Isolation and Characterisation of Two New Lactones from the Atacama Desert-Derived Fungus Chrysosporium merdarium
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of the Fungus
2.2. Cultivation of the Fungus and Isolation of the Compounds
2.3. Identification and Characterisation of the Compounds
2.4. Antimicrobial Assay
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monciardini, P.; Iorio, M.; Maffioli, S.; Sosio, M.; Donadio, S. Discovering new bioactive molecules from microbial sources. Microb. Biotechnol. 2014, 7, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, E.; Meslier, V.; Browning, A.; Treadgold, J.; DiRuggiero, J. Rock structure drives the taxonomic and functional diversity of endolithic microbial communities in extreme environments. Environ. Microbiol. 2021, 23, 3937–3956. [Google Scholar] [CrossRef]
- McKay, C.P.; Friedmann, E.I.; Gómez-Silva, B.; Cáceres-Villanueva, L.; Andersen, D.T.; Landheim, R. Temperature and Moisture Conditions for Life in the Extreme Arid Region of the Atacama Desert: Four Years of Observations Including the El Niño of 1997–1998. Astrobiology 2003, 3, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Dorador, C.; Fink, P.; Hengst, M.; Icaza, G.; Villalobos, A.S.; Vejar, D.; Meneses, D.; Zadjelovic, V.; Burmann, L.; Moelzner, J.; et al. Microbial community composition and trophic role along a marked salinity gradient in Laguna Puilar, Salar de Atacama, Chile. Antonie Van Leeuwenhoek 2018, 111, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Bull, A.T.; Andrews, B.A.; Dorador, C.; Goodfellow, M. Introducing the Atacama Desert. Antonie Van Leeuwenhoek 2018, 111, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Silva, B.; Rainey, F.; Warren-Rhodes, K.; McKay, C.; Navarro-Gonzalez, R. Atacama Desert Soil Microbiology. Microbiol. Extrem. Soils 2008, 13, 117–132. [Google Scholar] [CrossRef]
- Rateb, M.E.; Ebel, R.; Jaspars, M. Natural product diversity of actinobacteria in the Atacama Desert. Antonie Van Leeuwenhoek 2018, 111, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Astakala, R.V.; Preet, G.; Milne, B.F.; Tibyangye, J.; Razmilic, V.; Castro, J.F.; Asenjo, J.A.; Andrews, B.; Ebel, R.; Jaspars, M. Mutactimycin AP, a New Mutactimycin Isolated from an Actinobacteria from the Atacama Desert. Molecules 2022, 27, 7185. [Google Scholar] [CrossRef] [PubMed]
- Schulz, D.; Beese, P.; Ohlendorf, B.; Erhard, A.; Zinecker, H.; Dorador, C.; Imhoff, J.F. Abenquines A–D: Aminoquinone derivatives produced by Streptomyces sp. strain DB634. J. Antibiot. 2011, 64, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Trisuwan, K.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Modiolide and pyrone derivatives from the sea fan-derived fungus Curvularia sp. PSU-F22. Arch. Pharm. Res. 2011, 34, 709–714. [Google Scholar] [CrossRef]
- Tsuda, M.; Mugishima, T.; Komatsu, K.; Sone, T.; Tanaka, M.; Mikami, Y.; Kobayashi, J. Modiolides A and B, Two New 10-Membered Macrolides from a Marine-Derived Fungus. J. Nat. Prod. 2003, 66, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qu, Q.; Liu, X.; Cui, W.; Yu, F.; Chen, X.; Xing, X.; Zhou, Y.; Yang, Y.; Bello-Onaghise, G.; et al. 1-Hydroxyanthraquinone exhibited antibacterial activity by regulating glutamine synthetase of Staphylococcus xylosus as a virulence factor. Biomed. Pharmacother. 2020, 123, 109779. [Google Scholar] [CrossRef] [PubMed]
- Srikanta, D.; Santiago-Tirado, F.H.; Doering, T.L. Cryptococcus neoformans: Historical curiosity to modern pathogen. Yeast 2014, 31, 47–60. [Google Scholar] [CrossRef]
- Preet, G.; Astakala, R.V.; Rajakulendran, J.E.; Oluwabusola, E.T.; Ebel, R.; Jaspars, M. (E)-N-(3-(5-(3-Acetamidopropyl)-3,6-dioxopiperazin-2-yl)propyl)-5-hydroxy-3-methylpent-2-enamide. Molbank 2023, 2023, M1680. [Google Scholar] [CrossRef]
- Siddharth, S.; Vittal, R. Evaluation of Antimicrobial, Enzyme Inhibitory, Antioxidant and Cytotoxic Activities of Partially Purified Volatile Metabolites of Marine Streptomyces sp.S2A. Microorganisms 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Haj Hasan, A.; Preet, G.; Astakala, R.V.; Al-Adilah, H.; Oluwabusola, E.T.; Ebel, R.; Jaspars, M. Antibacterial activity of natural flavones against bovine mastitis pathogens: In vitro, SAR analysis, and computational study. In Silico Pharmacol. 2024, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Cimmino, A.; Berestetskiy, A.; Andolfi, A.; Motta, A. Stagonolides G−I and Modiolide A, Nonenolides Produced by Stagonospora cirsii, a Potential Mycoherbicide for Cirsium arvense. J. Nat. Prod. 2008, 71, 1897–1901. [Google Scholar] [CrossRef]
- Evidente, A. Putaminoxin, a phytotoxic nonenolide from Phoma putaminum. Phytochemistry 1995, 40, 1637–1641. [Google Scholar] [CrossRef]
- Evidente, A.; Lanzetta, R.; Capasso, R.; Andolfi, A.; Vurro, M.; Zonno, M.C. Putaminoxins B and C from Phoma putaminum. Phytochemistry 1997, 44, 1041–1045. [Google Scholar] [CrossRef]
- Rivero-Cruz, J.F.; Macías, M.; Cerda-García-Rojas, C.M.; Mata, R. A New Phytotoxic Nonenolide from Phoma herbarum. J. Nat. Prod. 2003, 66, 511–514. [Google Scholar] [CrossRef] [PubMed]
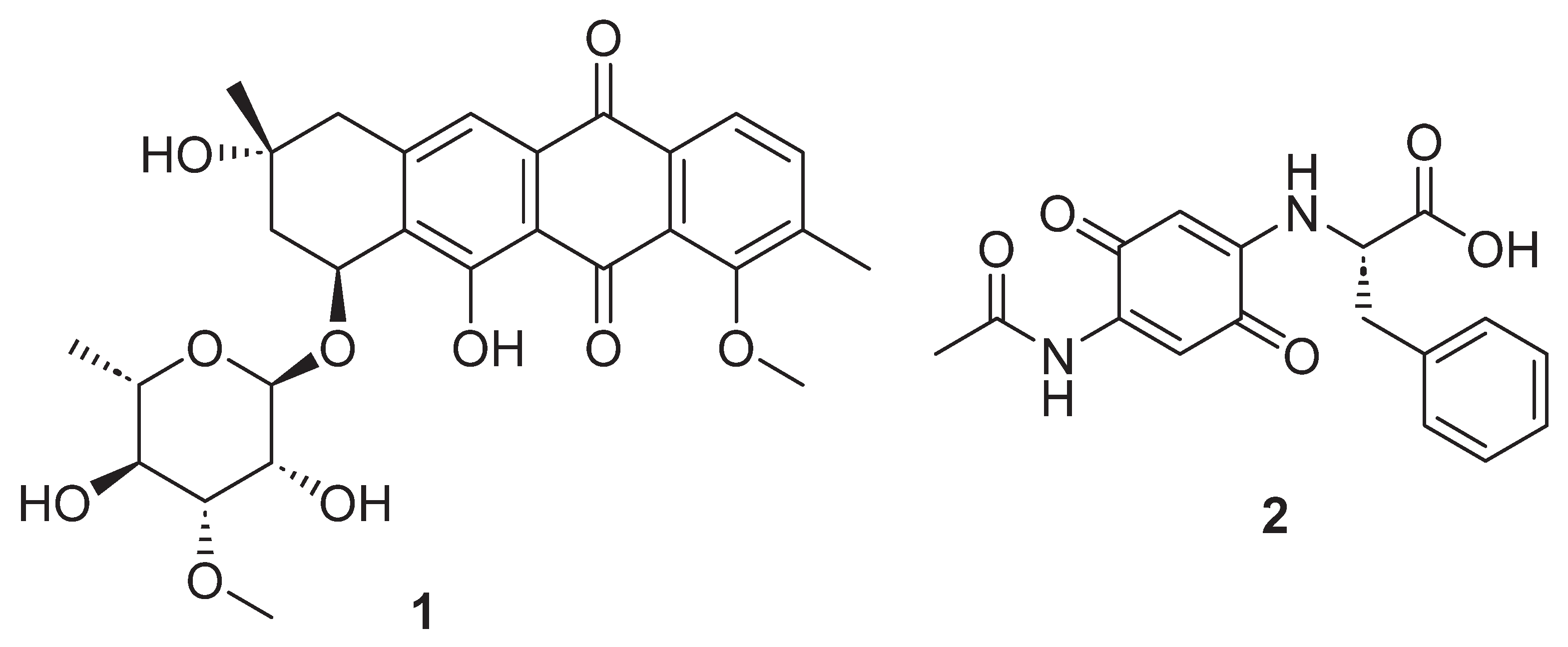
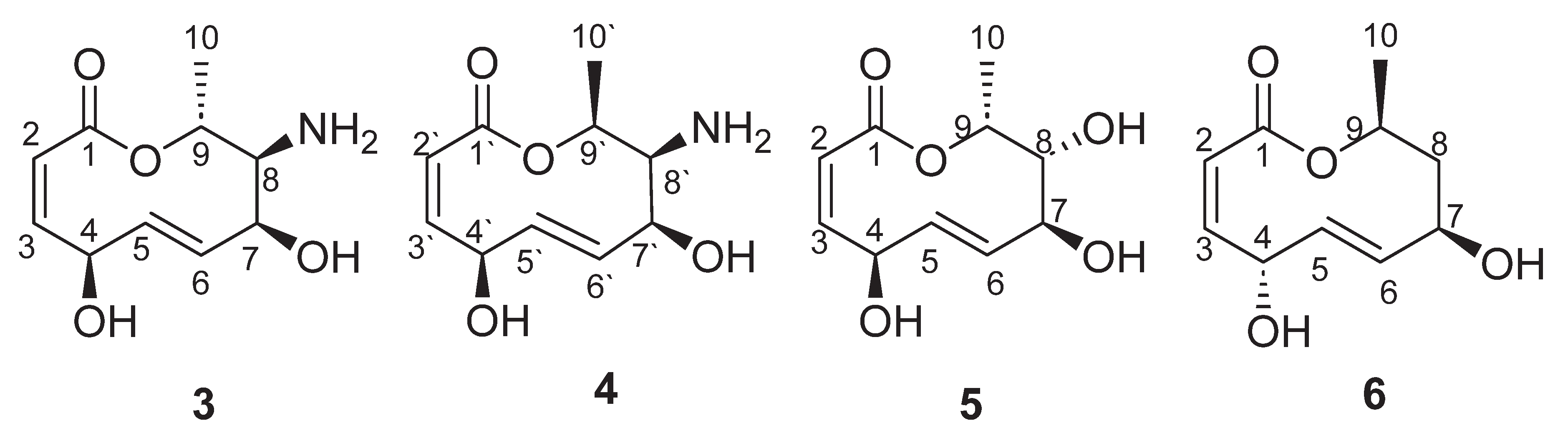

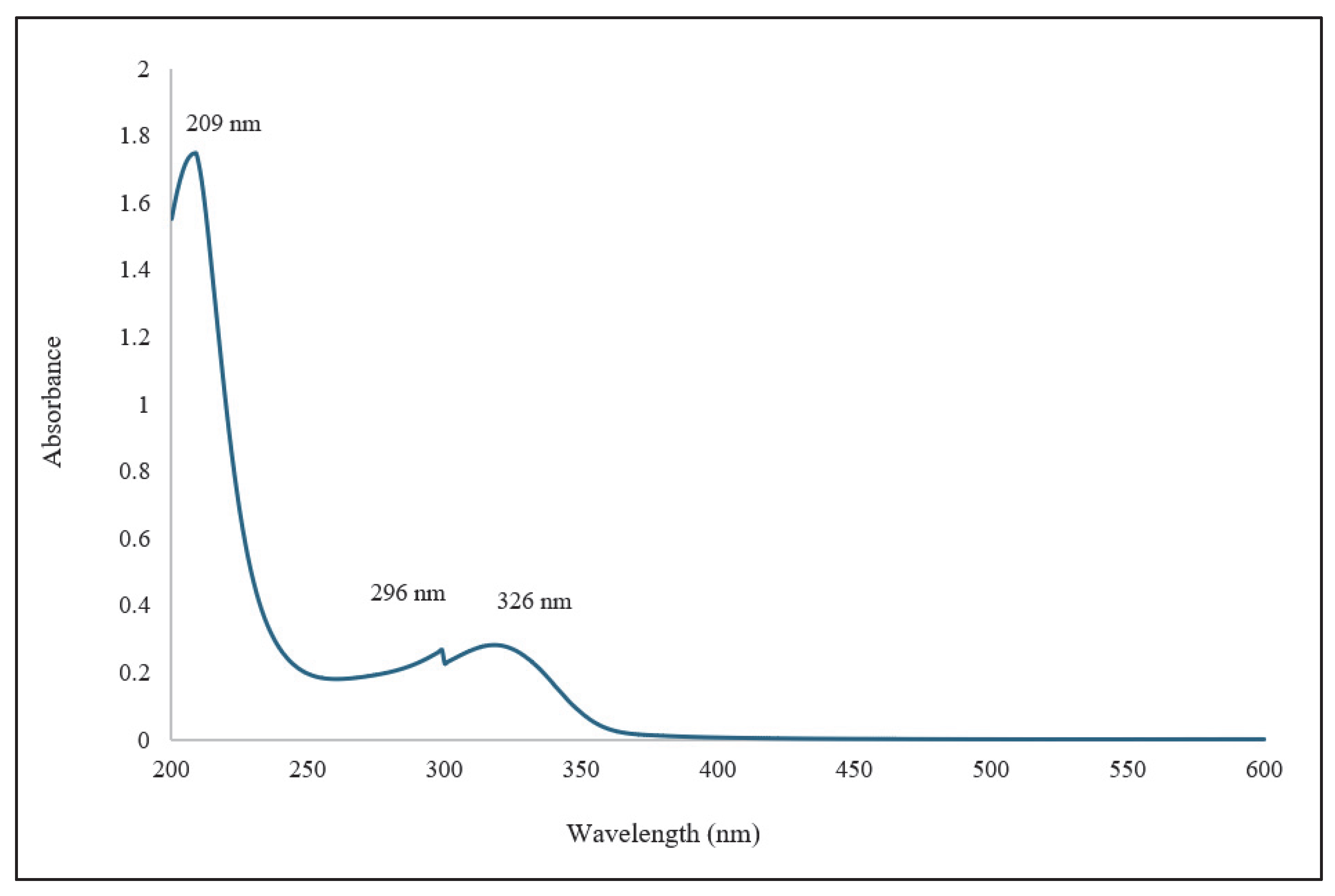
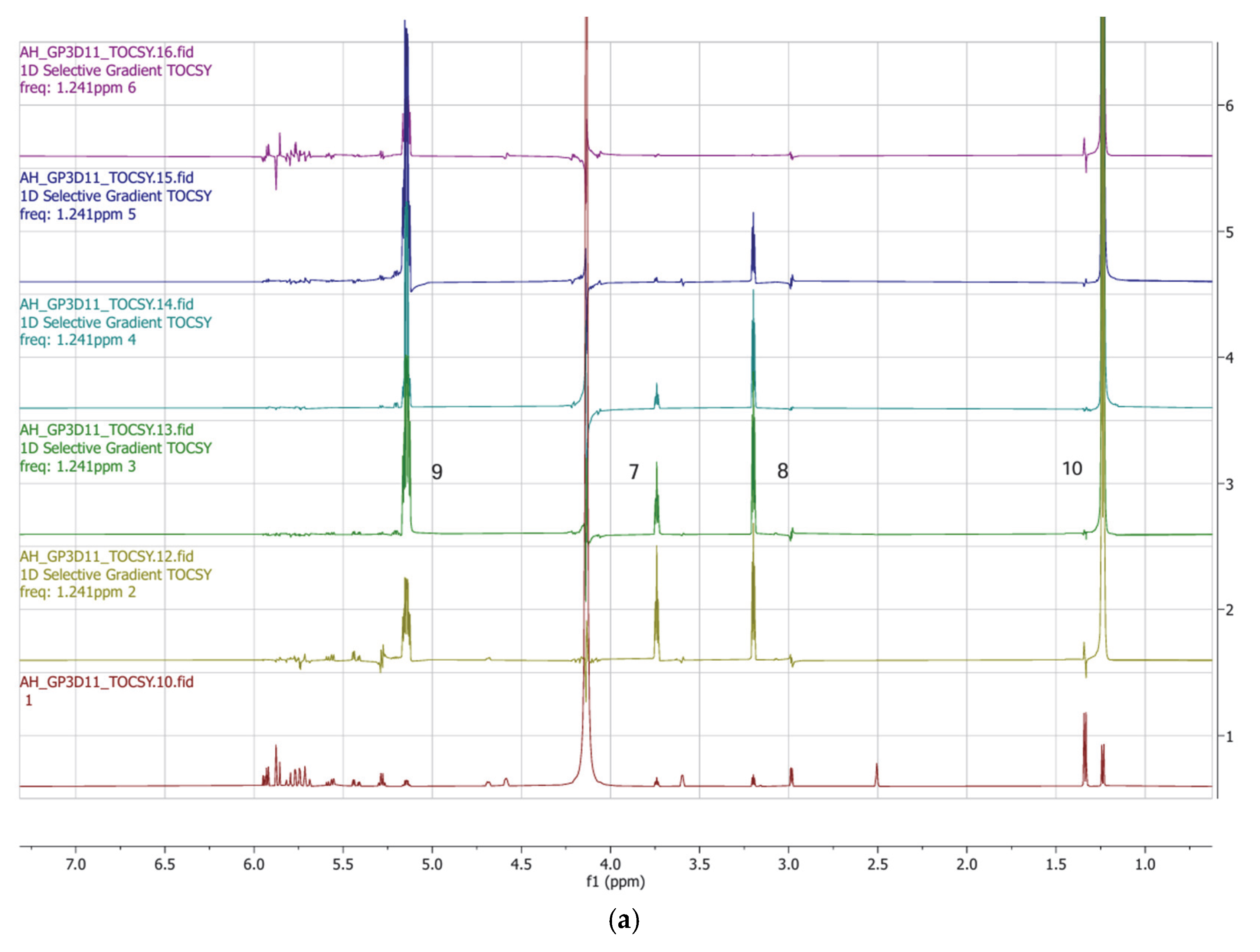
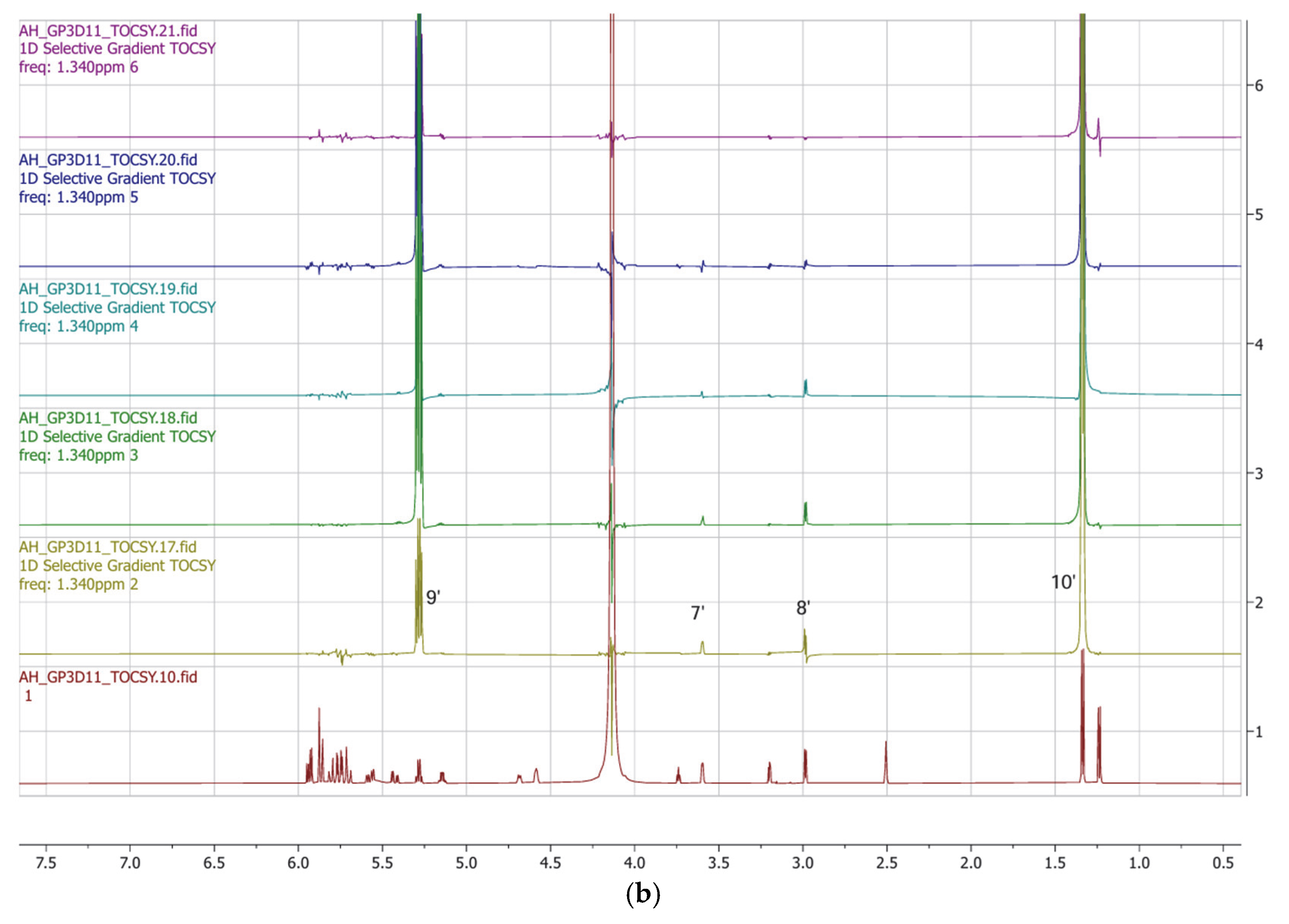
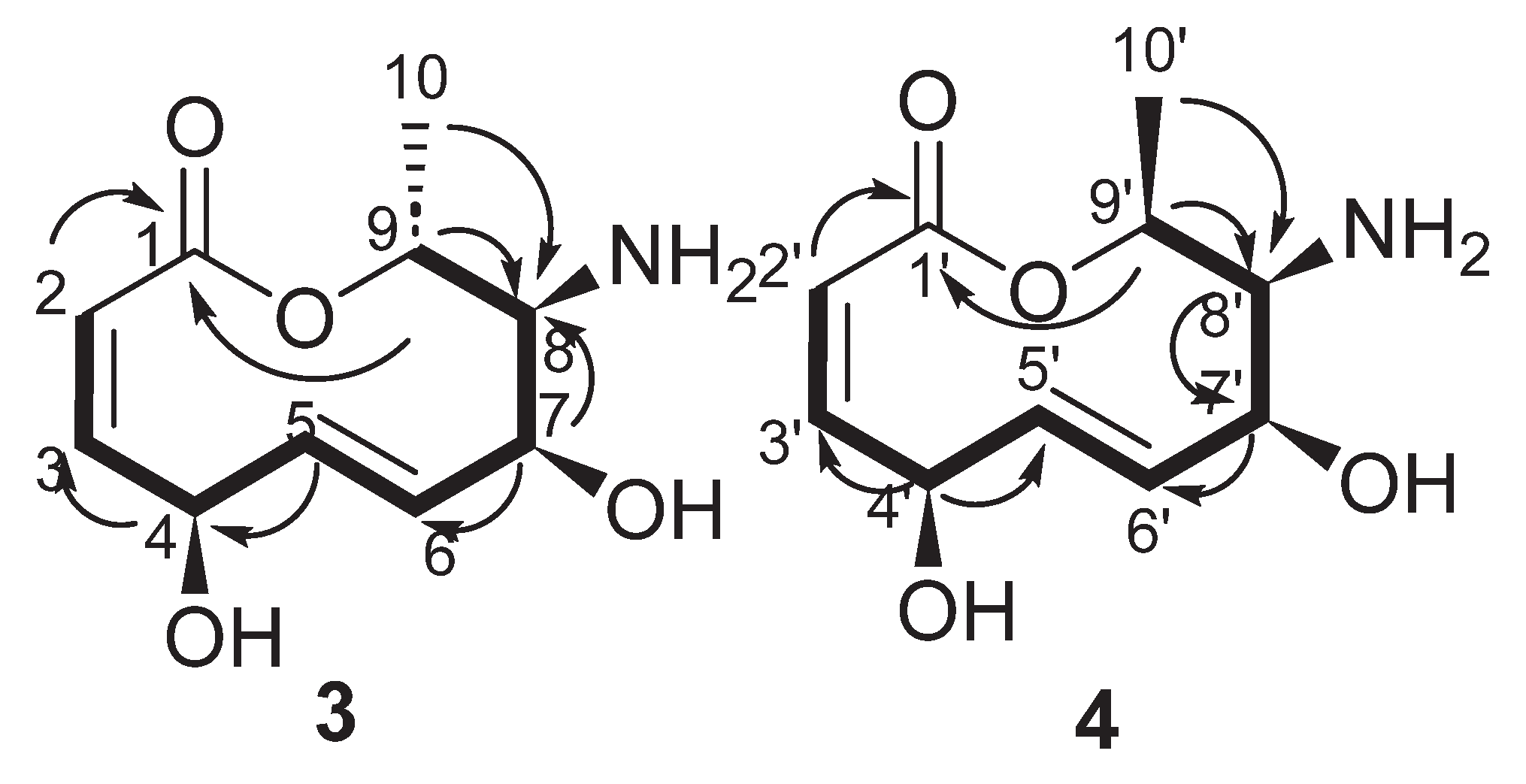
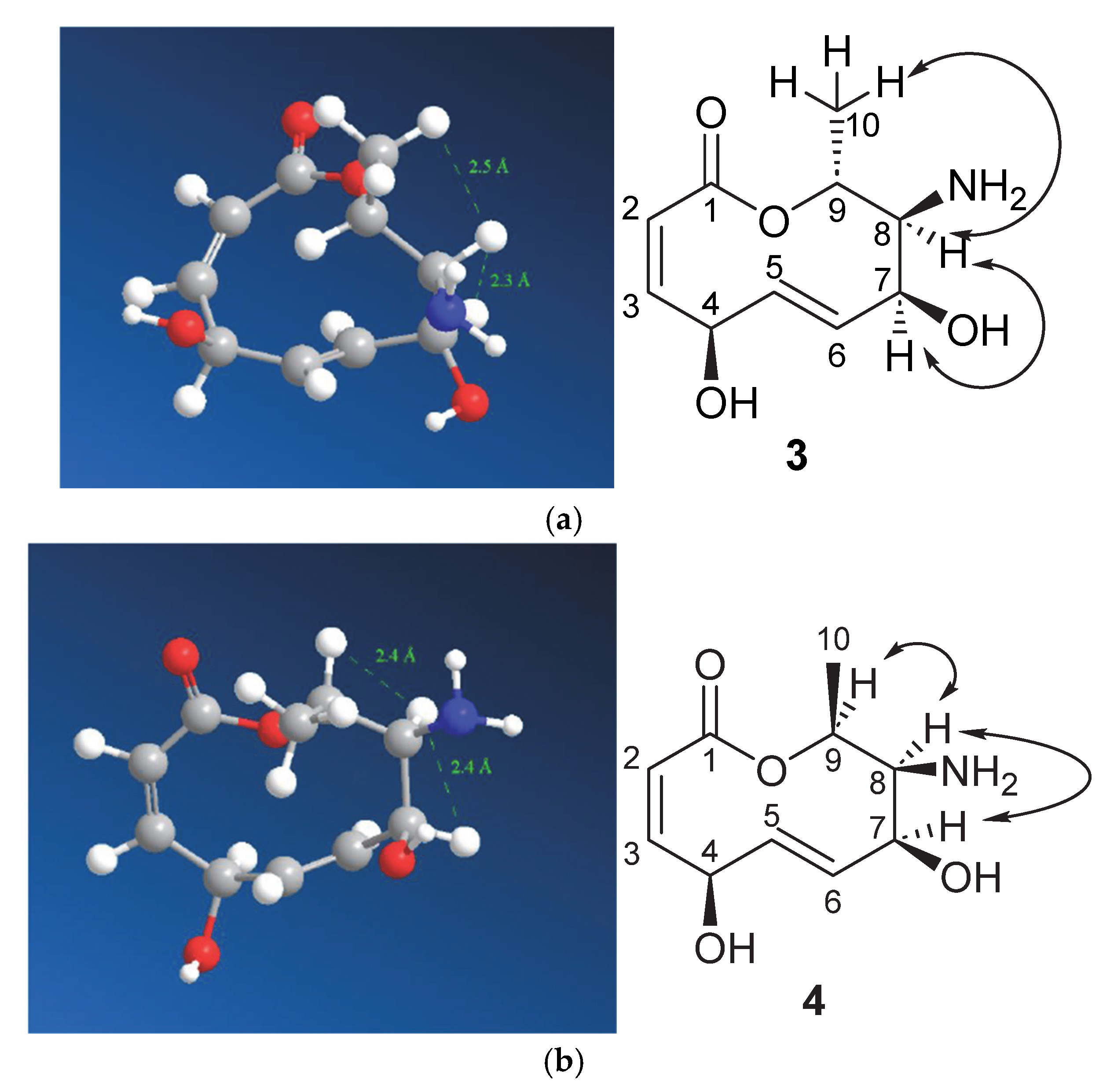
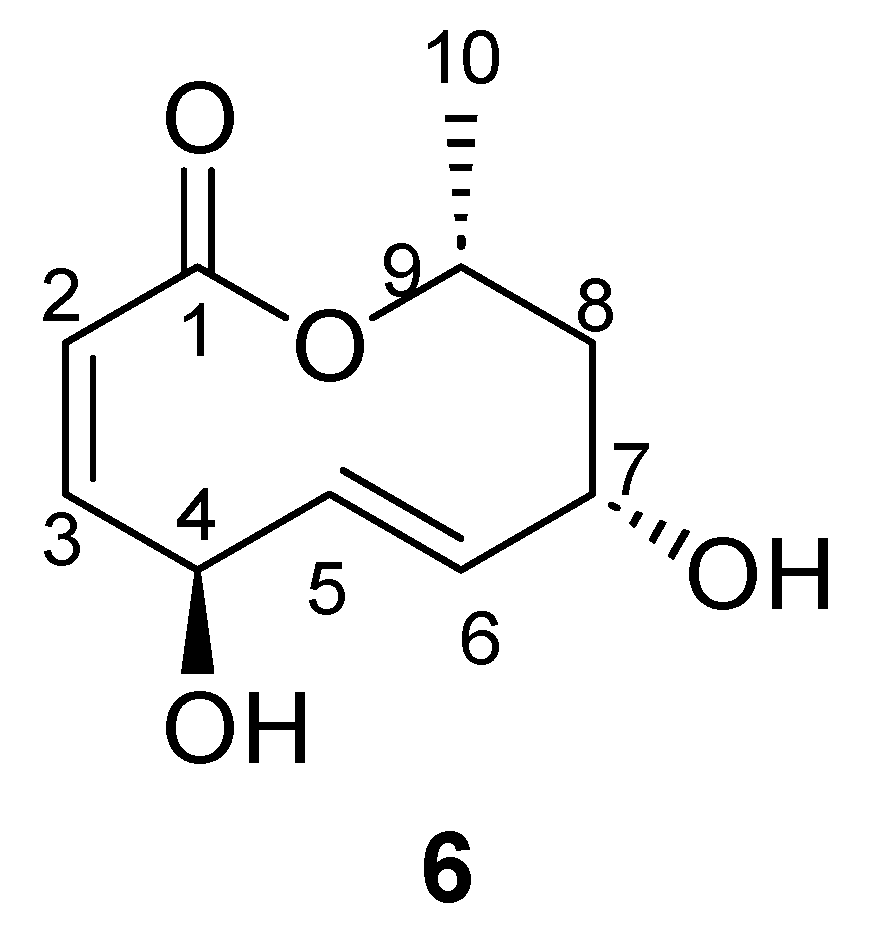
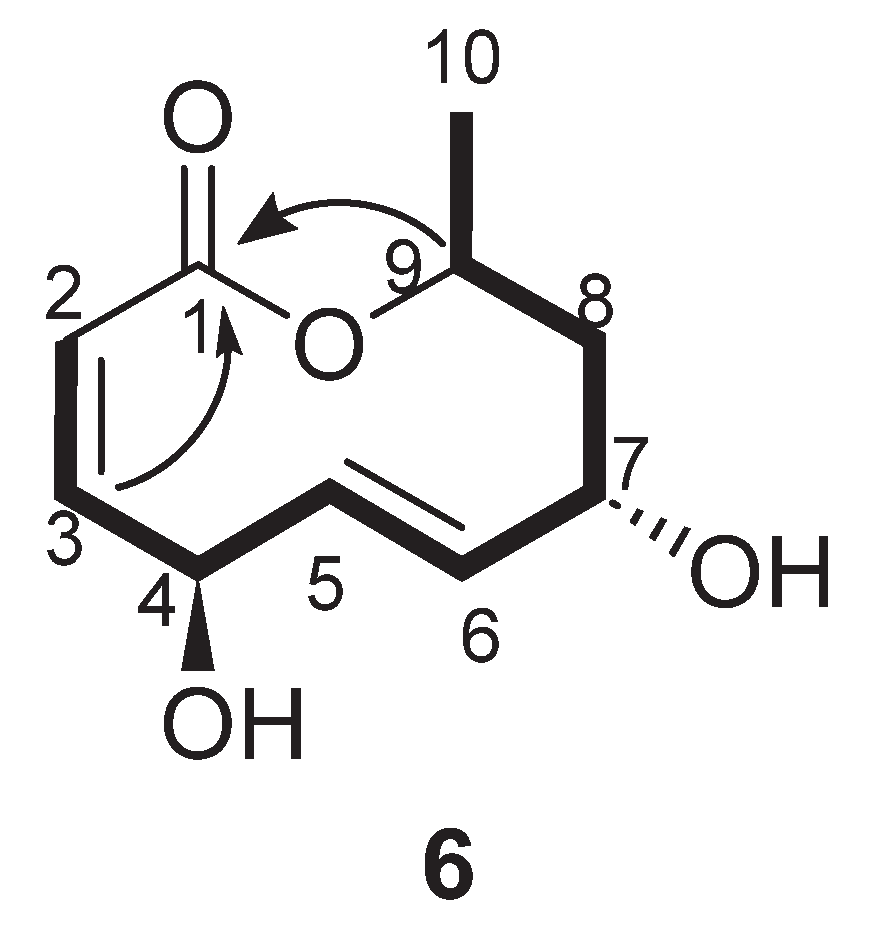
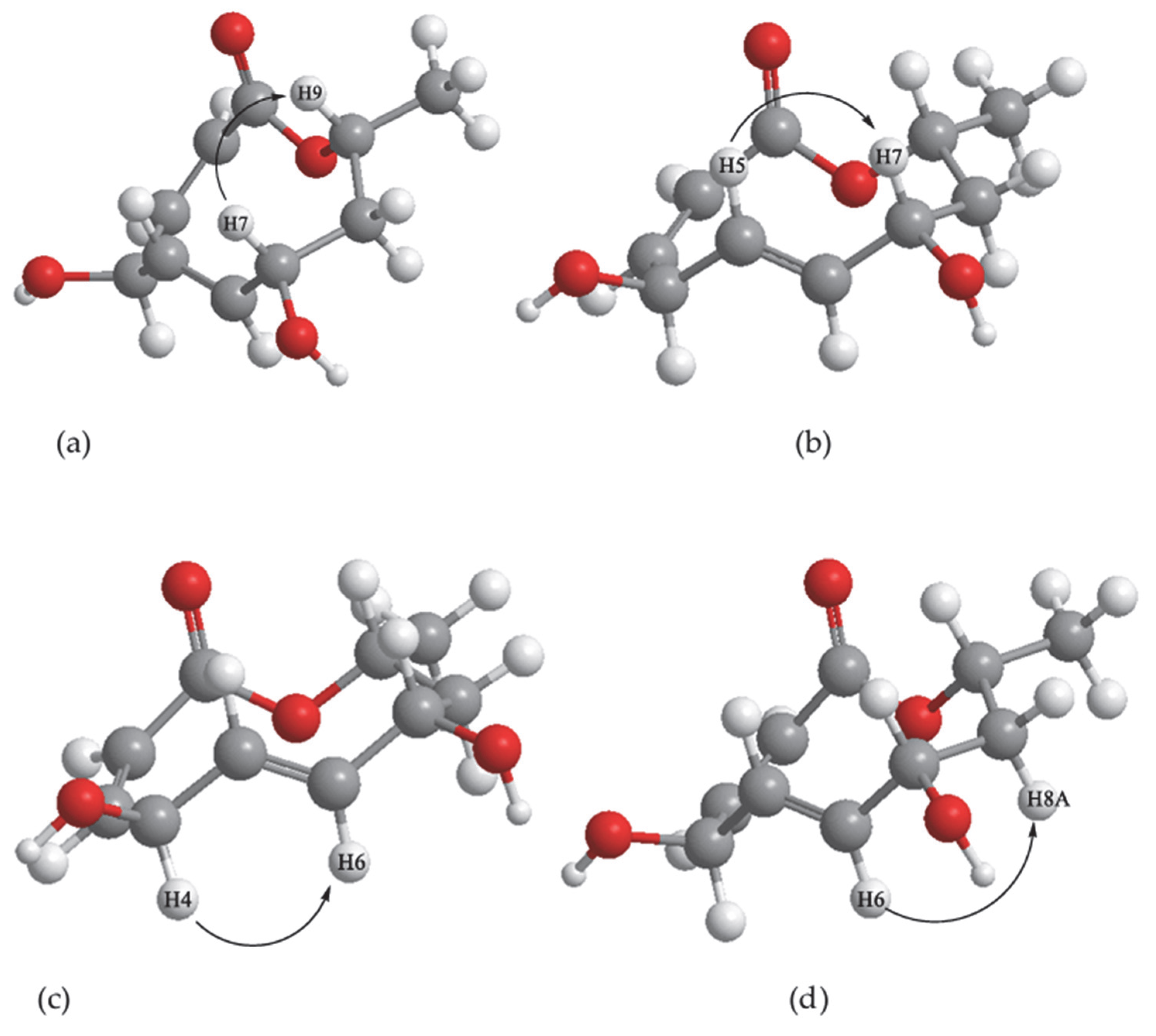
| Position | δ13C, Type | δ1H, Multiplicity (J in Hz) * | COSY (H→H) | HMBC (H→C) | Reference δ13C, Type ** [10] | Reference δ1H, Multiplicity (J in Hz) *** [10] |
|---|---|---|---|---|---|---|
| 1 | 167.8, C | 167.8, C | ||||
| 2 | 120.7, CH | 5.85, m | 3, 4 | 1, 4 | 122.4, CH | 5.84, dd (12.6, 1.5) |
| 3 | 138.6, CH | 5.78, m | 2, 4 | 1, 4 | 136.8, CH | 5.88, dd (12.6, 3.0) |
| 4 | 70.4, CH | 4.70, d (7.1) | 5, 6 | 3, 5, 6 | 71.2, CH | 5.01, d (7.5) |
| 5 | 133.5, CH | 5.61, dd (17.5, 7.2) | 4, 6 | 4 | 131.5, CH | 5.93, ddd (15.6, 7.5, 1.5) |
| 6 | 129.7, CH | 5.48, dd (17.5, 5.1) | 4, 5, 7 | 4 | 128.0, CH | 6.29, dd (15.6, 3.3) |
| 7 | 54.8, CH | 3.77, t (4.8) | 6, 8 | 5, 6, 8 | 58.8, CH | 4.87, d (5.3) |
| 7-OH | 4.00, m | 7 | ||||
| 8 | 55.5, CH | 3.23, t (4.1) | 7, 9 | 6, 7, 9 | 76.0, CH | 3.87, dd (5.3, 2.5) |
| 9 | 67.3, CH | 5.17, qd (6.8, 3.7) | 8, 10 | 1, 8, 10 | 68.1, CH | 5.64, dq (6.6, 2.5) |
| 10 | 15.7, CH3 | 1.27, d (7.0) | 9 | 8, 9 | 17.2, CH3 | 1.29, d (6.6) |
| Position | δ13C, Type | δ1H, Multiplicity (J in Hz) * | COSY (H→H) | HMBC (H→C) | Reference δ13C, Type ** [10] | Reference δ1H, Multiplicity (J in Hz) *** [10] |
|---|---|---|---|---|---|---|
| 1′ | 166.9, C | 167.8, C | ||||
| 2′ | 124.3, CH | 5.91, m | 4′ | 1′ | 122.4, CH | 5.84, dd (12.6, 1.5) |
| 3′ | 134.9, CH | 5.94, d (5.3) | 4′ | 1′ | 136.8, CH | 5.88, dd (12.6, 3.0) |
| 4′ | 65.1, CH | 4.49, br s | 3′, 5′ | 2′, 3′, 5′, 6′ | 71.2, CH | 5.01, d (7.5) |
| 5′ | 132.3, CH | 5.80, d (3.7) | 4′, 6′ | 6′ | 131.5, CH | 5.93, ddd (15.6, 7.5, 1.5) |
| 6′ | 119.4, CH | 5.78, m | 7′ | 5′ | 128.0, CH | 6.29, dd (15.6, 3.3) |
| 7′ | 55.3, CH | 3.61, m | 8′ | 5′, 6′ | 58.8, CH | 4.87, d (5.3) |
| 8′ | 55.8, CH | 3.00, dd (4.5, 0.8) | 7′ | 6′, 7′, 9′ | 76.0, CH | 3.87, dd (5.3, 2.5) |
| 9′ | 65.3, CH | 5.32, qd (6.8, 1.1) | 10′ | 1′, 8′ | 68.1, CH | 5.64, dq (6.6, 2.5) |
| 10′ | 18.2, CH3 | 1.37, d (7.0) | 9′ | 8′, 9′ | 17.2, CH3 | 1.29, d (6.6) |
| Position | δ13C, Type | δ1H, Multiplicity (J in Hz) | ROESY (H→H) | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 168.5, C | |||
| 2 | 121.6, CH | 5.80, dd (12.3, 2.0) | 4 | 1,3, 4, 5 |
| 3 | 138.7, CH | 5.85, dd (12.3, 2.9) | 4 | 5 |
| 4 | 71.7, CH | 4.80, d (6.5) | 2,3,5 | 2, 3, 6 |
| 4-OH | 4.58 brs | |||
| 5 | 133.8, CH | 5.69, ddd (17.3, 7.5, 1.2) | 4, 6, 7 | 7 |
| 6 | 130.6, CH | 5.52, ddd (17.3, 5.3, 1.0) | 4, 5, 7 | 4 |
| 7 | 55.3, CH | 3.72, td (5.5, 1.4) | 5, 6, 8 | 5, 6 |
| 8 | 55.8, CH | 3.20, t (4.0) | 7, 10 | 7, 9 |
| 9 | 67.8, CH | 5.22, qd (6.7, 3.6) | 10 | 10 |
| 10 | 15.7, CH3 | 1.32, d (6.8) | 9 | 9 |
| Position | δ13C, Type | δ1H, Multiplicity (J in Hz) | ROESY (H→H) | HMBC (H→C) |
|---|---|---|---|---|
| 1′ | 167.5, C | |||
| 2′ | 125.1, CH | 5.87, dd (12.1, 1.7) | 3′ | 1′,3′, 4′ |
| 3′ | 135.1, CH | 6.01, dd (12.1, 6.1) | 2′ | 1′, 2′, 5′ |
| 4′ | 66.5, CH | 4.71, brs | 5′ | 2′, 5′, 6′ |
| 4′-OH | 4.30 brs | 4′ | ||
| 5′ | 132.9, CH | 5.95, ddd (15.6, 3.1, 1.1) | 4′ | 2′, 4′, 6′ |
| 6′ | 120.3, CH | 5.86, m | 7′ | 5′ |
| 7′ | 55.9, CH | 3.58, m | 6′, 8′ | 5′, 6′. 8′ |
| 8′ | 56.6, CH | 2.99, dd (4.4, 1.1) | 7′, 9′ | 5′ |
| 9′ | 65.9, CH | 5.38, qd (6.8, 1.2) | 8′, 10′ | 1′, 8′, 10′ |
| 10′ | 18.3, CH3 | 1.42, d (6.8) | 9′ | 9′ |
| Position | δ13C, Type | δ1H, Multiplicity (J in Hz) * | COSY (H→H) | HMBC (H→C) | Reference δ13C, Type ** [11] | Reference δ1H, Multiplicity (J in Hz) ** [11] |
|---|---|---|---|---|---|---|
| 1 | 168.4, C | 170.0, C | ||||
| 2 | 121.5, CH | 5.84 dd (12.6, 1.8) | 3, | 1, 3, 4, 5 | 123.7, CH | 5.85, dd (1.5, 12.3) |
| 3 | 138.2, CH a | 5.76 dd (12.6, 3.2) | 2, 4 | 1, 4, 5 | 138.7, CH | 5.83, dd (3.5, 12.3) |
| 4 | 70.6, CH | 4.56 m | 3, 5 | 3, 5, 6 | 73.0, CH | 4.68, dd (3.5, 7.3) |
| 5 | 129.6, CH | 5.43 m | 4, 6 | 3, 4, 6, 7 | 131.8, CH | 5.61, dd (7.3, 15.8) |
| 6 | 138.2, CH a | 5.44 m | 5, 7 | 4, 5, 7, 8 | 139.4, CH | 5.56, dd (7.5, 15.8) |
| 7 | 71.2, CH | 3.97 ddd (11.0, 7.8, 3.3) | 6, 8 | 5 | 73.6, CH | 4.12, ddd (2.5, 7.5, 11.4) |
| 8 | 43.5, CH2 | A 1.78 mB 1.58, dt (13.9, 11.1) | 8B7, 8A, 9 | 6, 7, 9, 10 | 44.7, CH | A 1.71, dt (14.0, 11.4)B 1.87, dt (14.0, 2.5) |
| 9 | 68.9, CH | 5.11 m | 8B, 10 | 1, 7, 8, 10 | 70.9, CH | 5.25, ddq (2.5, 11.4, 6.7) |
| 10 | 21.7, CH3 | 1.14 d (6.3) | 9 | 7, 8, 9 | 22.4, CH3 | 1.22, d (6.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haj Hasan, A.; Preet, G.; Astakala, R.V.; Al-Furayh, M.; Oluwabusola, E.T.; Ebel, R.; Jaspars, M. Isolation and Characterisation of Two New Lactones from the Atacama Desert-Derived Fungus Chrysosporium merdarium. Chemistry 2025, 7, 101. https://doi.org/10.3390/chemistry7030101
Haj Hasan A, Preet G, Astakala RV, Al-Furayh M, Oluwabusola ET, Ebel R, Jaspars M. Isolation and Characterisation of Two New Lactones from the Atacama Desert-Derived Fungus Chrysosporium merdarium. Chemistry. 2025; 7(3):101. https://doi.org/10.3390/chemistry7030101
Chicago/Turabian StyleHaj Hasan, Ahlam, Gagan Preet, Rishi Vachaspathy Astakala, Meshari Al-Furayh, Emmanuel Tope Oluwabusola, Rainer Ebel, and Marcel Jaspars. 2025. "Isolation and Characterisation of Two New Lactones from the Atacama Desert-Derived Fungus Chrysosporium merdarium" Chemistry 7, no. 3: 101. https://doi.org/10.3390/chemistry7030101
APA StyleHaj Hasan, A., Preet, G., Astakala, R. V., Al-Furayh, M., Oluwabusola, E. T., Ebel, R., & Jaspars, M. (2025). Isolation and Characterisation of Two New Lactones from the Atacama Desert-Derived Fungus Chrysosporium merdarium. Chemistry, 7(3), 101. https://doi.org/10.3390/chemistry7030101







