Abstract
This work successfully prepared the Co3O4 NPs via simple galvanostatic deposition followed by annealing at 400 and 800 °C for two hours. The galvanostatic deposition was carried out from a modified Watts bath. We used Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), energy dispersive X-ray (EDX), scanning electron microscopy (SEM), and transmission electron microscopy (TEM) to examine the oxide’s characterization properties. The nature of the oxide formed was strongly dependent on the annealing temperature. The powder formed at room temperature (25 °C) is a mixture of Co(OH)2 and metallic Co. However, at 400 and 800 °C, and according to the XRD patterns, the powder consists of the Co3O4 phase and a slight quantity of Co(OH)2 phase. The average particle size measured by TEM ranged from 14.85 nm at room temperature to 90.19 nm at 800 °C. Moreover, the study examined how the operating deposition parameters affected the galvanostatic deposition process. Furthermore, these baths provide NPs, that demonstrate antibacterial activity against both Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria as well as antifungal activity against Aspergillus niger.
1. Introduction
Because of the special characteristics of their size, shape, dimension, and structure, metal and metal oxide nanoparticle synthesis and applications have attracted much attention [1,2,3,4]. High-purity metal and metal oxide nano-powders are produced chemically [5] and electrochemically [6,7] from aqueous solutions. Lithium ion batteries, gas sensing, ceramic pigments, heterogeneous catalysts, and electrochemical devices, like supercapacitors, are just a few of the many technologically significant uses for Co3O4 [8,9,10]. It can also be applied selectively as a coating material for high-temperature solar collectors, outperforming black chrome coatings [11]. Moreover, the synthesized Co3O4 NPs exhibit superlative antibacterial and antifungal activity in the viewpoint of various biomedical applications [12,13,14,15,16,17]. Although it is possible to prepare the nanostructured cobalt oxide powder in various ways, such as the thermal decomposition routes [16,18], the Taguchi method [19], and the hydrothermal synthesis method [20,21], most of these methods suffer from problems that may reduce the purity of the resulting powder and consequently affect their properties. Due to its ease of employment, rapid application, low cost, room temperature operation, and ability to predict chemical structure, the electrochemical deposition technique for synthesizing nanomaterials seems beneficial [22,23,24]. Additionally, neither the preparation nor the purification processes involved using surfactants, templates, or organic solvents. Cobalt oxides were directly electrodeposited anodically onto a glassy carbon electrode from a highly alkaline aqueous medium that contained gluconate ions as a ligand species [25,26]. The properties of these oxides during photoelectrochemical (PEC) water-splitting applications were determined. Observation showed that Co3O4 had the best photocatalytic properties among cobalt oxide phases produced by varying the annealing temperature for water-splitting reactions [27]. It is worth noting that the phases and the structure of the oxide formed depend strongly on the method of preparation and the annealing temperature [27,28,29].
Gram-positive organisms often have thicker cell walls and are more resistant to different antibacterial treatments than Gram-negative species. Certain chosen nanoparticles have appealing antibacterial properties. Following contact with the bacterial cell, they change the permeability of the cell, creating pits and gaps, which impact DNA replication and inhibit respiratory chain enzyme function, ultimately leading to cell death [30,31,32]. This research aims to synthesize cobalt oxide nanoparticles via galvanostatic electrodeposition from a modified Watts bath and study their morphology, composition, and antimicrobial activities. Moreover, the study examined how the operating deposition parameters, such as temperature, pH, current density, and the concentration of NO3− ions, affected the electrodeposition process.
2. Materials and Methods
2.1. The Synthesis of Co3O4 NPs
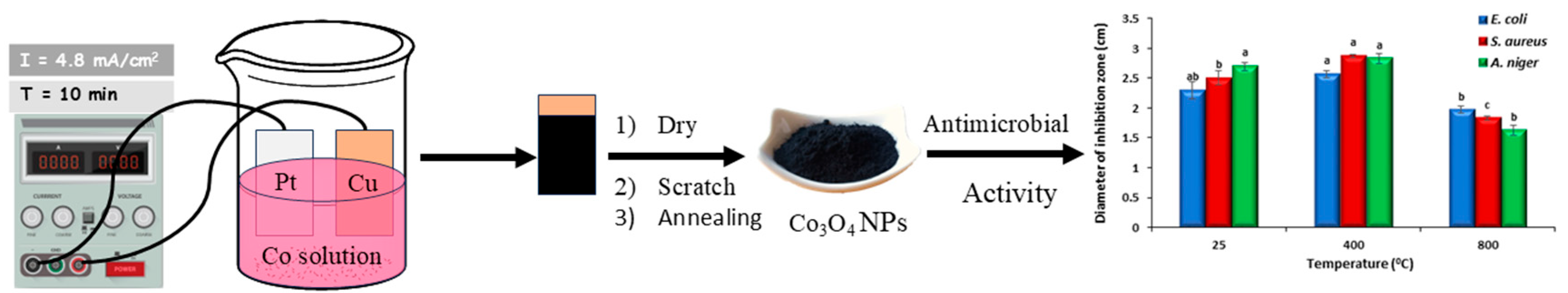
Electrochemically, the cobalt oxide was prepared via galvanostatic electrodeposition using a modified Watts bath at room temperature (25 °C). The Watts bath consisted of 0.63 M CoSO4, 0.09 M CoCl2, and 0.3M H3BO3 at pH 4.5 without stirring. The impact of various KNO3 concentrations (0.05, 0.1, 0.15, and 0.2 M) on the cobalt oxide deposition was examined in this work. All reagents used for the electrolytic solution and bi-distilled water were obtained from Sigma Aldrich, Cairo, Egypt without additional purification. Copper and platinum sheets served as the cathode and anode, respectively. Their respective dimensions were 2.7 cm × 2.3 cm × 1.0 cm. The distance between the copper and platinum sheets was 2.5 cm. Grades 800–3000 of abrasive sheets were used to mechanize the copper substrates. After polishing, copper sheets were immersed in a pickling solution. After that, they were ultrasonically cleaned in acetone for five minutes. Then, to facilitate the deposition of the cobalt oxide, these samples were promptly immersed in the electrolytic bath after washing with distilled H2O so that the surface was free of any contaminants. Finally, a direct current (4.8 mA/cm2) was supplied by a D.C. power supply unit (GW instek model: GPS-3030DD, Taipei, Taiwan) for 10 min. After that, the cobalt oxide powder was gathered and annealed at 400 (Co-400) and 800 (Co-800) degrees Celsius as a suitable range for cobalt oxide phase formation [27,28,29].
2.2. Characterization of the Cobalt Oxide
HRTEM (high-resolution transmission electron microscopy) (JEOL JEM-2100 EX II Electron Microscope, JEOL Ltd., Tokyo, Japan.) and HRSEM (high-resolution scanning electron microscopy) with Energy Dispersive X-ray analysis (EDS) were used to describe the powder’s morphology. Crystallinity and phase identification were assessed using an X-ray diffractometer (XRD) (Angstrom Advanced ADX-800 X-ray Diffraction Instrument). A Perkin Elmer 293 Spectrophotometer was used to record the samples’ Fourier transform infrared spectra (FTIR). Moreover, using an automatic surface area and pore size analyzer (BELSORP MINI X), the surface of the cobalt oxide samples was characterized by N2 adsorption/desorption at liquid nitrogen temperature (−196 °C). The samples were previously degassed until a constant weight was obtained.
2.3. Electrochemical Measurements
Electrochemical investigations were conducted using the 1000 Gamry Instrument Potentiostat/Galvanostat/ZRA. Silver/silver chloride (3 M KCl) served as the reference electrode. The LSV experiments were achieved in a cell containing a Pt disc (diameter of 0.25 cm) or copper sheet as a working electrode and a Pt wire as a counter electrode. Before every run, the Pt working electrode was polished with Al2O3 powder until a brilliant finish was obtained, and pure water was used to rinse it. The current–potential curves were obtained by moving the potential with a scan rate of 5 mVs−1 in an extra negative direction. Moreover, chronoamperometric analysis on the Pt working electrode was performed in a Watts’ bath at different deposition potentials.
2.4. Biological Activities (Well Diffusion Agar Method)
2.4.1. Antibacterial Assay
Gram-positive Staphylococcus aureus (ATCC 6538) and Gram-negative Escherichia coli (ATCC 25922) were obtained from Royal for Quality System, Egypt, and used as test bacterial strains. The bacterial strains were activated by growing overnight on the nutrient broth medium at 37 °C for 24 h in a shaking incubator at 180 rpm. The assay was performed by spreading 100 µL of the bacterial suspension (106 CFU mL−1 using spectrophotometer OD 660 nm) evenly over a Müller–Hinton agar plate using a sterile glass spreader, and then wells were made using a sterile cork borer (9 mm). Each well was loaded with 400 µL of cobalt oxide NPs (0.1 g mL−1 of sterile distilled water) using a micropipette and allowed to freely diffuse through the agar plates. All plates were kept at 4 °C for 2 h and then incubated overnight at 37 °C for 24 h. After incubation, the inhibition zones (clear zones) around the well were recorded, representing the antibacterial activity of cobalt oxide NPS against the tested bacterial strains. The assay was carried out in triplicate.
2.4.2. Antifungal Assay
Aspergillus niger (MT 103092) was obtained from Central Lab, Faculty of Science, Ain Shams University, Egypt. The spore suspension from the fungal culture was prepared in 0.89% saline solution and adjusted with a spectrophotometer at 520 nm (5 × 106 spores/mL). The assay was performed by spreading 100 µL of the fungal spore suspension evenly over the potato dextrose agar plates using a sterile glass spreader. A sterile cork borer was used to punch 9 mm wells into the agar plates. Four hundred microliters of cobalt oxide NPs (the same concentration as for bacteria) was transferred to the wells. All plates were kept at 4 °C for 2 h and then incubated at 28 °C for 5 days. After incubation, the inhibition zones were recorded, representing the antifungal activity of cobalt oxide NPS against the tested fungal strain. The assay was carried out in triplicate. The following schematic diagram, Scheme 1, provides an overview of the experimental protocol, encompassing the process from synthesis to biological evaluation.
Scheme 1.
Schematic overview of the electrochemical synthesis of Co3O2 NPs—method and its antibacterial and antifungal properties. Bars represent standard error; Different letters indicate significant differences in access treatment means from repeated-measures ANOVA.
3. Results and Discussion
3.1. Surface Characterization
3.1.1. SEM and EDS
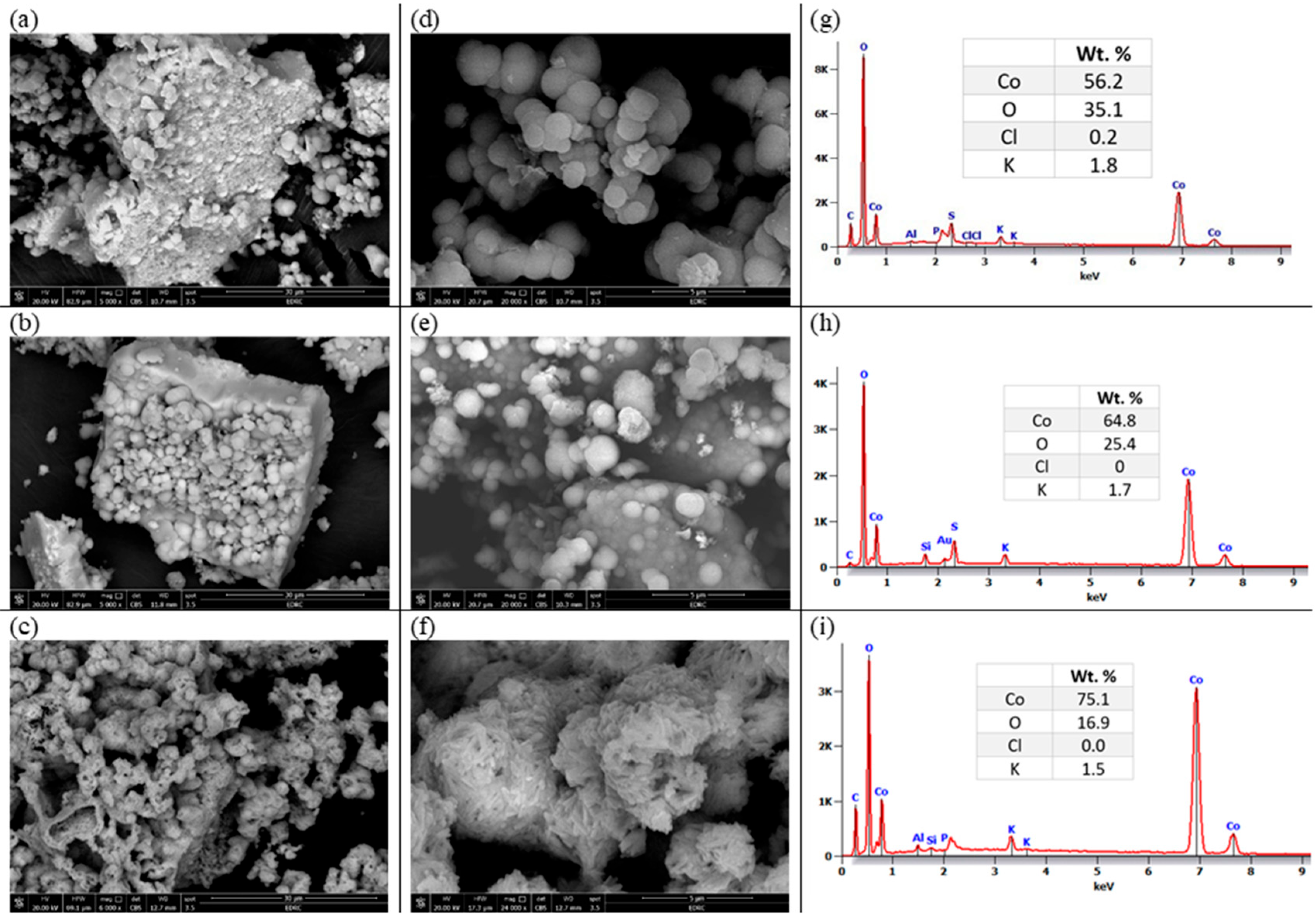
The morphology of the electrochemically prepared oxide nanoparticles is investigated using the HRSEM technique and illustrated in Figure 1. The powder obtained at room temperature (25 °C) was annealed at 400 and 800 °C. The studied cobalt oxide samples have a heterogeneous structure as shown in the zoomed-out images (a–c). Zooming in, we can describe particles of Co-25 and Co-400 samples as spheres of different particle sizes, as shown in Figure 1d,e. On the other hand, the Co-800 sample (Figure 1c,f) shows a drastic change in its morphology. The morphology changed into aggregated needle-like particles. The EDS and element percentages by weight of the prepared oxide samples are displayed in Figure 1g–i. All prepared oxide samples show the existence of Co, O, and some residue from the initial reactants, such as K and Cl. As shown in the tables inserted in Figure 1, as the temperature of annealing rises, the amount of Co increases and that of O decreases gradually, indicating the removal of H2O from the crystals and formation of cobalt oxides. In addition, with annealing, the Cl− ion is removed while K+ still exists.
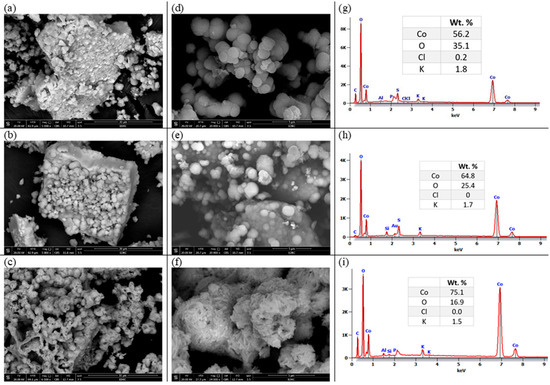
Figure 1.
The HRSEM images for Co-25 (a,d), Co-400 (b,e), and Co-800 (c,f), and their analogous EDS (g), (h), and (i), respectively.
3.1.2. XRD
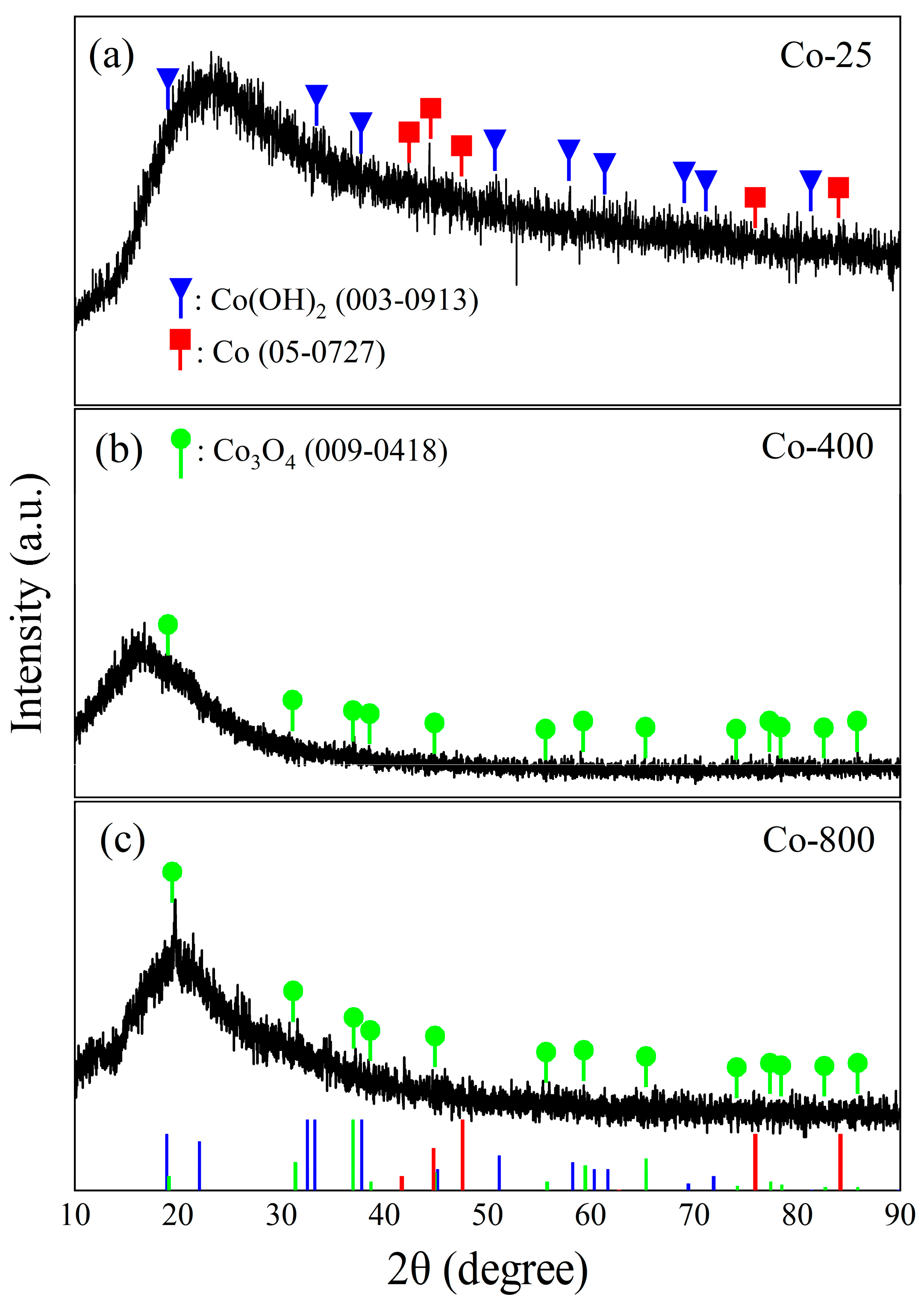
The crystal microstructure of the cobalt oxide samples is clarified in Figure 2. The XRD patterns of the oxide prepared at room temperature (Co-25) show the formation of a mixture of Co(OH)2 and metallic Co through the chosen method of preparation. The Co(OH)2 shows a hexagonal crystal with XRD peaks observed at 2θ: 19.02°, 32.14°, 37.76°, 51.71°, 57.71°, 61.34°, 69.23°, 71.15°, and 81.17° matching to (001), (100), (101), (102), (110), (111), (103), (201), and (202) planes, respectively (JPCDs#03-0913). The identified metallic Co is also a hexagonal crystal with main peaks observed at 2θ: 41.68°, 44.76°, 47.56°, 75.94°, and 84.19° corresponding to (100), (002), (101), (110), and (103) planes, respectively (JPCDs#05-0727). For Co-400 and Co-800 samples, the XRD peaks of the cobalt oxide (Co3O4) are displayed (JPCDs#09-0418). Co3O4 has a cubic crystal with unit cell parameters a = b = c = 8.084 Å with main XRD peaks at 2θ: 18.99°, 31.24°, 36.83°, 59.35°, and 65.22° corresponding to (111), (220), (311), (511), and (440) planes, respectively. The high background signal in XRD can arise from various factors, one of which is the heterogeneous nature of the prepared cobalt oxide samples. Moreover, the absence and suppression of some diffraction peaks indicate poor crystallinity, as supported by SEM analysis. This phenomenon is likely related to limited atomic mobility during deposition, low-temperature sample Co-25, the presence of amorphous regions contributing to background noise, and sample-related effects, such as preferred orientation, inhomogeneity, or macrostrain. These factors collectively reduce the intensity of characteristic reflections [33]. In contrast, the more pronounced Co3O4 peaks in Co-800 reflect a thermally induced transformation into a well-crystallized spinel structure, underscoring the incomplete crystallization in samples before annealing. A similar phenomenon has been obtained before [34].
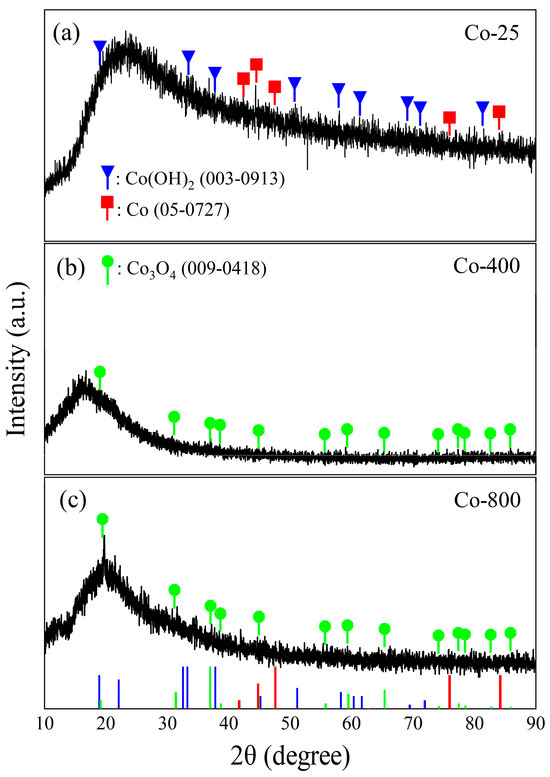
Figure 2.
XRD patterns for the prepared cobalt oxide samples. The red, blue, and green lines in subfigure (a–c) represent the reference XRD patterns for the different cobalt phases as indicated by the corresponding XRD cards.
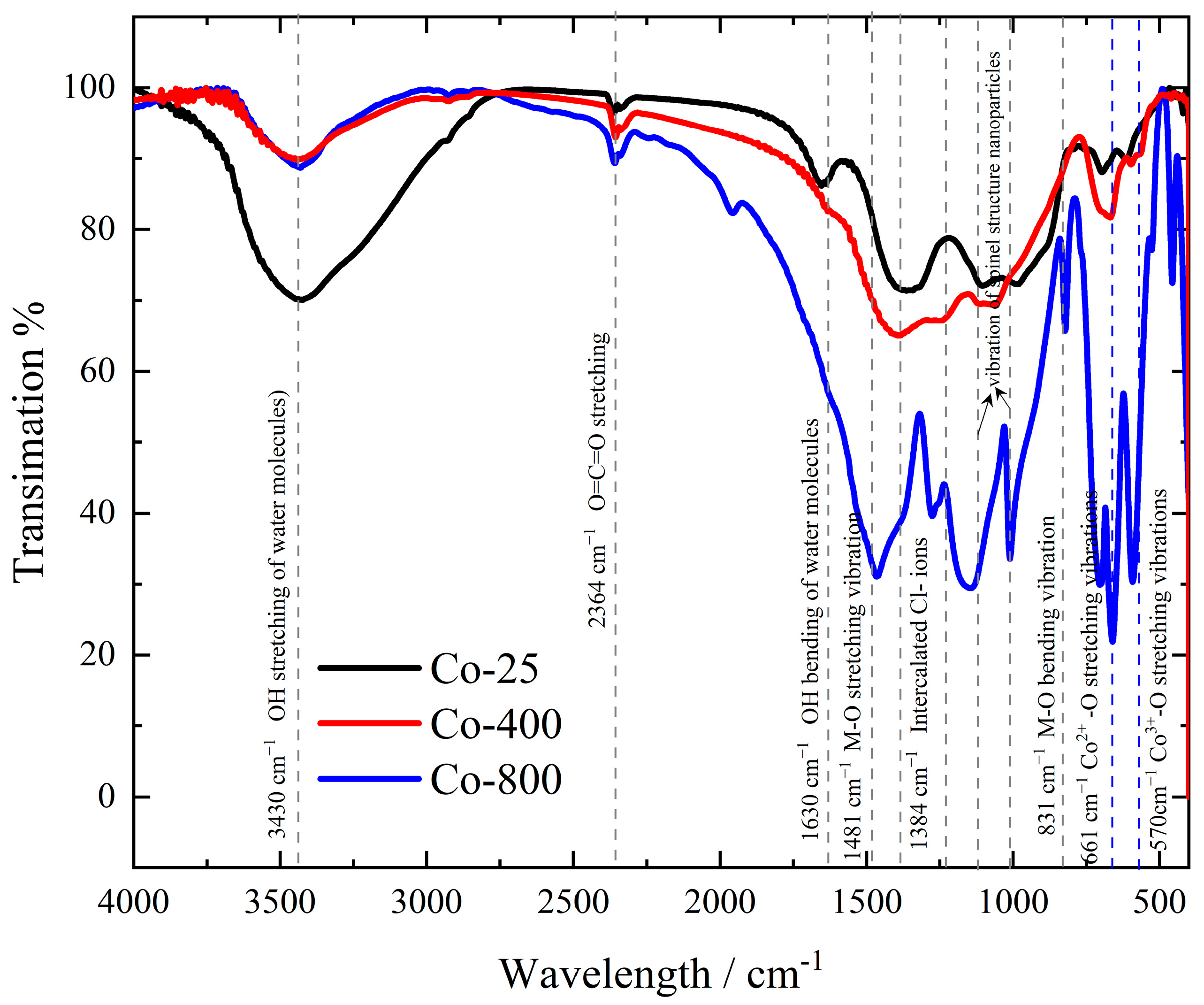
3.1.3. FTIR
Figure 3 shows the FTIR spectra of cobalt oxide samples. All studied cobalt oxide samples exhibit nearly similar characteristic absorption bands, even though with varying intensities based on the temperature of annealing employed. The broad peaks at 3430 cm−1 and 1630 cm−1 were ascribed to H–O stretching and bending, respectively [35]. The FTIR spectra of cobalt hydroxides made using alternative methods [36,37] often only show two vibration bands for water molecules, around 3500 and 1616 cm−1, indicating a weak hydrogen bond interaction between the hydroxide and water molecules [38]. The peak at 573 cm−1 corresponds to the Co3+ of octahedral sites, and 664 cm−1 is assigned to Co2+ of tetrahedral sites, confirming the formation of cobalt oxide nanoparticles (Co3O4) [10,39,40]. The face-centered cubic structure’s monodisperse phase purity is demonstrated by the occurrence of two significant M-O stretching and bending frequencies at 1481 and 831 cm−1 [41]. The intercalated Cl− ions may be responsible for the band at about 1384 cm−1 [36]. The small band detected at 2364 cm−1 refers to the existence of adsorbed CO2 molecules within the CO network, likely originating from residual air [42]. According to the FTIR spectra, the Co-25 sample has a huge absorption band of stretching H–O at 3430 cm−1 and a notable H–O bending band at 1630 cm−1 related to the electrochemically formed Co(OH)2 at room temperature. By annealing, the cobalt oxide nanoparticles are formed and enhanced gradually at the expense of Co(OH)2 as temperature increases. For Co-800, the characteristic absorption bands of Co3O4 at 573 and 664 cm−1 are significantly enhanced. Consequently, from the previous characterization study, it is evident that the primary phase formed during the electrodeposition process is Co(OH)₂, with a minor presence of metallic cobalt. Through annealing, the cobalt oxide (Co3O4) phase gradually develops as the Co(OH)2 phase diminishes due to the removal of H₂O. Consequently, the Co3O4 phase is more abundant in Co-800 compared to Co-400.
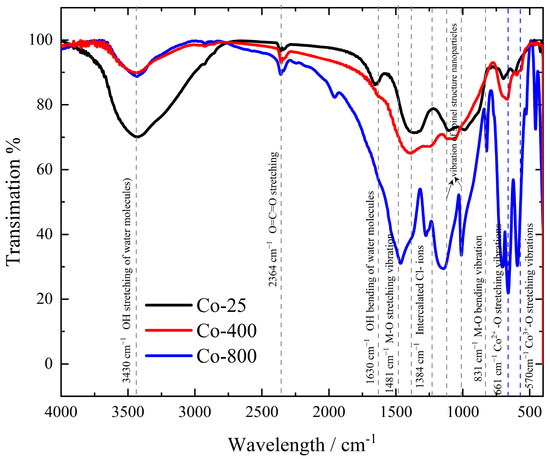
Figure 3.
FTIR spectra of cobalt oxide samples.
3.1.4. HRTEM
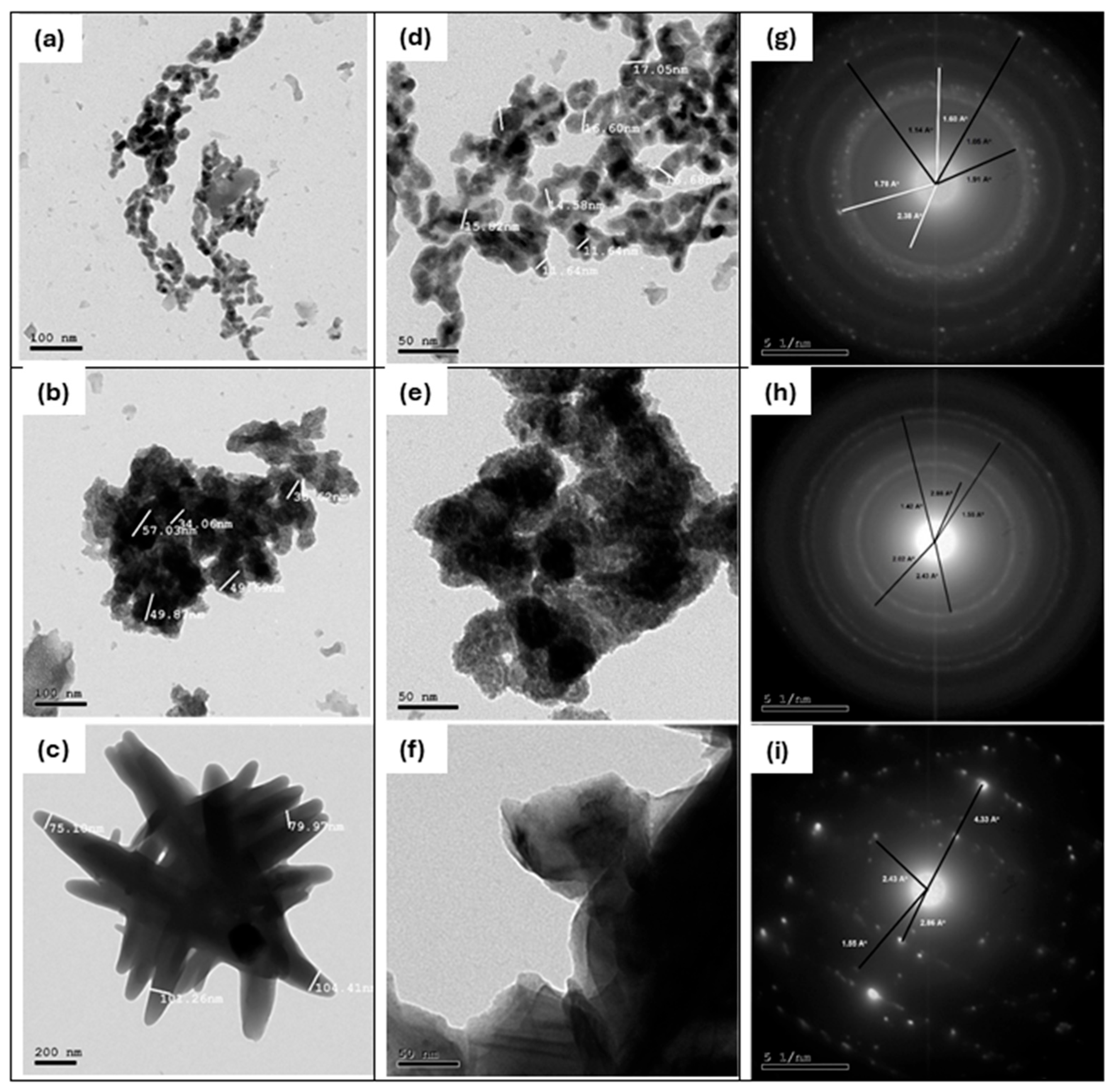
High-resolution transmission electron microscopy (HRTEM) investigations were performed on the prepared cobalt samples, along with their selected area electron diffraction (SAED) patterns, to confirm the presence of distinct cobalt phases and to investigate the morphology and crystal structures of the samples, as shown in Figure 4a–i. The HRTEM images of the Co-25 sample (Figure 4a,d) show irregular spherical particles of varying sizes, with an average particle size of around 14.85 nm. In contrast, the Co-400 sample (Figure 4b,e) exhibits larger aggregated particles with an average particle size of about 45.85 nm. Meanwhile, the Co-800 sample (Figure 4c,f) demonstrates an aggregated stick-like morphology, with an average particle size of approximately 90.19 nm.
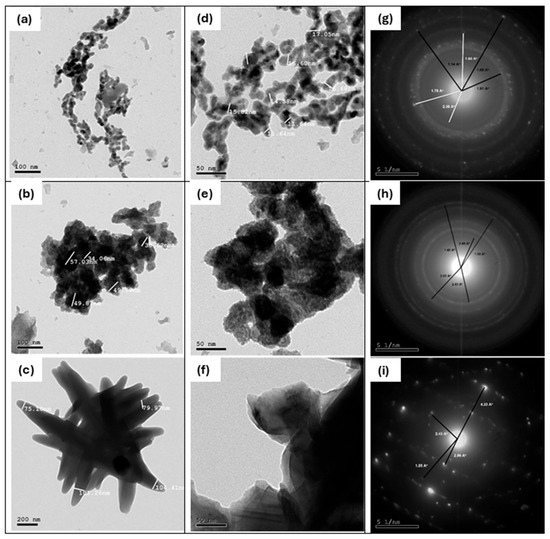
Figure 4.
HRTEM images of Co-25 (a,d), Co-400 (b,e), and Co-800 (c,f); and their SAED patterns (g–i), respectively.
The SAED patterns for these samples are presented in Figure 4g–i. The SAED pattern of the Co-25 sample confirms the coexistence of metallic cobalt (Co) and cobalt hydroxide (Co(OH)₂) phases, formed during the electrochemical deposition process. The metallic cobalt phase features a hexagonal crystal structure with unit cell parameters a = b = 2.503 A˚ and c = 4.060 A˚. Its main diffraction lines correspond to d-spacings of 1.91 Å, 1.15 Å, and 1.05 Å, attributed to the (101), (103), and (201) planes, respectively (JCPDS# 05-0727). The cobalt hydroxide (Co(OH)₂) phase also displays a hexagonal crystal structure with unit cell parameters a = b = 3.179 A˚ and c = 4.649 A˚, and key d-spacings of 2.38 Å, 3.48 Å, and 1.60 Å, corresponding to the (101), (102), and (110) planes (JCPDS# 03-0913).
For the Co-400 and Co-800 samples (Figure 4h,i), the SAED patterns reveal the presence of cubic cobalt oxide (Co3O4) with unit cell parameters a = b = c = 8.084 A˚. This phase is characterized by d-spacings of 2.43 Å, 1.43 Å, 2.86 Å, and 1.55 Å, corresponding to the (311), (440), (220), and (511) planes (JCPDS# 09-0418). Notably, the SAED pattern of the Co-800 sample exhibits spot patterns, indicative of single crystals or highly oriented polycrystalline materials. The phase structures identified through SAED analysis align with those observed in the XRD results.
3.1.5. Analysis of Surface Area and Pore Size
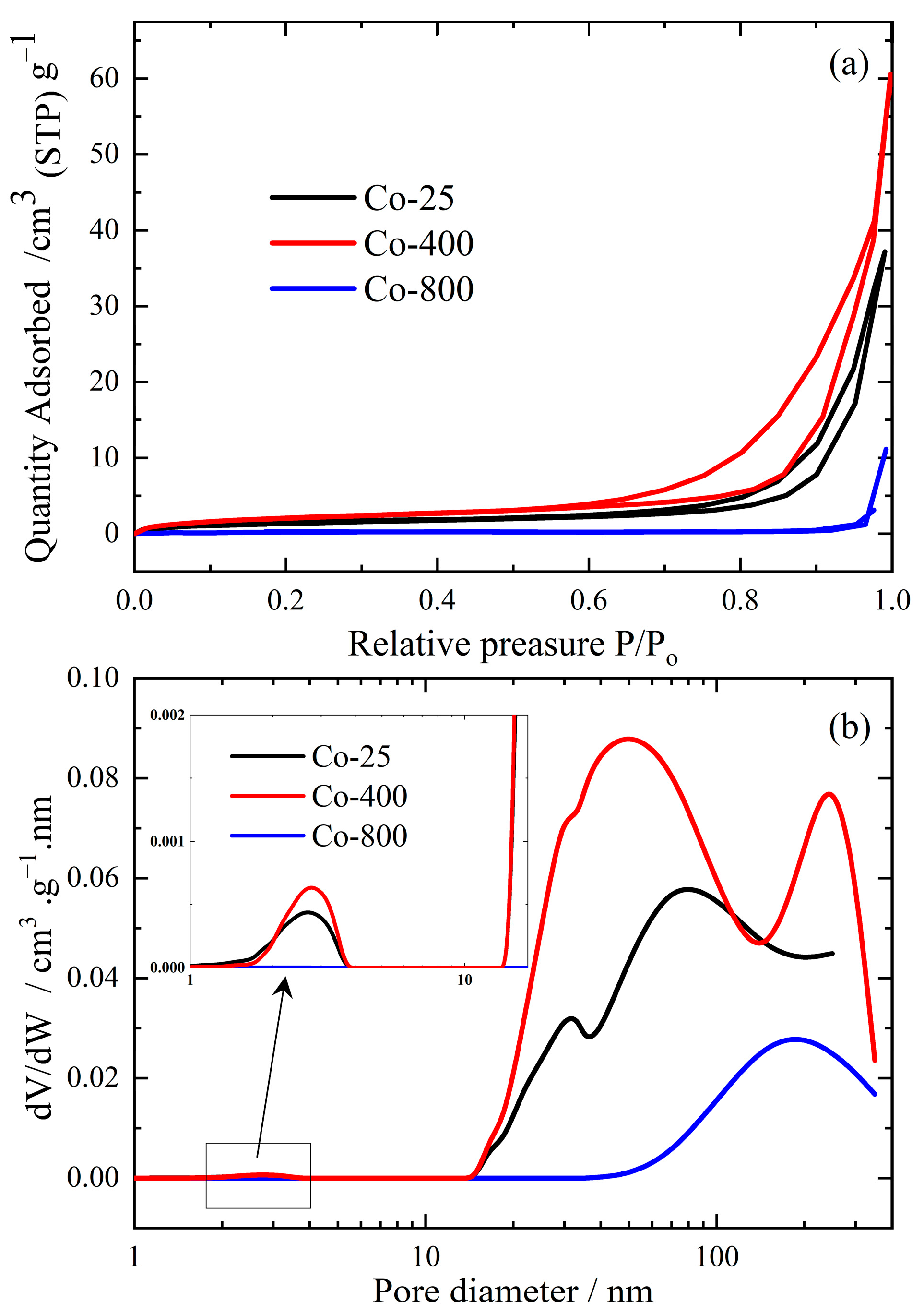
Conventional N2 adsorption/desorption isotherms, demonstrated in Figure 5a, were performed at −196 °C for the various prepared cobalt oxide samples. By applying the Brunauer–Emmet–Teller (BET) method [43], the main surface parameters, such as specific surface area (SBET), total pore volume (VP) at a relative vapor pressure (P/Po) ~ 0.95, and average pore width were evaluated at different annealing temperatures and listed in Table 1.
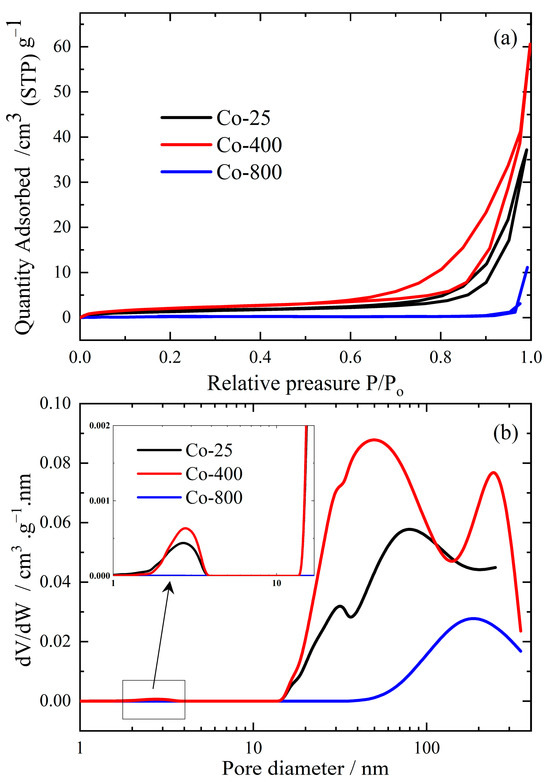
Figure 5.
N2 adsorption/desorption isotherms (a) and their corresponding pore size distribution (PSD) curves (b) for cobalt oxide samples.

Table 1.
Surface area and pore size analysis of the prepared cobalt oxide samples.
All cobalt oxide samples exhibited a typical type III adsorption/desorption isotherm [44], Figure 5a, with different hysteresis behavior. This difference in hysteresis behavior could be attributed to the different annealing temperatures. At a high temperature (800 °C), sintering and sealing of the mesoporous system occur, and consequently, a marked depression in the specific surface area is expected, as shown in Figure 5a. Samples Co-25 and Co-400 obey the type III isotherm with H3-hysteresis-loop that confirms the capillary condensation phenomenon inside the mesoporous structure. On the other hand, sample Co-800 obeys Type III without any hysteresis, which confirms the existence of macropores or a non-porous structure. The same observations are matched with BJH–pore size distribution (PSD) curves as clarified in Figure 5b. The figure illustrates that sample Co-400 possesses large numbers of mesopores of pore diameter 10–100 nm with a small number of micro- or macro-pores. Increasing the temperature to 800 °C destroyed the mesoporous system and converted it into a non-porous/macro-porous structure (pore diameter > 100 nm). The date from Table 1 confirms that Co-400 exhibits the highest texture parameters (SBET = 8.0094 m2/g, Vp = 0.08176 cm3/g, W = 40.49 nm). Therefore, this material is expected to have effective antibacterial and antifungal activity. In the same vein, many researchers have confirmed that excessively high temperatures can negatively affect tissue and morphological properties [45,46].
3.2. Electrochemical Studies
3.2.1. Potentiodynamic Polarization Behavior
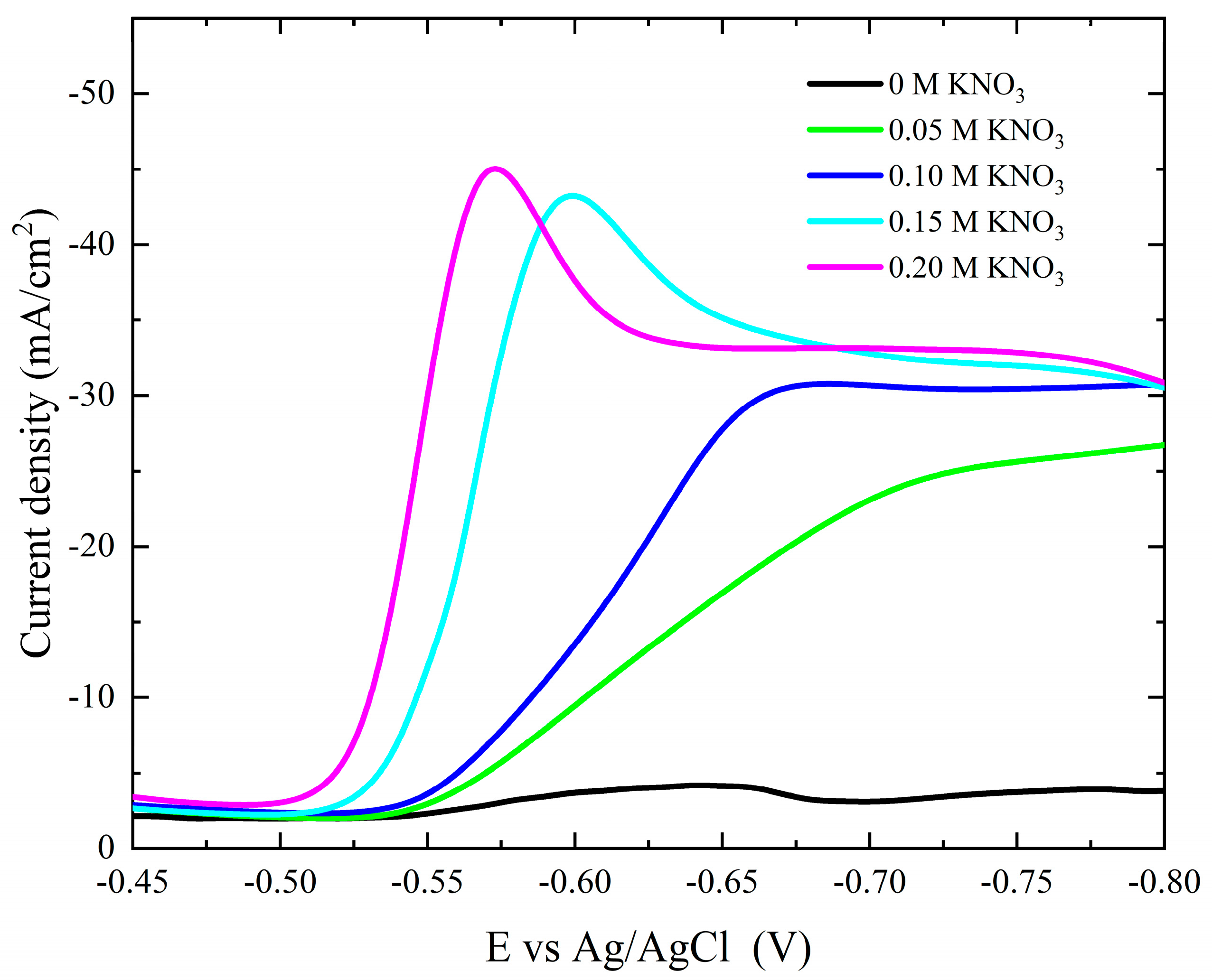
The characteristics of the (current–potential) polarization behavior for the cobalt deposition over copper sheets with different concentrations of KNO3 are shown in Figure 6. The addition of KNO3 to the electrolytic bath greatly increases the cathodic current, indicating the accelerating role of NO3− ions during the electrochemical reduction of Co2+ ions. As shown in the figure, as the KNO3 concentration increases, the onset potential and cathodic peak of deposition move towards the more noble potential, indicating the enhancement of the cobalt deposition.
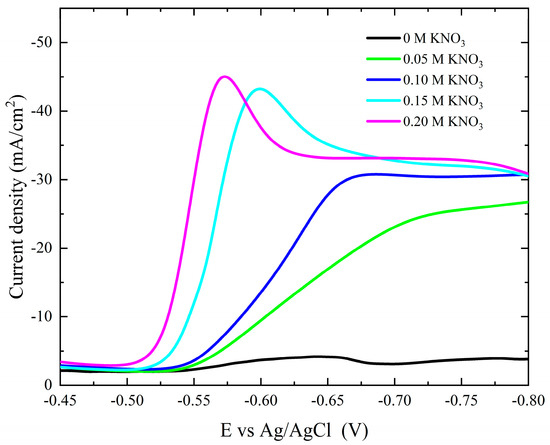
Figure 6.
Potentiodynamic cathodic polarization curves during cobalt deposition from the Watts bath in the absence and presence of different concentrations of NO3− ions.
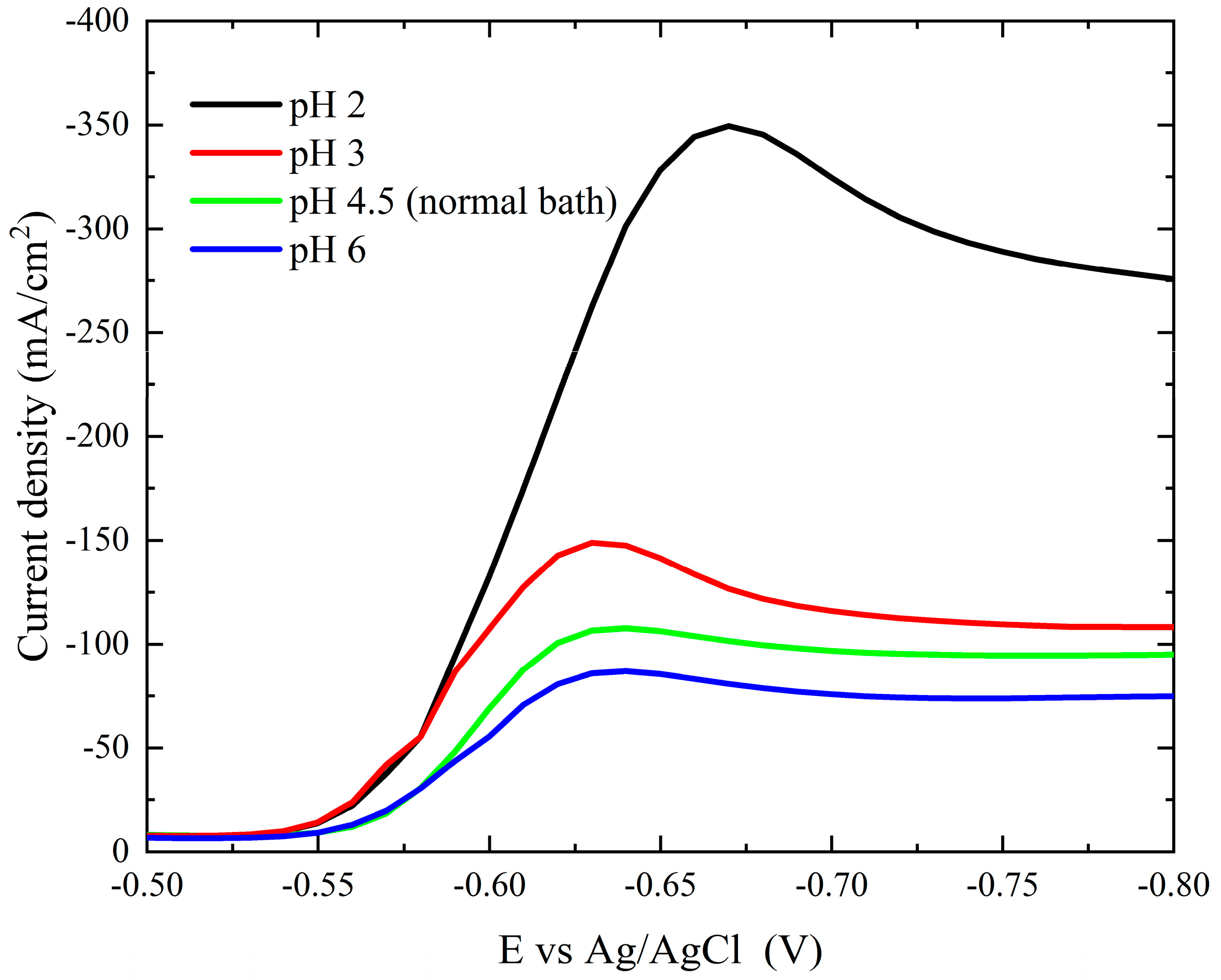
Two of the most important operating parameters are the pH value and bath temperature. Figure 7 illustrates how the cathodic polarization curves are affected by pH variations (2.0–6.0). The current polarization curves decrease as the pH of the bath increases. There is a huge drop in the cathodic polarization current when the pH changes from 2.0 to 3.0, and the deposition potential of the peak shifts to more noble potentials. This could be explained by the finding that elevated pH causes a reduction in the concentration of free ions Co2+.
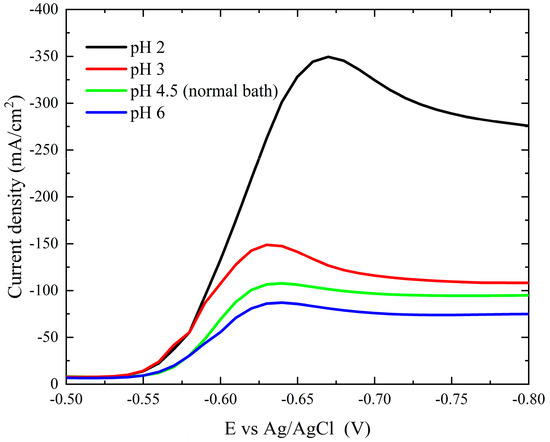
Figure 7.
Cathodic polarization curves during deposition of cobalt on a Pt electrode from the modified Watts bath at different pH.
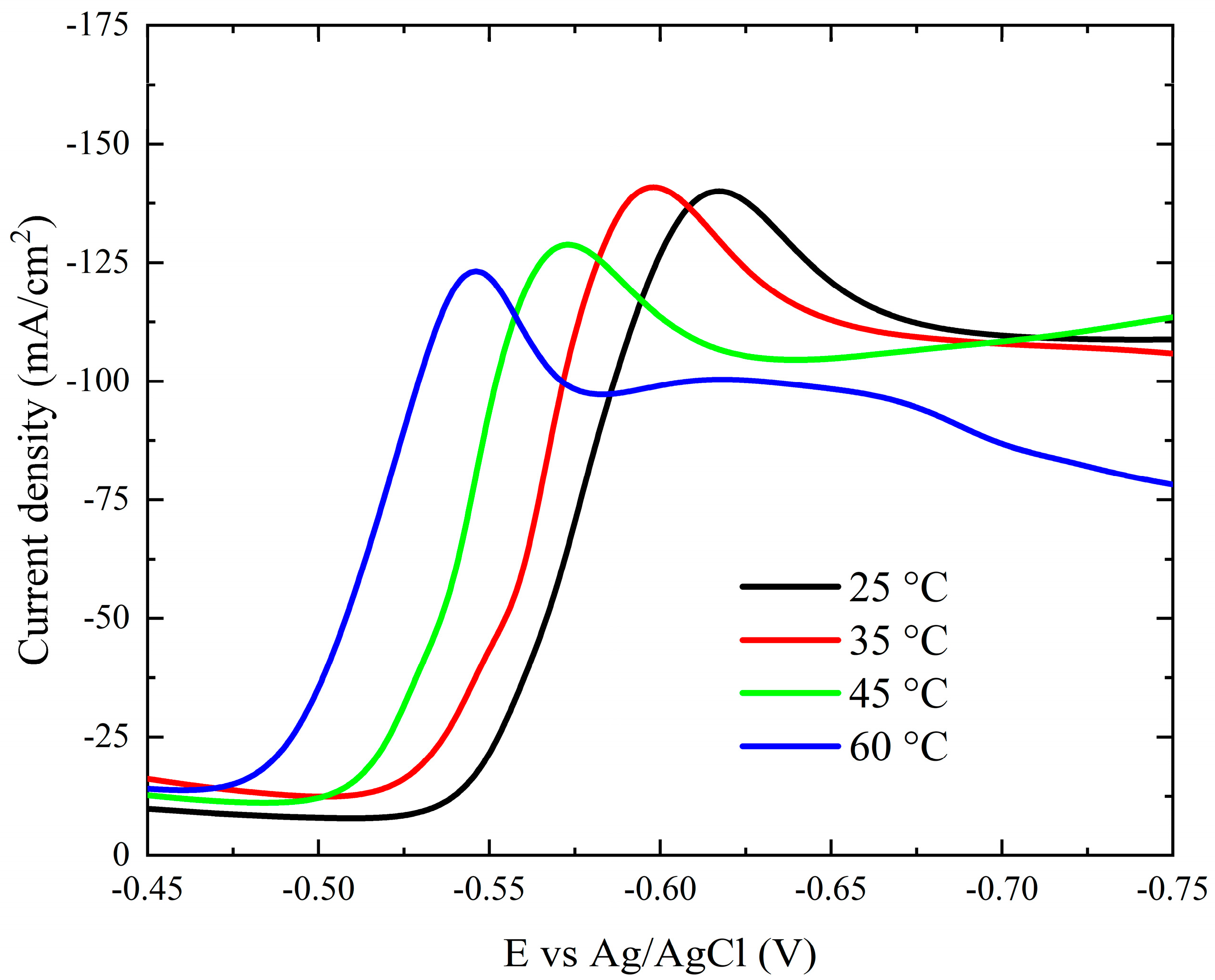
Figure 8 clearly shows how temperature (25–60 °C) affects the current–potential curves. As the bath temperature increases, the polarization curves and the onset potential of the deposition process shift to more noble potentials. A reduction in the activation overpotential of reducible species could be one reason for this. Furthermore, as the bath temperature rises, so do the diffusion coefficients of the reducible species.
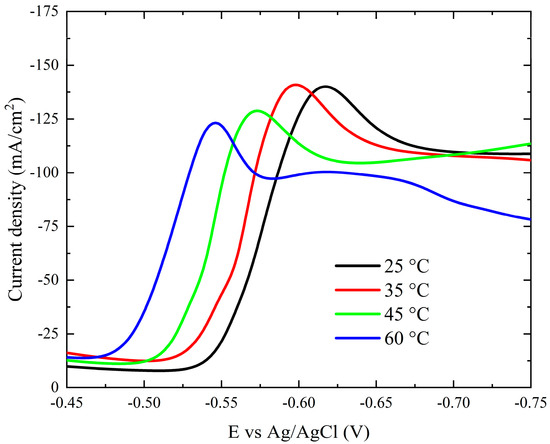
Figure 8.
Cathodic polarization curves during deposition of cobalt on Pt electrode from the modified Watts bath at different temperatures.
3.2.2. Chronoamperometric Analysis
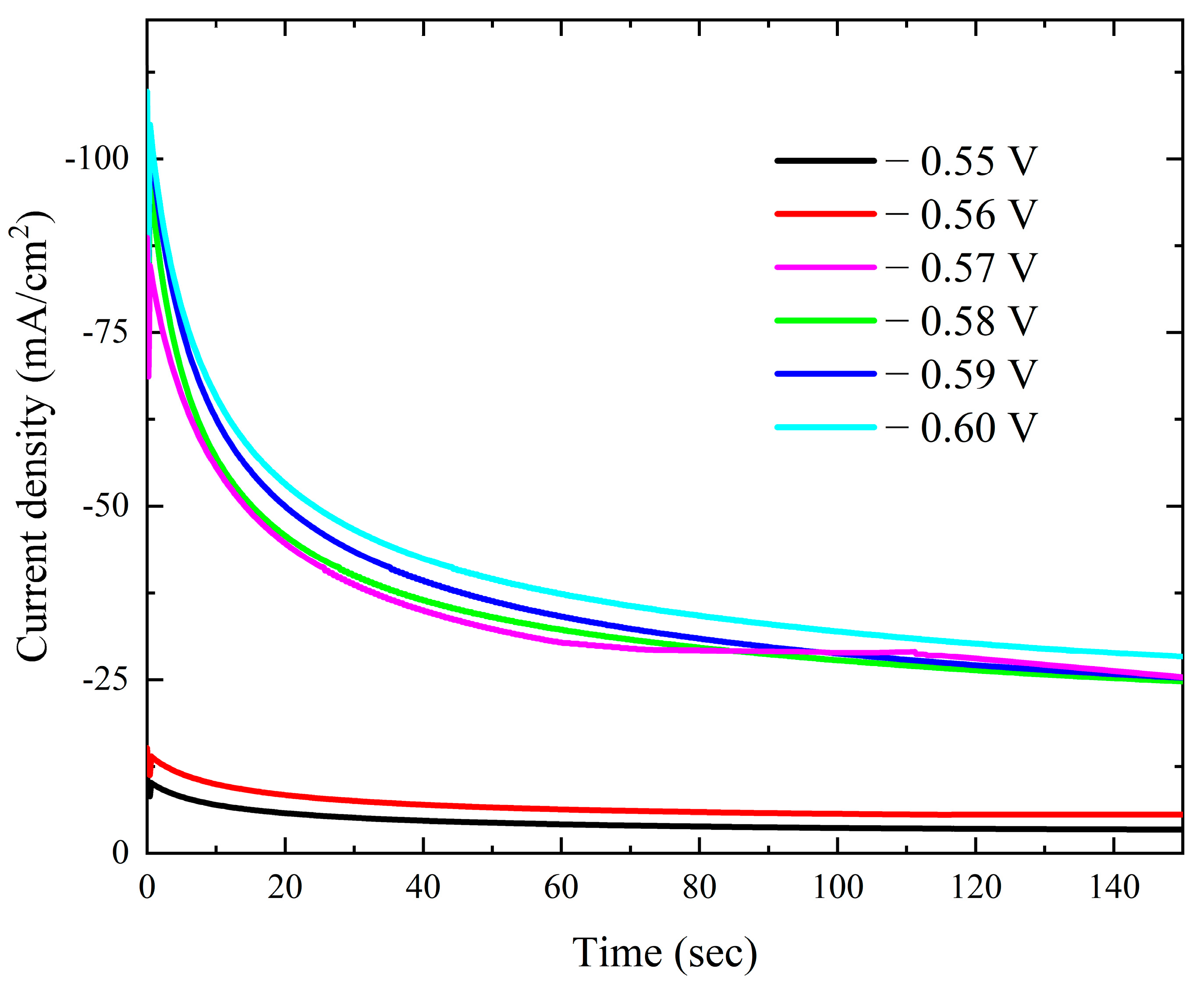
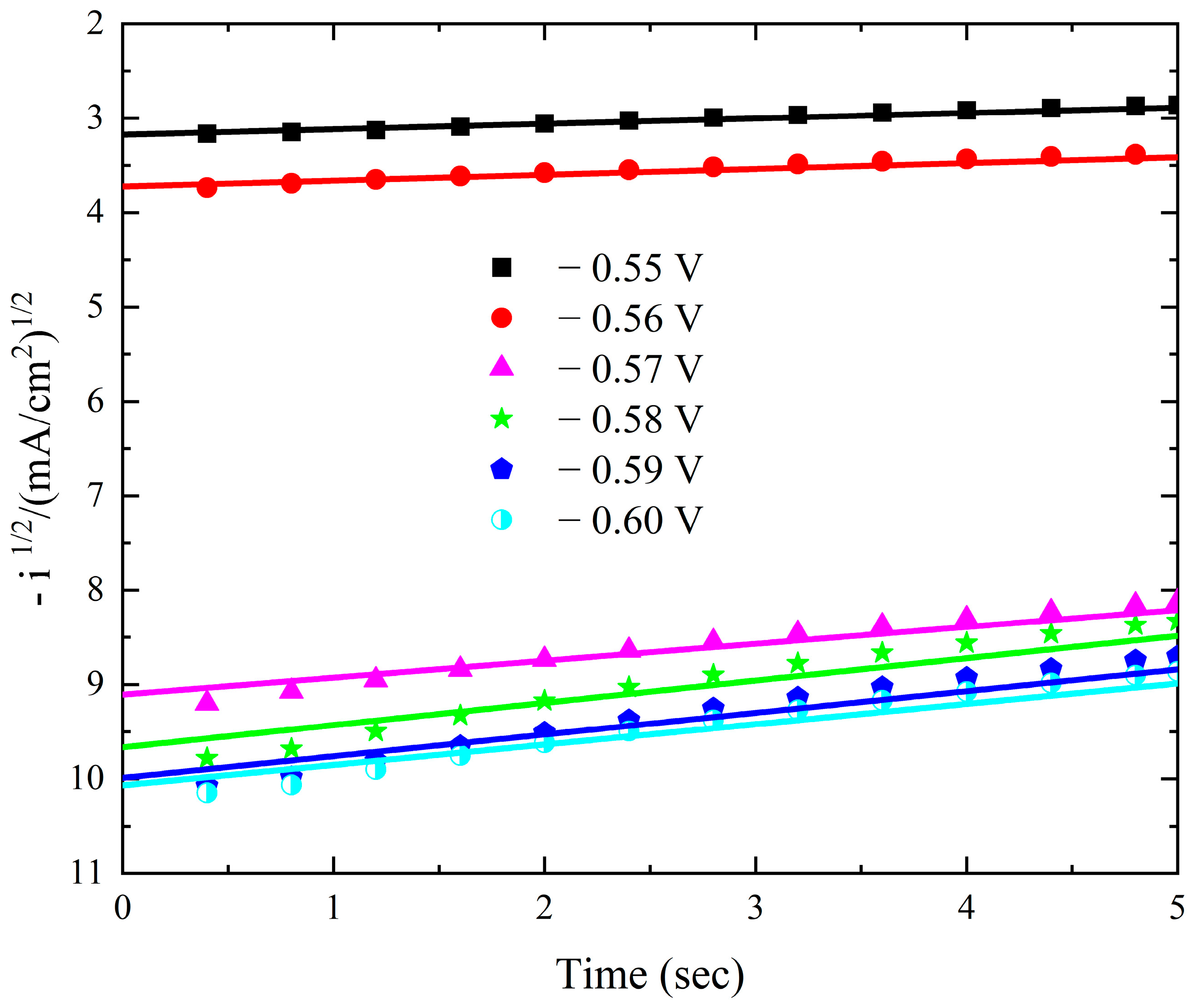
The current–time transients from the modified Watts bath with 0.15 M KNO3 over the Pt electrode at different deposition potentials are displayed in Figure 9. There is a gradual decrease in the current–time transients as the applied potential decreases. The current gradually drops at any potential until it reaches a nearly constant value. The initial current drop is caused by the double-layer charging process [47]. Additionally, the results verify that mass transfer controls the rate of nuclei growth by showing that the steady-state current rises as the potential becomes more negative. The Scharifker et al. models were employed to investigate the nucleation mechanism, specifically to determine whether it follows an instantaneous or progressive pathway. However, the experimental results obtained at all applied deposition potentials exhibited significant deviations from both theoretical models. This deviation is a well-documented characteristic of cobalt electrodeposition and is attributed to the concurrent occurrence of the oxygen reduction reaction (ORR) within the same potential range, which interferes with the nucleation and growth process [8,48]. Plotting –i1/2 against time t in Figure 10 demonstrates a strong linearity.
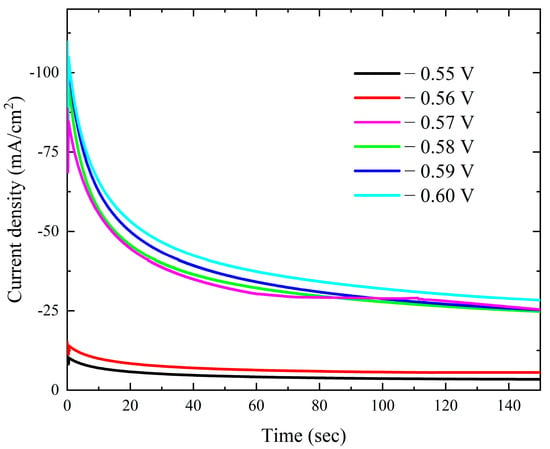
Figure 9.
Potentiostatic current–time transient curves for deposition of cobalt on Pt electrode from the modified Watts bath at different potentials.
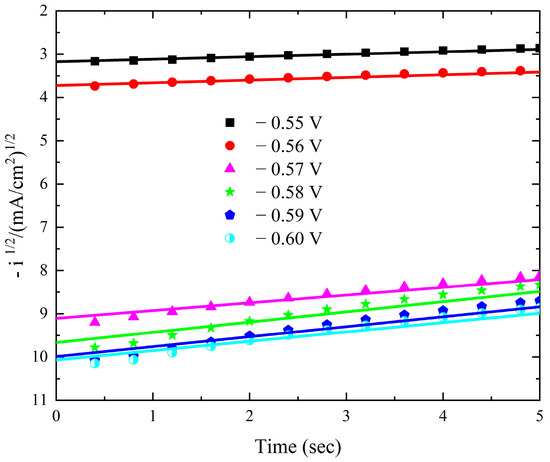
Figure 10.
Linear relationship between −i1/2 and t during deposition of cobalt oxide on Pt from the Watts bath with 0.15 M KNO3 at different deposition potentials.
The cobalt oxide deposit is caused by a mechanism that combines instantaneous nucleation with charge transfer-controlled three-dimensional growth. The slopes of these lines correspond to the charge transfer rate constant.
3.3. Biological Activities of Co3O4 NPs
3.3.1. Anti-Bacterial Activity
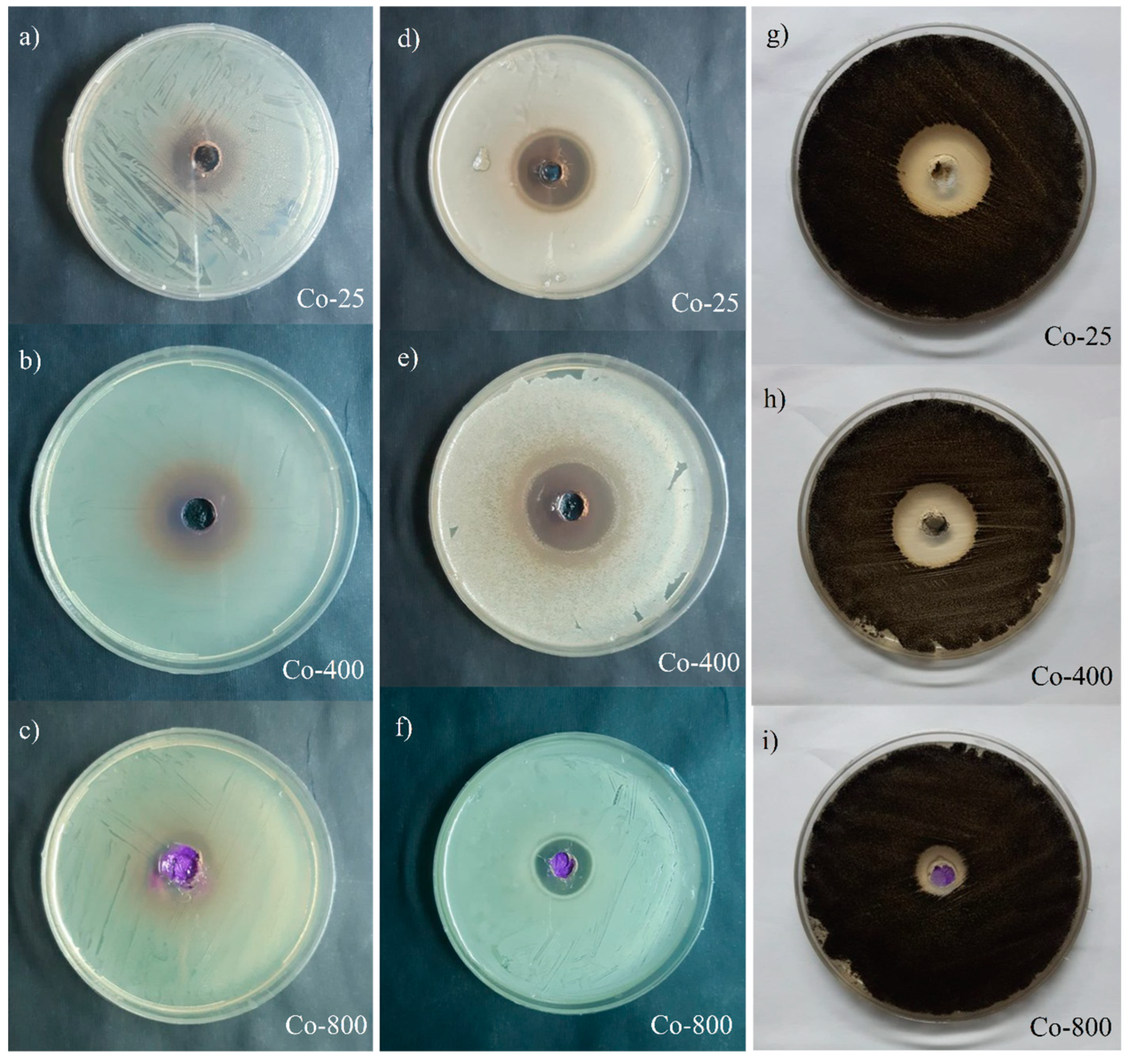
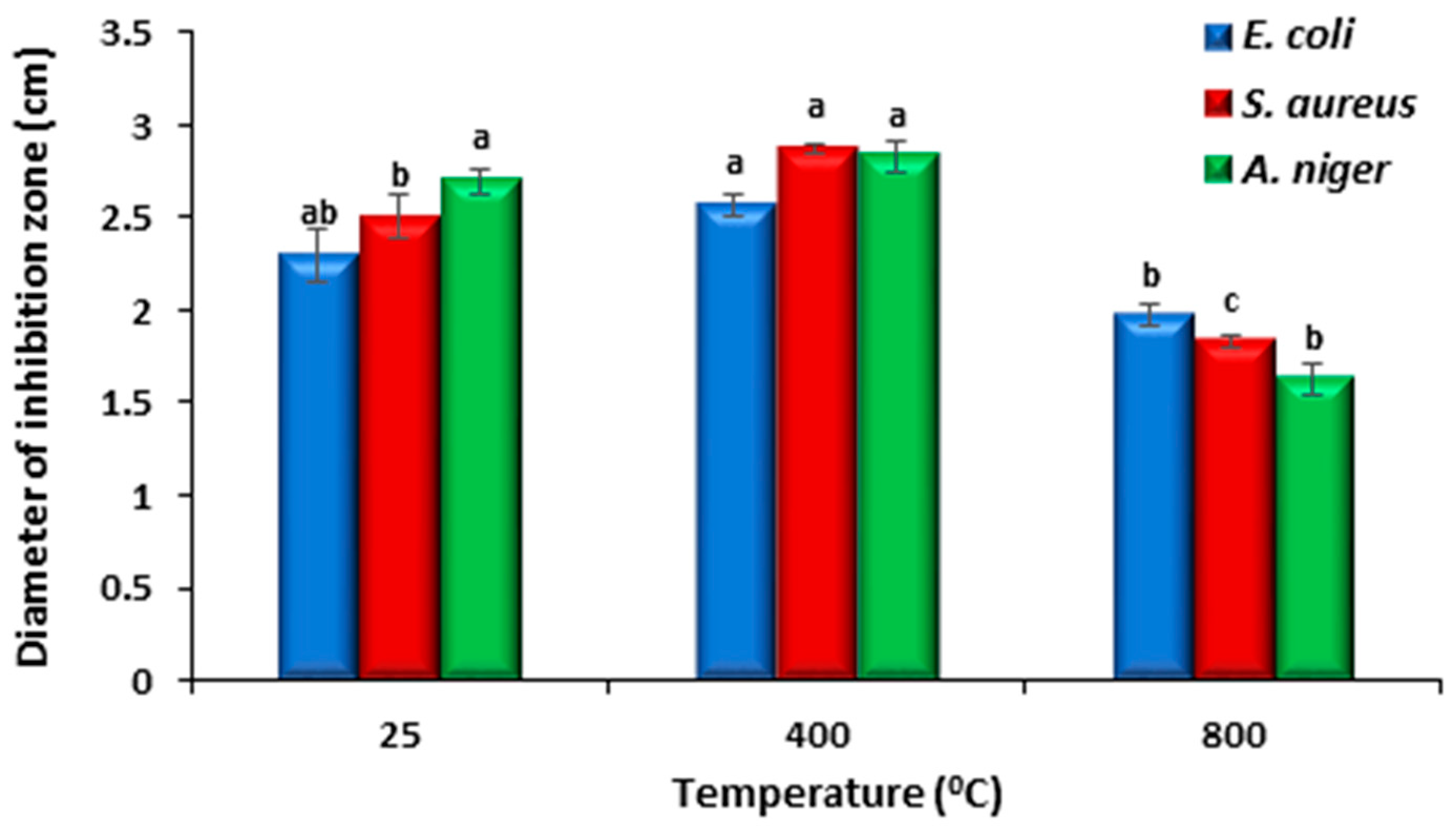
The agar well diffusion technique was used to assess the antibacterial activity of the generated cobalt oxide nanoparticles. The NPs have strong antibacterial properties because they are small and have a large surface area compared to larger molecules. By breaking down the cell membrane, the NPs prevent the bacterial production of proteins and exhibit dose-dependent membrane penetration [49,50]. A well diffusion assay was used to observe the antibacterial activity of the produced oxide NPs against S. aureus and E. coli. Figure 11 provides a representative example of an image displaying the inhibition zones. The presence of an inhibition zone indicates the antibacterial effect of the nanoparticles. A one-way ANOVA procedure was performed on the obtained data. Figure 12 represents the inhibition zone diameters of samples prepared at different annealing temperatures. The produced oxide nanoparticles (NPs) demonstrated exceptional antibacterial activity against S. aureus and E. coli, as indicated in Figure 12. The oxide made after annealing at 400 °C exhibits the best antibacterial activity because it has the largest surface area and total pore volume (8.0094 m2/g and 0.081076 m3/g, respectively). The outcome demonstrated that the NPs’ biocide efficacy against these bacteria is promising. The action of Co3O4 NPs against S. aureus was more effective in comparison to E. coli. This might be due to the difference in the structure of the bacteria. For comparison, the antibacterial activity of our samples is more efficient than that reported for Co3O4 prepared by a green method using Piper nigrum (P. nigrum) leaves extract and annealed at 100 °C [51]. Additionally, they demonstrated that Co3O4 is more efficient for Gram-negative bacteria than Gram-positive bacteria.
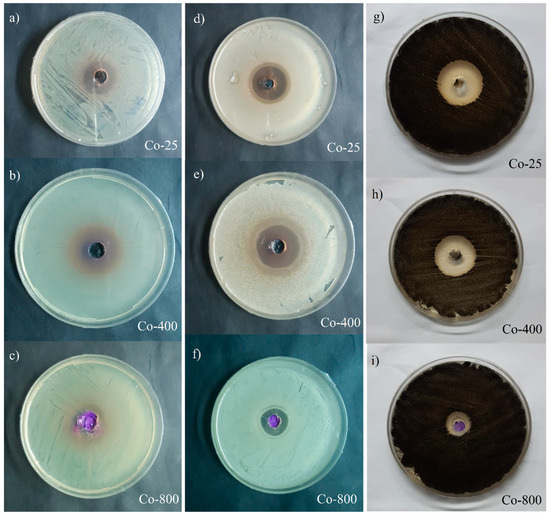
Figure 11.
Inhibition zone on an agar plate for Co-25 °C ((a) “E. coli ” and (d) “S. aureus”), Co-400 °C ((b) “E. coli” and (e) “S. aureus”, and Co-800 °C ((c) “E. coli ” and (f) “S. aureus”). A. niger (MT 103092) (g–i) for Co oxide samples.
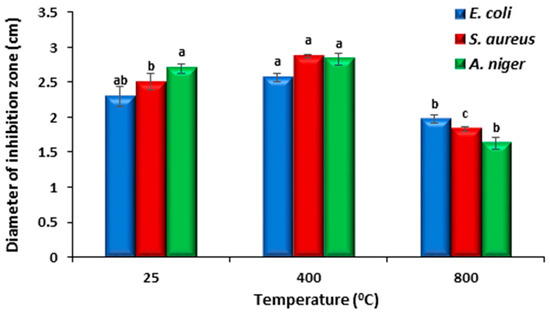
Figure 12.
The inhibition zone diameters of samples prepared at different annealing temperatures. Bars represent standard error; Different letters indicate significant differences in access treatment means from repeated-measures ANOVA.
The zone of inhibition in this investigation demonstrated the biocidal activity of Co3O4 NPs, which may be the result of bacterial membrane disruption. Furthermore, the nanoparticles’ large surface area may cause them to adhere tightly to the bacterial cells’ surface, further disrupting the bacterial membrane and allowing intracellular components to leak out, ultimately killing the bacterial cells [52]. These results are incongruence with the earlier published study in which E. coli has shown more sensitivity than S. aureus for Co3O4, CuO, and Co nanoparticles [53,54].
3.3.2. Antifungal Activity
The rate at which bacteria and fungi are becoming resistant to the available medications and antibiotics is concerning. Strong antifungal agents are therefore required to eradicate fungi that are resistant to the current medications [55]. The antifungal properties of cobalt and cobalt oxide nanoparticles are among their many biomedical uses. The synthesis of cobalt oxide nanoparticles (NPs) with H. rosa-sinensis flower extract and their antifungal properties have been documented. The outcome demonstrated that the produced NPs exhibited potent anti-A. niger and anti-A. flavus properties [56]. In our study, the produced cobalt oxide NPs demonstrated exceptional antifungal activity against Aspergillus niger, and the best antifungal effect is produced with the sample annealed at 400 °C as shown in Figure 12. The action of oxide NPs prepared at 25 °C is more efficient towards Aspergillus niger than that towards S. aureus and E. coli. The antifungal activity of the cobalt oxide (CO-400) is higher than the antibacterial activity of E. coli. However, for S. aureus, no significant difference was observed (Figure 12).
3.4. Mechanism of Antimirobial
Co3O4 NPs were found to exhibit good activity against both bacteria and fungi. The differences in susceptibility are explained by the different bacterial strains’ varying tolerance to oxidative stress. The primary factors influencing Co3O4 NPs’ antibacterial activity are their size and shape. There are multiple explanations for metal oxide nanoparticles’ antibacterial qualities. Reactive species, like hydroxyl radicals and superoxide radical anions, are created in aqueous solutions of Co3O4 NPs. The bacterium’s negatively charged surface interferes with essential biological functions, like respiration and cell division, by interacting with the radicals found in aqueous Co3O4 NPs. On the other hand, cobalt ions from cobalt oxides interact with the thiol group of essential bacterial enzymes, causing deactivation and cell death. An excited electron is produced when the surface of Co3O4 NPs interacts with absorbed O2. This electron combines with the oxygen ion to form the oxygen ion, which subsequently interacts with the H2O molecule to produce hydrogen peroxide. Consequently, H2O2 enters the cell, disrupting cytoplasmic functions and causing death [57,58].
4. Conclusions
Cobalt oxide NPs were successfully prepared by galvanostatic electrodeposition onto a copper substrate from a modified Watts bath, followed by annealing. The electrolytic bath contained CoSO4, CoCl2, H3BO3, and KNO3 and was operated at a pH of 4.5, with a low current density of 4.8 mA/cm2. The addition of KNO3 to the electrolytic bath significantly increases the cathodic current, indicating the accelerating role of NO3⁻ ions during the electrochemical reduction of Co2⁺ ions. The deposited cobalt was subsequently annealed at 400 °C and 800 °C to produce the cobalt oxide (Co3O4) phase. The surface morphology and characterization of the cobalt samples were analyzed using HRSEM, XRD, FTIR, and BET techniques. The nature of the oxide formed was strongly dependent on the annealing temperature. The powder formed at room temperature (25 °C) is a mixture of Co(OH)2 and metallic Co. However, at 400 and 800 °C and according to the XRD patterns, the powder is primarily composed of the Co3O4 phase and a slight quantity of Co(OH)2 phase. The average particle size measured by TEM ranged from 14.85 nm at room temperature to 90.19 nm at 800 °C. On the other hand, the produced oxide nanoparticles (NPs) demonstrated exceptional antibacterial activity against S. aureus and E. coli in addition to their effectiveness in terms of antifungal activity, and their activities are strongly dependent on the annealing temperature. The oxide made after annealing at 400 °C exhibits the best antibacterial and antifungal activity because it has the largest surface area and total pore volume.
Author Contributions
I.M.A.O.: Conceptualization, Data curation, Writing—original draft, Writing—review & editing, Investigation, Formal analysis, Methodology. M.E.S.: Conceptualization, Data curation, Writing—original draft, Writing—review & editing, Visualization, Investigation, Formal analysis, Methodology. M.A.M.I.: Conceptualization, Data curation, Writing—original draft, Writing—review & editing, Visualization, Investigation, Formal analysis, Methodology. M.A.E.-J.: Conceptualization, Visualization, Investigation, Formal analysis, Methodology, Writing—review & editing, Visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- El-Shafie, A.S.; Ahsan, I.; Radhwani, M.; Al-Khangi, M.A.; El-Azazy, M. Synthesis and application of cobalt oxide (Co3O4)-impregnated olive stones biochar for the removal of rifampicin and tigecycline: Multivariate controlled performance. Nanomaterials 2022, 12, 379. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, A.S.; Oyekunle, J.A.; Durosinmi, L.M.; Oluwafemi, O.S.; Olayanju, D.S.; Akinola, A.S.; Obisesan, O.R.; Akinyele, O.F.; Ajayeoba, T.A. Potential of cobalt and cobalt oxide nanoparticles as nanocatalyst towards dyes degradation in wastewater. Nano-Struct. Nano-Objects. 2020, 21, 100405. [Google Scholar] [CrossRef]
- El Boraei, N.; Ibrahim, M. Black binary nickel cobalt oxide nano-powder prepared by cathodic electrodeposition; characterization and its efficient application on removing the Remazol Red textile dye from aqueous solution. Mater. Chem. Phys. 2019, 238, 121894. [Google Scholar] [CrossRef]
- El Boraei, N.F.; Ibrahim, M.; Kamal, R. Facile synthesis of mesoporous ncCoW powder via electrodeposition; characterization and its efficient application on hydrogen generation and organic pollutants reduction. Surf. Interfaces 2024, 44, 103621. [Google Scholar] [CrossRef]
- Wahab, R.; Ahmad, N.; Alam, M.; Ahmad, J. The development of cobalt oxide nanoparticles based electrode to elucidate the rapid sensing of nitrophenol. Mater. Sci. Eng. B 2021, 265, 114994. [Google Scholar] [CrossRef]
- Samal, R.; Biswal, A.; Dash, B.; Sanjay, K.; Subbaiah, T.; Shin, S. Preparation and characterization of cobalt oxide by electrochemical technique. In Proceedings of the XIII International Seminar on Mineral Processing Technology (MPT-2013), Bhubaneswar, India, 10–12 December 2013. [Google Scholar]
- Abdel-Samad, H.S.; El-Jemni, M.A.; Abd El Rehim, S.S.; Hassan, H.H. Simply prepared α-Ni (OH)2-based electrode for efficient electrocatalysis of EOR and OER. Electrochim. Acta 2024, 503, 144896. [Google Scholar] [CrossRef]
- El-Jemni, M.A.; Abdel-Samad, H.S.; AlKordi, M.H.; Hassan, H.H. Normalization of the EOR catalytic efficiency measurements based on RRDE study for simply fabricated cost-effective Co/graphite electrode for DAEFCs. J. Electroanal. Chem. 2022, 918, 116488. [Google Scholar] [CrossRef]
- El-Jemni, M.A.; Abdel-Samad, H.S.; Essa, A.S.; Hassan, H.H. Controlled electrodeposited cobalt phases for efficient OER catalysis, RRDE and eQCM studies. Electrochim. Acta 2019, 313, 403–414. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Ponnalagi, A.K.; Nagamuthu, S.; Muralidharan, G. Microwave assisted synthesis of Co3O4 nanoparticles for high-performance supercapacitors. Electrochim. Acta 2013, 106, 500–505. [Google Scholar] [CrossRef]
- Guo, L.; Huang, Q.; Li, X.-Y.; Yang, S. Iron nanoparticles: Synthesis and applications in surface enhanced Raman scattering and electrocatalysis. Phys. Chem. Chem. Phys. 2001, 3, 1661–1665. [Google Scholar] [CrossRef]
- Sharma, B.K.; Shah, D.V.; Roy, D.R. Green synthesis of CuO nanoparticles using Azadirachta indica and its antibacterial activity for medicinal applications. Mater. Res. Express 2018, 5, 095033. [Google Scholar] [CrossRef]
- Saliani, M.; Jalal, R.; Goharshadi, E.K. Effects of pH and temperature on antibacterial activity of zinc oxide nanofluid against Escherichia coli O157: H7 and Staphylococcus aureus. Jundishapur J. Microbiol. 2015, 8, e17115. [Google Scholar] [CrossRef] [PubMed]
- Ayanwale, A.P.; Estrada-Capetillo, B.L.; Reyes-López, S.Y. Evaluation of antifungal activity by mixed oxide metallic nanocomposite against Candida spp. Processes 2021, 9, 773. [Google Scholar] [CrossRef]
- Anuradha, C.; Raji, P. Facile-synthesis and characterization of cobalt oxide (Co3O4) nanoparticles by using Arishta leaves assisted biological molecules and its antibacterial and antifungal activities. J. Mol. Struct. 2022, 1262, 133065. [Google Scholar] [CrossRef]
- Das, D.; Saikia, B.J. Synthesis, characterization and biological applications of cobalt oxide (Co3O4) nanoparticles. Chem. Phys. Impact. 2023, 6, 100137. [Google Scholar] [CrossRef]
- Gupta, V.; Kant, V.; Sharma, A.K.; Sharma, M. Comparative evaluation of antibacterial potentials of nano cobalt oxide with standard antimicrobials. J. Indian Chem. Soc. 2022, 99, 100533. [Google Scholar] [CrossRef]
- Farhadi, S.; Safabakhsh, J.; Zaringhadam, P. Synthesis, characterization, and investigation of optical and magnetic properties of cobalt oxide (Co3O4) nanoparticles. J. Nanostruct. Chem. 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Moradpoor, H.; Safaei, M.; Rezaei, F.; Golshah, A.; Jamshidy, L.; Hatam, R.; Abdullah, R.S. Optimisation of cobalt oxide nanoparticles synthesis as bactericidal agents. Open Access Maced. J. Med. Sci. 2019, 7, 2757. [Google Scholar] [CrossRef]
- Yetim, N.K. Hydrothermal synthesis of Co3O4 with different morphology: Investigation of magnetic and electrochemical properties. J. Mol. Struct. 2021, 1226, 129414. [Google Scholar] [CrossRef]
- Vattikuti, S.P.; Hoang Ngoc, C.T.; Nguyen, H.; Nguyen Thi, N.H.; Shim, J.; Dang, N.N. Carbon nitride coupled Co3O4: A pyrolysis-based approach for high-performance hybrid energy storage. J. Phys. Chem. Lett. 2023, 14, 9412–9423. [Google Scholar] [CrossRef]
- Zhu, T.; Chong, M.N.; Phuan, Y.W.; Ocon, J.D.; Chan, E.S. Effects of electrodeposition synthesis parameters on the photoactivity of nanostructured tungsten trioxide thin films: Optimisation study using response surface methodology. J. Taiwan Inst. Chem. Eng. 2016, 61, 196–204. [Google Scholar] [CrossRef]
- Petrović, M.M.; Slipper, I.J.; Antonijević, M.D.; Nikolić, G.S.; Mitrović, J.Z.; Bojić, D.V.; Bojić, A.L. Characterization of a Bi2O3 coat based anode prepared by galvanostatic electrodeposition and its use for the electrochemical degradation of Reactive Orange 4. J. Taiwan Inst. Chem. Eng. 2015, 50, 282–287. [Google Scholar] [CrossRef]
- He, S.; Xu, R.; Hu, G.; Chen, B. Electrosynthesis and performance of WC and Co3O4 co-doped α-PbO2 electrodes. RSC Adv. 2016, 6, 3362–3371. [Google Scholar] [CrossRef]
- Casella, I.G.; Di Fonzo, D.A. Anodic electrodeposition of cobalt oxides from an alkaline bath containing Co-gluconate complexes on glassy carbon. An electroanalytical investigation. Electrochim. Acta 2011, 56, 7536–7540. [Google Scholar] [CrossRef]
- Lisnund, S.; Blay, V.; Muamkhunthod, P.; Thunyanon, K.; Pansalee, J.; Monkrathok, J.; Maneechote, P.; Chansaenpak, K.; Pinyou, P. Electrodeposition of cobalt oxides on Carbon nanotubes for sensitive bromhexine sensing. Molecules 2022, 27, 4078. [Google Scholar] [CrossRef]
- Moridon, S.N.F.; Salehmin, M.I.; Mohamed, M.A.; Arifin, K.; Minggu, L.J.; Kassim, M.B. Cobalt oxide as photocatalyst for water splitting: Temperature-dependent phase structures. Int. J. Hydrogen Energy 2019, 44, 25495–25504. [Google Scholar] [CrossRef]
- Oh, S.W.; Bang, H.J.; Bae, Y.C.; Sun, Y.-K. Effect of calcination temperature on morphology, crystallinity and electrochemical properties of nano-crystalline metal oxides (Co3O4, CuO, and NiO) prepared via ultrasonic spray pyrolysis. J. Power Sources 2007, 173, 502–509. [Google Scholar] [CrossRef]
- Louardi, A.; Rmili, A.; Chtouki, T.; Elidrissi, B.; Erguig, H.; Bachiri, A.E.; Ammous, K.; Mejbri, H. Effect of annealing treatment on Co3O4 thin films properties prepared by spray pyrolysis. J. Mater. Environ. Sci 2017, 8, 485–493. [Google Scholar]
- Bao, Y.; Krishnan, K.M. Preparation of functionalized and gold-coated cobalt nanocrystals for biomedical applications. J. Magn. Magn. Mater. 2005, 293, 15–19. [Google Scholar] [CrossRef]
- Suganya, K.U.; Govindaraju, K.; Kumar, V.G.; Dhas, T.S.; Karthick, V.; Singaravelu, G.; Elanchezhiyan, M. Blue green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against Gram positive organisms. Mater. Sci. Eng. C 2015, 47, 351–356. [Google Scholar] [CrossRef]
- Reddy, N.J.; Vali, D.N.; Rani, M.; Rani, S.S. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater. Sci. Eng. C 2014, 34, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Rehman, W.; Waseem, M.; Elmnasri, K.; Hedfi, A.; Ben Ali, M.; Mahmoudi, E.; Rehman, M.U.; Khan, B. Variation in the crystallinity of cobalt oxide nanoparticles with increasing annealing temperature and pH. Dig. J. Nanomater. Bios. 2023, 18, 1079–1084. [Google Scholar] [CrossRef]
- Frost, R.L.; Kristof, J.; Paroz, G.N.; Kloprogge, J. Role of water in the intercalation of kaolinite with hydrazine. J. Colloid Interface Sci. 1998, 208, 216–225. [Google Scholar] [CrossRef]
- Hu, Z.-A.; Xie, Y.-L.; Wang, Y.-X.; Xie, L.-J.; Fu, G.-R.; Jin, X.-Q.; Zhang, Z.-Y.; Yang, Y.-Y.; Wu, H.-Y. Synthesis of α-cobalt hydroxides with different intercalated anions and effects of intercalated anions on their morphology, basal plane spacing, and capacitive property. J. Phys. Chem. C 2009, 113, 12502–12508. [Google Scholar] [CrossRef]
- Nethravathi, C.; Viswanath, B.; Sebastian, M.; Rajamathi, M. Exfoliation of α-hydroxides of nickel and cobalt in water. J. Colloid Interface Sci. 2010, 345, 109–115. [Google Scholar] [CrossRef]
- Cui, H.; Ma, W.; Wang, L.; Xue, J. Preparation of α-Co (OH)2 monolayer nanosheets by an intercalation agent-free exfoliation process. J. Solgel Sci. Technol. 2016, 78, 293–298. [Google Scholar] [CrossRef]
- Roth, W. The magnetic structure of Co3O4. J. Phys. Chem. Solids 1964, 25, 1–10. [Google Scholar] [CrossRef]
- Sundararajan, M.; Subramani, A.; Ubaidullah, M.; Shaikh, S.F.; Pandit, B.; Jesudoss, S.; Kamalakannan, M.; Yuvaraj, S.; Subudhi, P.S.; Dash, C.S. Synthesis, characterization and in vitro cytotoxic effects of Cu: Co3O4 nanoparticles via microwave combustion method. J. Clust. Sci. 2022, 33, 1821–1830. [Google Scholar] [CrossRef]
- Allaedini, G.; Muhammad, A. Study of influential factors in synthesis and characterization of cobalt oxide nanoparticles. J. Nanostruct. Chem. 2013, 3, 1–16. [Google Scholar] [CrossRef]
- Li, W.-C.; Lu, A.-H.; Guo, S.-C. Characterization of the microstructures of organic and carbon aerogels based upon mixed cresol–formaldehyde. Carbon 2001, 39, 1989–1994. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmet, P.; Teller, E. The adsorptiorrof gases in Multimolecular Layer. J. Am. Chem. Soc. 1938, 60, 39. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.; Pernicone, N.; Ramsay, J.D.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Mohsen, A.; Alharbi, Y.R.; Abadel, A.A.; Soliman, A.M.; Kohail, M.; Huang, H.; Ramadan, M. Facile synthesis and optimization of reactive bunsenite for the production of thermally stable geopolymeric composite. J. Mater. Res. Technol. 2023, 27, 876–893. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, H.; Zhang, Q. Preparation of NiO nanoflakes under different calcination temperatures and their supercapacitive and optical properties. Appl. Surf. Sci. 2017, 392, 1097–1106. [Google Scholar] [CrossRef]
- El Boraei, N.F.; Ibrahim, M.A.M.; Naghmash, M. Nanocrystalline FeNi alloy powder prepared by electrolytic synthesis; characterization and its high efficiency in removing Remazol Red dye from aqueous solution. J. Phys. Chem. Solids 2022, 167, 110714. [Google Scholar] [CrossRef]
- El-Jemni, M.A.; Abdel-Samad, H.S.; Hassan, H.H. On the deconvolution of the concurrent cathodic processes with cobalt deposition onto graphite from feebly acidic bath. J. Appl. Electrochem. 2021, 51, 1705–1719. [Google Scholar] [CrossRef]
- Shahzadi, T.; Zaib, M.; Riaz, T.; Shehzadi, S.; Abbasi, M.A.; Shahid, M. Synthesis of eco-friendly cobalt nanoparticles using Celosia argentea plant extract and their efficacy studies as antioxidant, antibacterial, hemolytic and catalytical agent. Arab. J. Sci. Eng. 2019, 44, 6435–6444. [Google Scholar] [CrossRef]
- Shriniwas, P.P.; Subhash, T.K. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem. Biophys. Rep. 2017, 10, 76–81. [Google Scholar]
- Haq, S.; Abbasi, F.; Ali, M.B.; Hedfi, A.; Mezni, A.; Rehman, W.; Waseem, M.; Khan, A.R.; Shaheen, H. Green synthesis of cobalt oxide nanoparticles and the effect of annealing temperature on their physiochemical and biological properties. Mater. Res. Express 2021, 8, 075009. [Google Scholar] [CrossRef]
- Arya, G.; Sharma, N.; Mankamna, R.; Nimesh, S. Antimicrobial silver nanoparticles: Future of nanomaterials. In Microbial Nanobionics: Volume 2, Basic Research and Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 89–119. [Google Scholar]
- Ghosh, T.; Dash, S.K.; Chakraborty, P.; Guha, A.; Kawaguchi, K.; Roy, S.; Chattopadhyay, T.; Das, D. Preparation of antiferromagnetic Co3O4 nanoparticles from two different precursors by pyrolytic method: In vitro antimicrobial activity. RSC Adv. 2014, 4, 15022–15029. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Khan, M.M.; Karuppiah, P.; Al-Dhabi, N.A. Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles. J. Nanomater. 2014, 2014, 637858. [Google Scholar] [CrossRef]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, C.; Raji, P. Facile synthesis and characterization of Co3O4 nanoparticles for high-performance supercapacitors using Camellia sinensis. Appl. Phys. A 2020, 126, 1–12. [Google Scholar] [CrossRef]
- Ali, M.; Abad, W.; Roomy, H.; Abd, A. Rapid synthesis of SeO2 nanoparticles and their activity against clinical isolates (gram positive, gram negative, and fungi). NanoWorld J. 2023, 9, 34–44. [Google Scholar]
- Ali, E.M.; Rasool, K.H.; Abad, W.K.; Abd, A.N. Green Synthesis, Characterization and Antimicrobial activity of CuO nanoparticles (NPs) Derived from Hibiscus sabdariffa a plant and CuCl. J. Phys. Conf. Ser. 2021, 1963, 012092. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).