Angular 6/6/5/6-Annelated Pyrrolidine-2,3-Diones: Growth-Regulating Activity in Chlorella vulgaris
Abstract
1. Introduction
2. Results and Discussion
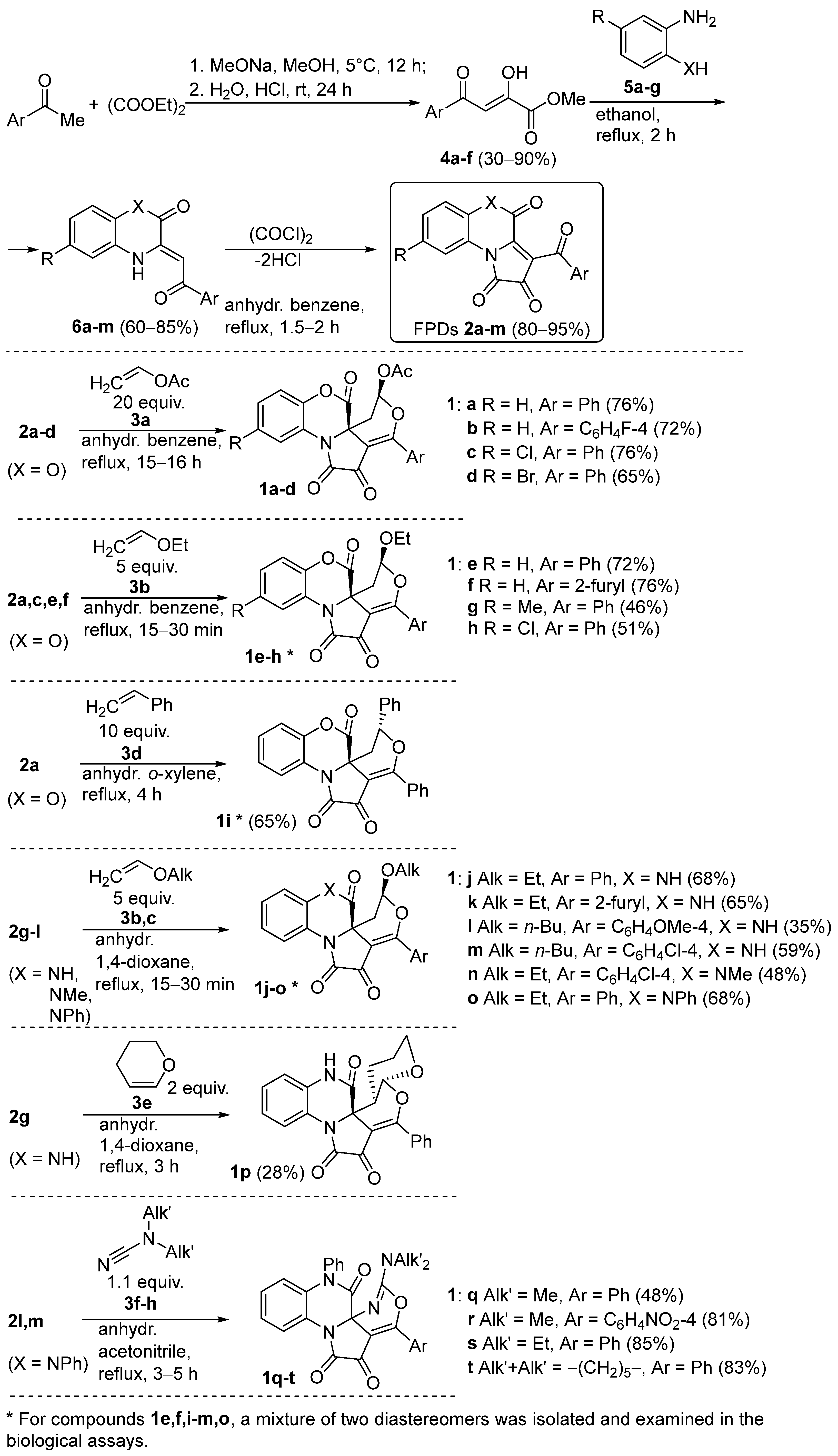
2.1. Synthesis of 6/6/5/6-Annelated Pyrrolidine-2,3-Diones 1
2.2. Biology
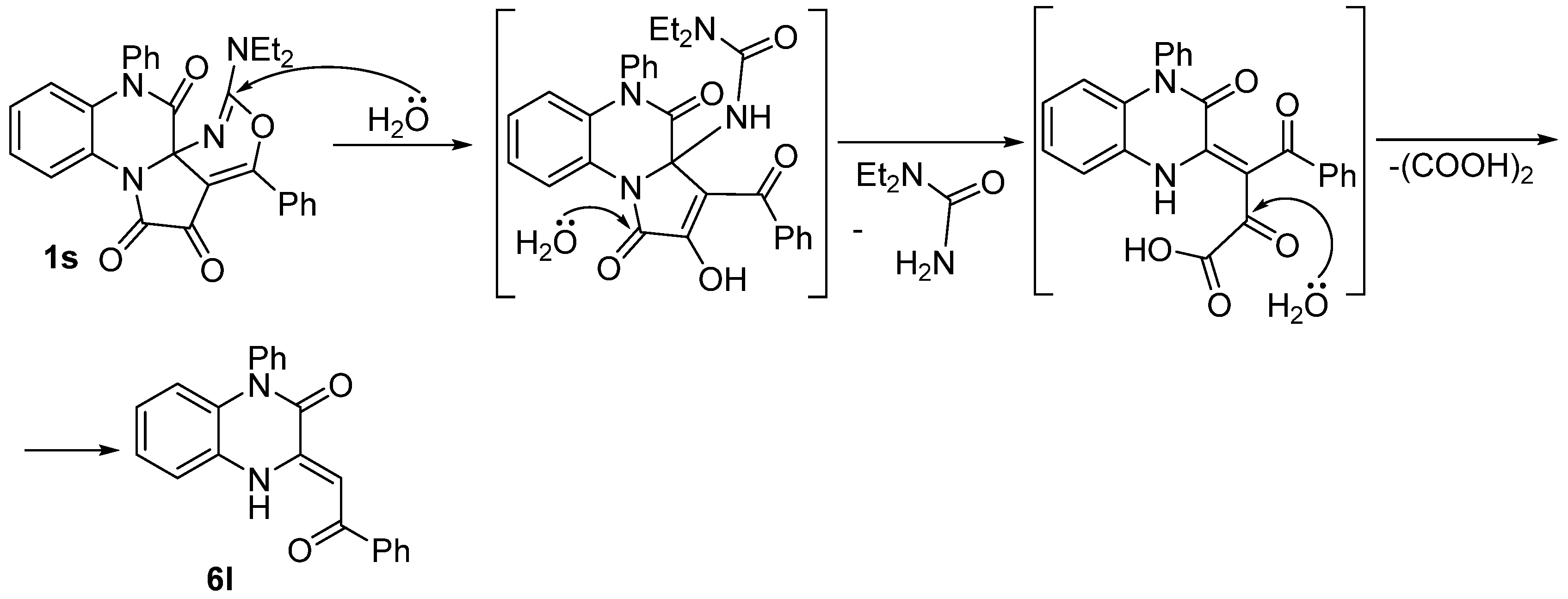
2.3. Hydrolysis Study of Compound 1s
3. Materials and Methods
3.1. Synthetic Methods and Analytic Data of Compounds
3.1.1. General Information
3.1.2. Analytic Data of Compounds 1a–t
3.2. Biology
3.2.1. Screening of Substances in 96-Well Plates
3.2.2. Evaluation of Compound 1s in 50 mL Flasks
3.2.3. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendes, A.R.; Spínola, M.P.; Lordelo, M.; Prates, J.A.M. Chemical Compounds, Bioactivities, and Applications of Chlorella vulgaris in Food, Feed and Medicine. Appl. Sci. 2024, 14, 10810. [Google Scholar] [CrossRef]
- Al-Hammadi, M.; Güngörmüşler, M. New insights into Chlorella vulgaris applications. Biotechnol. Bioeng. 2024, 121, 1486–1502. [Google Scholar] [CrossRef]
- Gallego, I.; Medic, N.; Pedersen, J.S.; Ramasamy, P.K.; Robbens, J.; Vereecke, E.; Romeis, J. The microalgal sector in Europe: Towards a sustainable bioeconomy. New Biotechnol. 2025, 86, 1–13. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.; Hill, R.T. Microalgal and bacterial auxin biosynthesis: Implications for algal biotechnology. Curr. Opin. Biotechnol. 2022, 73, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Ma, S.; Cao, Y.; Peng, S.; Zhang, X.; Kong, W. Effects of six phytohormones on the growth behavior and cellular biochemical components of Chlorella vulgaris 31. J. Appl. Phycol. 2023, 35, 1589–1602. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Jin, W.; Zhang, C.; Liang, Y.; He, Z.; Chen, Y.; Han, W.; Jiang, G. Enhancing cultivation of biodiesel-promising microalgae Chlorella pyrenoidosa using plant hormones in municipal wastewater. Biomass Conv. Bioref. 2023, 13, 9753–9763. [Google Scholar] [CrossRef]
- Bajguz, A. Brassinosteroid enhanced the level of abscisic acid in Chlorella vulgaris subjected to short-term heat stress. J. Plant Physiol. 2009, 166, 882–886. [Google Scholar] [CrossRef]
- Mendes, A.R.; Spínola, M.P.; Lordelo, M.; Prates, J.A. Advances in Bioprocess Engineering for Optimising Chlorella vulgaris Fermentation: Biotechnological Innovations and Applications. Foods 2024, 13, 4154. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Hu, H.Y.; Wu, Y.H.; Zhuang, L.L.; Xu, X.Q.; Wang, X.X.; Dao, G.H. Promising solutions to solve the bottlenecks in the large-scale cultivation of microalgae for biomass/bioenergy production. Renew. Sustain. Energy Rev. 2016, 60, 1602–1614. [Google Scholar] [CrossRef]
- Tate, J.J.; Gutierrez-Wing, M.T.; Rusch, K.A.; Benton, M.G. The effects of plant growth substances and mixed cultures on growth and metabolite production of green algae Chlorella sp.: A review. J. Plant Growth Regul. 2013, 32, 417–428. [Google Scholar] [CrossRef]
- Wase, N.; Black, P.; DiRusso, C. Innovations in improving lipid production: Algal chemical genetics. Prog. Lipid Res. 2018, 71, 101–123. [Google Scholar] [CrossRef]
- Wase, N.; Tu, B.; Allen, J.W.; Black, P.N.; DiRusso, C.C. Identification and metabolite profiling of chemical activators of lipid accumulation in green algae. Plant Physiol. 2017, 174, 2146–2165. [Google Scholar] [CrossRef]
- Bro, F.S.; Laraia, L. Unifying principles for the design and evaluation of natural product-inspired compound collections. Chem. Sci. 2025, 16, 2961–2979. [Google Scholar] [CrossRef] [PubMed]
- Maslivets, A.N.; Makhmudov, R.R.; Stepanova, E.E. Method of Producing 9-aryl-6,8-dioxa-13,20-diazapentacyclo [11.8.0.01,10.02,7014,19]heneicosa-9,14,16,18-tetraene-11,12,21-triones. Russia Patent RU2581271C1, 20 April 2016. [Google Scholar]
- Khramtsova, E.E.; Dmitriev, M.V.; Bormotov, N.I.; Serova, O.A.; Shishkina, L.N.; Maslivets, A.N. Alkaloid-like annulated pyrano [4,3-b]pyrroles: Antiviral activity and hydrolysis. Chem. Heterocycl. Compd. 2021, 57, 483–489. [Google Scholar] [CrossRef]
- Lovering, F. Escape from Flatland 2: Complexity and promiscuity. MedChemComm 2013, 4, 515–519. [Google Scholar] [CrossRef]
- Li Petri, G.; Raimondi, M.V.; Spanò, V.; Holl, R.; Barraja, P.; Montalbano, A. Pyrrolidine in drug discovery: A versatile scaffold for novel biologically active compounds. Top. Curr. Chem. 2021, 379, 34. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, J.P.; Pigni, N.B.; Torras-Claveria, L.; Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. Bioactive alkaloid extracts from Narcissus broussonetii: Mass spectral studies. J. Pharm. Biomed. Anal. 2012, 70, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.N.; Pande, H.; Bhakuni, D.S. Structure and stereochemistry of coccuvine (Cocculus laurifolius DC). Experientia 1976, 32, 1368–1369. [Google Scholar] [CrossRef]
- Stepanova, E.E.; Aliev, Z.G.; Maslivets, A.N. Five-membered 2,3-dioxoheterocycles: XCVIII. [4+2]-Cycloaddition of alkyl vinyl ethers to 3-aroyl-1H-pyrrolo[2,1-c][1,4]benzoxazine-1,2,4-triones. A new synthetic approach to heteroanalogs of 13(14 → 8)-abeo steroids. Russ. J. Org. Chem. 2013, 49, 1762–1767. [Google Scholar] [CrossRef]
- Miyata, Y.; Diyabalanage, T.; Amsler, C.D.; McClintock, J.B.; Valeriote, F.A.; Baker, B.J. Ecdysteroids from the Antarctic tunicate Synoicum adareanum. J. Nat. Prod. 2007, 70, 1859–1864. [Google Scholar] [CrossRef]
- Amagata, T.; Tanaka, M.; Yamada, T.; Doi, M.; Minoura, K.; Ohishi, H.; Yamori, T.; Numata, A. Variation in cytostatic constituents of a sponge-derived Gymnascella dankaliensis by manipulating the carbon source. J. Nat. Prod. 2007, 70, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Sano, T. Syntheses of heterocyclic compounds containing nitrogen utilizing dioxopyrrolines. J. Synth. Org. Chem. 1984, 42, 340–354. [Google Scholar] [CrossRef]
- Konovalova, V.V.; Shklyaev, Y.V.; Maslivets, A.N. Reactions of fused pyrrole-2,3-diones with dinucleophiles. ARKIVOC Online J. Org. Chem. 2015, 2015, 48–69. [Google Scholar] [CrossRef]
- Konovalova, V.V.; Maslivets, A.N. Synthesis of spiro compounds based on 1H-pyrrole-2,3-diones. Mini-Rev. Org. Chem. 2019, 16, 173–192. [Google Scholar] [CrossRef]
- Lystsova, E.A.; Khramtsova, E.E.; Maslivets, A.N. Acyl(imidoyl)ketenes: Reactive Bidentate Oxa/Aza-Dienes for Organic Synthesis. Symmetry 2021, 13, 1509. [Google Scholar] [CrossRef]
- Stepanova, E.E.; Maslivets, A.N. [4+2]-Cycloaddition of vinyl acetate to pyrrolobenzoxazinetriones. Diastereoselective synthesis of angularly fused pyrano[4,3-b]pyrroles. Russ. J. Org. Chem. 2016, 52, 879–882. [Google Scholar] [CrossRef]
- Kasatkina, S.O.; Titov, M.S.; Stepanova, E.E.; Dmitriev, M.V.; Maslivets, A.N. Synthesis of Pyrano[4′,3′:2,3]pyrrolo[1,2-a]quinoxalines by Reaction of Aroylpyrroloquinoxalines with Alkyl Vinyl Ethers. Russ. J. Org. Chem. 2018, 54, 626–632. [Google Scholar] [CrossRef]
- Stepanova, E.E.; Babenysheva, A.V.; Maslivets, A.N. Five-membered 2,3-dioxoheterocycles: LXXVII. [4+2]-Cycloaddition of alkyl vinyl ethers to 3-aroylpyrrolo[2,1-c][1,4]benzoxazine-1,2,4(4H)-triones. Russ. J. Org. Chem. 2011, 47, 937–940. [Google Scholar] [CrossRef]
- Stepanova, E.E.; Dmitriev, M.V.; Maslivets, A.N. Hetero-Diels–Alder reaction of 3-aroylpyrrolo[2,1-c][1,4]benzoxazines with styrene. Synthesis of pyrano[4′,3′:2,3]pyrrolo[2,1-c][1,4]benzoxazines. Russ. J. Org. Chem. 2017, 53, 1851–1856. [Google Scholar] [CrossRef]
- Kasatkina, S.O.; Stepanova, E.E.; Dmitriev, M.V.; Maslivets, A.N. Reactions of 3-Aroylpyrrolo[1,2-a]quinoxaline-1,2,4(5H)-triones with 2,3-Dihydrofuran and 3,4-Dihydro-2H-pyran. Russ. J. Org. Chem. 2018, 54, 1055–1060. [Google Scholar] [CrossRef]
- Khramtsova, E.E.; Krainov, A.D.; Dmitriev, M.V.; Maslivets, A.N. Cycloaddition of 4-Acyl-1H-pyrrole-2,3-diones Fused at [e]-Side and Cyanamides: Divergent Approach to 4H-1,3-Oxazines. Molecules 2022, 27, 5257. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.; Claisen, L. Ueber die Einführung von Säureradicalen in Ketone. Ber. Dtsch. Chem. Ges. 1887, 20, 2178–2188. [Google Scholar] [CrossRef]
- Bozdyreva, K.S.; Smirnova, I.V.; Maslivets, A.N. Five-Membered 2,3-Dioxoheterocycles: L. Synthesis and Thermolysis of 3-Aroyl-and 3-Hetaroyl-5-phenyl-1,2,4,5-tetrahydropyrrolo[1,2-a]quinoxalin-1,2,4-triones. Russ. J. Org. Chem. 2005, 41, 1081–1088. [Google Scholar] [CrossRef]
- Mashevskaya, I.V.; Mokrushin, I.G.; Bozdyreva, K.S.; Maslivets, A.N. Five-membered 2,3-dioxoheterocycles: LXXIII. Synthesis and thermolysis of 3-acylpyrrolo[1,2-a]quinoxaline-1,2,4(5H)-triones. Russ. J. Org. Chem. 2011, 47, 253–257. [Google Scholar] [CrossRef]
- Stepanova, E.E.; Lukmanova, D.N.; Kasatkina, S.O.; Dmitriev, M.V.; Maslivets, A.N. Facile Synthesis of Regioisomeric N-Alkyl Substituted 3-Methylene-3,4-dihydroquinoxalin-2(1H)-ones. ChemistrySelect 2019, 4, 12774–12778. [Google Scholar] [CrossRef]
- Maslivets, A.N.; Mashevskaya, I.V.; Smirnova, L.I.; Krasnykh, O.P.; Shurov, S.N.; Andreichikov, Y.S. Five-membered 2,3-dioxoheterocycles. Synthesis of 3-aroyl-1,2-dihydro-4H-pyrrolo[5,1-c][1,4]benzoxazine-1,2,4-triones and their reaction with water and alcohols. Zh. Org. Khim. 1992, 28, 2545–2553. (In Russian) [Google Scholar]
- Tolmacheva, I.A.; Mashevskaya, I.V.; Maslivets, A.N. Recyclization of 3-heteroylpyrrolo[2,1-c][1,4]benzoxazine-1,2,4-triones at treatment with o-phenylenediamine. Russ. J. Org. Chem. 2001, 37, 596–597. [Google Scholar] [CrossRef]
- Tolmacheva, I.A.; Mashevskaya, I.V.; Maslivets, A.N. Nucleophilic Transformations of Heterocyclic Derivatives of 4-Heteryl-2,4-dioxobutanoic Acids. Russ. J. Org. Chem. 2002, 38, 281–285. [Google Scholar] [CrossRef]
- Kasatkina, S.; Stepanova, E.; Dmitriev, M.; Mokrushin, I.; Maslivets, A. Divergent synthesis of (quinoxalin-2-yl)-1,3-oxazines and pyrimido[1,6-a]quinoxalines via the cycloaddition reaction of acyl(quinoxalinyl)ketenes. Tetrahedron Lett. 2019, 60, 151088. [Google Scholar] [CrossRef]
- Lin, B.; Ahmed, F.; Du, H.; Li, Z.; Yan, Y.; Huang, Y.; Cui, M.; Yin, Y.; Li, B.; Wang, M.; et al. Plant growth regulators promote lipid and carotenoid accumulation in Chlorella vulgaris. J. Appl. Phycol. 2018, 30, 1549–1561. [Google Scholar] [CrossRef]
- Bajguz, A.; Piotrowska-Niczyporuk, A. Synergistic effect of auxins and brassinosteroids on the growth and regulation of metabolite content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiol. Biochem. 2013, 71, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Spínola, M.P.; Costa, M.M.; Prates, J.A. Enhancing digestibility of Chlorella vulgaris biomass in monogastric diets: Strategies and insights. Animals 2023, 13, 1017. [Google Scholar] [CrossRef]
- Wang, C.A.; Onyeaka, H.; Miri, T.; Soltani, F. Chlorella vulgaris as a food substitute: Applications and benefits in the food industry. J. Food Sci. 2024, 89, 8231–8247. [Google Scholar] [CrossRef] [PubMed]
- Touloupakis, E.; Tartari, G.; Zittelli, G.C.; Torzillo, G. Growth and photosynthetic performance of Chlamydopodium fusiforme cells cultivated in BG11 and Bristol media. J. Appl. Phycol. 2020, 32, 145–152. [Google Scholar] [CrossRef]
- Chioccioli, M.; Hankamer, B.; Ross, I.L. Flow cytometry pulse width data enables rapid and sensitive estimation of biomass dry weight in the microalgae Chlamydomonas reinhardtii and Chlorella vulgaris. PLoS ONE 2014, 9, e97269. [Google Scholar] [CrossRef] [PubMed]
- Kondzior, P.; Butarewicz, A. Effect of heavy metals (Cu and Zn) on the content of photosynthetic pigments in the cells of algae Chlorella vulgaris. J. Ecol. Eng. 2018, 19, 18–28. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method for Protein Quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2002; pp. 15–21. [Google Scholar] [CrossRef]
- Weber, S.; Grande, P.M.; Blank, L.M.; Klose, H. Insights into cell wall disintegration of Chlorella vulgaris. PLoS ONE 2022, 17, e0262500. [Google Scholar] [CrossRef]


| Entry | Difference 1 in Algae Cell Concentration Between Cultures Containing Compounds Under Study and Control Cultures | ||
|---|---|---|---|
| Concentration of Compounds in Culture Medium | |||
| 0.1 μmol/L | 1 μmol/L | 10 μmol/L | |
| 1a | 9.65 | −3.09 | 2.70 |
| 1b | 0.77 | 1.93 | 3.47 |
| 1c | 5.02 | 6.18 | 1.93 |
| 1d | 5.79 | 0.00 | 4.63 |
| 1e | 9.65 | 0.77 | −1.93 |
| 1f | −2.32 | −1.93 | 9.65 |
| 1g | 6.83 | −1.84 | −1.45 |
| 1h | −0.66 | −4.20 | −0.66 |
| 1i | −4.20 | −1.05 | 1.71 |
| 1j | 3.47 | 6.56 | 4.25 |
| 1k | −0.77 | −1.93 | 8.11 |
| 1l | −8.05 | −4.94 | 2.86 |
| 1m | 4.42 | −4.94 | −6.49 |
| 1n | 2.50 | 4.47 | 13.532 |
| 1o | 9.20 | 6.83 | 5.26 |
| 1p | 2.08 | −1.82 | 1.30 |
| 1q | 16.23 | −0.31 | 6.13 |
| 1r | −0.27 | 2.95 | 12.60 |
| 1s | 16.62 | 23.06 | 29.49 |
| 1t | 13.03 | −0.18 | 2.99 |
| Glucose (2 g/L, 1.1 × 10−2 mol/L) | 128–193 | ||
| Concentration of 1s | Concentration of Cells, 106 Cell/mL | Chlorophyll a and b, μg/107 Cells | Carotenoids, μg/107 Cells | Carbohydrates, μg/106 Cells | Protein, μg/106 Cells |
|---|---|---|---|---|---|
| 100 μmol/L | 17.85 ± 0.76 3 | 3.558 ± 0.449 | 0.142 ± 0.013 | 3.29 ± 0.331 | 0.513 ± 0.041 |
| 10 μmol/L | 19.13 ± 0.98 | 3.520 ± 0.171 | 0.170 ± 0.032 | 3.52 ± 0.293 | 0.469 ± 0.056 |
| 1 μmol/L | 17.48 ± 0.23 | 3.552 ± 0.015 | 0.171 ± 0.023 | 3.97 ± 0.390 | 0.551 ± 0.065 4 |
| 0.1 μmol/L | 17.88 ± 0.74 | 3.476 ± 0.095 | 0.170 ± 0.026 | 3.27 ± 0.367 | 0.488 ± 0.044 |
| Negative control 1 | 18.68 ± 0.78 | 3.469 ± 0.084 | 0.185 ± 0.024 | 3.65 ± 0.239 | 0.462 ± 0.043 |
| Positive control 2 | 69.83 | 3.689 ± 0.042 | 0.111 ± 0.003 | 5.74 ± 0.312 | 0.370 ± 0.015 |
| Entry | Conditions 1 | Conversion 2 of 1s, % | Content 2 of 6l, % |
|---|---|---|---|
| 1 | DMSO with traces of H2O 3 | 1 | traces |
| 2 | DMSO/H2O, 3 8:1 v/v | 49 | ~5 |
| 3 | DMSO/BG-11, 3 8:1 v/v | 50 | ~5 |
| 4 | DMSO with traces of H2O, illumination 4 | 100 | 0 |
| 5 | DMSO/H2O, 8:1 v/v, illumination 4 | 100 | 0 |
| 6 | DMSO/BG-11, 8:1 v/v, illumination 4 | 100 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novokshonova, A.D.; Khramtsov, P.V.; Khramtsova, E.E. Angular 6/6/5/6-Annelated Pyrrolidine-2,3-Diones: Growth-Regulating Activity in Chlorella vulgaris. Chemistry 2025, 7, 102. https://doi.org/10.3390/chemistry7040102
Novokshonova AD, Khramtsov PV, Khramtsova EE. Angular 6/6/5/6-Annelated Pyrrolidine-2,3-Diones: Growth-Regulating Activity in Chlorella vulgaris. Chemistry. 2025; 7(4):102. https://doi.org/10.3390/chemistry7040102
Chicago/Turabian StyleNovokshonova, Anastasia D., Pavel V. Khramtsov, and Ekaterina E. Khramtsova. 2025. "Angular 6/6/5/6-Annelated Pyrrolidine-2,3-Diones: Growth-Regulating Activity in Chlorella vulgaris" Chemistry 7, no. 4: 102. https://doi.org/10.3390/chemistry7040102
APA StyleNovokshonova, A. D., Khramtsov, P. V., & Khramtsova, E. E. (2025). Angular 6/6/5/6-Annelated Pyrrolidine-2,3-Diones: Growth-Regulating Activity in Chlorella vulgaris. Chemistry, 7(4), 102. https://doi.org/10.3390/chemistry7040102







