Paddlewheel-Type Diruthenium(II) Naphthyridine Complex with Electron-Withdrawing Trifluoroacetate Ligands
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Synthesis of [Ru2(npc)2(O2CCF3)2] (2)
2.3. Theoretical Calculation Methods
2.4. Crystallography
3. Results
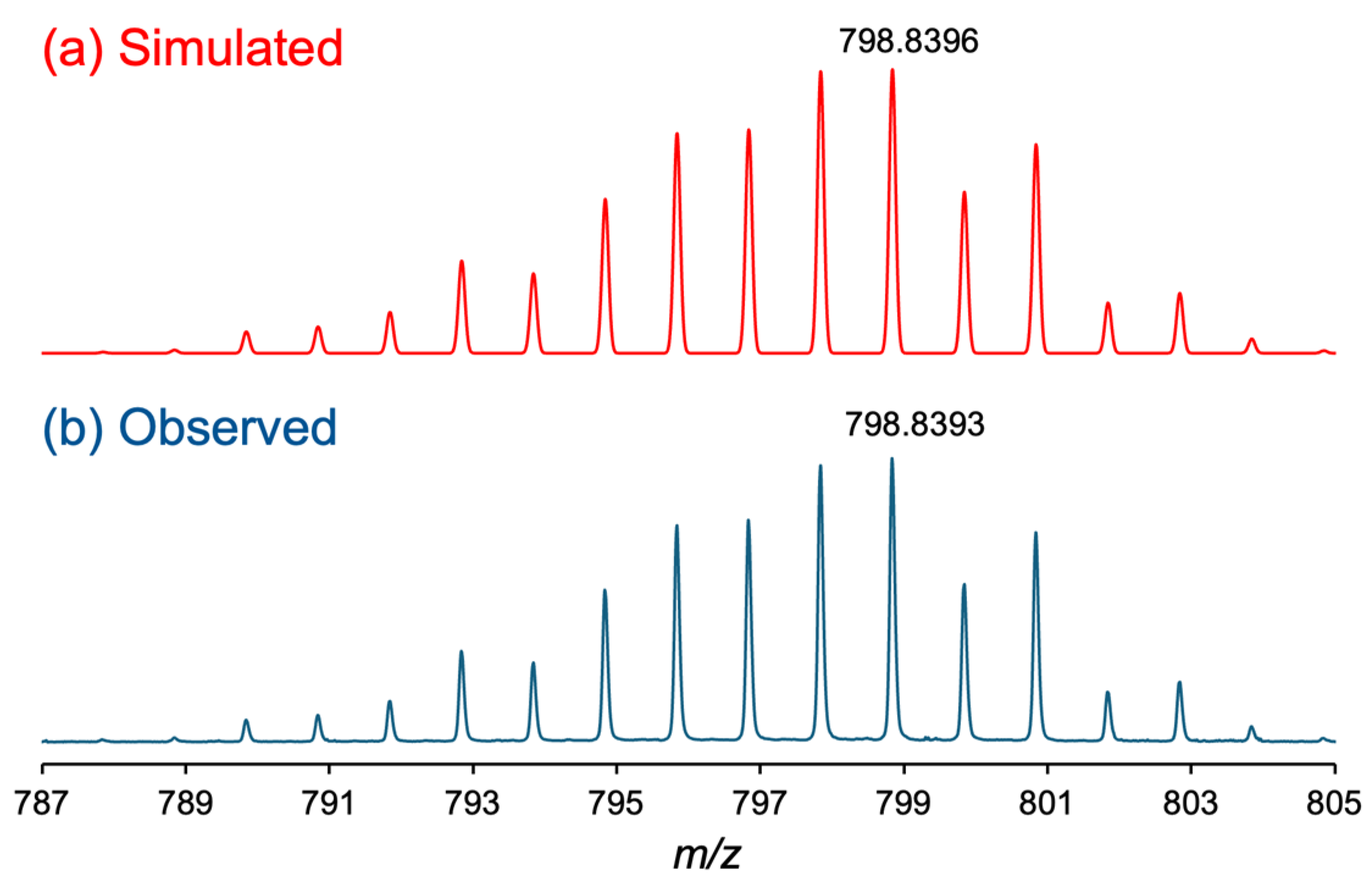
3.1. Synthesis and Chatacterization
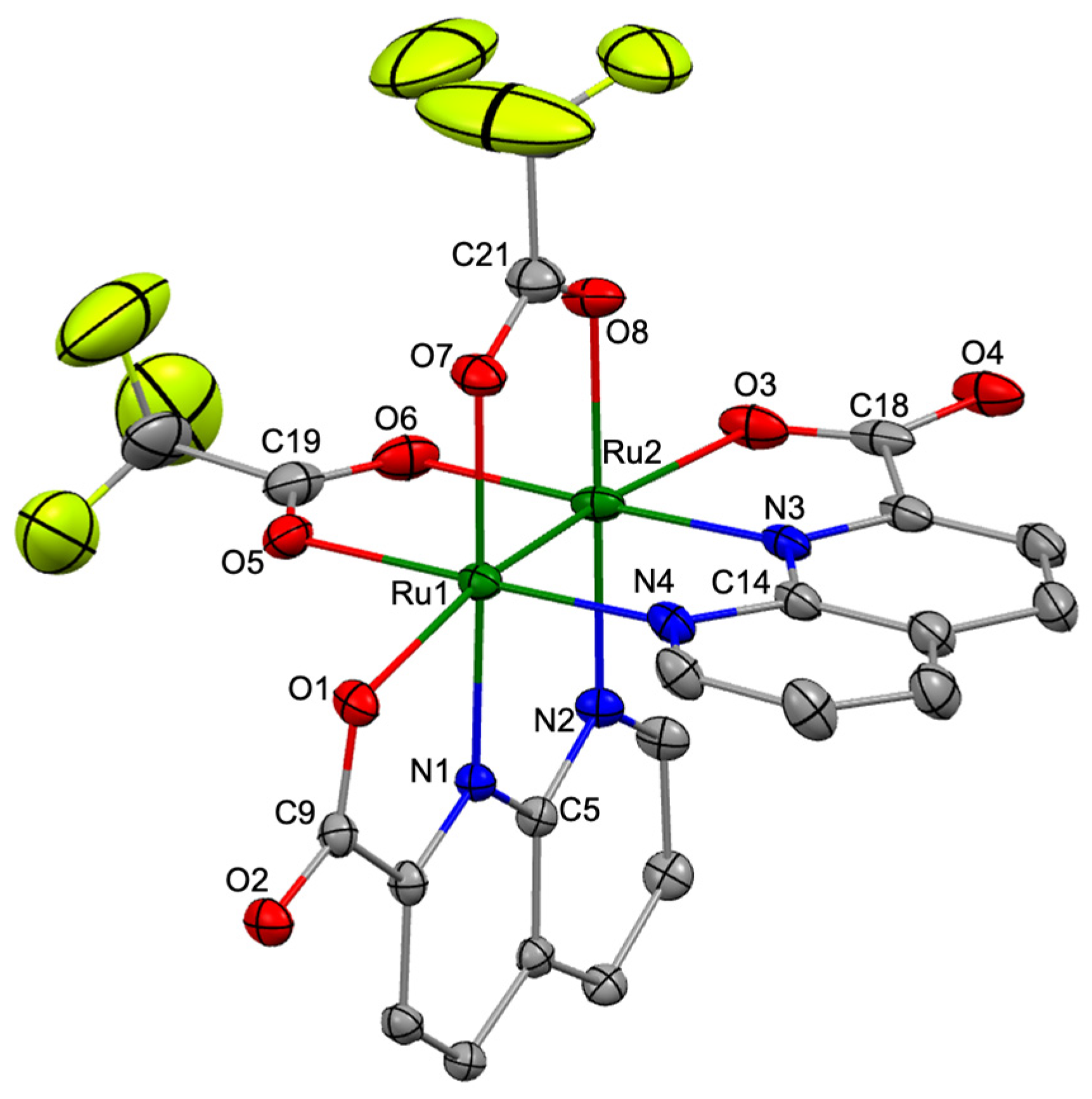
3.2. Crystal Structure
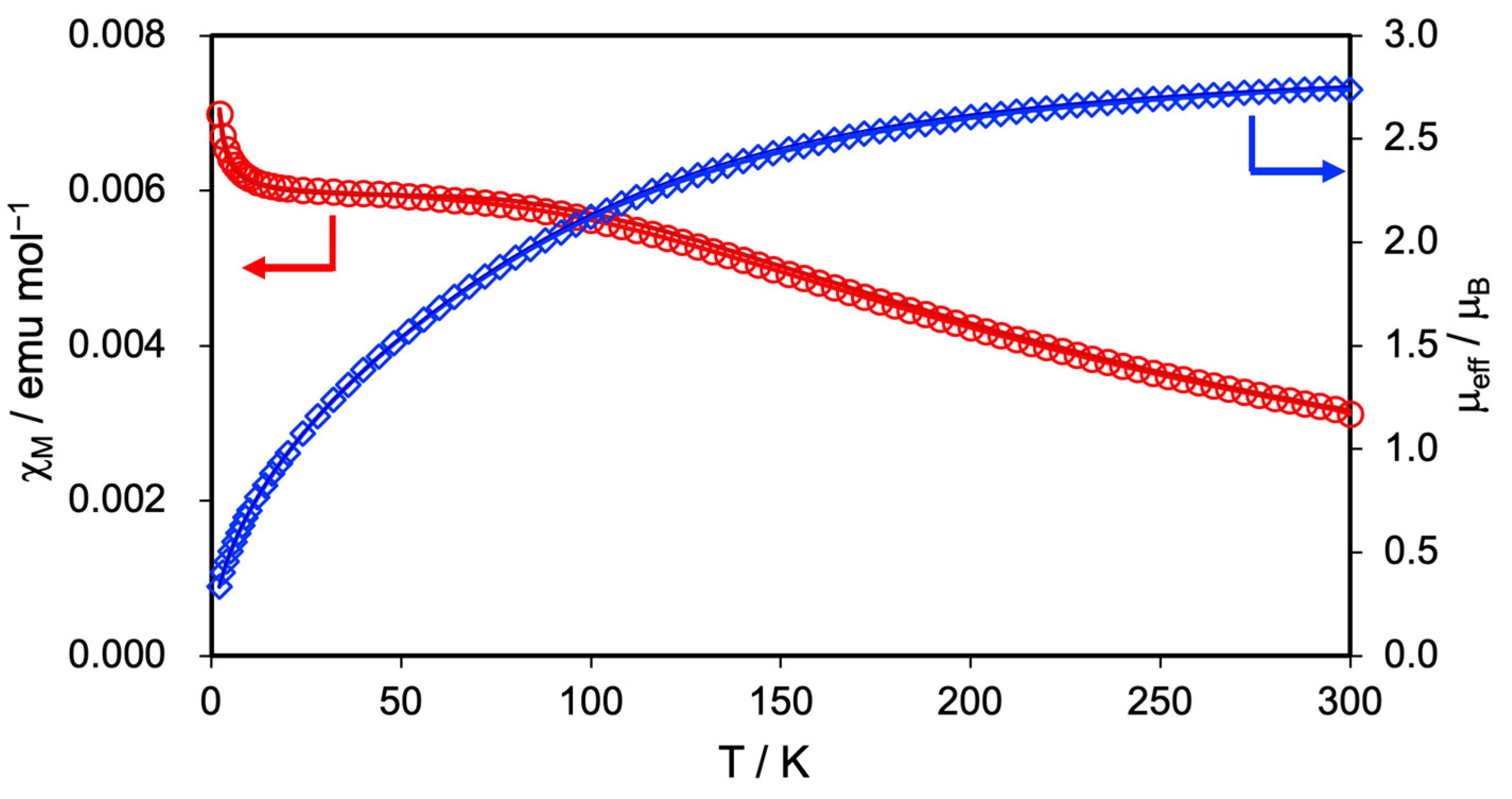
3.3. Magnetic Properties
3.4. Optimized Geometry and Electronic Structure
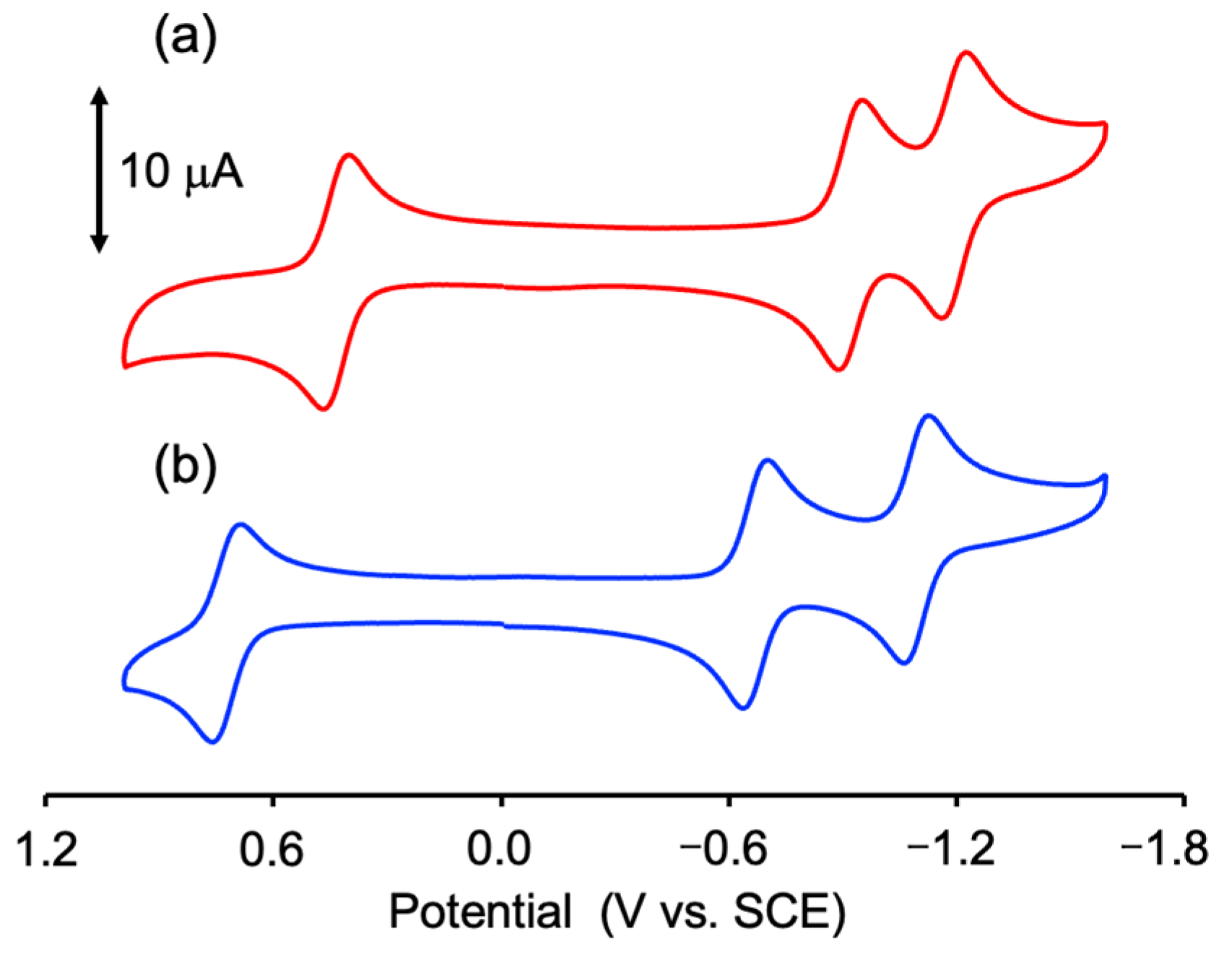
3.5. Electrochemical Property
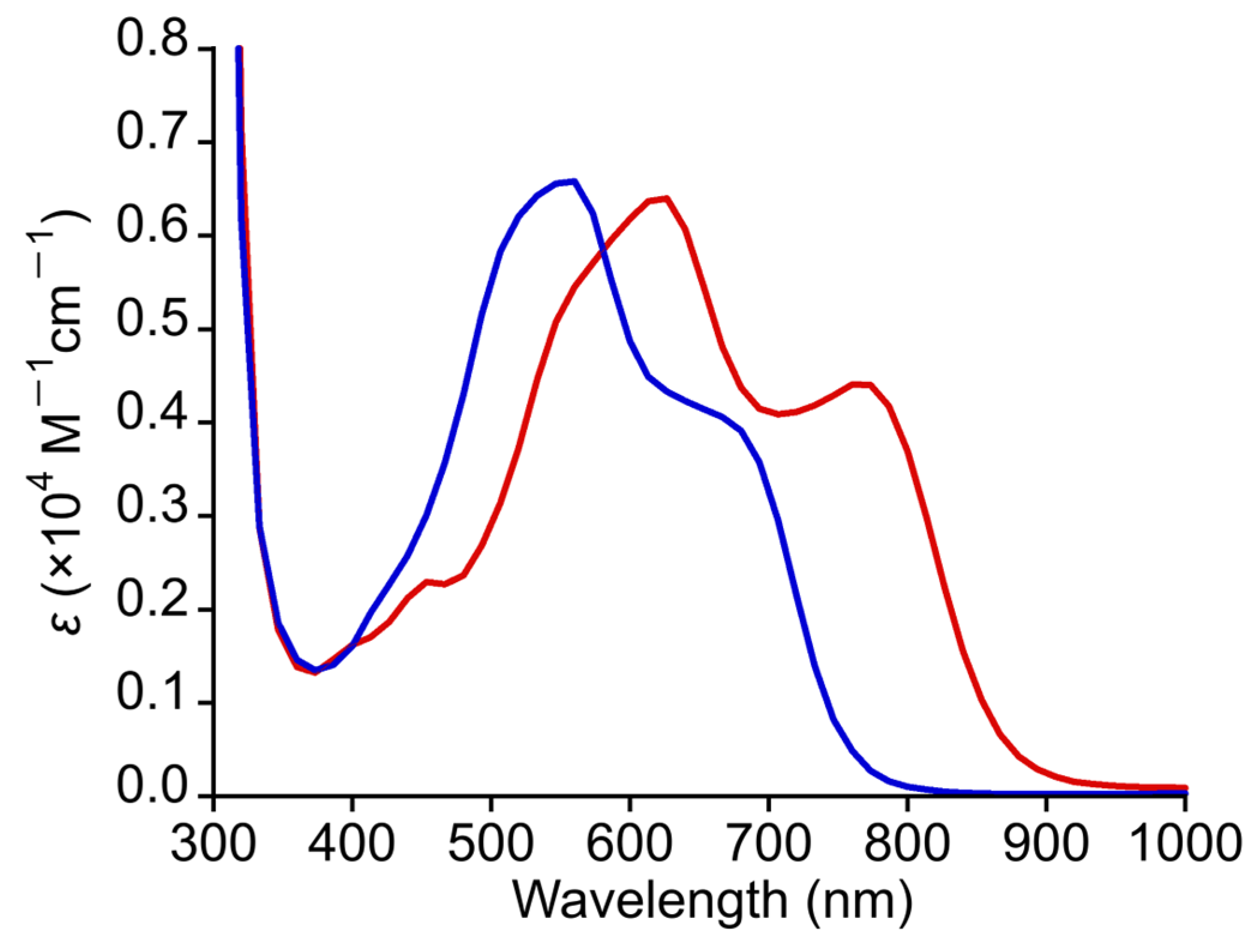
3.6. Absorption-Spectral Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotton, F.A.; Murillo, C.A.; Walton, R.A. Multiple Bonds Between Metal Atoms, 3rd ed.; Springer Science and Business Media: New York, NY, USA, 2005. [Google Scholar]
- Chisholm, M.H.; Patmore, N.J. Studies of electronic coupling and mixed valency in metal-metal quadruply bonded complexes linked by dicarboxylate and closely related ligands. Acc. Chem. Res. 2007, 40, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Murillo, C.A. Adventures in Divalent Early Transition Metal Coordination Chemistry: On the Way to Metal–Metal Bonded Species. Inorg. Chim. Acta 2017, 468, 3–15. [Google Scholar] [CrossRef]
- Ren, T. Substituent Effects in Dinuclear Paddlewheel Compounds: Electrochemical and Spectroscopic Investigations. Coord. Chem. Rev. 1998, 175, 43–58. [Google Scholar] [CrossRef]
- Hrdina, R. Dirhodium(II,II) Paddlewheel Complexes. Eur. J. Inorg. Chem. 2021, 6, 501–528. [Google Scholar] [CrossRef]
- Köberl, M.; Cokoja, M.; Herrmann, W.A.; Kühn, F.E. From Molecules to Materials: Molecular Paddle-Wheel Synthons of Macromolecules, Cage Compounds, and Metal–Organic Frameworks. Dalton Trans. 2011, 40, 6834–6859. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Yano, N.; Mikuriya, M.; Handa, M. Paddlewheel-type dirhodium complexes with N,N′-bridging ligands. Coord. Chem. Rev. 2023, 479, 214997. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; Groy, T.L.; Yaghi, O.M. Establishing Microporosity in Open Metal–Organic Frameworks: Gas Sorption Isotherms for Zn(BDC) (BDC = 1,4-Benzenedicarboxylate). J. Am. Chem. Soc. 1998, 120, 8571–8572. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Chun, H.; Kim, K. Rigid and Flexible: A Highly Porous Metal–Organic Framework with Unusual Guest-Dependent Dynamic Behavior. Angew. Chem. Int. Ed. 2004, 43, 5033–5036. [Google Scholar] [CrossRef]
- Mori, W.; Takamizawa, S.; Nozaki Kato, C.; Ohmura, T.; Sato, T. Molecular-Level Design of Efficient Microporous Materials Containing Metal Carboxylates: Inclusion Complex Formation with Organic Polymer, Gas-Occlusion Properties, and Catalytic Activities for Hydrogenation of Olefins. Microporous Mesoporous Mater. 2004, 73, 31–46. [Google Scholar] [CrossRef]
- Miyasaka, H. Charge Manipulation in Metal–Organic Frameworks: Toward Designer Functional Molecular Materials. Bull. Chem. Soc. Jpn. 2021, 94, 2929–2955. [Google Scholar] [CrossRef]
- Kataoka, Y.; Yano, N.; Mikuriya, M.; Handa, M. Coordination polymers and metal–organic frameworks based on paddlewheel-type dirhodium(II) tetracarboxylates. Coord. Chem. Rev. 2022, 472, 214796. [Google Scholar] [CrossRef]
- Aquino, M.A.S. Diruthenium and Diosmium Tetracarboxylates: Synthesis, Physical Properties, and Applications. Coord. Chem. Rev. 1998, 170, 141–202. [Google Scholar] [CrossRef]
- Aquino, M.A.S. Recent Developments in the Synthesis and Properties of Diruthenium Tetracarboxylates. Coord. Chem. Rev. 2004, 248, 1025–1045. [Google Scholar] [CrossRef]
- Van Caemelbecke, E.; Phan, T.; Osterloh, W.R.; Kadish, K.M. Electrochemistry of Metal-Metal Bonded Diruthenium Complexes. Coord. Chem. Rev. 2021, 434, 213706. [Google Scholar] [CrossRef]
- Cortijo, M.; González-Prieto, R.; Herrero, S.; Priego, J.L.; Jiménez-Aparicio, R. The Use of Amidinate Ligands in Paddlewheel Diruthenium Chemistry. Coord. Chem. Rev. 2019, 400, 213040. [Google Scholar] [CrossRef]
- Mikuriya, M.; Yoshioka, D.; Handa, M. Magnetic Interactions in One-, Two-, and Three-Dimensional Assemblies of Dinuclear Ruthenium Carboxylates. Coord. Chem. Rev. 2006, 250, 2194–2211. [Google Scholar] [CrossRef]
- Liao, Y.; Shum, W.W.; Miller, J.S. Synthesis and Magnetic Properties of 3-D [RuII/III2(O2CMe)4]3[MIII(CN)6] (M = Cr, Fe, Co). J. Am. Chem. Soc. 2002, 124, 9336–9337. [Google Scholar] [CrossRef]
- Angaridis, P.; Cotton, F.A.; Murillo, C.A.; Villagrán, D.; Wang, X. Structural and Magnetic Evidence Concerning Spin Crossover in Formamidinate Compounds with Ru25+ Cores. J. Am. Chem. Soc. 2005, 127, 5008–5009. [Google Scholar] [CrossRef]
- Miyasaka, H.; Motokawa, N.; Matsunaga, S.; Yamashita, M.; Sugimoto, K.; Mori, T.; Toyota, N.; Dunbar, K.R. Control of Charge Transfer in a Series of Ru22+/TCNQ Two-Dimensional Networks by Tuning the Electron Affinity of TCNQ Units: A Route to Synergistic Magnetic/Conducting Materials. J. Am. Chem. Soc. 2010, 132, 1532–1544. [Google Scholar] [CrossRef]
- Zhang, J.; Kosaka, W.; Liu, Q.; Amamizu, N.; Kitagawa, Y.; Miyasaka, H. CO2-Sensitive Porous Magnet: Antiferromagnet Creation from a Paramagnetic Charge-Transfer Layered Metal–Organic Framework. J. Am. Chem. Soc. 2023, 145, 26179–26189. [Google Scholar] [CrossRef]
- Trenerry, M.J.; Wallen, C.M.; Brown, T.R.; Park, S.V.; Berry, J.F. Spontaneous N2 Formation by a Diruthenium Complex Enables Electrocatalytic and Aerobic Oxidation of Ammonia. Nat. Chem. 2021, 13, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Sato, K.; Miyazaki, Y.; Masuda, K.; Tanaka, H.; Naito, S.; Mori, W. Photocatalytic Hydrogen Production from Water Using Porous Material [Ru2(p-BDC)2]n. Energy Environ. Sci. 2009, 2, 397–400. [Google Scholar] [CrossRef]
- Miyazawa, T.; Suzuki, T.; Kumagai, Y.; Takizawa, K.; Kikuchi, T.; Kato, S.; Onoda, A.; Hayashi, T.; Kamei, Y.; Kamiyama, F.; et al. Chiral Paddle-Wheel Diruthenium Complexes for Asymmetric Catalysis. Nature Catal. 2020, 3, 851–858. [Google Scholar] [CrossRef]
- Villalobos, L.; Barker Paredes, J.E.; Cao, Z.; Ren, T. tert-Butyl Hydroperoxide Oxygenation of Organic Sulfides Catalyzed by Diruthenium(II,III) Tetracarboxylates. Inorg. Chem. 2013, 52, 12545–12552. [Google Scholar] [CrossRef]
- Tolbatov, I.; Barresi, E.; Taliani, S.; La Mendola, D.; Marzo, T.; Marrone, A. Diruthenium(II,III) Paddlewheel Complexes: Effects of Bridging and Axial Ligands on Anticancer Properties. Inorg. Chem. Front. 2023, 10, 2226–2238. [Google Scholar] [CrossRef]
- Barresi, E.; Tolbatov, I.; Marzo, T.; Zappelli, E.; Marrone, A.; Re, N.; Pratesi, A.; Martini, C.; Taliani, S.; Da Settimo, F.; et al. Two Mixed-Valence Diruthenium(II,III) Isomeric Complexes Show Different Anticancer Properties. Dalton Trans. 2021, 50, 9643–9647. [Google Scholar] [CrossRef]
- Kataoka, Y.; Imasaki, N.; Yano, N.; Mitsumi, M.; Handa, M. Redox-Triggered Reversible Modulation of Intense Near-Infrared and Visible Absorption Using Paddlewheel-Type Diruthenium(III) Complex. Dalton Trans. 2021, 50, 9547–9553. [Google Scholar] [CrossRef]
- Stephenson, T.A.; Wilkinson, G. New Ruthenium Carboxylate Complexes. J. Inorg. Nucl. Chem. 1966, 28, 2285–2291. [Google Scholar] [CrossRef]
- Lindsay, A.J.; Tooze, R.P.; Motevalli, M.; Hursthouse, M.B.; Wilkinson, G. The Synthesis and Structure of Tetra-µ-acetatodiruthenium(II,II)–bis(tetrahydrofuran). J. Chem. Soc. Chem. Commun. 1984, 20, 1383b–1384. [Google Scholar] [CrossRef]
- Furukawa, S.; Kitagawa, S. Neutral Paddlewheel Diruthenium Complexes with Tetracarboxylates of Large π-Conjugated Substituents: Facile One-Pot Synthesis, Crystal Structures, and Electrochemical Studies. Inorg. Chem. 2004, 43, 6464–6472. [Google Scholar] [CrossRef]
- Kataoka, Y.; Tada, N.; Masamori, N.; Yano, N.; Moriyoshi, C.; Handa, M. Paddlewheel-Type and Half-Paddlewheel-Type Diruthenium(II,II) Complexes with 1,8-Naphthyridine-2-Carboxylate. Dalton Trans. 2025, 54, 3047–3056. [Google Scholar] [CrossRef]
- Miyasaka, H.; Motokawa, N.; Atsuumi, R.; Kamo, H.; Asai, Y.; Yamashita, M. Tuning of the Ionization Potential of Paddlewheel Diruthenium(II,II) Complexes with Fluorine Atoms on the Benzoate Ligands. Dalton Trans. 2011, 40, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, W.; Watanabe, Y.; Aliyah, K.H.; Miyasaka, H. Role of Intramolecular Hydrogen Bonding in the Redox Chemistry of Hydroxybenzoate-Bridged Paddlewheel Diruthenium(II,II) Complexes. Dalton Trans. 2022, 51, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, A.J.; Wilkinson, G.; Motevalli, M.; Hursthouse, M.B. The Synthesis, Magnetic, Electrochemical, and Spectroscopic Properties of Diruthenium(II,II) Tetra-µ-carboxylates and Their Adducts. X-Ray Structures of Ru2(O2CR)4L2 (R = Me, L = H2O or Tetrahydrofuran; R = Et, L = Me2CO). J. Chem. Soc. Dalton Trans. 1985, 11, 2321–2326. [Google Scholar] [CrossRef]
- Barral, M.C.; Jimenez-Aparicio, R.; Royer, E.C.; Ruiz-Valero, C.; Urbanos, F.A.; Gutierrez-Puebla, E.; Monge, A. Tertiary Phosphine Oxide Adducts of Diruthenium(II,III) Tetraacetate. Crystal Structure of [Ru2(μ-O2CCH3)4(OPPh3)2]PF6 · CH2ClCH2Cl. Polyhedron 1989, 8, 2571–2576. [Google Scholar] [CrossRef]
- Dikarev, E.V.; Filatov, A.S.; Clérac, R.; Petrukhina, M.A. Unligated Diruthenium(II,II) Tetra(trifluoroacetate): The First X-ray Structural Study, Thermal Compressibility, Lewis Acidity, and Magnetism. Inorg. Chem. 2006, 45, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Andrae, D.; Haeussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-Adjusted Ab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theor. Chem. Acc. 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The Calculations of Excited-State Properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro Software System; Rigaku Oxford Diffraction: Tokyo, Japan, 2018.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Lindsay, A.J.; Wilkinson, G.; Motevalli, M.; Hursthouse, M.B. Reactions of Tetra-µ-carboxylato-diruthenium(II,II) Compounds. X-Ray Crystal Structures of Ru2(µ-O2CCF3)4(THF)2, Ru2(µ-O2CR)4(NO)2 (R = Et or CF3), and {Na3[Ru2(µ-O2CO)4]·6H2O}n. J. Chem. Soc. Dalton Trans. 1987, 11, 2723–2736. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Sheldrick, W.S.; Mintert, M. Dimolybdenum(II) and Diruthenium(II) Complexes with 2-Substituted 7-Methyl-1,8-Naphthyridines as Bridging Ligands. Inorg. Chim. Acta 1994, 219, 23–29. [Google Scholar] [CrossRef]
- Shum, W.W.; Liao, Y.; Miller, J.S. Zero-Field Splitting, Field-Dependent Magnetization of Mixed-Valent S = 3/2 Diruthenium(II,III) Tetracarboxylates. J. Phys. Chem. A 2004, 108, 7460–7462. [Google Scholar] [CrossRef]
- Chen, W.-Z.; Cotton, F.A.; Dalal, N.S.; Murillo, C.A.; Ramsey, C.M.; Ren, T.; Wang, X. Proof of Large Positive Zero-Field Splitting in a Ru25+ Paddlewheel. J. Am. Chem. Soc. 2005, 127, 12691–12696. [Google Scholar] [CrossRef]
- Kataoka, Y.; Mikami, S.; Sakiyama, H.; Mitsumi, M.; Kawamoto, T.; Handa, M. A Neutral Paddlewheel-Type Diruthenium(III) Complex with Benzamidinato Ligands: Synthesis, Crystal Structure, Magnetism, and Electrochemical and Absorption Properties. Polyhedron 2017, 136, 87–92. [Google Scholar] [CrossRef]
- Cotton, F.A.; Miskowski, V.M.; Zhong, B. Chemistry, Structure, and Bonding in Diruthenium(II) Tetracarboxylates. J. Am. Chem. Soc. 1989, 111, 6177–6182. [Google Scholar] [CrossRef]
- Bonnet, L.; Cukiernik, F.D.; Maldivi, P.; Giroud-Godquin, A.-M.; Marchon, J.-C. Synthesis, X-Ray Diffraction, Differential Scanning Calorimetry, and Magnetic Susceptibility Studies of a Series of Binuclear Ruthenium(II) Carboxylates Giving Liquid-Crystalline Phases. Chem. Mater. 1994, 6, 31–38. [Google Scholar] [CrossRef]
- Cukiernik, F.D.; Giroud-Godquin, A.-M.; Maldivi, P.; Marchon, J.-C. Pyrazine-Mediated Antiferromagnetic Intermolecular Exchange in Mixed-Valent Diruthenium Tetracarboxylates. Inorg. Chim. Acta 1994, 215, 203. [Google Scholar] [CrossRef]
- Telser, J.; Drago, R.S. Reinvestigation of the Electronic and Magnetic Properties of Ruthenium Butyrate Chloride. Inorg. Chem. 1984, 23, 3114–3120. [Google Scholar] [CrossRef]
- Kataoka, Y.; Sato, K.; Yano, N.; Handa, M. Ferrocene-Bearing Homoleptic and Heteroleptic Paddlewheel-Type Dirhodium Complexes. Inorganics 2024, 12, 41. [Google Scholar] [CrossRef]
- Das, K.; Kadish, K.M.; Bear, J.L. Substituent and Solvent Effects on the Electrochemical Properties of Tetra-μ-carboxylato-dirhodium(II). Inorg. Chem. 1978, 17, 930–934. [Google Scholar] [CrossRef]
- Angaridis, P.; Berry, J.F.; Cotton, F.A.; Murillo, C.A.; Wang, X. Molecular Squares with Paramagnetic Diruthenium Corners: Synthetic and Crystallographic Challenges. J. Am. Chem. Soc. 2003, 125, 10327–10334. [Google Scholar] [CrossRef]
- Barral, M.C.; Gallo, T.; Herrero, S.; Jiménez-Aparicio, R.; Torres, M.R.; Urbanos, F.A. Equatorially Connected Diruthenium(II,III) Units toward Paramagnetic Supramolecular Structures with Singular Magnetic Properties. Inorg. Chem. 2006, 45, 3639–3647. [Google Scholar] [CrossRef]



| Complex | 2 |
|---|---|
| Radiation type | synchrotron |
| Radiation wavelength | 0.4108 |
| Chemical formula | C24H13F6N5O8Ru2 |
| Formula weight | 815.53 |
| Crystal system | Tetragonal |
| Space group | I 41/a |
| a (Å) | 18.66850 (10) |
| b (Å) | 18.66850 (10) |
| c (Å) | 33.7511 (2) |
| α (deg) | 90 |
| β (deg) | 90 |
| γ (deg) | 90 |
| V (Å3) | 11,762.69 (14) |
| Z | 16 |
| Dcalc (g cm−3) | 1.842 |
| μ (mm−1) | 1.483 |
| F (000) | 6368 |
| R1 (I > 2σ(I)) | 0.0379 |
| wR2 (I > 2σ(I)) | 0.1062 |
| R1 (all data) | 0.0384 |
| wR2 (all data) | 0.1067 |
| GOF on F2 | 1.072 |
| Complex | D Tensor (cm−1) | g Value | Ref |
|---|---|---|---|
| 1 | 227 | 2.13 | 32 |
| 2 | 243 | 2.03 | this study |
| [Ru2(O2CMe)4] | 244 | 2.08 | 54 |
| [Ru2(O2CCF3)4] | 220 | 2.0 | 37 |
| [Ru2(O2CC9H17)] | 283 | ― | 55 |
| [Ru2(O2CMe)4(H2O)2]BPh4 | 72 | 2.19 | 56 |
| [Ru2(O2CC3H7)4Cl] | 77 | 2.10 | 57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kataoka, Y.; Tada, N.; Omaki, J.; Matsubara, K.; Yano, N.; Handa, M. Paddlewheel-Type Diruthenium(II) Naphthyridine Complex with Electron-Withdrawing Trifluoroacetate Ligands. Chemistry 2025, 7, 72. https://doi.org/10.3390/chemistry7030072
Kataoka Y, Tada N, Omaki J, Matsubara K, Yano N, Handa M. Paddlewheel-Type Diruthenium(II) Naphthyridine Complex with Electron-Withdrawing Trifluoroacetate Ligands. Chemistry. 2025; 7(3):72. https://doi.org/10.3390/chemistry7030072
Chicago/Turabian StyleKataoka, Yusuke, Nozomi Tada, Junya Omaki, Kanami Matsubara, Natsumi Yano, and Makoto Handa. 2025. "Paddlewheel-Type Diruthenium(II) Naphthyridine Complex with Electron-Withdrawing Trifluoroacetate Ligands" Chemistry 7, no. 3: 72. https://doi.org/10.3390/chemistry7030072
APA StyleKataoka, Y., Tada, N., Omaki, J., Matsubara, K., Yano, N., & Handa, M. (2025). Paddlewheel-Type Diruthenium(II) Naphthyridine Complex with Electron-Withdrawing Trifluoroacetate Ligands. Chemistry, 7(3), 72. https://doi.org/10.3390/chemistry7030072








