Abstract
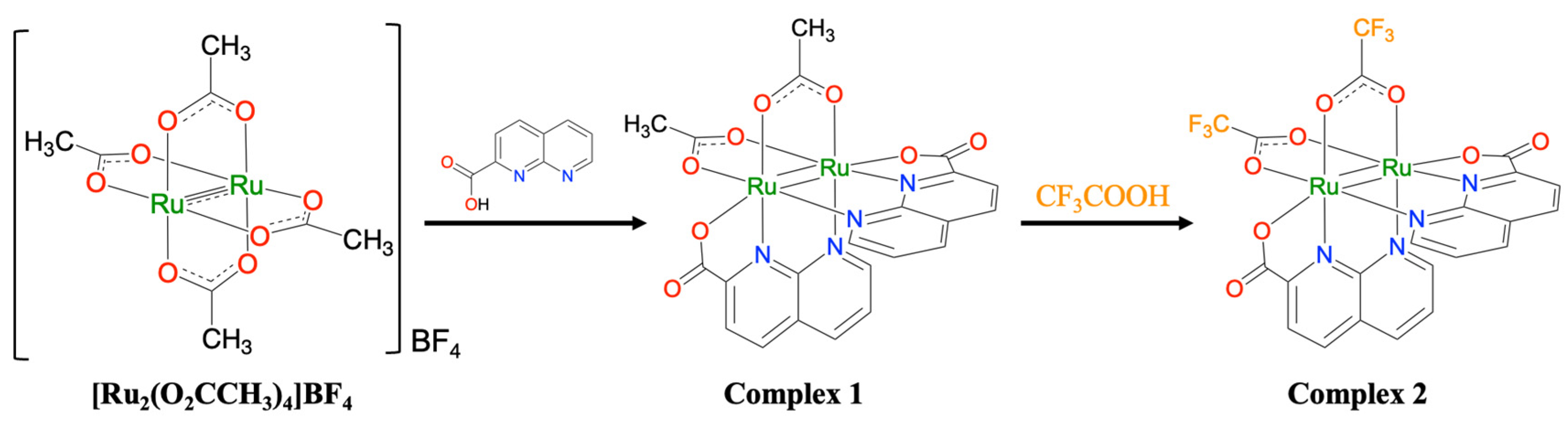
A ligand exchange reaction between [Ru2(npc)2(O2CMe)2] (1; npc = 1,8-naphthyridine-2-carboxylate) and trifluoroacetic acid yielded the diruthenium naphthyridine complex with two trifluoroacetate ligands, [Ru2(npc)2(O2CCF3)2] (2), which was structurally characterized by electrospray ionization mass spectrometry, elemental analysis, infrared spectrum, and synchrotron single-crystal X-ray diffraction. The crystal structure of 2 adopts a paddlewheel-type structure in which two npc and two O2CCF3 ligands are coordinated in a cis-2:2 arrangement around the Ru2 core. The temperature-dependent magnetic susceptibility measurements indicated that 2 has (i) an S = 1 spin state for the Ru24+ core and (ii) a large D value of 243 cm−1; characteristic of paddlewheel-type Ru2 complexes. The cyclic voltammetry measurements indicated that 2 exhibited one reversible oxidation wave (E1/2 = 0.72 V vs. SCE) and two reduction waves (E1/2 = −0.67 and −1.10 V vs. SCE); which were clearly positively shifted when compared with those of 1. Additionally, the absorption spectrum of 2 displayed intense absorption bands in the visible region; attributed to metal-to-ligand charge transfer from the Ru2 core to the npc ligands; which were blue-shifted by approximately 70–100 nm when compared with those of 1. These distinct shifts in redox potentials and absorption bands originated from the strong electron-withdrawing effect of the O2CCF3 ligands in 2.
1. Introduction
The paddlewheel-type dinuclear complexes, [M2(EL)4(AL)X] (EL = equatorial ligand, AL = axial ligand, x = 0–2), are one of the most well-known structural motifs in the field of polynuclear metal complexes [1,2,3,4,5,6,7]. This type of complex has been extensively investigated not only from the perspective of fundamental coordination chemistry, including molecular structure, metal–metal interactions, and multiple bonding but also for its applications such as functional secondary building units in metal-organic frameworks and its diverse functionalities arising from the selection of central metal ions [8,9,10,11,12]. Among these complexes, diruthenium (Ru2) complexes [1,13,14,15,16,17] are particularly interesting because they are redox-active species capable of forming three oxidation states (Ru24+, Ru25+, and Ru26+). Their rich oxidation states and the multiple Ru–Ru bonds endow them with intriguing functionalities, including molecular magnetism [18,19,20,21], catalysis [22,23,24,25], antitumor activity [26,27], and electrochromism [28]. The oxidation state of this type of Ru2 complex is greatly influenced by the type of bridging ligand; carboxylates, the most extensively studied bridging ligands, are known to stabilize the Ru25+ oxidation state with a quartet (S = 3/2) spin state in air [1,29]. By contrast, the Ru24+ and Ru26+, which have a triplet (S = 1) spin state, are generally unstable in air, and only a limited number of bridging ligands can stabilize these oxidation states under ambident conditions [1,30,31].
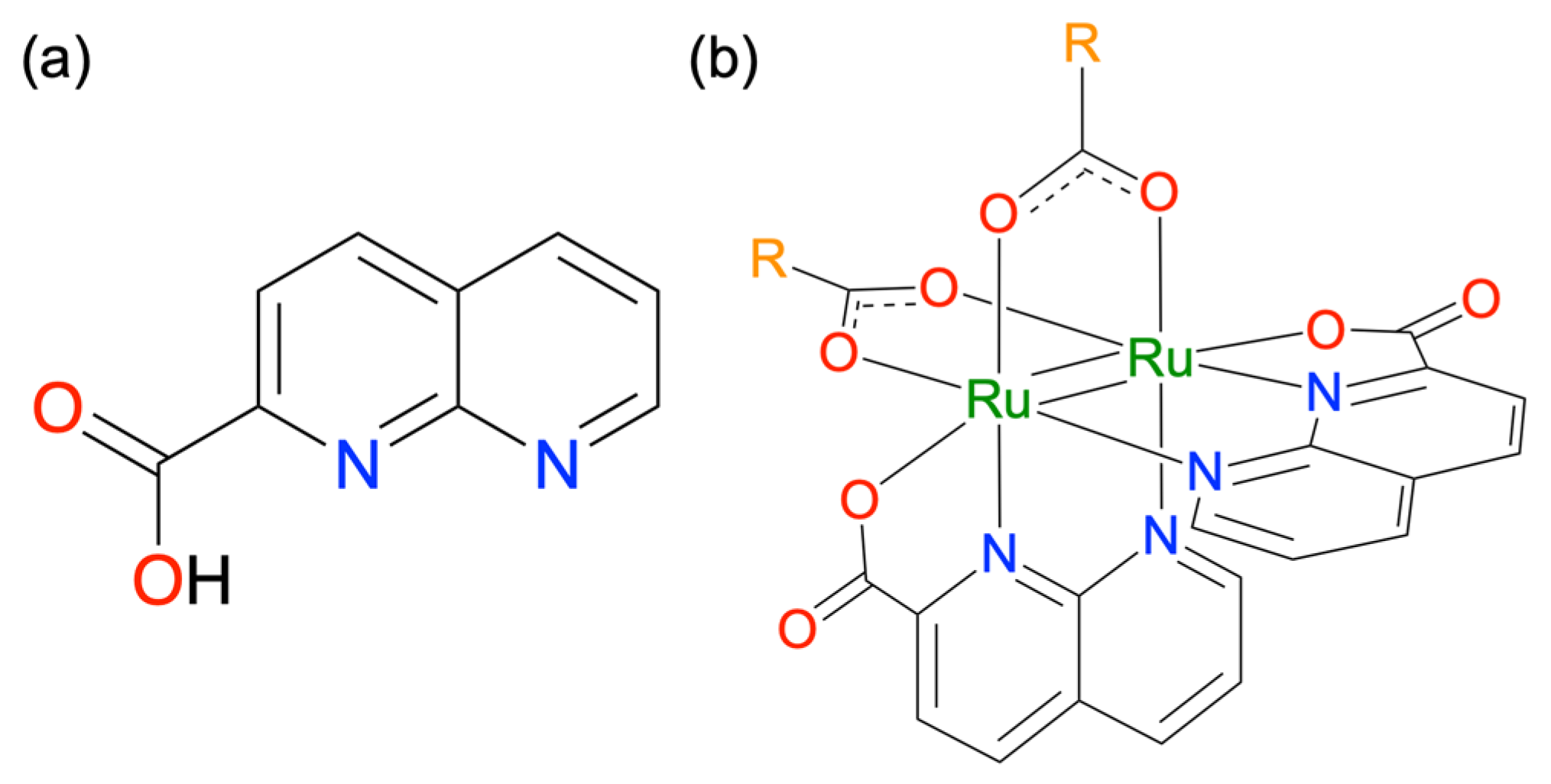
Based on this background, we recently developed a 1,8-naphthyridine-2-carboxylate (npc)-bridged Ru24+ complex [Ru2(npc)2(O2CMe)2] (1; Scheme 1), which is air-stable in both the solid and solution states [32]. Single crystal X-ray diffraction (SCXRD) analyses revealed that 1 forms a paddlewheel-type structure in which two npc and two acetate ligands coordinate to the Ru2 core in a cis-2:2-arrangement. In this structure, the naphthyridine moieties and carboxyl groups of npc ligands coordinate to the Ru2 core at the equatorial and axial positions, respectively. Density functional theory (DFT) calculation clarified that the highest-occupied molecular orbital (HOMO) and lowest-unoccupied MO (LUMO) of 1 are localized on the δ* (Ru2) and π* (npc) orbitals, respectively. Owing to its MO configuration, 1 exhibited the intense metal-to-ligand charge transfer (MLCT) transitions from the Ru2 core to the npc ligands in the visible light region, which were not typically observed in carboxylate-bridged paddlewheel-type Ru2 complexes. Additionally, reacting 1 with an excess amount of benzoic acid selectively replaced the acetate ligands of 1, yielding [Ru2(npc)2(O2CC6H5)2] with a high yield. In general, the absorption and redox properties of paddlewheel-type Ru2 complexes drastically change when electron-withdrawing or donating groups are introduced into the bridging-carboxylate moieties [33,34,35,36]. For example, Ru2 complexes with electron-withdrawing trifluoroacetate as bridging ligands [37] are expected to exhibit a positive shift in redox potential and a blue shift in absorption due to the stabilization of MOs of Ru2 cores.
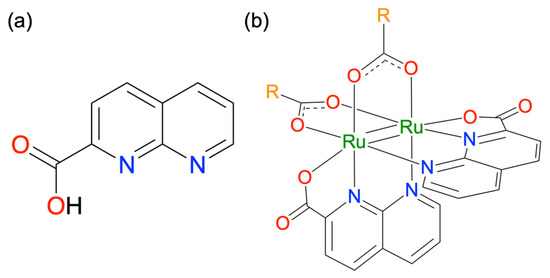
Scheme 1.
Molecular structures of (a) Hnpc and (b) [Ru2(npc)2(O2CR)2].
In this study, a paddlewheel-type Ru2 naphthyridine complex with trifluoroacetate bridging ligands, [Ru2(npc)2(O2CCF3)2] (2), was synthesized and characterized by electrospray ionization mass spectroscopy (ESI-MS), infrared spectrum, elemental analysis, and SCXRD analysis. The electrochemical and absorption spectral properties of 2 were investigated, and their results were compared with those of 1. Furthermore, DFT and time-dependent DFT (TDDFT) calculations were also performed to clarify the electronic features of 2 in detail.
2. Materials and Methods
2.1. Chemicals and Instruments
Complex 1 was synthesized according to a literature procedure [32]. 1,8-naphthyridine-2-carboxylic acid (Hnpc) was purchased from ChemScene LLC (Monmouth Junction, NJ, USA), and trifluoroacetic acid was purchased from Kanto Chemical Co. (Tokyo, Japan). All other chemicals were purchased from Fujifilm-Wako Pure Chemical Industry (Osaka, Japan).
Positive-ion mode ESI-MS spectra were measured with a Bruker (Billerica, MA, USA) micrOTOFII using sodium formate as a mass calibrant. Elemental analysis was performed using a Yanaco (Tokyo, Japan) CHN corder MT-6 instrument at Shimane University. The infrared spectrum was measured with a JASCO (Tokyo, Japan) FT/IR-6300 spectrophotometer using a KBr disk. The temperature dependence of the magnetic susceptibility was measured with a Quantum Design (San Diego, CA, USA) MPMS-3 SQUID magnetometer at a magnetic field of 5000 Oe in the temperature range of 2–300 K. The absorption spectrum was measured using a JASCO (Tokyo, Japan) V-670 spectrophotometer. Diffuse reflectance spectra were recorded using a JASCO V-670 spectrophotometer equipped with an ISN-923 integrating sphere. Cyclic voltammetry (CV) measurement was performed with a scan rate of 100 mV/s in degassed propylene carbonate (PC) solution containing 0.10 M tetrabutylammonium hexafluorophosphate (TBAPF6) using a HOKUTO DENKO (Tokyo, Japan) HZ-7000 system with a 3.0 mm glassy carbon disk, platinum wire, and saturated calomel electrode (SCE).
2.2. Synthesis of [Ru2(npc)2(O2CCF3)2] (2)
Complex 1 (67.6 mg, 0.100 mmol) was dissolved in trifluoroacetic acid (30.0 mL) and refluxed under nitrogen for 12 h. After cooling to room temperature, the solution was evaporated and then purified by silica gel column chromatography (eluent: 4:1 CH2Cl2–MeOH). The resultant solution was evaporated to dryness, and the obtained black powder was washed with diethyl ether (Et2O) and dried under vacuum at 393 K for 3 h. Yield: 68.9 mg (0.0879 mmol, 87.9%). Anal. Calc. for C22H10N4O8F6Ru2·0.5H2O: C, 33.73; H, 1.41; N, 7.15%. Found: C, 33.82; H, 1.90; N, 7.08%. ESI-MS: calc. for [M + Na]+: 798.8396 m/z; found 798.8393 m/z. FT-IR (cm−1): 622 (w), 737 (m), 780 (m), 826 (vw), 864 (m), 916 (w), 1039 (w), 1158 (s), 1200 (vs), 1317 (w), 1352 (m), 1621 (vs), 1645 (vs), 3073 (w), 3431 (s).
2.3. Theoretical Calculation Methods
All DFT calculations in this study were performed using the uB3LYP functional [38], with the SDD basis set for Ru atoms, the aug-cc-pVDZ for N, O, and F atoms, and the cc-pVDZ basis set for other atoms, on the Gaussian 16 C.01 suite program [39,40,41]. This combination of functional method and basis sets theoretically reproduced the experimental results of 1 (molecular structure and absorption spectrum) well [32]. The polarizable continuum model was applied to consider the effect of N,N-dimethylformamide (DMF) [42]. Geometry optimization was conducted without symmetry constraints, and the obtained geometry was verified by frequency analysis. TDDFT calculation [43] was conducted to estimate the absorption wavelengths, oscillator strengths, and excitation characters.
2.4. Crystallography
Synchrotron SCXRD data of 2 was collected at 100 K using a Pilatus3 X CdTe 1M detector, and monochromated synchrotron radiation (λ = 0.4139 Å) installed at the BL02B1 beamline of the SPring-8 synchrotron radiation facility. Diffraction data were collected and processed using the CrysAlisPro program (version 1.171.42.94a) [44]. The structure was solved by the SHELXT program [45] with the intrinsic phasing method and then refined with full-matrix least squares on F2 using the SHELXL program [46] in the Olex2 software (version 1.5) [47]. All non-hydrogen atoms were refined with anisotropic thermal parameters, and all hydrogen atoms were fixed at the calculated positions and refined as riding models. The electron densities of the disordered Et2O solvents were removed using the Olex2 solvent mask. The crystallographic data for the final refined structure of 2 is summarized in Table 1, and the structural parameters of 2 are summarized in Table S1 in Supplementary Materials. The crystallographic data can be obtained free of charge from the Cambridge Crystallographic Data Centre (CCDC); deposition number of 2 is CCDC-2427167.

Table 1.
Crystallographic data of 2.
3. Results
3.1. Synthesis and Chatacterization
The synthetic scheme for complex 2 is shown in Scheme 2. By reacting 1 with an excess amount of trifluoroacetic acid under nitrogen for 12 h and purifying the resulting solution through silica-gel column chromatography, a black powder of 2 was obtained with a good yield of 87.9%. The resulting complex 2 was paramagnetic and remained stable in both solution and solid states in the air. 2 dissolved in common organic solvents such as CH2Cl2, CHCl3, alcohols, DMF, and PC. Additionally, 1 was found to dissolve in tetrahydrofuran, whereas 2 did not.
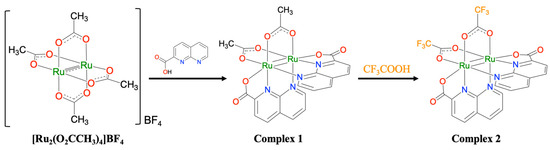
Scheme 2.
Synthetic scheme for complex 2.
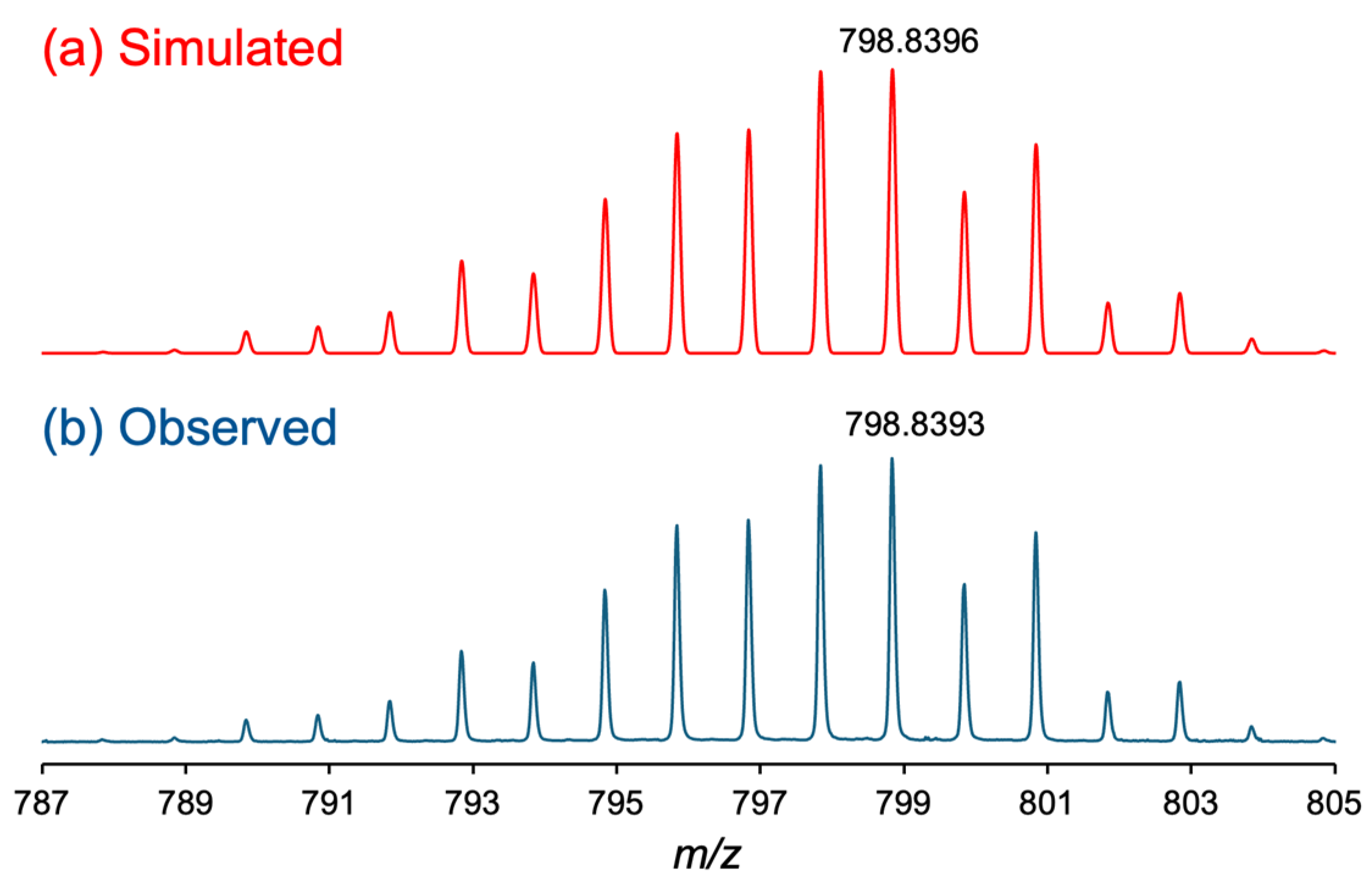
The positive-ion ESI-MS spectrum of 2 displayed an intense signal at 798.8393 m/z, corresponding to the calculated [M + Na]+ value of [Ru2(npc)2(O2CCF3)2] (798.8396 m/z). As shown in Figure 1, the observed isotope distribution pattern of 2 is consistent with the simulated pattern. No signal originating from the precursor complex 1 or the ligand exchange intermediate species of 2, i.e., [Ru2(npc)2(O2CMe)(O2CCF3)], were observed. The CHN elemental analysis result of 2 showed good agreement with the calculated values for 2 with 0.5 H2O. In the infrared spectrum (Figure S1), the asymmetric stretching modes of the CO2− group, i.e., νasym (CO2−), of bridging −O2CCF3 ligands in 2 was observed at 1644 cm−1, which was consistent with that of [Ru2(O2CCF3)4] (1624 cm−1) [48]. In addition, the νasym (CO2−) of the carboxylate moieties of the npc ligands in 2 was found at 1620 cm−1. Because the vibration mode of the carboxyl (COOH) group, which generally appears around 1700 cm−1 [49], was not found in the infrared spectrum of 2, the carboxyl groups were understood to be fully deprotonated in 2.
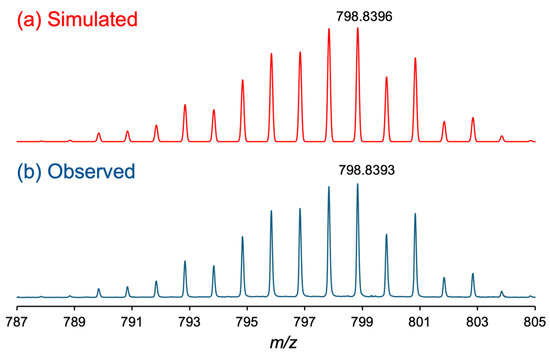
Figure 1.
Isotope distribution patterns of (a) simulated and (b) observed ESI-MS spectra of 2.
3.2. Crystal Structure
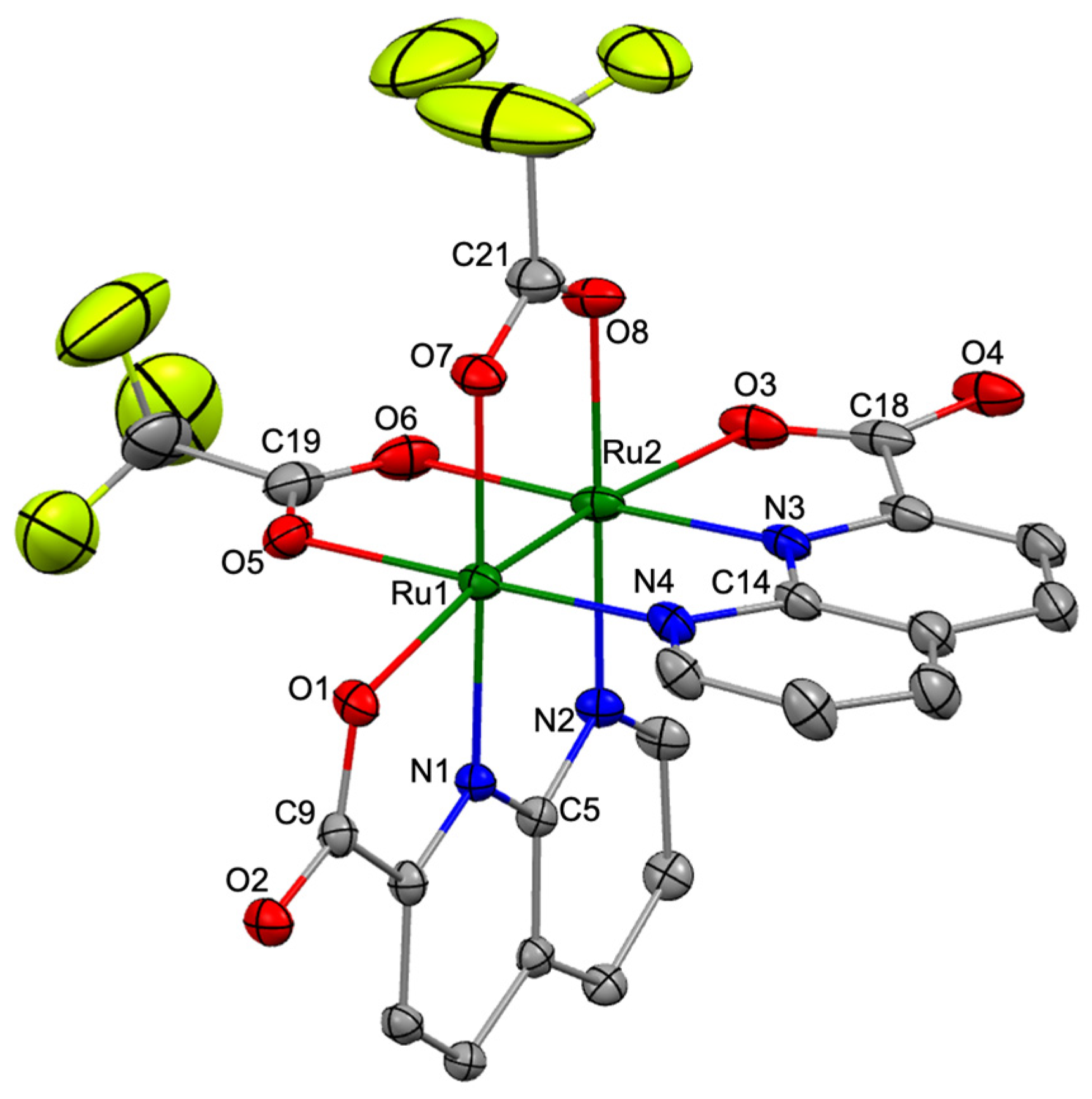
Single crystals of 2, suitable for X-ray diffraction analyses, were obtained by slow diffusion of Et2O into an acetonitrile solution of 2. The diffraction analysis of a black block-shaped crystal of 2 was performed at 100 K using the high-energy synchrotron source at the BL02B1 beamline of the SPring-8 synchrotron facility in Japan, and this analysis revealed that 2 crystallized in the tetragonal system with the I 41/a space group. Figure 2 shows the crystal structure (thermal ellipsoid view) of 2 with a selected numbering scheme. Selected bond lengths and angles in 2 are summarized in Table S1.
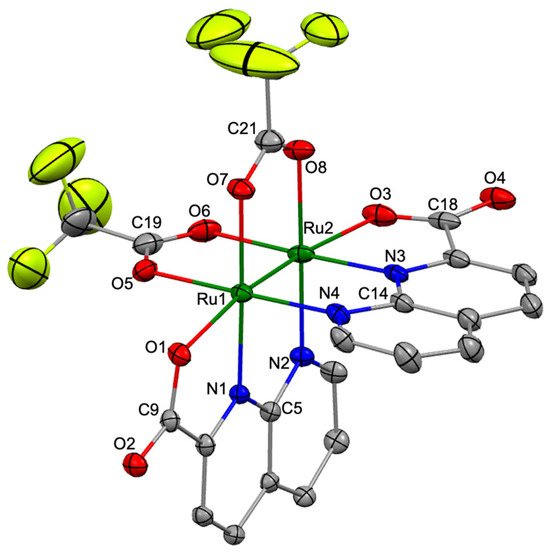
Figure 2.
Crystal structure of 2 [Ru: green, C: gray, N: blue, O: red, and F: yellow]. Thermal ellipsoids were set at 30% probability level, and hydrogen atoms and solvent molecules are omitted for clarity.
The asymmetric unit of the crystal contained one crystallographically independent molecule of 2 and co-crystallized solvent molecules (one CH3CN and one disordered Et2O). The molecular structure of 2 formed the paddlewheel structure in which two npc and two O2CCF3 bridging ligands were coordinated to the Ru2 core in a cis-2:2 arrangement, similar to that of 1 [32]. The naphthyridine and carboxylate moieties in npc ligands were coordinated to the equatorial and axial positions of the Ru2 core in 2. The Ru–Ru bond length in 2 was 2.2978 (4) Å, which was slightly longer than those in 1 (2.2893 (4) Å) [32], [Ru2(O2CCF3)4(THF)2] (2.276 (3) Å) [48], and [Ru2(monp)4] (2.258 (2) Å; monp = 7-methyl-1,8-naphthyridin-2-one) [50], indicating that a double bond was formed between the two Ru atoms in 2. The averaged Ru–N(npc) and Ru–O(npc) bond lengths in 2 were 2.046 and 2.219 Å, respectively, which were slightly shorter than those in 1 (Ru–N(npc): 2.051 Å Ru–O(npc): 2.243 Å), whereas the average bridging Ru–O bond length in 2 was 2.087 Å, which was slightly longer than that in 1 (2.076 Å) [32].
3.3. Magnetic Properties
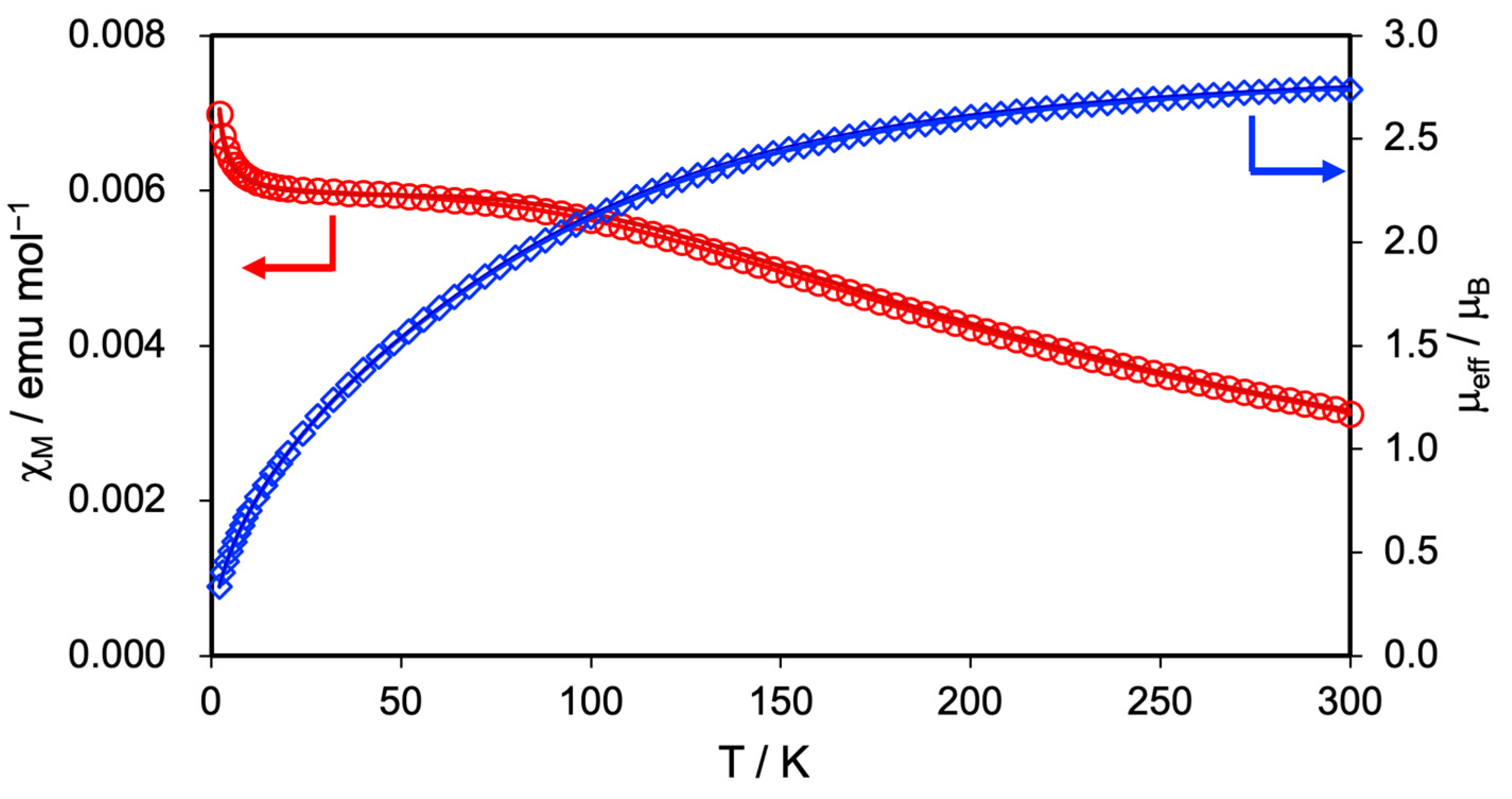
The magnetic susceptibility was measured at a magnetic field of 5000 Oe in the temperature range of 2–300 K to clarify the spin state and magnetic property of 2. Figure 3 shows the temperature dependence of the molar magnetic susceptibility (χM) and effective moments (μeff) of 2. At 300 K, the μeff value of 2 was estimated to be 2.73 μB, which aligned well with the spin-only value of 2.83 μB predicted for the triplet spin (S = 1) state of the Ru24+ complex, while it significantly deviated from the 3.87 μB predicted for the quartet spin (S = 3/2) state of the Ru25+ complex. With decreasing temperature, 2 exhibited a gradual increase in χM value and a corresponding decrease in μeff value. The χM and μeff values of 2 at 2.0 K reached 7.07 × 10−3 emu/mol and 0.34 μB, respectively.
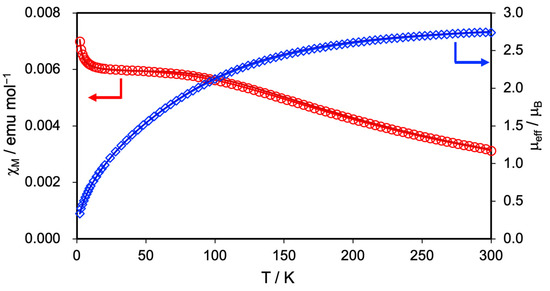
Figure 3.
Temperature-dependent magnetic susceptibility (red circle) and effective magnetic moment (blue rhombus) of 2. The solid lines represent the best fit of the data using Equation (1).
This magnetic behavior of 2 is similar to that of 1, suggesting that zero-field splitting (ZFS) characteristics of the Ru2 complexes occur [51,52]. Therefore, by fitting the measurement data of 2 using Equation (1), we determined the ZFS parameter (the D tensor) and g-value [53].
In this equation, ρ is the correction term for a small amount of paramagnetic impurity with S = 1/2 (= 0.0012), N is the Avogadro’s number, β is the Bohr magneton, k is the Boltzmann constant, and gimp value is assumed to equal the average g-value. The best-fitting results indicated that 2 has a D tensor of 243 cm−1 and a g-value of 2.03. Table 2 summarizes the D tensor and g values of 1, 2, and related Ru2 complexes. The D tensor of 2 is close to those of 1 and other Ru24+ complexes such as [Ru2(O2CMe)4] (244 cm−1) [54], [Ru2(O2CCF3)4] (220 cm−1) [37], and [Ru2(O2CC9H17)] (283 cm−1) [55], but significantly different from those of Ru25+ complexes such as [Ru2(O2CMe)4(H2O)2]BPh4 (71.8 cm−1) [56] and [Ru2(O2CC3H7)4Cl] (77 cm−1) [57]. This result strongly supports that 2 adopts an oxidation state of Ru24+ with S = 1.

Table 2.
D tensors and g-values of 1, 2, and related Ru2 complexes.
3.4. Optimized Geometry and Electronic Structure
Unrestricted DFT calculations were performed to gain a deeper understanding of the molecular geometry and electronic structure of 2. The results of full geometry optimization indicated that the optimized geometry of 2 adopts a paddlewheel-type structure with a cis-2:2 arrangement, consistent with the experimental structure. The optimized Ru–Ru bond length in 2 was 2.337 Å, which was only 0.04 Å longer than the experimental value (2.2978 (4) Å). Additionally, as shown in Table S2, the averaged bond lengths between the Ru2 core and the primary coordination sphere [Ru–N(npc), Ru–O(npc), and Ru–O(O2CCF3)] in the optimized geometry of 2 were approximately 0.01 to 0.04 Å longer than the experimental values. The slight elongation of these bond lengths in the optimized geometry compared with the crystal structure is likely due to the absence of crystal packing stress [58].
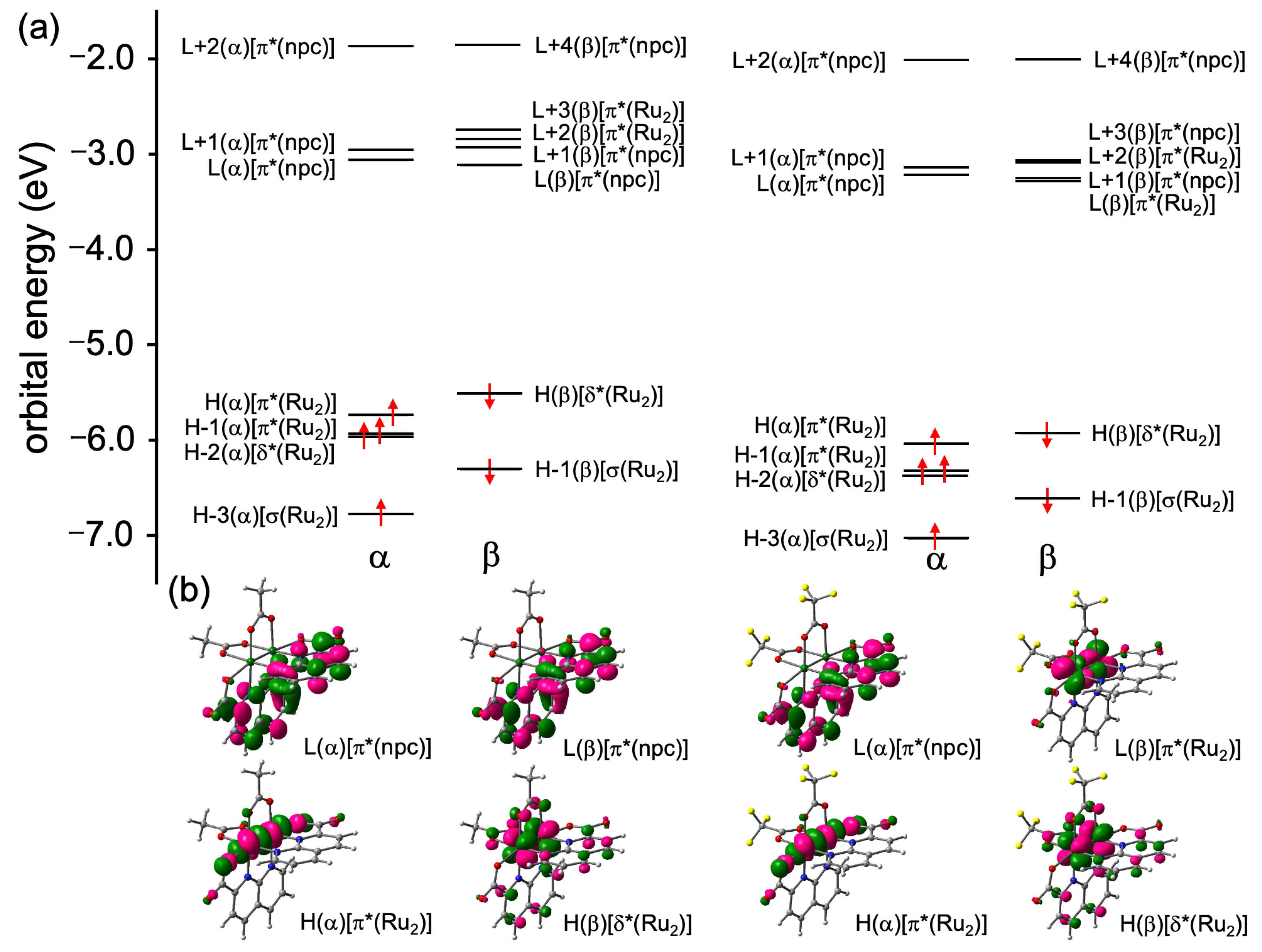
Figure 4 depicts the MO diagrams near the frontier MOs, as well as the HOMOs and LUMOs of 1 and 2. The occupied MO features of 2 are very similar to those of 1, but the orbital energies of 2 are relatively more stabilized than those of 1. Specifically, the two singly unoccupied MOs, i.e., HOMO (α) and HOMO-1 (α), of 2 are localized on the π* (Ru2) orbitals and are stabilized by 0.30 and 0.39 eV, respectively, compared with those of 1. The δ* (Ru2) orbitals were observed in HOMO-2 (α) and HOMO (β), while the σ (Ru2) orbitals, which are the most unstable bonding d–d orbital interaction, were found in HOMO-3 (α) and HOMO-1 (β). The HOMO (α) and HOMO (β) orbitals of 2 were stabilized by 0.40 and 0.42 eV, respectively, compared with those of 1. In the unoccupied MO spaces, clear differences in orbital stability were observed between 1 and 2 due to the electron-withdrawing effect of the O2CCF3 ligands; while the π* (npc) orbitals of 2 were also stabilized by the electron-withdrawing nature of the O2CCF3 ligands, the energy differences between 1 and 2 were relatively smaller than those of π* (Ru2) orbital. Consequently, although LUMO (α) was localized on the π* (npc) orbitals in both complexes, the LUMO (β) orbital in 1 was localized on the π* (npc) orbital, while the LUMO (β) orbital in 2 was localized on the π* (Ru2) orbital (see Figure 4b). Based on the results of these MO analyses, the electronic configurations between two Ru ions in 2 can be described as π4δ2σ2δ*2π*2, which is the same as the case of 1 [32].
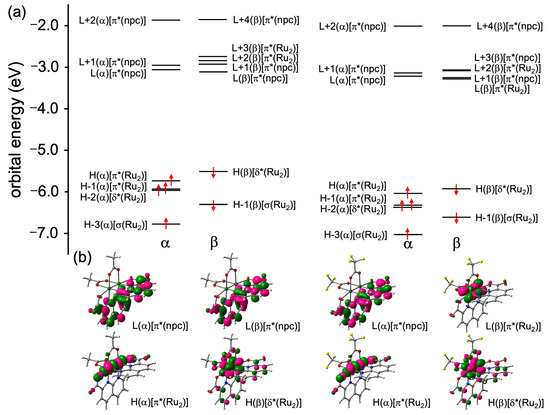
Figure 4.
(a) MO diagrams and (b) HOMOs [H] and LUMOs [L] of 1 and 2.
3.5. Electrochemical Property
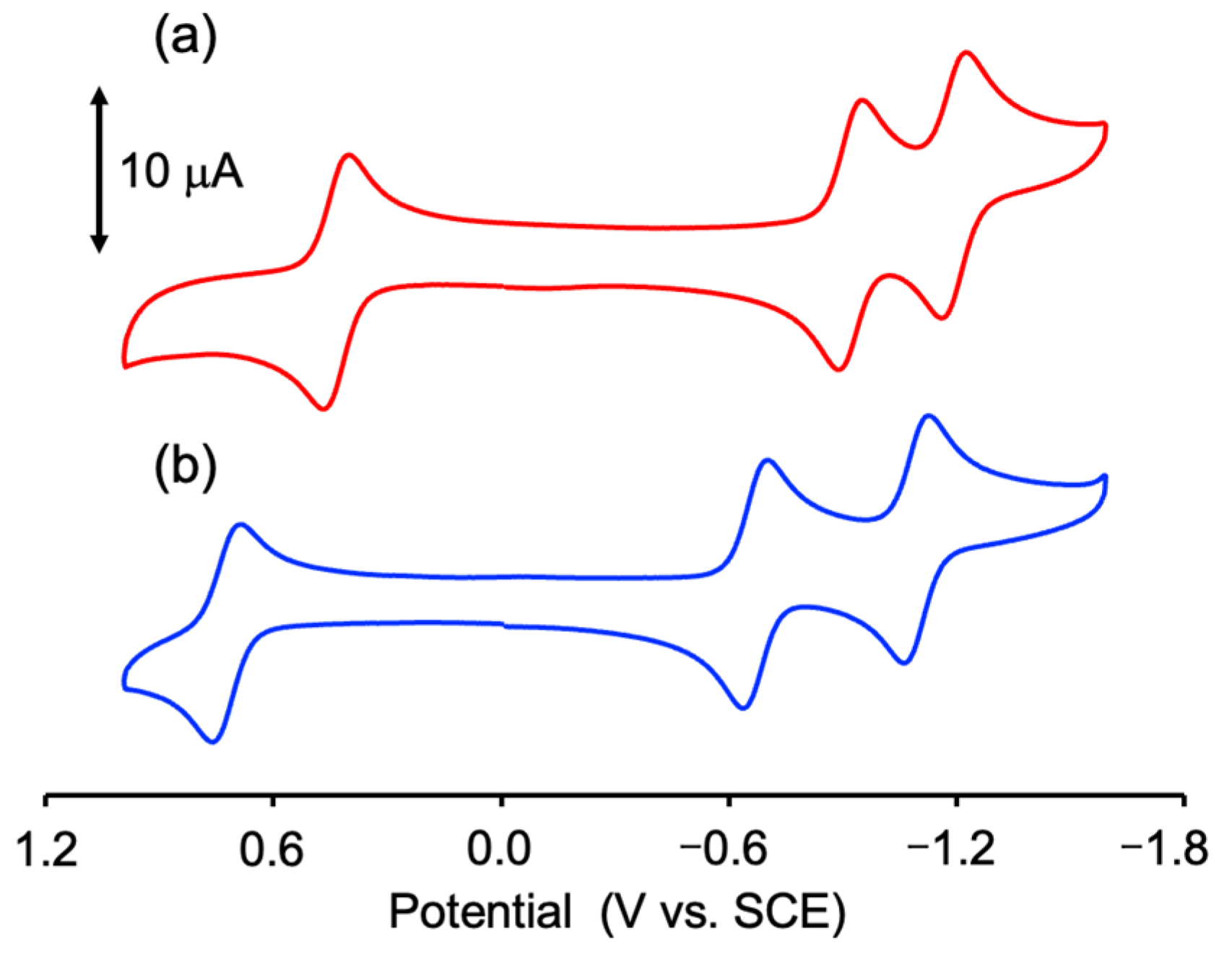
CV measurement was performed in a degassed PC solution containing 0.10 M TBAPF6 to investigate the electrochemical properties of 2. The obtained redox potential (E1/2) and the separation of the oxidation and reduction waves (DE) are summarized in Table S4. As shown in Figure 5, 2 exhibited one reversible wave at E1/2 = 0.72 V vs. SCE on the oxidation side and two reversible waves at E1/2 = −0.67 and −1.10 V vs. SCE on the reduction side. The CV diagram of 2 was similar to that of 1, but the E1/2 values of 2 were shifted in the positive direction relative to those of 1 (E1/2 = 0.43, −0.92, and −1.19 V vs. SCE) [32]. This large positive shift of 2 originated from the electron-withdrawing effect of the two O2CCF3 ligands and is consistent with the stabilization of the HOMO (α) orbital of 2 compared to that of 1. Further, 2 was easily oxidized compared with [Ru2(O2CCF3)4] (E1/2 = 1.8 V vs. SCE) [59]. From the results of the electronic structure of 2 calculated by DFT and CV result of Hnpc (E1/2 = −0.91 V vs. SCE; Figure S2), the oxidation wave was attributed to the Ru25+/Ru24+ process, while the two reduction waves were associated with the reduction of two npc ligands (i.e., npc/npc− processes).
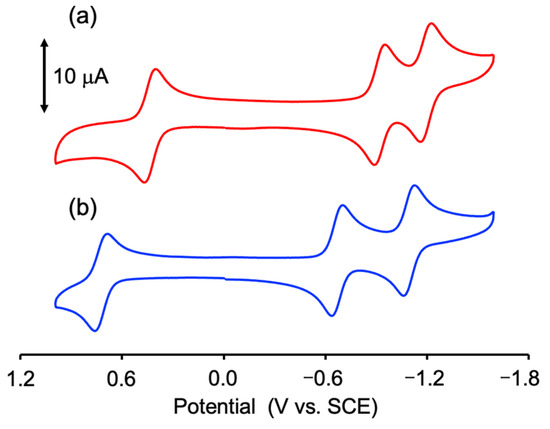
Figure 5.
CV diagrams of (a) 1 [red line] and (b) 2 [blue line] in degassed PC/TBAPF6.
3.6. Absorption-Spectral Property
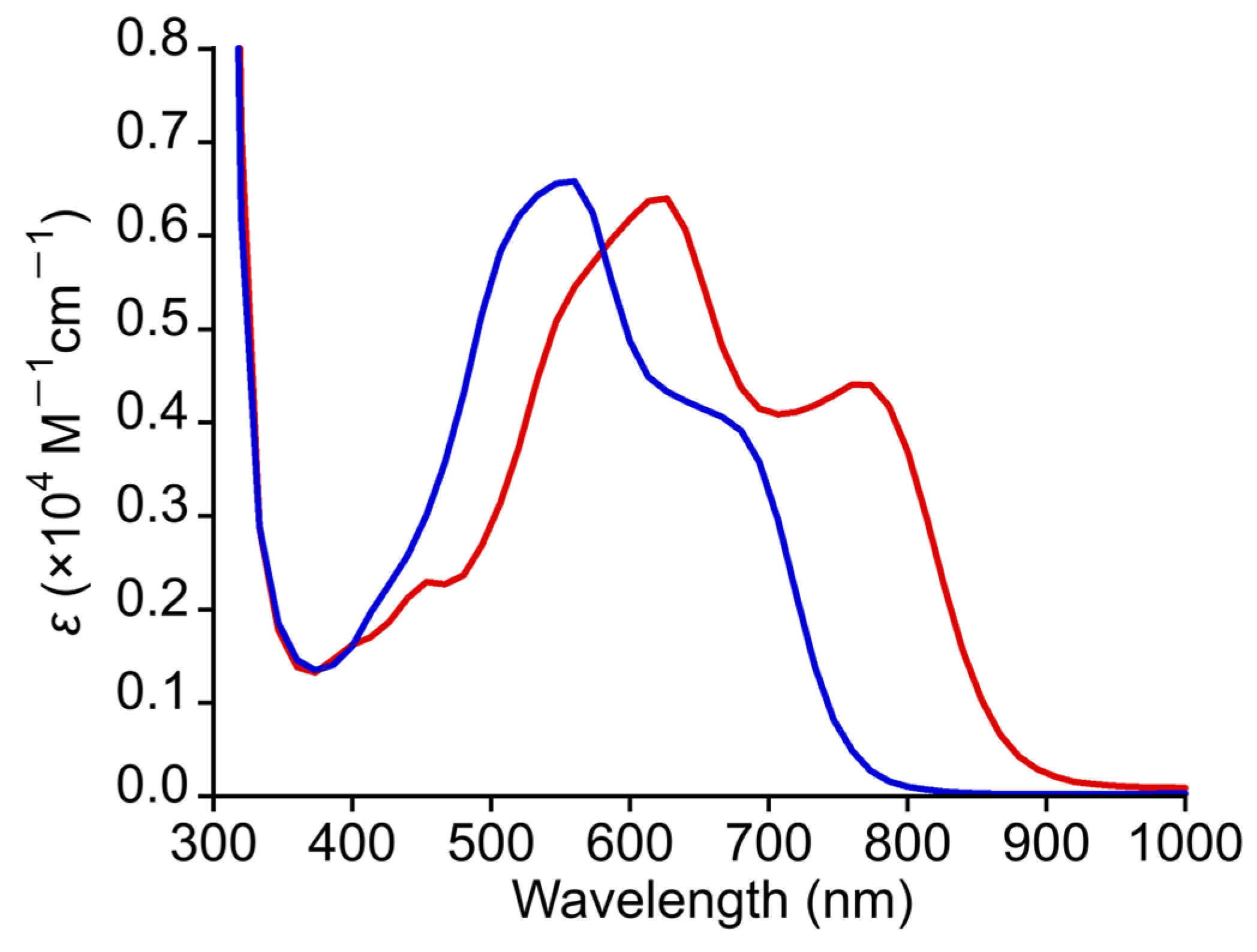
The steady-state absorption spectrum of 2 was measured in DMF at 300 K. As shown in Figure 6, 2 displayed intense absorption bands in the visible light region similar to those of 1 but with a clear blue shift [32]. Specifically, 2 exhibited a shoulder band around 680–700 nm and an intense band at 555 nm (ε = 3780 M−1cm−1). The absorption bands of 2 were found to be blue-shifted by approximately 70–100 nm when the absorption bands of 2 were compared with the corresponding bands of 1. The significant blue shift of these absorption bands in 2 was considered to be due to the electron-withdrawing effect of trifluoroacetate ligands in 2. As shown in Table S3, TDDFT calculations revealed that the main contributions of the shoulder and intense bands of 2 in the visible light region are MLCTs from the δ* (Ru2) to the π* (npc) orbitals. Additionally, the d–d transitions and MLCTs from the π* (Ru2), σ (Ru2), or δ (Ru2) to the π* (npc) orbitals are also involved as minor contributions in their absorption bands. Therefore, the blue shift in the absorption bands of 2 compared with that of 1 was concluded to originate from the stabilization of δ* (Ru2) orbital in 2 due to the electron-withdrawing effect of the CF3COO− ligands. The absorption bands in the solid-state diffuse reflectance spectra of 1 and 2 appear at approximately the same positions as those in solution (see Figure S3).
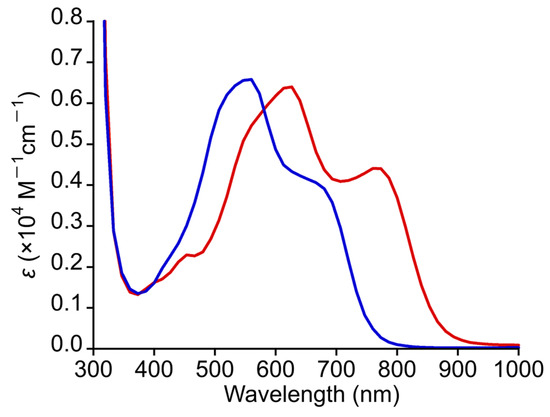
Figure 6.
Absorption spectra of 1 (red line) and 2 (blue line) in DMF.
4. Conclusions
A new paddlewheel-type Ru2 naphthyridine complex with two trifluoroacetate ligands, [Ru2(npc)2(O2CCF3)2] (2), was synthesized and structurally characterized. SCXRD analyses revealed that 2 adopted a paddlewheel-type structure, in which two npc ligands and two O2CCF3 ligands were coordinated in a cis-2:2 arrangement around the Ru2 core. The Ru–Ru and average bridging Ru–O bond lengths in 2 were 2.2978(4) and 2.087 Å, which were slightly longer than those in 1 (2.2893 (4) and 2.076 Å, respectively), whereas the averaged Ru–N(npc) and Ru–O(npc) bond lengths in 2 were 2.046 and 2.219 Å, respectively, which were slightly shorter than those in 1 (Ru–N(npc): 2.051 Å Ru–O(npc): 2.243 Å). Based on the results from magnetic susceptibility measurements and DFT calculations, 2 had a Ru2+ oxidation state and possessed unpaired spins in the two π*(Ru2) orbitals, resulting in a spin state of S = 1. The paddlewheel-type Ru24+ complex was generally unstable in the air; however, 2 was a rare compound that remained stable in both solid and solution states. As expected, 2 was a redox-active compound and exhibited MLCT transitions from the Ru2 core to the npc ligands in the visible light region; (i) the absorption bands of 2 were found to be blue-shifted by approximately 70–100 nm relative to those of 1, and (ii) the E1/2 values of 2 (E1/2 = 0.72, −0.67 and −1.10 V vs. SCE) were shifted in the positive direction relative to those of 1 (E1/2 = 0.43, −0.92, and −1.19 V vs. SCE). This study has revealed that the absorption band positions and redox potentials of the Ru2 naphthyridine complex could be modified through the appropriate selection of bridging carboxylate ligands. We are planning to carry out further research on the development of electrochromic [28] and/or supramolecular complexes [60,61] using this Ru2 core structure.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry7030072/s1, Figure S1: Infrared spectrum of 2.; Figure S2: CV diagram of Hnpc (1.0 mM) in PC/TBAPF6.; Figure S3: Diffuse reflectance spectra of 1 (red line) and 2 (blue line).; Table S1: Selected bond lengths (Å) and angles (°) of crystal structure of 2.; Table S2: Averaged bond lengths between the Ru2 core and the primary coordination sphere in the crystal structure and optimized geometry of 2.; Table S3: TDDFT results (absorption wavelengths, oscillator strengths (f), and assignments of excitation character) for 2.; Table S4: Redox potentials (E1/2; V vs. SCE) and the separation of the oxidation and reduction waves (ΔE; mV) of 1 and 2.
Author Contributions
Conceptualization, Y.K.; Validation, Y.K., N.Y. and M.H.; Formal analysis, Y.K., N.T., J.O., K.M., N.Y. and M.H.; Investigation, Y.K., N.T., J.O., K.M. and N.Y.; Resources, Y.K., N.Y. and M.H.; Data curation, Y.K., N.Y. and M.H.; Writing—original draft preparation, Y.K.; Writing—review and editing, Y.K., N.T., J.O., K.M., N.Y. and M.H.; Visualization, Y.K. and N.Y.; Supervision, Y.K.; Project administration, Y.K.; Funding acquisition, Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Society for the Promotion of Science (JSPS KAKENHI; Grant Number 24K08494, 22K14765, and 24K08363) and SDGs research project of Shimane University.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
The synchrotron radiation experiments were performed at the BL02B1 of SPring–8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2022B1647, 2023A1741, 2023B1829, 2024A1713, and 2024B1920).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cotton, F.A.; Murillo, C.A.; Walton, R.A. Multiple Bonds Between Metal Atoms, 3rd ed.; Springer Science and Business Media: New York, NY, USA, 2005. [Google Scholar]
- Chisholm, M.H.; Patmore, N.J. Studies of electronic coupling and mixed valency in metal-metal quadruply bonded complexes linked by dicarboxylate and closely related ligands. Acc. Chem. Res. 2007, 40, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Murillo, C.A. Adventures in Divalent Early Transition Metal Coordination Chemistry: On the Way to Metal–Metal Bonded Species. Inorg. Chim. Acta 2017, 468, 3–15. [Google Scholar] [CrossRef]
- Ren, T. Substituent Effects in Dinuclear Paddlewheel Compounds: Electrochemical and Spectroscopic Investigations. Coord. Chem. Rev. 1998, 175, 43–58. [Google Scholar] [CrossRef]
- Hrdina, R. Dirhodium(II,II) Paddlewheel Complexes. Eur. J. Inorg. Chem. 2021, 6, 501–528. [Google Scholar] [CrossRef]
- Köberl, M.; Cokoja, M.; Herrmann, W.A.; Kühn, F.E. From Molecules to Materials: Molecular Paddle-Wheel Synthons of Macromolecules, Cage Compounds, and Metal–Organic Frameworks. Dalton Trans. 2011, 40, 6834–6859. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Yano, N.; Mikuriya, M.; Handa, M. Paddlewheel-type dirhodium complexes with N,N′-bridging ligands. Coord. Chem. Rev. 2023, 479, 214997. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; Groy, T.L.; Yaghi, O.M. Establishing Microporosity in Open Metal–Organic Frameworks: Gas Sorption Isotherms for Zn(BDC) (BDC = 1,4-Benzenedicarboxylate). J. Am. Chem. Soc. 1998, 120, 8571–8572. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Chun, H.; Kim, K. Rigid and Flexible: A Highly Porous Metal–Organic Framework with Unusual Guest-Dependent Dynamic Behavior. Angew. Chem. Int. Ed. 2004, 43, 5033–5036. [Google Scholar] [CrossRef]
- Mori, W.; Takamizawa, S.; Nozaki Kato, C.; Ohmura, T.; Sato, T. Molecular-Level Design of Efficient Microporous Materials Containing Metal Carboxylates: Inclusion Complex Formation with Organic Polymer, Gas-Occlusion Properties, and Catalytic Activities for Hydrogenation of Olefins. Microporous Mesoporous Mater. 2004, 73, 31–46. [Google Scholar] [CrossRef]
- Miyasaka, H. Charge Manipulation in Metal–Organic Frameworks: Toward Designer Functional Molecular Materials. Bull. Chem. Soc. Jpn. 2021, 94, 2929–2955. [Google Scholar] [CrossRef]
- Kataoka, Y.; Yano, N.; Mikuriya, M.; Handa, M. Coordination polymers and metal–organic frameworks based on paddlewheel-type dirhodium(II) tetracarboxylates. Coord. Chem. Rev. 2022, 472, 214796. [Google Scholar] [CrossRef]
- Aquino, M.A.S. Diruthenium and Diosmium Tetracarboxylates: Synthesis, Physical Properties, and Applications. Coord. Chem. Rev. 1998, 170, 141–202. [Google Scholar] [CrossRef]
- Aquino, M.A.S. Recent Developments in the Synthesis and Properties of Diruthenium Tetracarboxylates. Coord. Chem. Rev. 2004, 248, 1025–1045. [Google Scholar] [CrossRef]
- Van Caemelbecke, E.; Phan, T.; Osterloh, W.R.; Kadish, K.M. Electrochemistry of Metal-Metal Bonded Diruthenium Complexes. Coord. Chem. Rev. 2021, 434, 213706. [Google Scholar] [CrossRef]
- Cortijo, M.; González-Prieto, R.; Herrero, S.; Priego, J.L.; Jiménez-Aparicio, R. The Use of Amidinate Ligands in Paddlewheel Diruthenium Chemistry. Coord. Chem. Rev. 2019, 400, 213040. [Google Scholar] [CrossRef]
- Mikuriya, M.; Yoshioka, D.; Handa, M. Magnetic Interactions in One-, Two-, and Three-Dimensional Assemblies of Dinuclear Ruthenium Carboxylates. Coord. Chem. Rev. 2006, 250, 2194–2211. [Google Scholar] [CrossRef]
- Liao, Y.; Shum, W.W.; Miller, J.S. Synthesis and Magnetic Properties of 3-D [RuII/III2(O2CMe)4]3[MIII(CN)6] (M = Cr, Fe, Co). J. Am. Chem. Soc. 2002, 124, 9336–9337. [Google Scholar] [CrossRef]
- Angaridis, P.; Cotton, F.A.; Murillo, C.A.; Villagrán, D.; Wang, X. Structural and Magnetic Evidence Concerning Spin Crossover in Formamidinate Compounds with Ru25+ Cores. J. Am. Chem. Soc. 2005, 127, 5008–5009. [Google Scholar] [CrossRef]
- Miyasaka, H.; Motokawa, N.; Matsunaga, S.; Yamashita, M.; Sugimoto, K.; Mori, T.; Toyota, N.; Dunbar, K.R. Control of Charge Transfer in a Series of Ru22+/TCNQ Two-Dimensional Networks by Tuning the Electron Affinity of TCNQ Units: A Route to Synergistic Magnetic/Conducting Materials. J. Am. Chem. Soc. 2010, 132, 1532–1544. [Google Scholar] [CrossRef]
- Zhang, J.; Kosaka, W.; Liu, Q.; Amamizu, N.; Kitagawa, Y.; Miyasaka, H. CO2-Sensitive Porous Magnet: Antiferromagnet Creation from a Paramagnetic Charge-Transfer Layered Metal–Organic Framework. J. Am. Chem. Soc. 2023, 145, 26179–26189. [Google Scholar] [CrossRef]
- Trenerry, M.J.; Wallen, C.M.; Brown, T.R.; Park, S.V.; Berry, J.F. Spontaneous N2 Formation by a Diruthenium Complex Enables Electrocatalytic and Aerobic Oxidation of Ammonia. Nat. Chem. 2021, 13, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Sato, K.; Miyazaki, Y.; Masuda, K.; Tanaka, H.; Naito, S.; Mori, W. Photocatalytic Hydrogen Production from Water Using Porous Material [Ru2(p-BDC)2]n. Energy Environ. Sci. 2009, 2, 397–400. [Google Scholar] [CrossRef]
- Miyazawa, T.; Suzuki, T.; Kumagai, Y.; Takizawa, K.; Kikuchi, T.; Kato, S.; Onoda, A.; Hayashi, T.; Kamei, Y.; Kamiyama, F.; et al. Chiral Paddle-Wheel Diruthenium Complexes for Asymmetric Catalysis. Nature Catal. 2020, 3, 851–858. [Google Scholar] [CrossRef]
- Villalobos, L.; Barker Paredes, J.E.; Cao, Z.; Ren, T. tert-Butyl Hydroperoxide Oxygenation of Organic Sulfides Catalyzed by Diruthenium(II,III) Tetracarboxylates. Inorg. Chem. 2013, 52, 12545–12552. [Google Scholar] [CrossRef]
- Tolbatov, I.; Barresi, E.; Taliani, S.; La Mendola, D.; Marzo, T.; Marrone, A. Diruthenium(II,III) Paddlewheel Complexes: Effects of Bridging and Axial Ligands on Anticancer Properties. Inorg. Chem. Front. 2023, 10, 2226–2238. [Google Scholar] [CrossRef]
- Barresi, E.; Tolbatov, I.; Marzo, T.; Zappelli, E.; Marrone, A.; Re, N.; Pratesi, A.; Martini, C.; Taliani, S.; Da Settimo, F.; et al. Two Mixed-Valence Diruthenium(II,III) Isomeric Complexes Show Different Anticancer Properties. Dalton Trans. 2021, 50, 9643–9647. [Google Scholar] [CrossRef]
- Kataoka, Y.; Imasaki, N.; Yano, N.; Mitsumi, M.; Handa, M. Redox-Triggered Reversible Modulation of Intense Near-Infrared and Visible Absorption Using Paddlewheel-Type Diruthenium(III) Complex. Dalton Trans. 2021, 50, 9547–9553. [Google Scholar] [CrossRef]
- Stephenson, T.A.; Wilkinson, G. New Ruthenium Carboxylate Complexes. J. Inorg. Nucl. Chem. 1966, 28, 2285–2291. [Google Scholar] [CrossRef]
- Lindsay, A.J.; Tooze, R.P.; Motevalli, M.; Hursthouse, M.B.; Wilkinson, G. The Synthesis and Structure of Tetra-µ-acetatodiruthenium(II,II)–bis(tetrahydrofuran). J. Chem. Soc. Chem. Commun. 1984, 20, 1383b–1384. [Google Scholar] [CrossRef]
- Furukawa, S.; Kitagawa, S. Neutral Paddlewheel Diruthenium Complexes with Tetracarboxylates of Large π-Conjugated Substituents: Facile One-Pot Synthesis, Crystal Structures, and Electrochemical Studies. Inorg. Chem. 2004, 43, 6464–6472. [Google Scholar] [CrossRef]
- Kataoka, Y.; Tada, N.; Masamori, N.; Yano, N.; Moriyoshi, C.; Handa, M. Paddlewheel-Type and Half-Paddlewheel-Type Diruthenium(II,II) Complexes with 1,8-Naphthyridine-2-Carboxylate. Dalton Trans. 2025, 54, 3047–3056. [Google Scholar] [CrossRef]
- Miyasaka, H.; Motokawa, N.; Atsuumi, R.; Kamo, H.; Asai, Y.; Yamashita, M. Tuning of the Ionization Potential of Paddlewheel Diruthenium(II,II) Complexes with Fluorine Atoms on the Benzoate Ligands. Dalton Trans. 2011, 40, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, W.; Watanabe, Y.; Aliyah, K.H.; Miyasaka, H. Role of Intramolecular Hydrogen Bonding in the Redox Chemistry of Hydroxybenzoate-Bridged Paddlewheel Diruthenium(II,II) Complexes. Dalton Trans. 2022, 51, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, A.J.; Wilkinson, G.; Motevalli, M.; Hursthouse, M.B. The Synthesis, Magnetic, Electrochemical, and Spectroscopic Properties of Diruthenium(II,II) Tetra-µ-carboxylates and Their Adducts. X-Ray Structures of Ru2(O2CR)4L2 (R = Me, L = H2O or Tetrahydrofuran; R = Et, L = Me2CO). J. Chem. Soc. Dalton Trans. 1985, 11, 2321–2326. [Google Scholar] [CrossRef]
- Barral, M.C.; Jimenez-Aparicio, R.; Royer, E.C.; Ruiz-Valero, C.; Urbanos, F.A.; Gutierrez-Puebla, E.; Monge, A. Tertiary Phosphine Oxide Adducts of Diruthenium(II,III) Tetraacetate. Crystal Structure of [Ru2(μ-O2CCH3)4(OPPh3)2]PF6 · CH2ClCH2Cl. Polyhedron 1989, 8, 2571–2576. [Google Scholar] [CrossRef]
- Dikarev, E.V.; Filatov, A.S.; Clérac, R.; Petrukhina, M.A. Unligated Diruthenium(II,II) Tetra(trifluoroacetate): The First X-ray Structural Study, Thermal Compressibility, Lewis Acidity, and Magnetism. Inorg. Chem. 2006, 45, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Andrae, D.; Haeussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-Adjusted Ab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theor. Chem. Acc. 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The Calculations of Excited-State Properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro Software System; Rigaku Oxford Diffraction: Tokyo, Japan, 2018.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Lindsay, A.J.; Wilkinson, G.; Motevalli, M.; Hursthouse, M.B. Reactions of Tetra-µ-carboxylato-diruthenium(II,II) Compounds. X-Ray Crystal Structures of Ru2(µ-O2CCF3)4(THF)2, Ru2(µ-O2CR)4(NO)2 (R = Et or CF3), and {Na3[Ru2(µ-O2CO)4]·6H2O}n. J. Chem. Soc. Dalton Trans. 1987, 11, 2723–2736. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Sheldrick, W.S.; Mintert, M. Dimolybdenum(II) and Diruthenium(II) Complexes with 2-Substituted 7-Methyl-1,8-Naphthyridines as Bridging Ligands. Inorg. Chim. Acta 1994, 219, 23–29. [Google Scholar] [CrossRef]
- Shum, W.W.; Liao, Y.; Miller, J.S. Zero-Field Splitting, Field-Dependent Magnetization of Mixed-Valent S = 3/2 Diruthenium(II,III) Tetracarboxylates. J. Phys. Chem. A 2004, 108, 7460–7462. [Google Scholar] [CrossRef]
- Chen, W.-Z.; Cotton, F.A.; Dalal, N.S.; Murillo, C.A.; Ramsey, C.M.; Ren, T.; Wang, X. Proof of Large Positive Zero-Field Splitting in a Ru25+ Paddlewheel. J. Am. Chem. Soc. 2005, 127, 12691–12696. [Google Scholar] [CrossRef]
- Kataoka, Y.; Mikami, S.; Sakiyama, H.; Mitsumi, M.; Kawamoto, T.; Handa, M. A Neutral Paddlewheel-Type Diruthenium(III) Complex with Benzamidinato Ligands: Synthesis, Crystal Structure, Magnetism, and Electrochemical and Absorption Properties. Polyhedron 2017, 136, 87–92. [Google Scholar] [CrossRef]
- Cotton, F.A.; Miskowski, V.M.; Zhong, B. Chemistry, Structure, and Bonding in Diruthenium(II) Tetracarboxylates. J. Am. Chem. Soc. 1989, 111, 6177–6182. [Google Scholar] [CrossRef]
- Bonnet, L.; Cukiernik, F.D.; Maldivi, P.; Giroud-Godquin, A.-M.; Marchon, J.-C. Synthesis, X-Ray Diffraction, Differential Scanning Calorimetry, and Magnetic Susceptibility Studies of a Series of Binuclear Ruthenium(II) Carboxylates Giving Liquid-Crystalline Phases. Chem. Mater. 1994, 6, 31–38. [Google Scholar] [CrossRef]
- Cukiernik, F.D.; Giroud-Godquin, A.-M.; Maldivi, P.; Marchon, J.-C. Pyrazine-Mediated Antiferromagnetic Intermolecular Exchange in Mixed-Valent Diruthenium Tetracarboxylates. Inorg. Chim. Acta 1994, 215, 203. [Google Scholar] [CrossRef]
- Telser, J.; Drago, R.S. Reinvestigation of the Electronic and Magnetic Properties of Ruthenium Butyrate Chloride. Inorg. Chem. 1984, 23, 3114–3120. [Google Scholar] [CrossRef]
- Kataoka, Y.; Sato, K.; Yano, N.; Handa, M. Ferrocene-Bearing Homoleptic and Heteroleptic Paddlewheel-Type Dirhodium Complexes. Inorganics 2024, 12, 41. [Google Scholar] [CrossRef]
- Das, K.; Kadish, K.M.; Bear, J.L. Substituent and Solvent Effects on the Electrochemical Properties of Tetra-μ-carboxylato-dirhodium(II). Inorg. Chem. 1978, 17, 930–934. [Google Scholar] [CrossRef]
- Angaridis, P.; Berry, J.F.; Cotton, F.A.; Murillo, C.A.; Wang, X. Molecular Squares with Paramagnetic Diruthenium Corners: Synthetic and Crystallographic Challenges. J. Am. Chem. Soc. 2003, 125, 10327–10334. [Google Scholar] [CrossRef]
- Barral, M.C.; Gallo, T.; Herrero, S.; Jiménez-Aparicio, R.; Torres, M.R.; Urbanos, F.A. Equatorially Connected Diruthenium(II,III) Units toward Paramagnetic Supramolecular Structures with Singular Magnetic Properties. Inorg. Chem. 2006, 45, 3639–3647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).