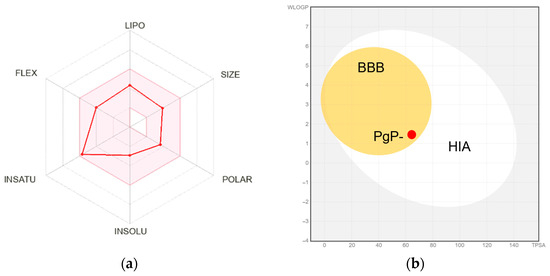
Figure 3.
Drug-likeness and pharmacokinetic profiling of piplartine. (a) Bioavailability radar analysis of piplartine, depicting key physicochemical properties (lipophilicity, molecular size, polarity, solubility, flexibility, and saturation) within ideal ranges for oral bioavailability. (b) Dual evaluation of passive gastrointestinal absorption (HIA) and blood–brain barrier (BBB) permeability. The white region represents a high probability of passive gastrointestinal absorption (HIA), while the yellow region (yolk) indicates high BBB penetration potential. The red data point reflects piplartine classification as a non-substrate of P-glycoprotein (PgP-). Predictions were based on the molecule’s position in the WLOGP (lipophilicity) vs. TPSA (topological polar surface area) reference space.
Piplartine adhered to all major pharmaceutical drug-likeness criteria, including Lipinski’s Rule of Five, as well as Ghose, Veber, Egan, and Muegge filters, confirming its suitability as a lead compound (Figure 4). The absence of PAINS alerts suggests a low risk of nonspecific interactions, while a single Brenk alert highlighted the α,β-unsaturated ketone moiety as a Michael acceptor—a structural feature linked to covalent target binding. While this reactivity may contribute to the antiparasitic activity of piplartine, it warrants further structural optimization to balance efficacy and safety.
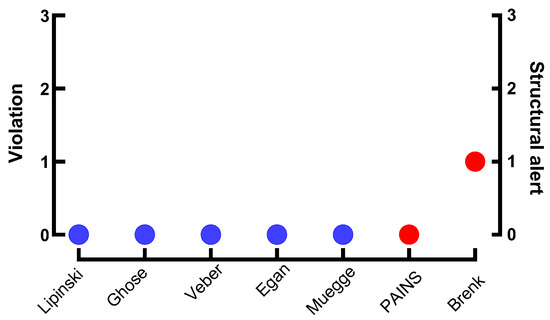
Figure 4.
Drug-likeness compliance with established pharmaceutical criteria (blue): Lipinski (Pfizer), Ghose (Amgen), Veber (GSK), Egan (Pharmacia), and Muegge (Bayer). Blue indicates adherence to all five criteria. Structural risk assessment (red) using PAINS (pan-assay interference compounds) and Brenk (structural toxicity alerts) filters. A Brenk alert identifies a Michael acceptor moiety, a structural feature associated with potential electrophilic reactivity and off-target effects.
4. Discussion
The growing burden of zoonotic helminthiasis, coupled with the limitations of current anthelmintics, underscores the urgent need for novel therapeutics [6,28]. In this study, piplartine—a bioactive amide isolated from P. truncatum and other Piper species [10,11,12,13]—emerged as a potent and selective agent against A. cantonensis, out-performing the reference drug albendazole while demonstrating no toxicity in the free-living nematode C. elegans. EC50 values of 8.3 µM (L1) and 10.4 µM (L3) for piplartine represent 1.7- and 1.5-fold improvements, respectively, over albendazole, positioning it as one of the most promising natural-product-derived anthelmintics reported to date. Notably, its exceptional selectivity profile (LD50 > 500 µM in C. elegans) contrasts sharply with albendazole’s toxicity (LD50 = 14.8 µM), suggesting a mechanism of action that preferentially targets parasitic nematodes. This selectivity may arise from structural differences in piplartine molecular targets, such as β-tubulin isoforms or redox-regulatory proteins, which are known to vary between parasitic and free-living nematodes [6].
The genus Piper has long been recognized as a reservoir of antiparasitic compounds, particularly against flatworms. For example, piplartine isolated from P. tuberculatum exhibits potent activity against Schistosoma mansoni by inducing tegumental damage and impairing egg viability [17]. Cardamonin, a chalcone isolated from P. aduncum, exhibits schistosomicidal effects through the inhibition of ATP diphosphohydrolase [29]. Expanding this pharmacopeia further, uvangoletin, a dihydrochalcone from P. aduncum, disrupts schistosome viability, while lignans from P. cubeba exhibit marked antischistosomal effects by inhibiting egg production [30]. However, until now, the anthelmintic potential of Piper-derived compounds against parasitic nematodes remained largely unexplored. Our findings bridge this gap, revealing piplartine unique efficacy against A. cantonensis, a phylogenetically distant roundworm, and underscoring the broader therapeutic potential of Piper amides across helminth classes.
The computational profiling of piplartine further supports its therapeutic potential. SwissADME predictions revealed favorable physicochemical properties, including optimal lipophilicity and topological polar surface area, aligning with high gastrointestinal absorption and blood–brain barrier permeability. The latter is particularly significant given A. cantonensis’ neurotropic behavior in accidental hosts [2,5]. Piplartine compliance with all major drug-likeness criteria (Lipinski, Ghose, and Veber) and absence of PAINS alerts further validate its suitability as a lead compound. However, the identification of a Michael acceptor moiety via Brenk analysis warrants caution, as this electrophilic group may confer reactivity toward host proteins. Interestingly, such moieties are also implicated in the antiparasitic activity of other natural products, such as plumbagin and thymoquinone [31,32], suggesting a potential dual role in efficacy and off-target effects.
The superior efficacy of piplartine over albendazole may reflect differences in their mechanisms of action. While albendazole primarily disrupts microtubule polymerization by binding to β-tubulin [6], the presence of an α,β-unsaturated carbonyl system in the structure of piplartine could induce oxidative stress or inhibit redox-sensitive enzymes like thioredoxin reductase—a mechanism reported in its anticancer and anti-Leishmania activity [14,33]. Indeed, several studies have shown that piplartine induces oxidative stress and genotoxicity selectively in cancer cells, while sparing normal tissues [34,35,36]. This oxidative mechanism may similarly underlie its selective toxicity against parasitic nematodes. In support of this, biomimetic oxidation studies suggest that piplartine is metabolized at both its lactam ring and trimethoxyphenyl moiety by cytochrome P450-like enzymes, which may contribute to its bioactivation [36,37]. Furthermore, piplartine lack of P-glycoprotein substrate status may reduce the likelihood of resistance, a critical advantage over albendazole in regions where benzimidazole resistance is emerging [38,39]. These observations warrant further investigation to delineate the precise targets and pathways involved in helminthicidal activity. This includes preparing derivatives that modify the α,β-unsaturated carbonyl system to reduce potential electrophilicity while preserving piplartine anthelmintic efficacy.
Toxicological and pharmacokinetic data available in the literature support the potential safety and druggability of piplartine. Acute toxicity studies in rats and mice have shown no significant clinical signs at doses up to 3000 mg/kg orally. Moreover, subacute toxicity evaluations in healthy mice treated with 50 mg/kg for 7 days revealed no significant alterations in hematological or biochemical parameters, and only mild, reversible renal histopathology [36]. These findings suggest a favorable therapeutic window. Our results in C. elegans further support this profile, as piplartine exhibited no toxicity at high concentrations, reinforcing its selective antiparasitic action. In pharmacokinetic studies, piplartine demonstrated excellent oral bioavailability in mice (up to 76.4%), with a relatively short half-life ranging from 0.84 to 1.6 h depending on the route and dose [35,36]. These data are consistent with the in silico predictions of good gastrointestinal absorption and suggest that oral administration may be feasible in future in vivo efficacy studies.
Despite these advances, several challenges remain. First, while in silico and preliminary in vivo data suggest oral bioavailability and safety, additional pharmacokinetic and toxicity studies are needed to confirm absorption, metabolism, and tissue distribution, particularly in the context of neurotropic infections. Second, the structural alert associated with a Michael acceptor in the structure of piplartine necessitates medicinal chemistry optimization to mitigate potential toxicity without compromising efficacy.
5. Conclusions
In conclusion, the results of the present study demonstrated the anthelmintic activity against the zoonotic nematode A. cantonensis of piplartine isolated from P. truncatum. In vitro assays demonstrated that piplartine showed superior efficacy to albendazole and exhibited no toxicity in the C. elegans model. Therefore, this work identifies piplartine as a naturally derived anthelmintic compound with dual advantages—bioactivity rooted in traditional medicine and computational validation of drug-likeness—highlighting the enduring value of ethnopharmacology in modern drug discovery. Future studies should prioritize in vivo efficacy validation, mechanistic elucidation, and structural refinement to advance piplartine toward clinical development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry7040105/s1, Figure S1: 1H NMR spectrum of piplartine; Figure S2: 13C NMR spectrum of piplartine.
Author Contributions
L.F.-S., S.C.S., B.L.L., C.S.A., M.M.G., J.d.M., and J.H.G.L., data curation, visualization, investigation, writing—original draft preparation; J.d.M. and J.H.G.L., conceptualization, methodology, writing, reviewing, and editing the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP (grants 23/08418-6 and 23/12447-1). J.d.M. and J.H.G.L. also received the Established Investigator Fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (Brazil). E.U. and R.A.C. received a post-graduate fellowship from the Coordenação de Aperfeiçoa-mento de Pessoal de Nível Superior—CAPES (Finance Code 001, Brazil).
Data Availability Statement
Data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the Multiuser Central Facilities at UFABC for their experimental support. We are also grateful to Mariana B. Silva for her valuable technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADME | Absorption, Distribution, Metabolism, and Excretion |
| BBB | Blood–Brain Barrier |
| CC | Column Chromatography |
| CDCl3 | Deuterated Chloroform |
| CONCEA | Conselho Nacional de Controle de Experimentação Animal/National Council for the Control of Animal Experimentation |
| d | Doublet |
| dd | Double-Doublet |
| ddt | Double-Double-Triplet |
| DMSO | Dimethylsulfoxide |
| EC50 | Effective Concentration at 50% |
| EtOAc | Ethyl Acetate |
| GSK | GlaxoSmithKline |
| HIA | Passive Gastrointestinal Absorption |
| HPLC | High Performance Liquid Chromatography |
| J | Coupling Constant |
| L1 | First-Stage Larvae |
| L3 | Infective Third-Stage Larvae |
| LD50 | Lethal Dose 50% |
| m | Multiplet |
| MeOH | Methanol |
| MHz | Megahertz |
| mM | Micromolar |
| NGM | Nematode Growth Medium |
| NMR | Nuclear Magnetic Resonance |
| PAINS | Pan-Assay Interference |
| PDA | Photodiode Array |
| P-gp | P-Glycoprotein |
| RPMI | Roswell Park Memorial Institute |
| s | Singlet |
| SISGEN | Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado/National System For The Management of Genetic Heritage and Associated Traditional Knowledge |
| SMILES | Simplified Molecular Input Line Entry System |
| t | Triplet |
| TLC | Thin Layer Chromatography |
| TMS | Tetramethyl Silane |
| TPSA | Topological Polar Surface Area |
| WLOGP | Wildman Lipophilicity |
References
- King, C.H. Helminthiasis Epidemiology and Control: Scoring Successes and Meeting the Remaining Challenges. Adv. Parasitol. 2019, 103, 11–30. [Google Scholar] [PubMed]
- Griffin, C.D.; Ezenwa, V.O.; Cowie, R.H. Insights into the Biology of the Rat Lungworm, Angiostrongylus cantonensis. Parasites Vectors 2025, 18, 163. [Google Scholar] [CrossRef]
- Tian, X.; Chen, S.; Duan, L.; Qian, Y.; Li, H.; Lv, S. The Global Spread Pattern of Rat Lungworm Based on Mitochondrial Genetics. Pathogens 2023, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.J.; Gilotra, T.S.; Nguyen, A.D.; Lui, F. Angiostrongyliasis; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Jacob, J.; Steel, A.; Howe, K.; Jarvi, S. Management of Rat Lungworm Disease (Neuroangiostrongyliasis) Using Anthelmintics: Recent Updates and Recommendations. Pathogens 2022, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Mengarda, A.C.; Silva, T.C.; Silva, A.S.; Roquini, D.B.; Fernandes, J.P.S.; de Moraes, J. Toward Anthelmintic Drug Candidates for Toxocariasis: Challenges and Recent Developments. Eur. J. Med. Chem. 2023, 251, 115268. [Google Scholar] [CrossRef]
- Shang, X.; Dai, L.; Cao, X.; Ma, Y.; Gulnaz, I.; Miao, X.; Li, X.; Yang, X. Natural products in antiparasitic drug discovery: Advances, opportunities and challenges. Nat. Prod. Rep. 2025. [Google Scholar] [CrossRef]
- Moraes, J.; Lago, J.H.G. Natural Products as Lead Compounds for Treatment of Neglected Tropical Diseases: Dream or Reality? Future Med. Chem. 2022, 14, 1607–1609. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef]
- Araújo-Vilges, K.M.; Oliveira, S.V.; Couto, S.C.P.; Fokoue, H.H.; Romero, G.A.S.; Kato, M.J.; Romeiro, L.A.S.; Leite, J.R.S.A.; Kuckelhaus, S.A.S. Effect of Piplartine and Cinnamides on Leishmania amazonensis, Plasmodium falciparum and on Peritoneal Cells of Swiss Mice. Pharm. Biol. 2017, 55, 1601–1607. [Google Scholar] [CrossRef]
- Ticona, J.C.; Bilbao-Ramos, P.; Flores, N.; Dea-Ayuela, M.A.; Bolás-Fernàndez, F.; Jiménez, I.A.; Bazzocchi, I.L. (E)-Piplartine Isolated from Piper pseudoarboreum, a Lead Compound Against Leishmaniasis. Foods 2020, 9, 1250. [Google Scholar] [CrossRef]
- Jyothi, D.; Vanathi, P.; Gowri, P.M.; Rao, V.R.S.; Rao, J.M.; Sreedhar, A.S. Diferuloylmethane Augments the Cytotoxic Effects of Piplartine Isolated from Piper chaba. Toxicol. Vitr. 2009, 23, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Bodiwala, H.S.; Singh, G.; Singh, R.; Dey, C.S.; Sharma, S.S.; Bhutani, K.K.; Singh, I.P. Antileishmanial Amides and Lignans from Piper cubeba and Piper retrofractum. J. Nat. Med. 2007, 61, 418–421. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K. Historical Spice as a Future Drug: Therapeutic Potential of Piperlongumine. Curr. Pharm. Des. 2016, 22, 4151–4159. [Google Scholar] [CrossRef]
- Parmar, V.S.; Jain, S.C.; Bisht, K.S.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.D.; Prasad, A.K.; Wengel, J.; Olesen, C.E.; et al. Phytochemistry of the genus Piper. Phytochemistry 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Peixoto, J.F.; Ramos, Y.J.; de Lima Moreira, D.; Alves, C.R.; Gonçalves-Oliveira, L.F. Potential of Piper spp. as a Source of New Compounds for the Leishmaniases Treatment. Parasitol. Res. 2021, 120, 2731–2747. [Google Scholar] [CrossRef]
- de Moraes, J.; Keiser, J.; Ingram, K.; Nascimento, C.; Yamaguchi, L.F.; Bittencourt, C.R.; Bemquerer, M.P.; Leite, J.R.; Kato, M.J.; Nakano, E. In Vitro Synergistic Interaction between Amide Piplartine and Antimicrobial Peptide Dermaseptin against Schistosoma mansoni Schistosomula and Adult Worms. Curr. Med. Chem. 2013, 20, 301–309. [Google Scholar] [CrossRef]
- Campelo, Y.D.M.; Mafud, A.C.; Véras, L.M.C.; Guimarães, M.A.; Yamaguchi, L.F.; Lima, D.F.; Arcanjo, D.D.R.; Kato, M.J.; Mendonça, R.Z.; Pinto, P.L.S.; et al. Synergistic Effects of In Vitro Combinations of Piplartine, Epiisopiloturine and Praziquantel against Schistosoma mansoni. Biomed. Pharmacother. 2017, 88, 488–499. [Google Scholar] [CrossRef]
- Brenner, S. The Genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Rugai, E.; Mattos, T.; Brisola, A. Nova Técnica para Isolar Larvas de Nematóides das Fezes–Modificação do Método de Baermann. Rev. Inst. Adolfo Lutz 1954, 14, 5–8. [Google Scholar] [CrossRef]
- Roquini, D.B.; Silva, G.L.; Ferreira, L.L.G.; Andricopulo, A.D.; Wilairatana, P.; de Moraes, J. Susceptibility of Angiostrongylus cantonensis Larvae to Anthelmintic Drugs. Front. Pharmacol. 2022, 13, 901459. [Google Scholar] [CrossRef]
- Roquini, D.B.; Lemes, B.L.; Kreutz, A.L.B.; Spoladore, S.C.; Amaro, M.C.; Lopes, F.B.; Fernandes, J.P.S.; de Moraes, J. Antihistamines H1 as Potential Anthelmintic Agents against the Zoonotic Parasite Angiostrongylus cantonensis. ACS Omega 2024, 9, 31159–31165. [Google Scholar] [CrossRef]
- da Mota, D.J.G.; de Melo, L.C.V.; Pereira-Chioccola, V.L.; Gava, R.; Pinto, P.L.S. First Record of Natural Infection by Angiostrongylus cantonensis (Nematoda: Metastrongyloidea) in Belocaulus willibaldoi and Rattus norvegicus in an Urban Area of São Paulo City, SP, Brazil. Heliyon 2020, 6, e05150. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.C.S.; Totini, C.H.; Cajás, R.A.; Teixeira, T.R.; Oliveira, E.A.; Cirino, M.E.; Souza, M.C.; Salvadori, M.C.; Teixeira, F.S.; de Moraes, J.; et al. In Vivo Antischistosomal Efficacy of Porcelia ponderosa γ-Lactones. Phytomedicine 2024, 135, 156045. [Google Scholar] [CrossRef] [PubMed]
- Cirino, M.E.; Teixeira, T.R.; Silva, A.M.H.; Borges, A.C.C.; Fukui-Silva, L.; Wagner, L.G.; Fernandes, C.; McCann, M.; Santos, A.L.S.; de Moraes, J. Anthelmintic Activity of 1,10-Phenanthroline-5,6-Dione-Based Metallodrugs. Sci. Rep. 2025, 15, 4699. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Xavier, R.P.; Mengarda, A.C.; Silva, M.P.; Roquini, D.B.; Salvadori, M.C.; Teixeira, F.S.; Pinto, P.L.; Morais, T.R.; Ferreira, L.L.G.; Andricopulo, A.D.; et al. H1-antihistamines as antischistosomal drugs: In vitro and in vivo studies. Parasites Vectors 2020, 13, 278. [Google Scholar] [CrossRef]
- Mengarda, A.C.; Iles, B.; Longo, J.P.F.; de Moraes, J. Recent Trends in Praziquantel Nanoformulations for Helminthiasis Treatment. Expert Opin. Drug Deliv. 2022, 19, 383–393. [Google Scholar] [CrossRef]
- de Castro, C.C.; Costa, P.S.; Laktin, G.T.; de Carvalho, P.H.; Geraldo, R.B.; de Moraes, J.; Pinto, P.L.; Couri, M.R.; Pinto, P.F.; Da Silva Filho, A.A. Cardamonin, a Schistosomicidal Chalcone from Piper aduncum L. (Piperaceae) That Inhibits Schistosoma mansoni ATP Diphosphohydrolase. Phytomedicine 2015, 22, 921–928. [Google Scholar] [CrossRef]
- Ferreira, E.A.; Campos, I.M.; Cajás, R.A.; Costa, D.S.; Carvalho, L.S.A.; Franklin, P.F.C.; de Nigro, N.P.D.; Pinto, P.F.; Capriles, P.S.Z.; de Moraes, J.; et al. In Vivo Efficacy of Uvangoletin from Piper aduncum (Piperaceae) against Schistosoma mansoni and In Silico Studies Targeting SmNTPDases. Exp. Parasitol. 2025, 269, 108897. [Google Scholar] [CrossRef]
- Singh, A.P.; Sharma, A. Structural Insights and Pharmaceutical Relevance of Plumbagin in Parasitic Disorders: A Comprehensive Review. Recent Adv. Antiinfect. Drug Discov. 2022, 17, 187–198. [Google Scholar] [CrossRef]
- Qureshi, K.A.; Imtiaz, M.; Al Nasr, I.; Koko, W.S.; Khan, T.A.; Jaremko, M.; Mahmood, S.; Fatmi, M.Q. Antiprotozoal Activity of Thymoquinone (2-Isopropyl-5-methyl-1,4-benzoquinone) for the Treatment of Leishmania major-Induced Leishmaniasis: In Silico and In Vitro Studies. Antibiotics 2022, 11, 1206. [Google Scholar] [CrossRef]
- Duarte, A.B.S.; Gomes, R.C.; Nunes, V.R.V.; Gonçalves, J.C.R.; Correia, C.A.; Dos Santos, A.Z.G.; de Sousa, D.P. The Antitumor Activity of Piplartine: A Review. Pharmaceuticals 2023, 16, 1246. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Min, X.; Zhuang, C.; Li, J.; Yu, Z.; Dong, G.; Yao, J.; Wang, S.; Liu, Y.; Wu, S.; et al. Design, synthesis and biological activity of piperlongumine derivatives as selective anticancer agents. Eur. J. Med. Chem. 2014, 82, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Shen, Y.; Xu, X.; Yao, Y.; Fu, C.; Yan, Z.; Wu, Q.; Cao, J.; Sang, W.; Zeng, L.; et al. Piperlongumine selectively suppresses ABC-DLBCL through inhibition of NF-κB p65 subunit nuclear import. Biochem. Biophys Res. Commun. 2015, 462, 326–331. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Pessoa, C.; de Moraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm. Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F.L.; Habenschus, M.D.; Barth, T.; Marques, L.M.; Pilon, A.C.; Bolzani, V.S.; Vessecchi, R.; Lopes, N.P.; Oliveira, A.R. Metabolic profile and safety of piperlongumine. Sci. Rep. 2016, 6, 33646. [Google Scholar]
- Pawłowska, M.; Mila-Kierzenkowska, C.; Szczegielniak, J.; Woźniak, A. Oxidative Stress in Parasitic Diseases—Reactive Oxygen Species as Mediators of Interactions between the Host and the Parasites. Antioxidants 2023, 13, 38. [Google Scholar] [CrossRef]
- Collins, J.B.; Stone, S.A.; Koury, E.J.; Paredes, A.G.; Shao, F.; Lovato, C.; Chen, M.; Shi, R.; Li, A.Y.; Candal, I.; et al. Quantitative tests of albendazole resistance in Caenorhabditis elegans beta-tubulin mutants. Int. J. Parasitol. Drugs Drug Resist. 2024, 25, 100556. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).